Recycling Waste Tires into Ground Tire Rubber (GTR)/Rubber Compounds: A Review
Abstract
:1. Introduction
Tire Composition
2. Tire Recycling
2.1. Retreading
2.2. Incineration
2.3. Pyrolysis
2.4. Material Composite
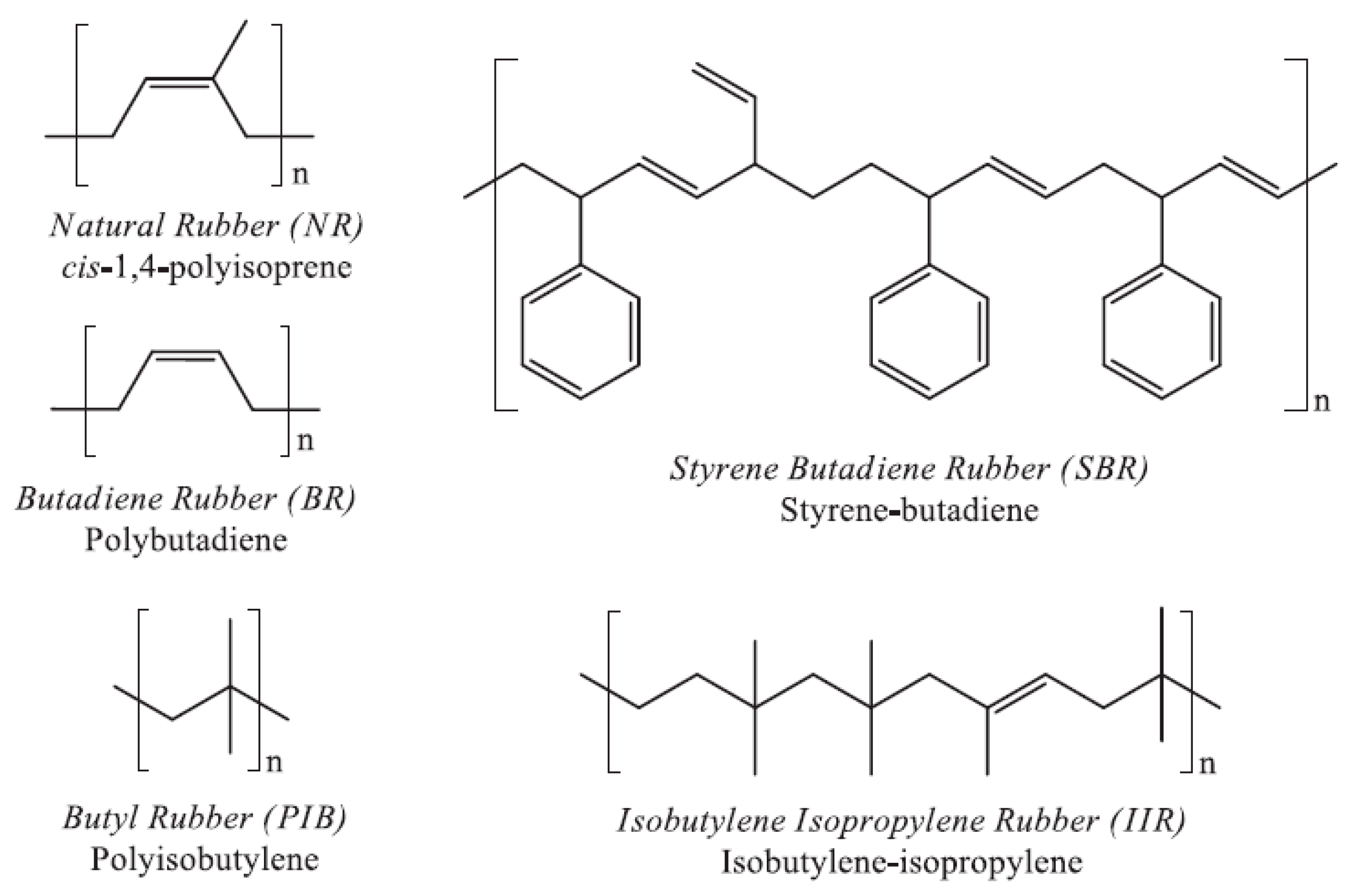
3. GTR in Blends
3.1. Modification of GTR
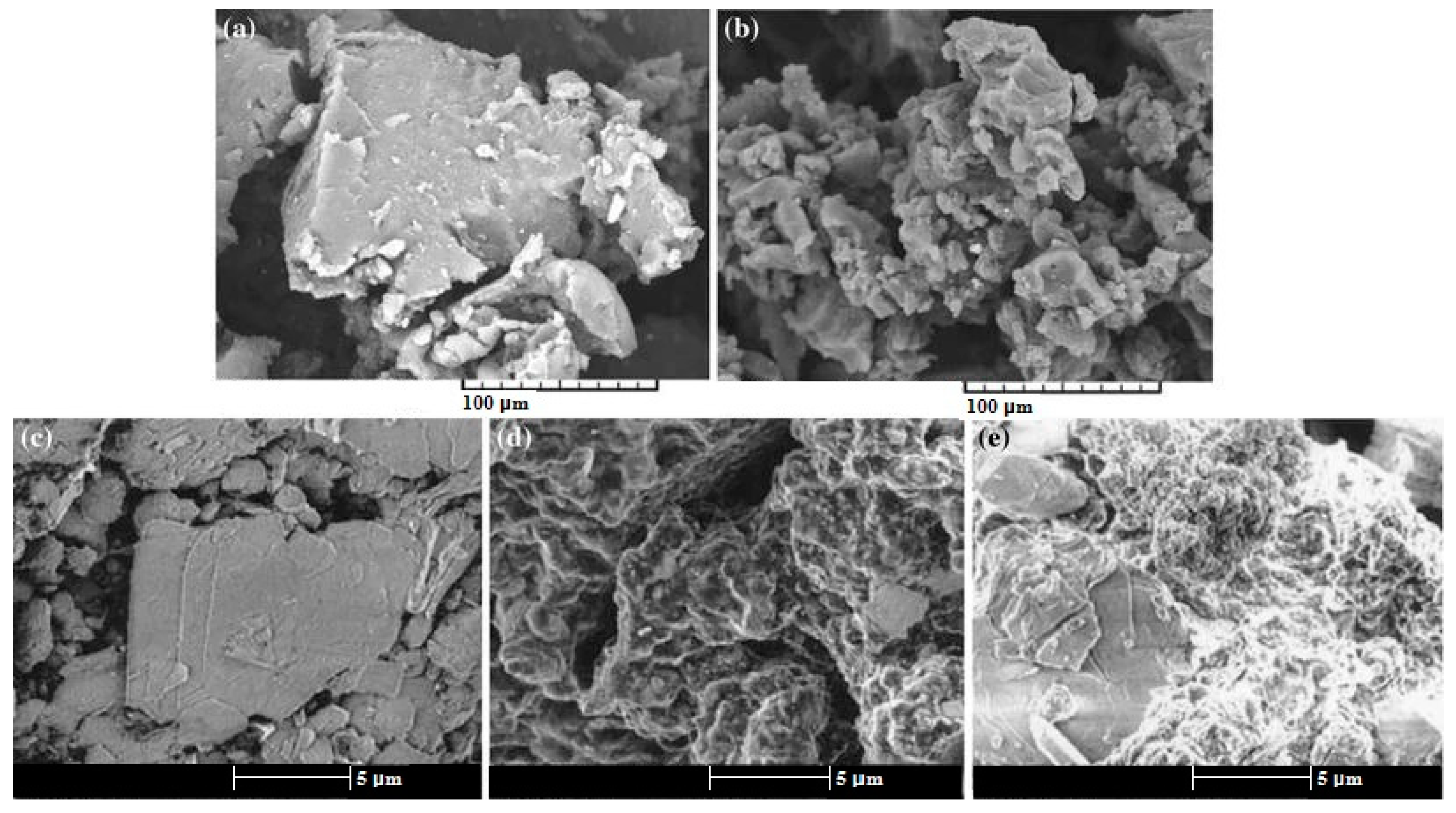
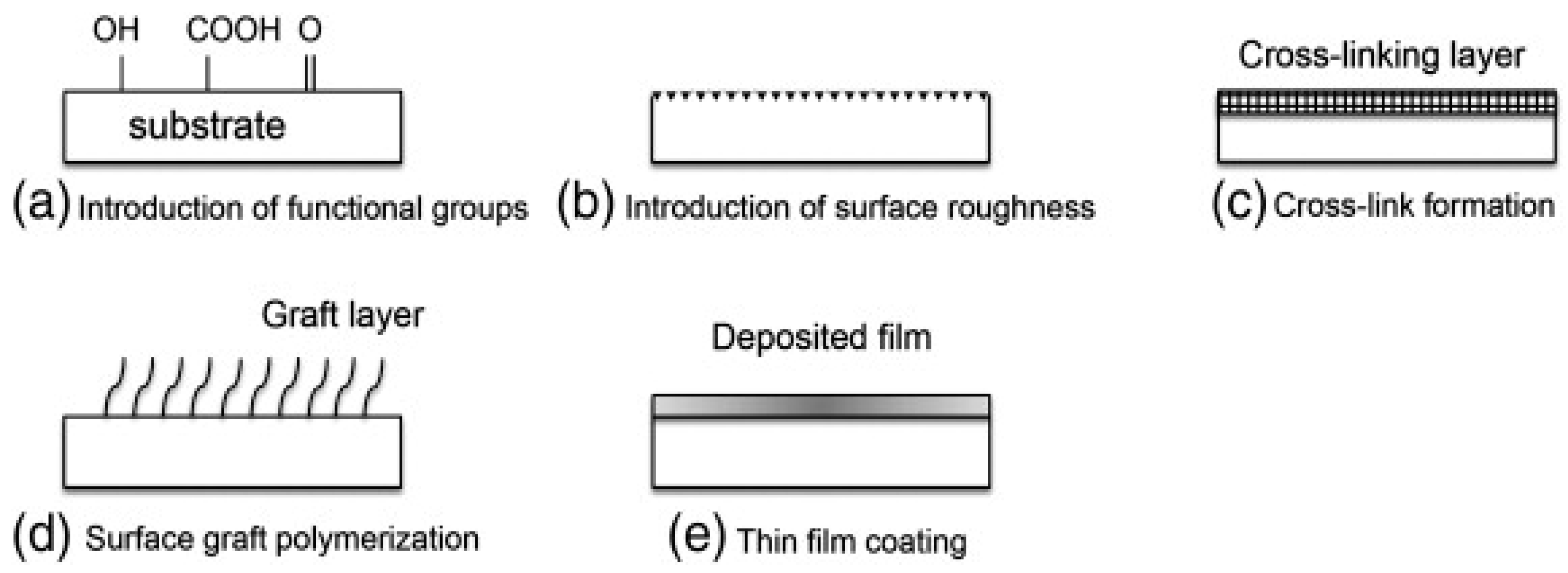
3.1.1. Physical Methods
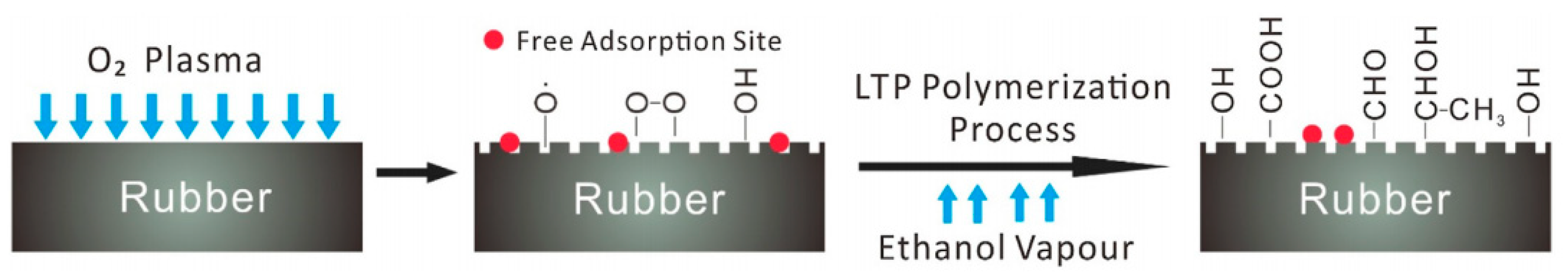
3.1.2. Chemical Methods
3.2. Regeneration of GTR
3.2.1. Thermo-Mechanical Processes
3.2.2. Microwave Method
3.2.3. Ultrasonic Method
3.2.4. Thermo-Chemical Processes
3.3. GTR in Curable Rubbers
3.3.1. Cure Characteristics
3.3.2. Rheological Properties
3.3.3. Mechanical Properties
3.3.4. Aging Properties
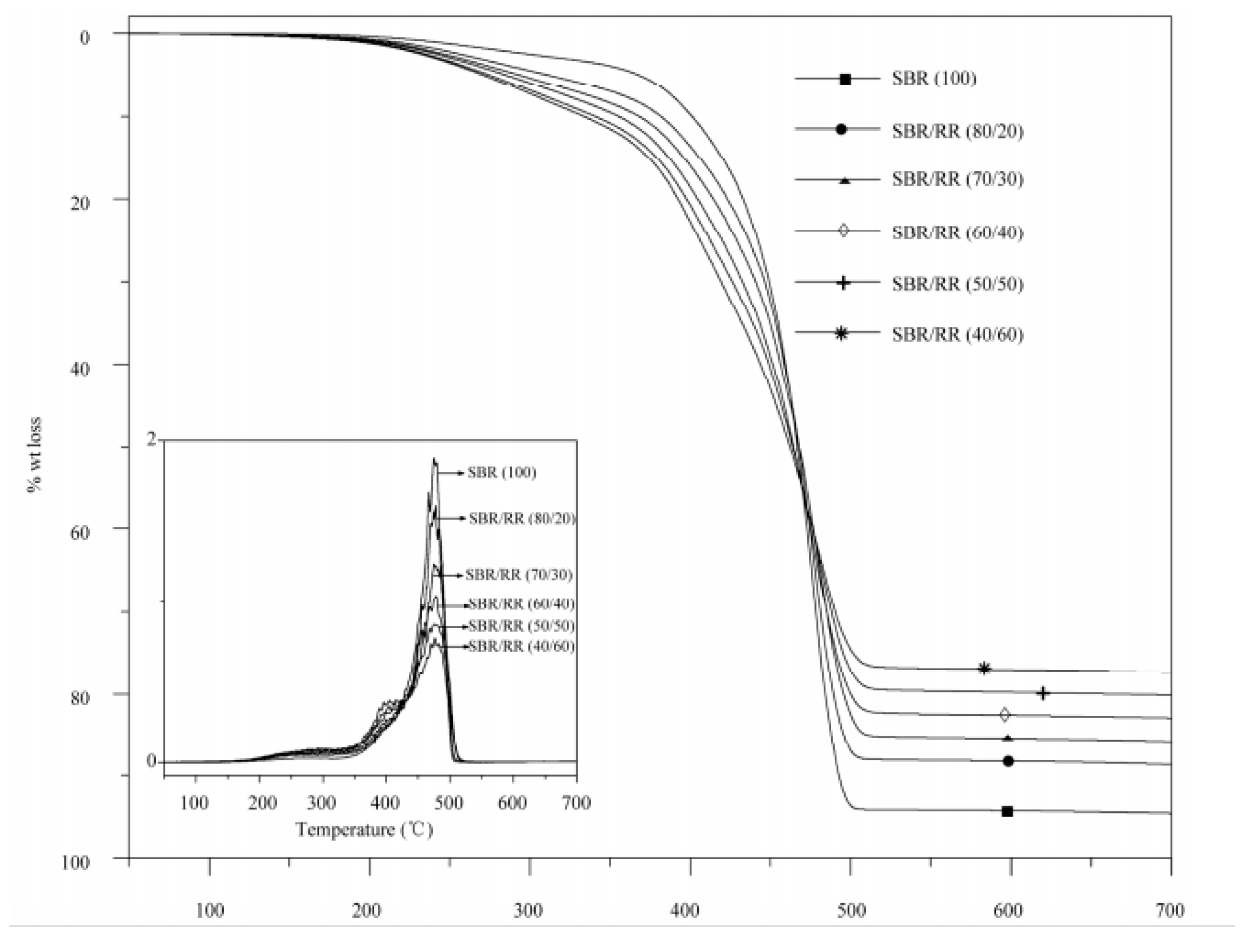
3.3.5. Thermal Properties
3.3.6. Dynamic Mechanical Properties
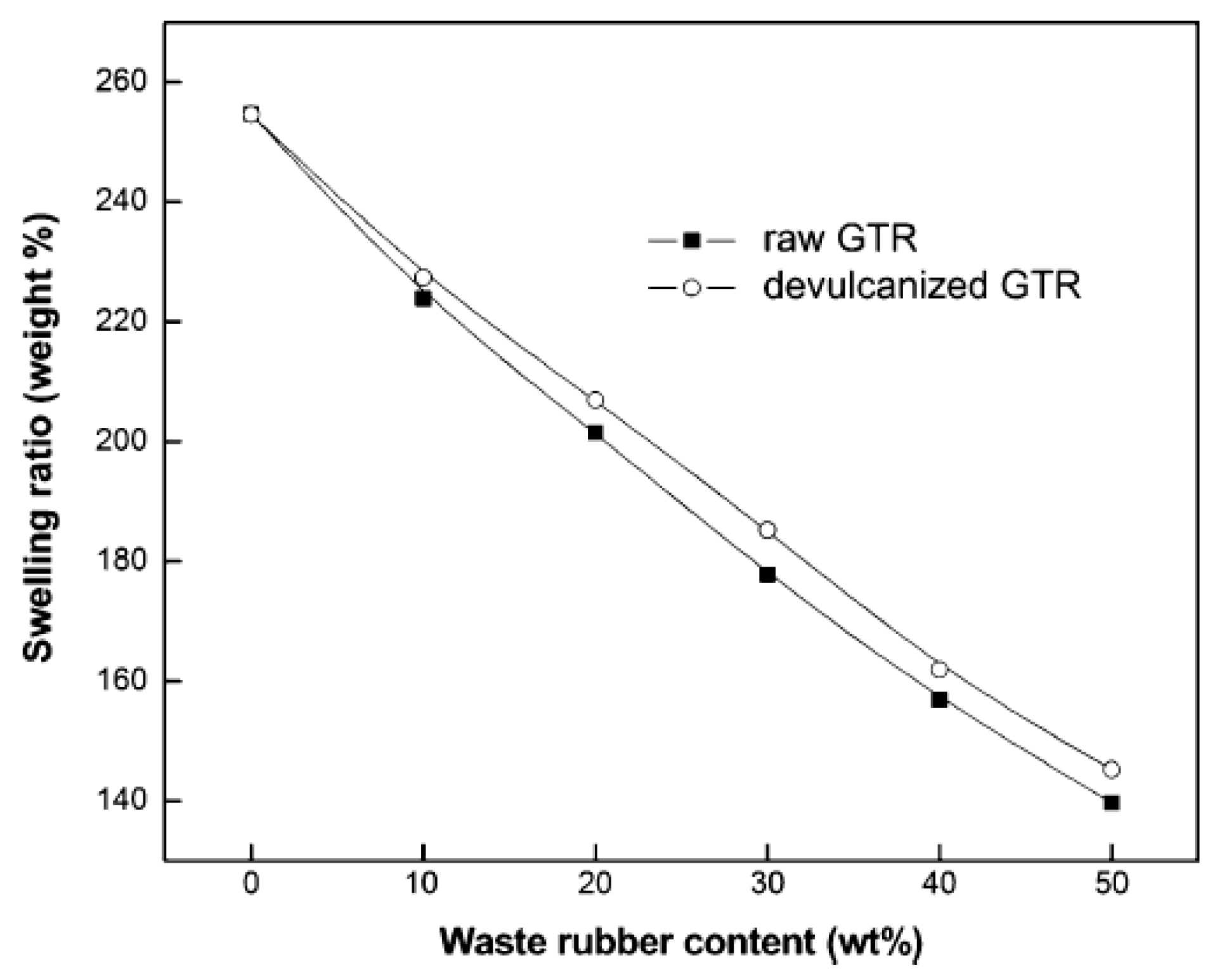
3.3.7. Swelling Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McKeen, L.W. The Effect of Long Term Thermal Exposure on Plastics and Elastomers; William Andrew: Norwich, NY, USA, 2014. [Google Scholar]
- Nuzaimah, M.; Sapuan, S.M.; Nadlene, R.; Jawaid, M. Recycling of waste rubber as fillers: A review. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Selangor, Malaysia, 21–23 November 2017; Volume 368, p. 012016. [Google Scholar]
- Rubber, S.N. Natural rubber and reclaimed Rubber composites–A Systematic Review. Polymer 2016, 2, 7. [Google Scholar]
- Fiksel, J.; Bakshi, B.R.; Baral, A.; Guerra, E.; DeQuervain, B. Comparative life cycle assessment of beneficial applications for scrap tires. Clean Technol. Environ. Policy 2010, 13, 19–35. [Google Scholar] [CrossRef]
- De Sadhan, K.; Isayev, A.; Khait, K. (Eds.) Rubber Recycling; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Sienkiewicz, M.; Kucinska-Lipka, J.; Janik, H.; Balas, A. Progress in used tyres management in the European Union: A review. Waste Manag. 2012, 32, 1742–1751. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R.; Zeeshan, M.; Masood, A. Enhancement of hydrocarbons production through co-pyrolysis of acid-treated biomass and waste tire in a fixed bed reactor. Waste Manag. 2020, 106, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Banar, M.; Akyıldız, V.; Ozkan, A.; Cokaygil, Z.; Onay, O. Characterization of pyrolytic oil obtained from pyrolysis of TDF (Tire Derived Fuel). Energy Convers. Manag. 2012, 62, 22–30. [Google Scholar] [CrossRef]
- Shu, X.; Huang, B. Recycling of waste tire rubber in asphalt and portland cement concrete: An overview. Constr. Build. Mater. 2014, 67, 217–224. [Google Scholar] [CrossRef]
- Ge, D.; Yan, K.; You, Z.; Xu, H. Modification mechanism of asphalt binder with waste tire rubber and recycled polyethylene. Constr. Build. Mater. 2016, 126, 66–76. [Google Scholar] [CrossRef]
- Yung, W.H.; Yung, L.C.; Lee, H.H. A study of the durability properties of waste tire rubber applied to self-compacting concrete. Constr. Build. Mater. 2013, 41, 665–672. [Google Scholar] [CrossRef]
- Aiello, M.; Leuzzi, F. Waste tyre rubberized concrete: Properties at fresh and hardened state. Waste Manag. 2010, 30, 1696–1704. [Google Scholar] [CrossRef]
- Kakroodi, A.R.; Rodrigue, D. Highly filled thermoplastic elastomers from ground tire rubber, maleated polyethylene and high density polyethylene. Plast. Rubber Compos. 2013, 42, 115–122. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Chen, Y.-K.; Rodrigue, D. Production of Thermoplastic Elastomers Based on Recycled PE and Ground Tire Rubber: Morphology, Mechanical Properties and Effect of Compatibilizer Addition. Int. Polym. Process. 2018, 33, 525–534. [Google Scholar] [CrossRef]
