Antibacterial Activity of TiO2- and ZnO-Decorated with Silver Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Silver-Decorated Nanoparticles
2.3. Characterization
2.4. Antibacterial Tests
3. Results and Discussions
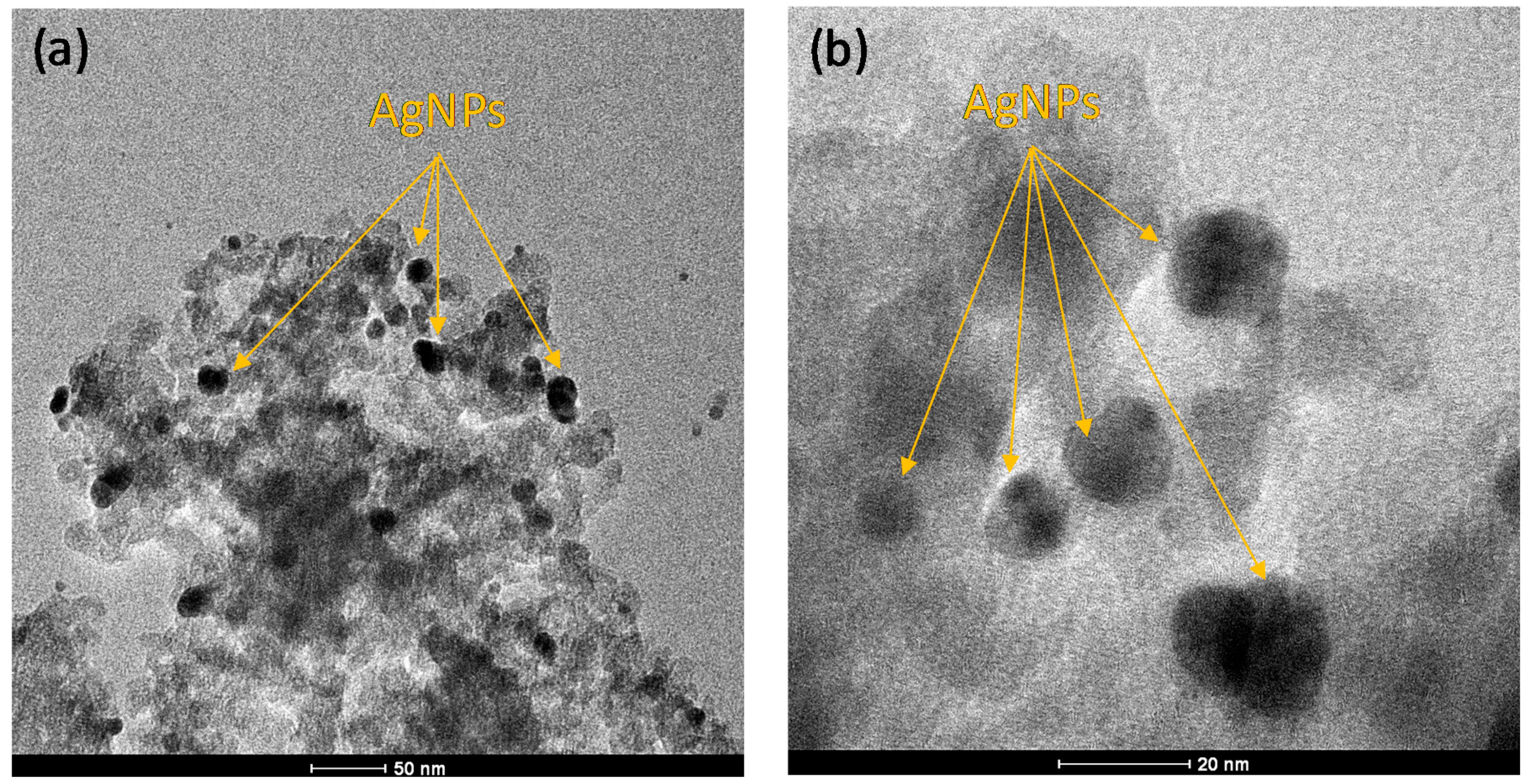
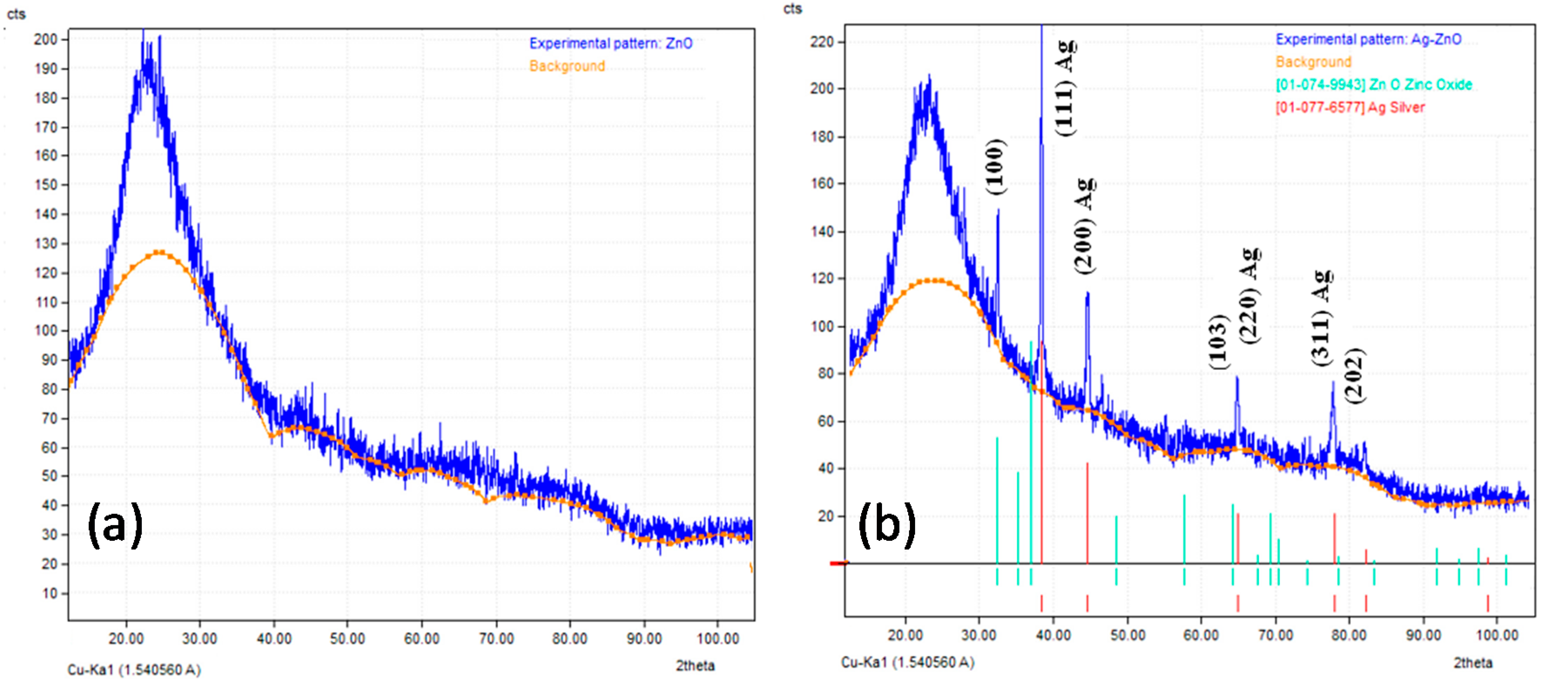
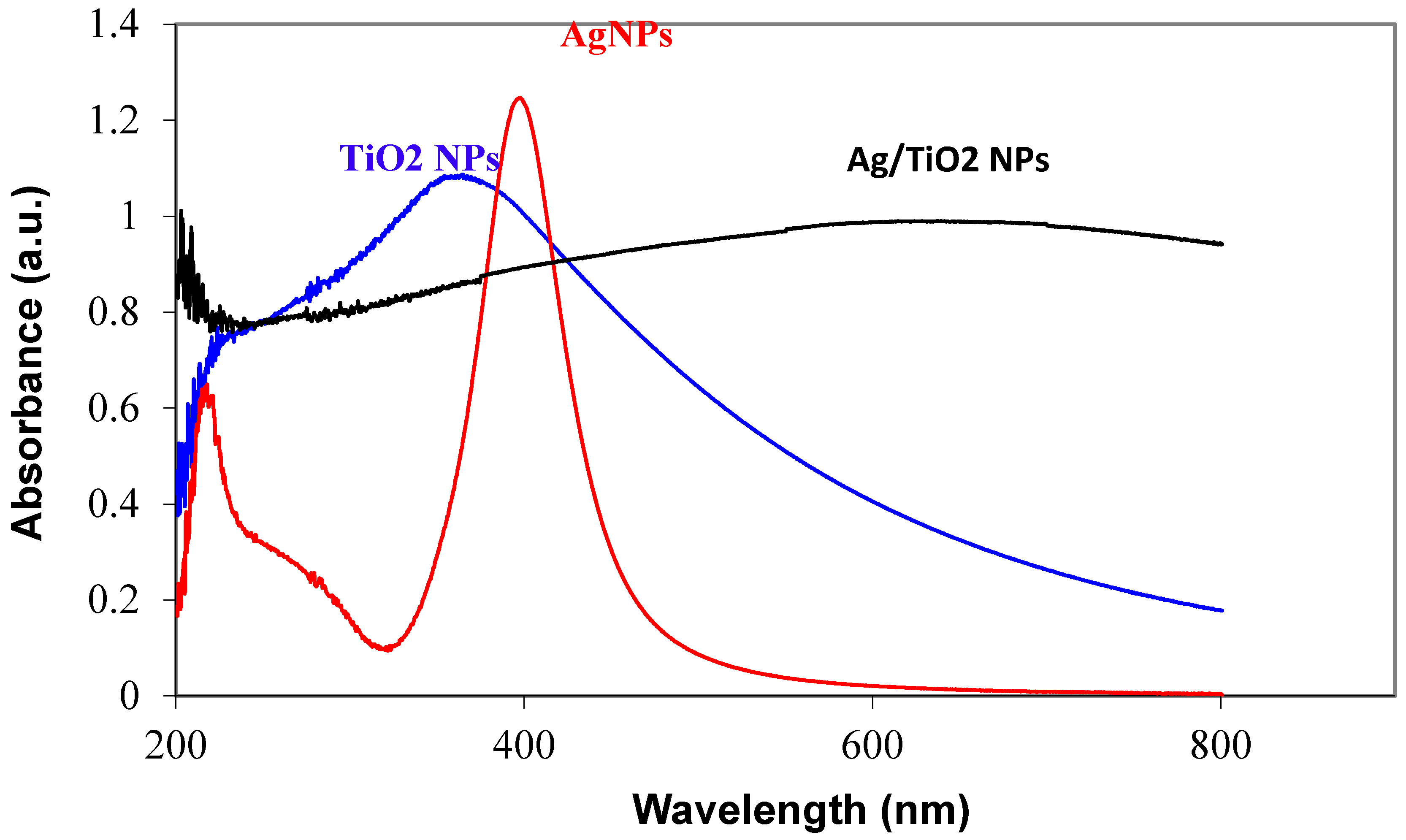
3.1. Characterization of Prepared Ag/TiO2 and Ag/ZnO Nanoparticles
3.2. Antibaterial Tests
3.2.1. TiO2 and Ag/TiO2 Nanoparticles
3.2.2. ZnO and Ag/ZnO Nanoparticles
4. Conclusions
- Silver-decorated oxide nanoparticles were successfully prepared using sodium borohydride as a reducing agent, with the weight ratio of Ag precursors:oxide nanoparticles = 1:30.
- The TEM images indicated that AgNPs (5–10 nm) were deposited on the surface of nano-TiO2 particles (30–60 nm), whereas the bigger AgNPs (<20 nm) were dispersed on the surface