- Araujo-Morera, J.; Hernández, M.; Verdejo, R.; López-Manchado, M.A. Giving a Second Opportunity to Tire Waste: An Alternative Path for the Development of Sustainable Self-Healing Styrene–Butadiene Rubber Compounds Overcoming the Magic Triangle of Tires. Polymers 2019, 11, 2122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramarad, S.; Khalid, M.; Ratnam, C.T.; Abdullah, L.; Rashmi, W. Waste tire rubber in polymer blends: A review on the evolution, properties and future. Prog. Mater. Sci. 2015, 72, 100–140. [Google Scholar] [CrossRef]
- Shaker, R.; Rodrigue, D. Rotomolding of Thermoplastic Elastomers Based on Low-Density Polyethylene and Recycled Natural Rubber. Appl. Sci. 2019, 9, 5430. [Google Scholar] [CrossRef] [Green Version]
- Moghaddamzadeh, S.; Rodrigue, D. The effect of polyester recycled tire fibers mixed with ground tire rubber on polyethylene composites. Part II. Prog. Rubber Plast. Recycl. Technol. 2018, 34, 128–142. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, X.; Liang, M.; Lu, C. Improvement of the properties of ground tire rubber (GTR)-filled nitrile rubber vulcanizates through plasma surface modification of GTR powder. J. Appl. Polym. Sci. 2009, 114, 1118–1125. [Google Scholar] [CrossRef]
- Aggour, Y.A.; Al-Shihri, A.S.; Bazzt, M.R. Surface Modification of Waste Tire by Grafting with Styrene and Maleic Anhydride. Open J. Polym. Chem. 2012, 2, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Garcia, P. Devulcanization of ground tire rubber: Physical and chemical changes after different microwave exposure times. Express Polym. Lett. 2015, 9, 1015–1026. [Google Scholar] [CrossRef]
- Esmizadeh, E.; Bakhshandeh, G.R.; Fasaie, M.R.; Ahmadi, S.; Naderi, G. Reactively compatibilized and dynamically vulcanized thermoplastic elastomers based on high-density polyethylene and reclaimed rubber. Polym. Sci. Ser. B 2017, 59, 362–371. [Google Scholar] [CrossRef]
- Wang, L.; Lang, F.; Li, S.; Du, F.; Wang, Z. Thermoplastic elastomers based on high-density polyethylene and waste ground rubber tire composites compatibilized by styrene–butadiene block copolymer. J. Thermoplast. Compos. Mater. 2013, 27, 1479–1492. [Google Scholar] [CrossRef]
- Thomas, B.S.; Gupta, R.C. A comprehensive review on the applications of waste tire rubber in cement concrete. Renew. Sustain. Energy Rev. 2016, 54, 1323–1333. [Google Scholar] [CrossRef]
- Mohajerani, A.; Burnett, L.; Smith, J.V.; Markovski, S.; Rodwell, G.; Rahman, T.; Kurmus, H.; Mirzababaei, M.; Arulrajah, A.; Horpibulsuk, S.; et al. Recycling waste rubber tyres in construction materials and associated environmental considerations: A review. Resour. Conserv. Recycl. 2020, 155, 104679. [Google Scholar] [CrossRef]
- Sathiskumar, C.; Karthikeyan, S. Recycling of waste tires and its energy storage application of by-products —A review. Sustain. Mater. Technol. 2019, 22, e00125. [Google Scholar] [CrossRef]
- Fazli, A.; Rodrigue, D. Waste Rubber Recycling: A Review on the Evolution and Properties of Thermoplastic Elastomers. Materials 2020, 13, 782. [Google Scholar] [CrossRef] [Green Version]
- Bockstal, L.; Berchem, T.; Schmetz, Q.; Richel, A. Devulcanisation and reclaiming of tires and rubber by physical and chemical processes: A review. J. Clean. Prod. 2019, 236, 117574. [Google Scholar] [CrossRef]
- Shulman, V. Chapter 21-Tyre Recycling; Waste, Academic Press: Boston, MA, USA, 2011; pp. 297–320. [Google Scholar]