of nano-ZnO particles (30–50 nm). XRD patterns confirmed the presence of AgNPs in both Ag-decorated TiO2 and Ag-decorated ZnO nanoparticles.
- UV-vis spectra indicated that the hybridization of Ag and oxide nanoparticles led to a shift in the absorption edge of oxide nanoparticles to the lower energy region (visible region).
- The antibacterial tests indicated that both oxide nanoparticles did not exhibit inhibitory against bacteria, with or without light irradiation. However, the presence of AgNPs in their hybrids (at a concentration <40 mg/mL) exhibited higher inhibition zones under light irradiation, as compared to that in dark. At a high concentration of 40 mg/mL, the antibacterial behavior of these nanohybrids under light irradiation is similar to that in dark, indicating the dominated contribution of AgNPs to the antibacterial activity of these nanohybrids (at this high concentration).
- In the comparative study, under light irradiation at a low concentration (8 mg/mL), Ag/ZnO nanohybrids exhibited higher antibacterial activity against both bacteria than the Ag–Ag/TiO2 nanohybrids.
Author Contributions
Funding
Conflicts of Interest
References
- Rai, V.R.; Bai, A.J. Nanoparticles and their potential application as antimicrobials. In Science against microbial pathogens: Communicating current research and technological advances, Formatex, Microbiology Series No.3; Méndez-Vilas, A., Ed.; Elsevier: Badajoz, Spain, 2011; Volume 1, pp. 97–209. [Google Scholar]
- BECON. Nanoscience and Nanotechnology Symposium Report; National Institutes of Health Bioengineering Consortium: Bethesda, MD, USA, June 2000. Available online: http://www.uta.edu/rfmems/060515-NSF-NUE/Info/biomed/nanotechsympreport.pdf (accessed on 10 June 2019).