- Evans, A.; Evans, R. The Composition of a Tyre: Typical Components; The Waste & Resources Action Programme: Banbury, UK, 2006; p. 5. [Google Scholar]
- Shulman, V.L. Tire Recycling. Waste 2019, 489–515. [Google Scholar] [CrossRef]
- Akiba, M. Vulcanization and crosslinking in elastomers. Prog. Polym. Sci. 1997, 22, 475–521. [Google Scholar] [CrossRef]
- Vergnaud, J.-M.; Rosca, I.-D. Rubber Curing and Properties; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Morin, J.E.; Farris, R.J. Recycling of 100% Cross Linked Rubber Powder by High Temperature High Pressure Sintering. Encycl. Polym. Eng. 2000, 37, 95–101. [Google Scholar]
- Association, United States Tire Manufacturers, U.S. Scrap Tire Disposition 2017. Available online: https://www.ustires.org/ (accessed on 30 July 2020).
- Imbernon, L.; Norvez, S. From landfilling to vitrimer chemistry in rubber life cycle. Eur. Polym. J. 2016, 82, 347–376. [Google Scholar] [CrossRef]
- Czajczynska, D.; Krzyżyńska, R.; Jouhara, H.; Spencer, N. Use of pyrolytic gas from waste tire as a fuel: A review. Energy 2017, 134, 1121–1131. [Google Scholar] [CrossRef]
- Karger-Kocsis, J.; Mészáros, L.; Bárány, T. Ground tyre rubber (GTR) in thermoplastics, thermosets, and rubbers. J. Mater. Sci. 2012, 48, 1–38. [Google Scholar] [CrossRef]
- Zebala, J.; Ciepka, P.; Reza, A.; Janczur, R. Influence of rubber compound and tread pattern of retreaded tyres on vehicle active safety. Forensic Sci. Int. 2007, 167, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, B.; Tuma, A. A quantitative approach to assessing the profitability of car and truck tire remanufacturing. Int. J. Prod. Econ. 2006, 104, 639–652. [Google Scholar] [CrossRef]
- Gieré, R.; Smith, K.; Blackford, M. Chemical composition of fuels and emissions from a coal+tire combustion experiment in a power station. Fuel 2006, 85, 2278–2285. [Google Scholar] [CrossRef]
- Tsang, H.-H. Uses of scrap rubber tires. In Rubber: Types, Properties and Uses; Nova Science Publisher: New York, NY, USA, 2013; pp. 477–491. [Google Scholar]
- Saha, P. Rubber Recycling: Challenges and Developments; Royal Society of Chemistry: London, UK, 2018. [Google Scholar]
- Oriaku, E. Waste to wealth through the incineration of waste tyres and recovery of carbon black. Int. J. Multidiscip. Sci. Eng. 2013, 4, 30–36. [Google Scholar]
- Singh, S.; Nimmo, W.; Gibbs, B.; Williams, P. Waste tyre rubber as a secondary fuel for power plants. Fuel 2009, 88, 2473–2480. [Google Scholar] [CrossRef]
- Yu, Q. Application of nanomaterials in alkali-activated materials. In Nanotechnology in Eco-Efficient Construction; Elsevier: Amsterdam, The Netherlands, 2019; pp. 97–121. [Google Scholar]
- Gnanaraj, J.S.; Lee, R.J.; Levine, A.M.; Wistrom, J.L.; Wistrom, S.L.; Li, Y.; Li, J.; Akato, K.; Naskar, A.K.; Paranthaman, M. Sustainable Waste Tire Derived Carbon Material as a Potential Anode for Lithium-Ion Batteries. Sustainability 2018, 10, 2840. [Google Scholar] [CrossRef] [Green Version]
- Navarro, F. Thermo-rheological behaviour and storage stability of ground tire rubber-modified bitumens. Fuel 2004, 83, 2041–2049. [Google Scholar] [CrossRef]
- Medina, N.F.; Garcia, R.; Hajirasouliha, I.; Pilakoutas, K.; Guadagnini, M.; Raffoul, S. Composites with recycled rubber aggregates: Properties and opportunities in construction. Constr. Build. Mater. 2018, 188, 884–897. [Google Scholar] [CrossRef]
- Benazzouk, A.; Douzane, O.; Langlet, T.; Mezreb, K.; Roucoult, J.; Quéneudéc, M. Physico-mechanical properties and water absorption of cement composite containing shredded rubber wastes. Cem. Concr. Compos. 2007, 29, 732–740. [Google Scholar] [CrossRef]
- Oikonomou, N.; Mavridou, S. Improvement of chloride ion penetration resistance in cement mortars modified with rubber from worn automobile tires. Cem. Concr. Compos. 2009, 31, 403–407. [Google Scholar] [CrossRef]
- Hejna, A.; Korol, J.; Przybysz-Romatowska, M.; Zedler, Ł.; Chmielnicki, B.; Formela, K. Waste tire rubber as low-cost and environmentally-friendly modifier in thermoset polymers—A review. Waste Manag. 2020, 108, 106–118. [Google Scholar] [CrossRef]
- Sunthonpagasit, N.; Duffey, M.R. Scrap tires to crumb rubber: Feasibility analysis for processing facilities. Resour. Conserv. Recycl. 2004, 40, 281–299. [Google Scholar] [CrossRef]
- Fernández, A.; Barriocanal, C.; Alvarez, R. Pyrolysis of a waste from the grinding of scrap tyres. J. Hazard. Mater. 2012, 203, 236–243. [Google Scholar] [CrossRef]
- Hassan, M.M.; Mahmoud, G.A.; Hegazy, E.-S.A.; El-Nahas, H.H. Reinforced material from reclaimed rubber/natural rubber, using electron beam and thermal treatment. J. Appl. Polym. Sci. 2007, 104, 2569–2578. [Google Scholar] [CrossRef]
- Dierkes, W. Untreated and treated rubber powder. In Rubber Recycling; CRC Press: Boca Raton, FL, USA, 2005; pp. 151–175. [Google Scholar]
- Darrel, R., Sr. Process for Recycling Vehicle Tires. U.S. Patent 5,115,983, 26 May 1992. [Google Scholar]
- Dufton, P. End-Of-Life Tyres: Exploiting Their Value; iSmithers Rapra Publishing: Akron, OH, USA, 2001. [Google Scholar]
- Adhikari, J.; Das, A.; Sinha, T.K.; Saha, P.; Kim, J.K. Grinding of Waste Rubber. Green Chem. Ser. 2018, 59, 1–23. [Google Scholar]
- Kumar, C.; Fuhrmann, I.; Karger-Kocsis, J. LDPE-based thermoplastic elastomers containing ground tire rubber with and without dynamic curing. Polym. Degrad. Stab. 2002, 76, 137–144. [Google Scholar] [CrossRef]
- Sonnier, R.; Leroy, E.; Clerc, L.; Bergeret, A.; Lopez-Cuesta, J. Polyethylene/ground tyre rubber blends: Influence of particle morphology and oxidation on mechanical properties. Polym. Test. 2007, 26, 274–281. [Google Scholar] [CrossRef]
- Yehia, A.; Mull, M.A.; Ismail, M.N.; Hefny, Y.A.; Abdel-Bary, E.M. Effect of chemically modified waste rubber powder as a filler in natural rubber vulcanizates. J. Appl. Polym. Sci. 2004, 93, 30–36. [Google Scholar] [CrossRef]
- Colom, X.; Cañavate, J.; Carrillo, F.; Velasco, J.I.; Pagès, P.; Mujal, R.; Nogués, F. Structural and mechanical studies on modified reused tyres composites. Eur. Polym. J. 2006, 42, 2369–2378. [Google Scholar] [CrossRef]
- Martínez-Barrera, G.; Del Coz-Díaz, J.J.; Álvarez-Rabanal, F.P.; Gayarre, F.L.; Martínez-López, M.; Cruz-Olivares, J. Waste tire rubber particles modified by gamma radiation and their use as modifiers of concrete. Case Stud. Constr. Mater. 2020, 12, e00321. [Google Scholar] [CrossRef]
- Feng, W.; Isayev, A.I. Recycling of tire-curing bladder by ultrasonic devulcanization. Polym. Eng. Sci. 2005, 46, 8–18. [Google Scholar] [CrossRef]
- Seghar, S.; Asaro, L.; Rolland-Monnet, M.; Hocine, N. Thermo-mechanical devulcanization and recycling of rubber industry waste. Resour. Conserv. Recycl. 2019, 144, 180–186. [Google Scholar] [CrossRef]
- Fan, P.; Lu, C. A Study on Functionalization of Waste Tire Rubber Powder Through Ozonization. J. Polym. Environ. 2011, 19, 943–949. [Google Scholar] [CrossRef]
- Maris, J.; Bourdon, S.; Brossard, J.-M.; Cauret, L.; Fontaine, L.; Montembault, V. Mechanical recycling: Compatibilization of mixed thermoplastic wastes. Polym. Degrad. Stab. 2018, 147, 245–266. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, C.; Liang, M. Preparation of rubber composites from ground tire rubber reinforced with waste-tire fiber through mechanical milling. J. Appl. Polym. Sci. 2006, 103, 4087–4094. [Google Scholar] [CrossRef]