- Rtimi, S.; Nadtochenko, V.; Khmel, I.; Konstantinidis, S.; Britun, N.; Kiwi, J. Monitoring the energy of the metal ion-content plasma-assisted deposition and its implication for bacterial inactivation. Appl. Sur. Sci. 2019, 467, 749–752. [Google Scholar] [CrossRef]
- Oesterling, E.; Chopra, N.; Gavalas, V.; Arzuaga, X.; Lim, E.J.; Sultana, R.; Butterfield, D.A.; Bachas, L.; Hennig, B. Alumina nanoparticles induce expression of endothelial cell adhesion molecules. Toxicol. Lett. 2008, 178, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Bakthavatchalu, V.; Tseng, M.T.; Wu, P.; Florence, R.L.; Grulke, E.A.; Yokel, R.A.; Dhar, S.K.; Yang, H.-S.; Chen, Y.; et al. Interactions between SIRT1 and AP-1 reveal a mechanistic insight into the growth promoting properties of alumina (Al2O3) nanoparticles in mouse skin epithelial cells. Carcinogenesis 2008, 29, 1920–1929. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-N.; Zhang, M.; Xia, L.; Zhang, J.; Xing, G. The Toxic Effects and Mechanisms of CuO and ZnO Nanoparticles. Materials 2012, 5, 2850–2871. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Möller, L. Copper Oxide Nanoparticles Are Highly Toxic: A Comparison between Metal Oxide Nanoparticles and Carbon Nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Kovochich, M.; Liong, M.; Mädler, L.; Gilbert, B.; Shi, H.; Yeh, J.I.; Zink, J.I.; Nel, A.E. Comparison of the Mechanism of Toxicity of Zinc Oxide and Cerium Oxide Nanoparticles Based on Dissolution and Oxidative Stress Properties. ACS Nano 2008, 2, 2121–2134. [Google Scholar] [CrossRef] [PubMed]
- Rtimi, S.; Dionysiou, D.; Pillai, S.C.; Kiwi, J. Advances in catalytic/photocatalytic bacterial inactivation by nano Ag and Cu coated surfaces and medical devices. Appl. Catal. B Environ. 2019, 240, 291–318. [Google Scholar] [CrossRef]
- Ren, G.; Hu, D.; Cheng, E.W.; Vargas-Reus, M.A.; Reip, P.; Allaker, R.P. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int. J. Antimicrob. Agents 2009, 33, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Niskanen, J.; Shan, J.; Tenhu, H.; Jiang, H.; Kauppinen, E.; Barranco, V.; Picó, F.; Yliniemi, K.; Kontturi, K. Synthesis of copolymer-stabilized silver nanoparticles for coating materials. Colloid Polym. Sci. 2010, 288, 543–553. [Google Scholar] [CrossRef]
- Guggenbichler, J.-P.; Böswald, M.; Lugauer, S.; Krall, T. A new technology of microdispersed silver in polyurethane induces antimicrobial activity in central venous catheters. Infect 1999, 27, S16–S23. [Google Scholar] [CrossRef]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.; Memic, A. Size-dependent antimicrobial properties of CuO nanoparticles against Gram-positive and -negative bacterial strains. Int. J. Nanomed. 2012, 7, 3527–3535. [Google Scholar] [CrossRef] [PubMed]
- Allaker, R.P. The use of nanoparticles to control oral biofilm formation. J. Dent. Res. 2010, 89, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.L.; Albuquerque, A.J.R.; Sampaio, F.C.; Keyson, D. Nanomaterials with Antimicrobial Properties: Applications in Health Sciences. In Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education; Méndez-Vilas, A., Ed.; Formatex: Badajoz, Spain, 2013. [Google Scholar]
- Huang, H.H.; Ni, X.P.; Loy, G.L.; Chew, C.H.; Tan, K.L.; Loh, F.C.; Deng, J.F.; Xu, G.Q. Photochemical Formation of Silver Nanoparticles in Poly(N-vinylpyrrolidone). Langmuir 1996, 12, 909–912. [Google Scholar] [CrossRef]
- Weir, E.; Lawlor, A.; Whelan, A.; Regan, F. The use of nanoparticles in anti-microbial materials and their characterization. Analyst 2008, 133, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Shenashen, M.A.; El-Safty, S.A.; Elshehy, E.A. Synthesis, morphological control, and properties of silver nanoparticles in potential applications. Part. Part. Syst. Charact. 2014, 31, 293–316. [Google Scholar] [CrossRef]
- Li, Q.; Mahendra, S.; Lyon, D.Y.; Brunet, L.; Liga, M.V.; Li, D.; Alvarez, P.J. Antimicrobial nanomaterials for water disinfection and microbial control: Potential applications and implications. Water Res. 2008, 42, 4591–4602. [Google Scholar] [CrossRef]
- Nguyen-Tri, P.; Nguyen, V.T.; Nguyen, T.A. Biological activity and nanostructuration of Fe3O4-Ag/polyethyelene nanocomposites. J. Compos. Sci. 2019, 3. [Google Scholar] [CrossRef]
- Hussain, S.M.; Hess, K.L.; Gearhart, J.M.; Geiss, K.T.; Schlager, J.J. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol. Vitr. 2005, 19, 975–983. [Google Scholar] [CrossRef]
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Dhanalekshmi, K.; Van Thang Nguyen, I.; Magesan, P. Chapter 26: Nanosilver loaded oxide nanoparticles for antibacterial application. In Smart Nanocontainers: Fundamentals and Emerging Applications; Nguyen-Tri, P., Do, T.-O., Nguyen, T.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128167700. [Google Scholar]