- Cañavate, J. Influence of microwave treatment conditions of GTR on physico-mechanical and structural properties of NBR/NR/GTR composites. Afinidad 2019, 76, 171–179. [Google Scholar]
- Shanmugharaj, A.; Kim, J.K.; Ryu, S.H. UV surface modification of waste tire powder: Characterization and its influence on the properties of polypropylene/waste powder composites. Polym. Test. 2005, 24, 739–745. [Google Scholar] [CrossRef]
- Romero-Sánchez, M.D.; Pastor-Blas, M.M.; Martın-Martınez, J.M. Treatment of a styrene-butadiene-styrene rubber with corona discharge to improve the adhesion to polyurethane adhesive. Int. J. Adhes. Adhes. 2003, 23, 49–57. [Google Scholar] [CrossRef]
- Satyanarayana, M.; Bhowmick, A.K.; Kumar, K.D. Preferentially fixing nanoclays in the phases of incompatible carboxylated nitrile rubber (XNBR)-natural rubber (NR) blend using thermodynamic approach and its effect on physico mechanical properties. Polymer 2016, 99, 21–43. [Google Scholar] [CrossRef]
- Akca, E.; Gursel, A.; Sen, N. A Review on Devulcanization of Waste Tire Rubber. Period. Eng. Nat. Sci. 2018, 6, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Long, D.; Huang, S.; Li, Z.; Guo, X. Time effectiveness of the low-temperature plasma surface modification of ground tire rubber powder. J. Adhes. Sci. Technol. 2015, 29, 1330–1340. [Google Scholar] [CrossRef]
- Yoshida, S.; Hagiwara, K.; Hasebe, T.; Hotta, A. Surface modification of polymers by plasma treatments for the enhancement of biocompatibility and controlled drug release. Surf. Coatings Technol. 2013, 233, 99–107. [Google Scholar] [CrossRef]
- Xiaowei, C.; Sheng, H.; Xiaoyang, G.; Wenhui, D. Crumb waste tire rubber surface modification by plasma polymerization of ethanol and its application on oil-well cement. Appl. Surf. Sci. 2017, 409, 325–342. [Google Scholar] [CrossRef]
- Park, H.B.; Han, D.W.; Lee, Y.M. Effect of a UV/Ozone Treatment on Siloxane-Containing Copolyimides: Surface Modification and Gas Transport Characteristics. Chem. Mater. 2003, 15, 2346–2353. [Google Scholar] [CrossRef]
- Diaz-Quijada, G.A.; Wayner, D.D.M. A Simple Approach to Micropatterning and Surface Modification of Poly(dimethylsiloxane). Langmuir 2004, 20, 9607–9611. [Google Scholar] [CrossRef]
- Cataldo, F.; Ursini, O.; Angelini, G. Surface oxidation of rubber crumb with ozone. Polym. Degrad. Stab. 2010, 95, 803–810. [Google Scholar] [CrossRef]
- Formela, K.; Korol, J.; Saeb, M.R. Interfacially modified LDPE/GTR composites with non-polar elastomers: From microstructure to macro-behavior. Polym. Test. 2015, 42, 89–98. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Zhang, Y. Morphology and mechanical properties of HDPE/SRP/elastomer composites: Effect of elastomer polarity. Polym. Test. 2004, 23, 83–90. [Google Scholar] [CrossRef]
- Naderi, G.; LaFleur, P.G.; Dubois, C. The influence of matrix viscosity and composition on the morphology, rheology, and mechanical properties of thermoplastic elastomer nanocomposites based on EPDM/PP. Polym. Compos. 2008, 29, 1301–1309. [Google Scholar] [CrossRef]
- Amani, M.; Sharif, M.; Kashkooli, A.; Rahnama, N.; Fazli, A. Effect of mixing conditions on the selective localization of graphite oxide and the properties of polyethylene/high-impact polystyrene/graphite oxide nanocomposite blends. RSC Adv. 2015, 5, 77723–77733. [Google Scholar] [CrossRef]
- Sahakaro, K. Mechanism of reinforcement using nanofillers in rubber nanocomposites. In Progress in Rubber Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2017; pp. 81–113. [Google Scholar]
- Shan, C.; Gu, Z.; Wang, L.; Li, P.; Song, G.; Gao, Z.; Yang, X. Preparation, characterization, and application of NR/SBR/Organoclay nanocomposites in the tire industry. J. Appl. Polym. Sci. 2010, 119, 1185–1194. [Google Scholar] [CrossRef]
- Zhang, C.-L.; Feng, L.-F.; Gu, X.-P.; Hoppe, S.; Hu, G.-H. Efficiency of graft copolymers as compatibilizers for immiscible polymer blends. Polymer 2007, 48, 5940–5949. [Google Scholar] [CrossRef]
- Botros, S. Preparation and characteristics of NR/EPDM rubber blends. Polym. Technol. Eng. 2002, 41, 341–359. [Google Scholar] [CrossRef]
- Abou-Helal, M.O.; El-Sabbagh, S. A Study on the Compatibility of NR-EPDM Blends Using Electrical and Mechanical Techniques. J. Elastomers Plast. 2005, 37, 319–346. [Google Scholar] [CrossRef]
- Adhikari, B. Reclamation and recycling of waste rubber. Prog. Polym. Sci. 2000, 25, 909–948. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J. Statistical Mechanics of Cross-Linked Polymer Networks I. Rubberlike Elasticity. J. Chem. Phys. 1943, 11, 512. [Google Scholar] [CrossRef]
- Markl, E.; Lackner, M. Devulcanization Technologies for Recycling of Tire-Derived Rubber: A Review. Materials 2020, 13, 1246. [Google Scholar] [CrossRef] [Green Version]
- Formela, K.; Cysewska, M. Efficiency of thermomechanical reclaiming of ground tire rubber conducted in counter-rotating and co-rotating twin screw extruder. Polimery 2014, 59, 231–238. [Google Scholar] [CrossRef]
- Zanchet, A.; Carli, L.N.; Giovanela, M.; Crespo, J.D.S.; Scuracchio, C.H.; Nunes, R.C. Characterization of Microwave-Devulcanized Composites of Ground SBR Scraps. J. Elastomers Plast. 2009, 41, 497–507. [Google Scholar] [CrossRef]
- Yun, J.; Yashin, V.V.; Isayev, A.I. Ultrasonic devulcanization of carbon black-filled ethylene propylene diene monomer rubber. J. Appl. Polym. Sci. 2003, 91, 1646–1656. [Google Scholar] [CrossRef]
- Isayev, A.I.; Liang, T.; Lewis, T.M. Effect of particle size on ultrasonic devulcanization of tire rubber in twin-screw extruder. Rubber Chem. Technol. 2014, 87, 86–102. [Google Scholar] [CrossRef]
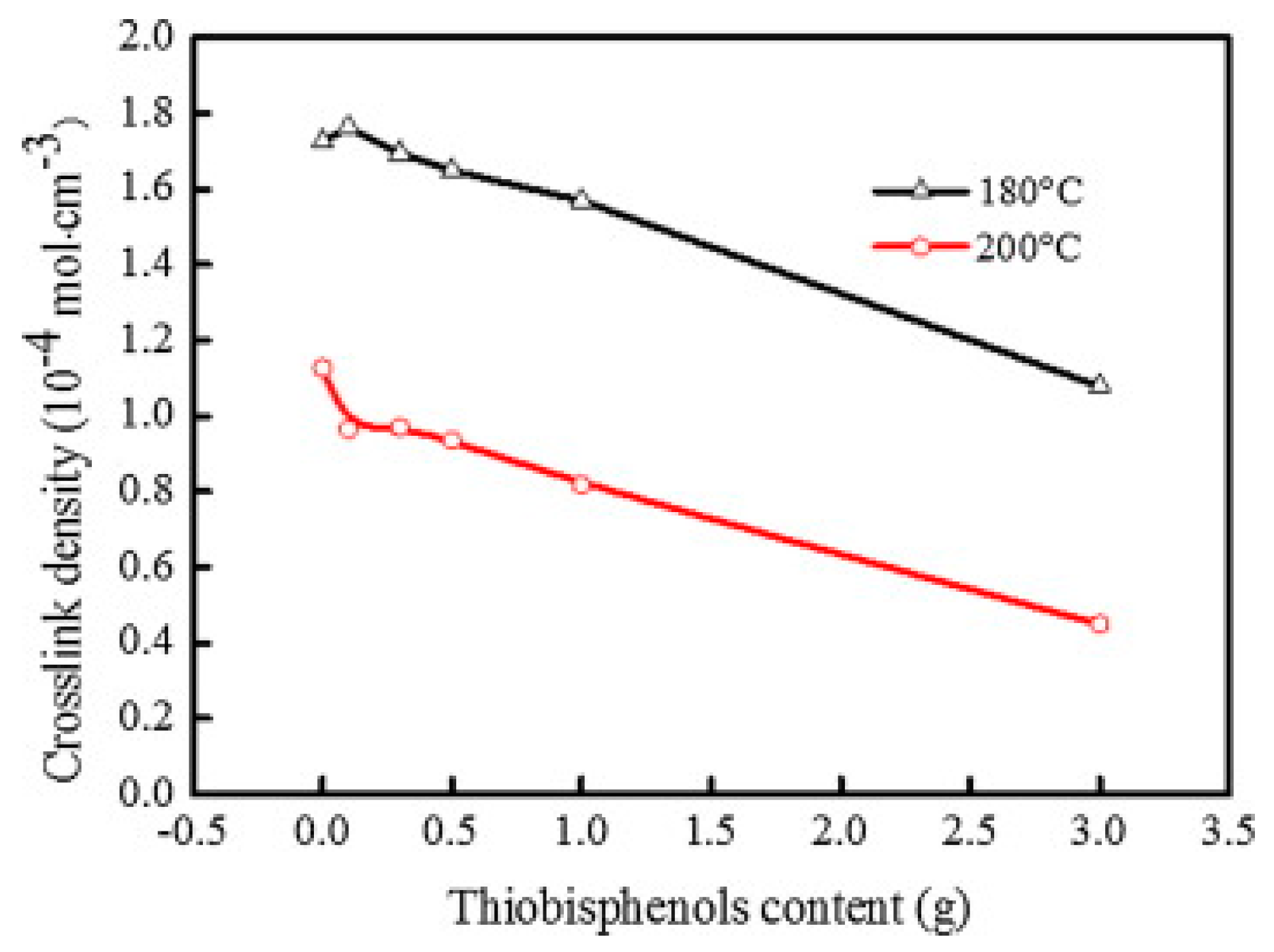
- Zhang, X.; Saha, P.; Cao, L.; Li, H.; Kim, J.K. Devulcanization of waste rubber powder using thiobisphenols as novel reclaiming agent. Waste Manag. 2018, 78, 980–991. [Google Scholar] [CrossRef]
- Wang, X.; Shi, C.; Zhang, L.; Zhang, Y. Effects of shear stress and subcritical water on devulcanization of styrene-butadiene rubber based ground tire rubber in a twin-screw extruder. J. Appl. Polym. Sci. 2013, 130, 1845–1854. [Google Scholar] [CrossRef]
- Yazdani, H.; Karrabi, M.; Ghasmi, I.; Azizi, H.; Bakhshandeh, G.R. Devulcanization of waste tires using a twin-screw extruder: The effects of processing conditions. J. Vinyl Addit. Technol. 2011, 17, 64–69. [Google Scholar] [CrossRef]
- Tao, G.; He, Q.; Xia, Y.; Jia, G.; Yang, H.; Ma, W. The effect of devulcanization level on mechanical properties of reclaimed rubber by thermal-mechanical shearing devulcanization. J. Appl. Polym. Sci. 2013, 129, 2598–2605. [Google Scholar] [CrossRef]
- Horikx, M.M. Chain scissions in a polymer network. J. Polym. Sci. 1956, 19, 445–454. [Google Scholar] [CrossRef]
- Seghar, S.; Asaro, L.; Hocine, N.A. Experimental Validation of the Horikx Theory to be Used in the Rubber Devulcanization Analysis. J. Polym. Environ. 2019, 27, 2318–2323. [Google Scholar] [CrossRef]