- Akhavan, O. Lasting antibacterial activities of Ag–TiO2/Ag/a-TiO2 nanocomposite thin film photocatalysts under solar light irradiation. J. Colloid Interface Sci. 2009, 336, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Gholap, H.; Warule, S.; Sangshetti, J.; Kulkarni, G.; Banpurkar, A.; Satpute, S.; Patil, R. Hierarchical nanostructures of Au@ZnO: Antibacterial and antibiofilm agent. Appl. Microbiol. Biotechnol. 2016, 100, 5849–5858. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, Y.; Hara, K.; Katoh, R.; Tachiya, M. A Furub Femtosecond visible-to-IR spectroscopy of TiO2 nanocrystalline films: Elucidation of the electron mobility before deep trapping. J. Phys. Chem. C 2009, 113, 11741–11746. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, J.; Wang, C.; Li, S.; Lai, Y.-K.; Chen, H.; Lin, C. Ultrasound aided photochemical synthesis of Ag loaded TiO2 nanotube arrays to enhance photocatalytic activity. J. Hazard. Mater. 2009, 171, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Abdulla-Al-Mamun, M.; Kusumoto, Y.; Zannat, T.; Islam, M.S. Synergistic enhanced photocatalytic and photothermal activity of Au@TiO2 nanopellets against human epithelial carcinoma cells. Phys. Chem. Chem. Phys. 2011, 13, 21026. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.S.; Choi, S.H.; Kim, H.G.; Lee, J.S. Location and State of Pt in Platinized CdS/TiO2 Photocatalysts for Hydrogen Production from Water under Visible Light. J. Phys. Chem. C 2008, 112, 17200–17205. [Google Scholar] [CrossRef]
- Sarkart, D.; Ghosh, C.K.; Mukherjee, S.; Chattopadhyay, K.K. Three dimensional Ag2O/TiO2 type-II (p-n) nanoheterojunctions for superior photocatalytic activity. ACS Appl. Mater. Interfaces 2013, 5, 331–337. [Google Scholar] [CrossRef]
- Ji, Y.F.; Guo, W.; Chen, H.H.; Zhang, L.S.; Chen, S.; Hua, M.T.; Long, Y.H.; Chen, Z. Surface Ti3+/Ti4+ redox shuttle enhancing photocatalytic H2 production in ultrathin TiO2 nanosheets/CdSe quantum dots. J. Phys. Chem. C 2015, 119, 27053–27059. [Google Scholar] [CrossRef]
- Deng, Q.; Tang, H.; Liu, G.; Song, X.; Xu, G.; Li, Q.; Ng, D.H.; Wang, G. The fabrication and photocatalytic performances of flower-like Ag nanoparticles/ZnO nanosheets-assembled microspheres. Appl. Surf. Sci. 2015, 331, 50–57. [Google Scholar] [CrossRef]
- Liang, Y.M.; Guo, N.; Li, L.L.; Li, R.Q.; Ji, G.J.; Gan, S.C. Fabrication of porous 3D flower-like Ag/ZnO heterostructure composites with enhanced photocatalytic performance. Appl. Surf. Sci. 2015, 332, 32–39. [Google Scholar] [CrossRef]
- Ubonchonlakate, K.; Sikong, L.; Saito, F.P. aeruginosa inactivation with silver and nickel doped TiO2 films coated on glass fiber roving. Adv. Mater. Res. 2011, 150–151, 1726–1731. [Google Scholar]
- Umebayashi, T.; Yamaki, T.; Tanaka, S.; Asai, K. Visible light-induced degradation of methylene blue on S-doped TiO2. Chem. Lett. 2003, 32, 330–331. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A Review on the Visible Light Active Titanium Dioxide Photocatalysts for Environmental Applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Mohapatra, S.; Nguyen, T.A.; Nguyen-Tri, P. Noble Metal-Metal Oxide Hybrid Nanoparticles: Fundamentals and Applications; Elsevier: Amsterdam, The Netherlands, 2018; Volume 1. [Google Scholar]
- Fageria, P.; Gangopadhyay, S.; Pande, S. Synthesis of ZnO/Au and ZnO/Ag nanoparticles and their photocatalytic application using UV and visible light. RSC Adv. 2014, 4, 24962–24972. [Google Scholar] [CrossRef]
- Xu, C.; Chen, P.; Liu, J.; Yin, H.; Gao, X.; Mei, X. Fabrication of visible-light-driven Ag/TiO2 heterojunction composites induced by shock wave. J. Alloy. Compd. 2016, 679, 463–469. [Google Scholar] [CrossRef]
- Xu, F.; Mei, J.; Zheng, M.; Bai, D.; Wu, D.; Gao, Z.; Jiang, K. Au nanoparticles modified branched TiO2 nanorod array arranged with ultrathin nanorods for enhanced photoelectrochemical water splitting. J. Alloy. Compd. 2017, 693, 1124–1132. [Google Scholar] [CrossRef]
- Chang, Y.; Xu, J.; Zhang, Y.; Ma, S.; Xin, L.; Zhu, L.; Xu, C. Optical Properties and Photocatalytic Performances of Pd Modified ZnO Samples. J. Phys. Chem. C 2009, 113, 18761–18767. [Google Scholar] [CrossRef]
- Tri, P.N.; Nguyen, T.A.; Nguyen, T.H.; Carriere, P. Antibacterial Behavior of Hybrid Nanoparticles (Chapter 7). In Noble Metal-Metal Oxide Hybrid Nanoparticles: Fundamentals and Applications; Mohapatra, S., Nguyen, T.H., Nguyen-Tri, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 141–155. [Google Scholar] [CrossRef]
- Standard and White LED Basics and Operation. Available online: https://www.maximintegrated.com/en/app-notes/index.mvp/id/3070 (accessed on 10 June 2019).