- Shi, J.; Jiang, K.; Ren, D.; Zou, H.; Wang, Y.; Lv, X.; Zhang, L. Structure and performance of reclaimed rubber obtained by different methods. J. Appl. Polym. Sci. 2012, 129, 999–1007. [Google Scholar] [CrossRef]
- Zanchet, A.; Carli, L.; Giovanela, M.; Brandalise, R.; Crespo, J.D.S. Use of styrene butadiene rubber industrial waste devulcanized by microwave in rubber composites for automotive application. Mater. Des. 2012, 39, 437–443. [Google Scholar] [CrossRef]
- Rooj, S.; Basak, G.C.; Maji, P.K.; Bhowmick, A.K. New Route for Devulcanization of Natural Rubber and the Properties of Devulcanized Rubber. J. Polym. Environ. 2011, 19, 382–390. [Google Scholar] [CrossRef]
- Mandal, S.K.; Alam, N.; Debnath, S.C. Reclaiming of ground rubber tire by safe multifunctional rubber additives: I. tetra benzyl thiuram disulfide. Rubber Chem. Technol. 2012, 85, 629–644. [Google Scholar] [CrossRef]
- Kojima, M.; Tosaka, M.; Ikeda, Y. Chemical recycling of sulfur-cured natural rubber using supercritical carbon dioxide. Green Chem. 2004, 6, 84. [Google Scholar] [CrossRef]
- Thaicharoen, P.; Thamyongkit, P.; Poompradub, S. Thiosalicylic acid as a devulcanizing agent for mechano-chemical devulcanization. Korean J. Chem. Eng. 2010, 27, 1177–1183. [Google Scholar] [CrossRef]
- Diaz, R.; Colomines, G.; Peuvrel-Disdier, E.; Deterre, R. Thermo-mechanical recycling of rubber: Relationship between material properties and specific mechanical energy. J. Mater. Process. Technol. 2018, 252, 454–468. [Google Scholar] [CrossRef]
- Li, X.; Deng, X.-Q.; Dong, C. Effect of Temperature on Devulcanization of Waste Sidewall Rubber by Supercritical Ethanol. J. Braz. Chem. Soc. 2018, 29, 2169–2179. [Google Scholar] [CrossRef]
- Ismail, H.; Awang, M.; Hazizan, M.A. Effect of Waste Tire Dust (WTD) Size on the Mechanical and Morphological Properties of Polypropylene/Waste Tire Dust (PP/WTD) Blends. Polym. Technol. Eng. 2006, 45, 463–468. [Google Scholar] [CrossRef]
- Sreeja, T.D.; Kutty, S.K.N. Cure characteristics and mechanical properties of natural rubber/reclaimed rubber blends. Polym. Technol. Eng. 2000, 39, 501–512. [Google Scholar] [CrossRef]
- Li, S.; Lamminmäki, J.; Hanhi, K. Effect of ground rubber powder and devulcanizates on the properties of natural rubber compounds. J. Appl. Polym. Sci. 2005, 97, 208–217. [Google Scholar] [CrossRef]
- Yehia, A.; Ismail, M.N.; Hefny, Y.A.; Abdel-Bary, E.M.; Mull, M.A. Mechano-Chemical Reclamation of Waste Rubber Powder and its Effect on the performance of NR and SBR Vulcanizates. J. Elastomers Plast. 2004, 36, 109–123. [Google Scholar] [CrossRef]
- Nelson, P.; Kutty, S. Studies on Maleic Anhydride Grafted Reclaimed Rubber/Acrylonitrile Butadiene Rubber Blends. Prog. Rubber Plast. Recycl. Technol. 2003, 19, 171–188. [Google Scholar] [CrossRef]
- Sreeja, T.; Kutty, S. Studies on acrylonitrile butadiene rubber. J. Elastomers Plast. 2002, 34, 145–155. [Google Scholar] [CrossRef]
- Sreeja, T.D.; Kutty, S.K.N. Styrene butadiene rubber/reclaimed rubber blends. Int. J. Polym. Mater. 2003, 52, 599–609. [Google Scholar] [CrossRef]
- Lamminmäki, J.; Li, S.; Hanhi, K. Feasible incorporation of devulcanized rubber waste in virgin natural rubber. J. Mater. Sci. 2006, 41, 8301–8307. [Google Scholar] [CrossRef]
- Han, S.-C.; Han, M.-H. Fracture behavior of NR and SBR vulcanizates filled with ground rubber having uniform particle size. J. Appl. Polym. Sci. 2002, 85, 2491–2500. [Google Scholar] [CrossRef]