- Reyes-Coronado, D.; Gattorno, G.R.; E Espinosa-Pesqueira, M.; Cab, C.; De Coss, R.; Oskam, G. Phase-pure TiO2 nanoparticles: Anatase, brookite and rutile. Nanotechnology 2008, 19. [Google Scholar] [CrossRef]
- Alshamsi, H.A.H.; Hussein, B.S. Hydrothermal Preparation of Silver Doping Zinc Oxide Nanoparticles: Studys, Characterization and Photocatalytic Activity. Orient. J. Chem. 2018, 34, 1898–1907. [Google Scholar] [CrossRef]
- Anandalakshmi, K.; Venugobal, J.; Ramasamy, V. Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl. Nanosci. 2016, 6, 399–408. [Google Scholar] [CrossRef]
- Kuriakose, S.; Choudhary, V.; Satpati, B.; Mohapatra, S.; Xu, R. Enhanced photocatalytic activity of Ag–ZnO hybrid plasmonic nanostructures prepared by a facile wet chemical method. Beilstein J. Nanotechnol. 2014, 5, 639–650. [Google Scholar] [CrossRef]
- Kuriakose, S.; Choudhary, V.; Satpati, B.; Mohapatra, S. Facile synthesis of Ag-ZnO hybrid nanospindles for highly efficient photocatalytic degradation of methyl orange. Phys. Chem. Chem Phys. 2014, 16, 17560–17568. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Tatsuma, T. Plasmon-induced photoelectrochemistry at metal nanoparticles supported on nanoporous TiO2. Chem. Commun. 2004, 16, 1810. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Hashimoto, K.; Itoh, K.; Kubota, Y.; Fujishima, A. Photokilling of malignant cells with ultrafine TiO2 powder. Bull. Chem. Soc. Jpn. 1991, 64, 1268–1273. [Google Scholar] [CrossRef]
- Ubonchonlakate, K.; Sikong, L.; Saito, F. Photocatalytic disinfection of P. aeruginosa bacterial Ag-doped TiO2 film. Procedia Eng. 2012, 32, 656–662. [Google Scholar] [CrossRef]
- Sakthivel, S.; Shankar, M.; Palanichamy, M.; Arabindoo, B.; Bahnemann, D.; Murugesan, V. Enhancement of photocatalytic activity by metal deposition: Characterisation and photonic efficiency of Pt, Au and Pd deposited on TiO2 catalyst. Water Res. 2004, 38, 3001–3008. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Wang, Q.; Zhang, X.; Wang, Z.; Xia, W.; Dong, Y. Synthesis of Ag/TiO2 Core/Shell Nanoparticles with Antibacterial Properties. Bull. Korean Chem. Soc. 2011, 32, 2607–2610. [Google Scholar] [CrossRef]
- Dhanalekshmi, K.I.; Meen, K.S.; Ramesh, I. Synthesis and Characterization of Ag@TiO2 Core-shell nanoparticles and study of its antibacterial activity. Int. J. Nanotechnol. Appl. 2013, 3, 5–14. [Google Scholar]
- Zhang, H.; Chen, G. Potent Antibacterial Activities of Ag/TiO2 Nanocomposite Powders Synthesized by a One-Pot Sol−Gel Method. Environ. Sci. Technol. 2009, 43, 2905–2910. [Google Scholar] [CrossRef]
- Hidayati, N.; Barudin, A.; Sreekantan, S.; Thong, O.M.; Sahgal, G. Antibacterial Activity of Ag-TiO2 Nanoparticles with Various Silver Contents. Mater. Sci. Forum 2013, 756, 238–245. [Google Scholar]
- Jin, T.; Sun, D.; Su, J.Y.; Zhang, H.; Sue, H.J. Antimicrobial Efficacy of Zinc Oxide Quantum Dots against Listeria monocytogenes, Salmonella Enteritidis, and Escherichia coli O157:H7. J. Food Sci. 2009, 74, M46–M52. [Google Scholar] [CrossRef]
- Kumar, K.M.; Mandal, B.K.; Naidu, E.A.; Sinha, M.; Kumar, K.S.; Reddy, P.S. Synthesis and Characterization of Flower Shaped Zinc Oxide Nanostructures and Its Antimicrobial Activity. Spectrochim. Actapart A 2013, 104, 171–174. [Google Scholar] [CrossRef]
- Lipovsky, A.; Nitzan, Y.; Gedanken, A.; Lubart, R. Antifungal activity of ZnO nanoparticles—The role of ROS mediated cell injury. Nanotechnology 2011, 22, 105101. [Google Scholar] [CrossRef]
- Stoimenov, P.K.; Klinger, R.L.; Marchin, G.L.; Klabunde, K.J. Metal Oxide Nanoparticles as Bactericidal Agents. Langmuir 2002, 18, 6679–6686. [Google Scholar] [CrossRef]
- Ibănescu, M.; Muşat, V.; Textor, T.; Badilita, V.; Mahltig, B. Photocatalytic and antimicrobial Ag/ZnO nanocomposites for functionalization of textile fabrics. J. Alloy. Compd. 2014, 610, 244–249. [Google Scholar] [CrossRef]
- Nagaraju, G.; Udayabhanu; Shivaraj; Prashanth, S.A.; Shastri, M.; Yathish, K.V.; Anupama, C.; Rangappa, D. Electrochemical heavy metal detection, photocatalytic, photoluminescence, biodiesel production and antibacterial activities of Ag–ZnO nanomaterial. Mater. Res. Bull. 2017, 94, 54–63. [Google Scholar] [CrossRef]
- Wei, Y.; Chong, Y.B.; Du, H.; Kong, J.; He, C. Loose Yarn of Ag-ZnO-PAN/ITO Hybrid Nanofibres: Preparation, Characterization and Antibacterial Evaluation. Mater. Des. 2018, 139, 153–161. [Google Scholar] [CrossRef]
- Phuong, N.-T.; Rtimi, S. Claudiane Ouellet Plamondon. In Nanomaterials Based Coatings: Fundamentals and Applications; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128158845. [Google Scholar]
- Tri, P.N.; Guinault, A.; Sollogoub, C. Élaboration et propriétés des composites polypropylène recyclé/fibres de bambou. Matériaux Tech. 2012, 100, 413–423. [Google Scholar]
- Azizi, S.; David, E.; Fréchette, M.F.; Nguyen-Tri, P.; Ouellet-Plamondon, C.M. Electrical and thermal conductivity of ethylene vinyl acetate composite with graphene and carbon black filler. Polym. Test. 2018, 72, 24–31. [Google Scholar] [CrossRef]
- Azizi, S.; David, E.; Fréchette, M.F.; Nguyen-Tri, P.; Ouellet-Plamondon, C.M. Electrical and thermal phenomena in low-density polyethylene/carbon black composites near the percolation threshold. J. Appl. Polym. Sci. 2018, 136, 47043. [Google Scholar] [CrossRef]
- Boukehili, H.; Tri, P.N. Helium gas barrier and water absorption behavior of bamboo fiber reinforced recycled polypropylene. J. Reinf. Plast. Compos. 2012, 31, 1638–1651. [Google Scholar] [CrossRef]
- Phuong, N.T.; Gilbert, V. Non-isothermal Crystallization Kinetics of Short Bamboo Fiber-reinforced Recycled Polypropylene Composites. J. Reinf. Plast. Compos. 2010, 29, 2576–2591. [Google Scholar] [CrossRef]
- Phuong, N.T.; Sollogoub, C.; Guinault, A. Relationship between fiber chemical treatment and properties of recycled pp/bamboo fiber composites. J. Reinf. Plast. Compos. 2010, 29, 3244–3256. [Google Scholar] [CrossRef]
- Tri, P.N.; Rtimi, S.; Nguyen, T.A.; Vu, M.T. Physics, Electrochemistry, Photochemistry, and Photoelectrochemistry of Hybrid Nanoparticles. In Noble Metal-Metal Oxide Hybrid Nanoparticles; Woodhead Publishing: Sawston, UK, 2019; pp. 95–123. [Google Scholar]
- Tri, P.N.; Ouellet-Plamondon, C.; Rtimi, S.; Assadi, A.A.; Nguyen, T.A. Methods for Synthesis of Hybrid Nanoparticles. In Noble Metal-Metal Oxide Hybrid Nanoparticles; Woodhead Publishing: Sawston, UK, 2019; pp. 51–63. [Google Scholar]
- Nguyen, T.V.; Tri, P.N.; Nguyen, T.D.; El Aidani, R.; Trinh, V.T.; Decker, C. Accelerated degradation of water borne acrylic nanocomposites used in outdoor protective coatings. Polym. Degrad. Stab. 2016, 128, 65–76. [Google Scholar] [CrossRef]
- Tri, P.N.; Prud’Homme, R.E. Crystallization and Segregation Behavior at the Submicrometer Scale of PCL/PEG Blends. Macromolecules 2018, 51, 7266–7727. [Google Scholar]
- Nguyen, T.P. Nanoscale analysis of the photodegradation of Polyester fibers by AFM-IR. J. Photochem. Photobiol. A Chem. 2018, 371, 196–204. [Google Scholar] [CrossRef]
- Tri, P.N.; Prud’homme, R.E. Nanoscale Lamellar Assembly and Segregation Mechanism of Poly(3-hydroxybutyrate)/Poly(ethylene glycol) Blends. Macromolecules 2018, 51, 181–188. [Google Scholar] [CrossRef]
- El Aidani, R.; Nguyen-Tri, P.; Malajati, Y.; Lara, J.; Vu-Khanh, T. Photochemical aging of an e-PTFE/NOMEX® membrane used in firefighter protective clothing. Polym. Degrad. Stab. 2013, 98, 1300–1310. [Google Scholar] [CrossRef]
- Zeb, G.; Tri, P.N.; Le, X.T.; Palacin, S. Pulse potential deposition of thick polyvinylpyridine-like film on the surface of titanium nitride. RSC Adv. 2016, 6, 80825–80829. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Le, X.H.; Dao, P.H.; Decker, C.; Nguyen-Tri, P. Stability of acrylic polyurethane coatings under accelerated aging tests and natural outdoor exposure: The critical role of the used photo-stabilizers. Prog. Org. Coat. 2018, 124, 137–146. [Google Scholar] [CrossRef]
- Nguyen-Tri, P.; Nguyen, T.H.; Ouellet Plamondon, C.; Vo Dai-Viet, N.; Nanda, S.; Mishra, A.; Chao, H.P.; Bajpai, A.K. Recent Progress in the Preparation, Properties and Applications of Superhydrophobic Nano-based Coatings and Surfaces: A review. Prog. Org. Coat. 2019, 132, 235–256. [Google Scholar] [CrossRef]
- Nguyen-Tri, P.; Altiparmak, F.; Nguyen, N.; Tuduri, L.; Ouellet-Plamondon, C.M.; Prud’Homme, R.E. Robust Superhydrophobic Cotton Fibers Prepared by Simple Dip-Coating Approach Using Chemical and Plasma-Etching Pretreatments. ACS Omega 2019, 4, 7829–7837. [Google Scholar] [CrossRef]
- Nguyen-Tri, P.; Triki, E.; Nguyen, T.A. Butyl Rubber-Based Composite: Thermal Degradation and Prediction of Service Lifetime. J. Compos. Sci. 2019, 3, 48. [Google Scholar] [CrossRef]
- Nguyen-Tri, P.; Nguyen, T.A.; Carriere, P.; Xuan, C.N. Nanocomposite Coatings: Preparation, Characterization, Properties, and Applications. Int. J. Corros. 2018, 2018, 4749501. [Google Scholar] [CrossRef]







| Concentrations (mg/mL) | Inhibition Zone (mm) | |||
|---|---|---|---|---|
| Without Light Irradiation | Under Light Irradiation | |||
| TiO2 Nanoparticles | Ag-Decorated TiO2 Nanoparticles | TiO2 Nanoparticles | Ag-Decorated TiO2 Nanoparticles | |
| 8 | 0 | 0 | 0 | 0 |
| 16 | 0 | 0 | 0 | 2 |
| 40 | 0 | 4 | 0 | 4 |
| Concentrations (mg/mL) | Inhibition Zone (mm) | |||
|---|---|---|---|---|
| Without Light Irradiation | Under Light Irradiation | |||
| TiO2 Nanoparticles | Ag-Decorated TiO2 Nanoparticles | TiO2 Nanoparticles | Ag-Decorated TiO2 Nanoparticles | |
| 8 | 0 | 2 | 0 | 6 |
| 16 | 0 | 6 | 0 | 8 |
| 40 | 0 | 8 | 0 | 8 |
| Concentrations (mg/mL) | Inhibition Zone (mm) | |||
|---|---|---|---|---|
| Without Light Irradiation | With Light Irradiation | |||
| ZnO Nanoparticles | Ag-Decorated ZnO Nanoparticles | ZnO nanoparticles | Ag-Decorated ZnO Nanoparticles | |
| 8 | 0 | 0 | 0 | 2 |
| 16 | 0 | 2 | 0 | 2 |
| 40 | 0 | 4 | 0 | 4 |
| Concentrations (mg/mL) | Inhibition Zone (mm) | |||
|---|---|---|---|---|
| Without Light Irradiation | With Light Irradiation | |||
| ZnO Nanoparticles | Ag-Decorated ZnO Nanoparticles | ZnO Nanoparticles | Ag-Decorated ZnO Nanoparticles | |
| 8 | 0 | 2 | 0 | 7 |
| 16 | 0 | 4 | 0 | 8 |
| 40 | 0 | 6 | 0 | 8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, V.T.; Vu, V.T.; Nguyen, T.H.; Nguyen, T.A.; Tran, V.K.; Nguyen-Tri, P. Antibacterial Activity of TiO2- and ZnO-Decorated with Silver Nanoparticles. J. Compos. Sci. 2019, 3, 61. https://doi.org/10.3390/jcs3020061
Nguyen VT, Vu VT, Nguyen TH, Nguyen TA, Tran VK, Nguyen-Tri P. Antibacterial Activity of TiO2- and ZnO-Decorated with Silver Nanoparticles. Journal of Composites Science. 2019; 3(2):61. https://doi.org/10.3390/jcs3020061
Chicago/Turabian StyleNguyen, Van Thang, Viet Tien Vu, The Huu Nguyen, Tuan Anh Nguyen, Van Khanh Tran, and Phuong Nguyen-Tri. 2019. "Antibacterial Activity of TiO2- and ZnO-Decorated with Silver Nanoparticles" Journal of Composites Science 3, no. 2: 61. https://doi.org/10.3390/jcs3020061
APA StyleNguyen, V. T., Vu, V. T., Nguyen, T. H., Nguyen, T. A., Tran, V. K., & Nguyen-Tri, P. (2019). Antibacterial Activity of TiO2- and ZnO-Decorated with Silver Nanoparticles. Journal of Composites Science, 3(2), 61. https://doi.org/10.3390/jcs3020061