- De, D.; Maiti, S.; Adhikari, B. Reclaiming of rubber by a renewable resource material (RRM). III. Evaluation of properties of NR reclaim. J. Appl. Polym. Sci. 2000, 75, 1493–1502. [Google Scholar] [CrossRef]
- De, D.; Panda, P.K.; Roy, M.; Bhunia, S. Reinforcing effect of reclaim rubber on natural rubber/polybutadiene rubber blends. Mater. Des. 2013, 46, 142–150. [Google Scholar] [CrossRef]
- Sombatsompop, N.; Kumnuantip, C. Rheology, cure characteristics, physical and mechanical properties of tire tread reclaimed rubber/natural rubber compounds. J. Appl. Polym. Sci. 2003, 87, 1723–1731. [Google Scholar] [CrossRef]
- Vlachopoulos, J. Die Swell and Normal Stresses: An Explanation. Rubber Chem. Technol. 1978, 51, 133–138. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, C.; Liang, M. Properties of natural rubber vulcanizates containing mechanochemically devulcanized ground tire rubber. J. Polym. Res. 2008, 16, 411–419. [Google Scholar] [CrossRef]
- Rattanasom, N.; Poonsuk, A.; Makmoon, T. Effect of curing system on the mechanical properties and heat aging resistance of natural rubber/tire tread reclaimed rubber blends. Polym. Test. 2005, 24, 728–732. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, S.; Wang, Y. Microbial Desulfurization of Ground Tire Rubber by Sphingomonas sp.: A Novel Technology for Crumb Rubber Composites. J. Polym. Environ. 2011, 20, 372–380. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, S.; Wang, Y. Improvement of the properties of natural rubber/ground tire rubber composites through biological desulfurization of GTR. J. Polym. Res. 2012, 19, 9864. [Google Scholar] [CrossRef]
- De, D.; De, D. Processing and material characteristics of a reclaimed ground rubber tire reinforced styrene butadiene rubber. Mater. Sci. Appl. 2011, 2, 486. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Berridi, M.J.; González, N.; Mugica, A.; Bernicot, C. Pyrolysis-FTIR and TGA techniques as tools in the characterization of blends of natural rubber and SBR. Thermochim. Acta 2006, 444, 65–70. [Google Scholar] [CrossRef]
- Formela, K.; Formela, M.; Thomas, S.; Haponiuk, J. Styrene-Butadiene Rubber/Modified Ground Tire Rubber Blends Co-Vulcanization: Effect of Accelerator Type. Macromol. Symp. 2016, 361, 64–72. [Google Scholar] [CrossRef]
- Colom, X.; Marin-Genesca, M.; Mujal, R.; Formela, K.; Cañavate, J. Structural and physico-mechanical properties of natural rubber/GTR composites devulcanized by microwaves: Influence of GTR source and irradiation time. J. Compos. Mater. 2018, 52, 3099–3108. [Google Scholar] [CrossRef]
- De, D.; De, D.; Singharoy, G. Reclaiming of ground rubber tire by a novel reclaiming agent. I. virgin natural rubber/reclaimed GRT vulcanizates. Polym. Eng. Sci. 2007, 47, 1091–1100. [Google Scholar] [CrossRef]
- Kumnuantip, C.; Sombatsompop, N. Dynamic mechanical properties and swelling behaviour of NR/reclaimed rubber blends. Mater. Lett. 2003, 57, 3167–3174. [Google Scholar] [CrossRef]
- Balasubramanian, M. Cure modeling and mechanical properties of counter rotating twin screw extruder devulcanized ground rubber tire—Natural rubber blends. J. Polym. Res. 2008, 16, 133–141. [Google Scholar] [CrossRef]
- Kraus, G. Modification of Asphalt by Block Polymers of Butadiene and Styrene. Rubber Chem. Technol. 1982, 55, 1389–1402. [Google Scholar] [CrossRef]
- Igwe, I.O.; Ezeani, O.E. Studies on the Transport of Aromatic Solvents through Filled Natural Rubber. Int. J. Polym. Sci. 2012, 2012, 1–11. [Google Scholar] [CrossRef] [Green Version]



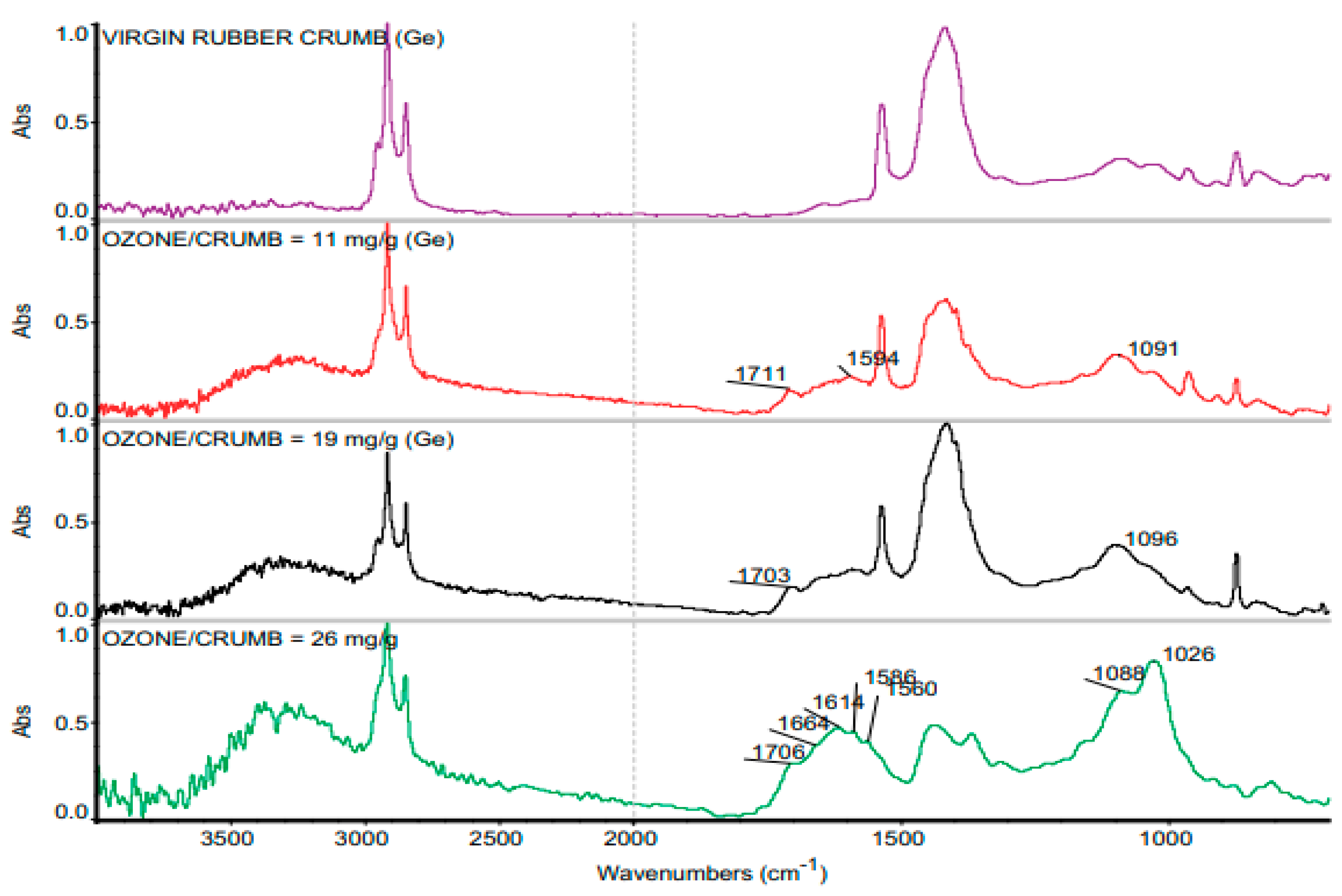
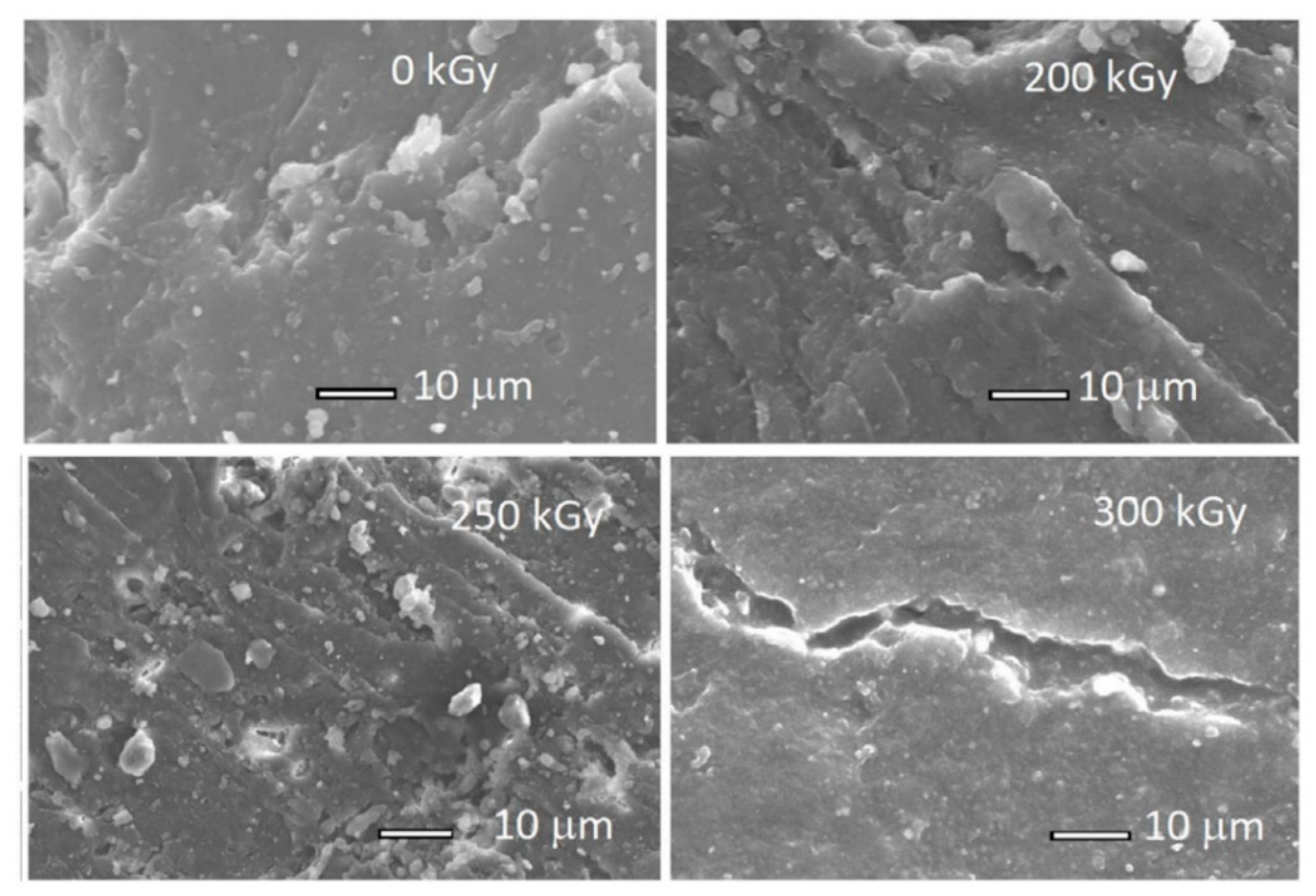

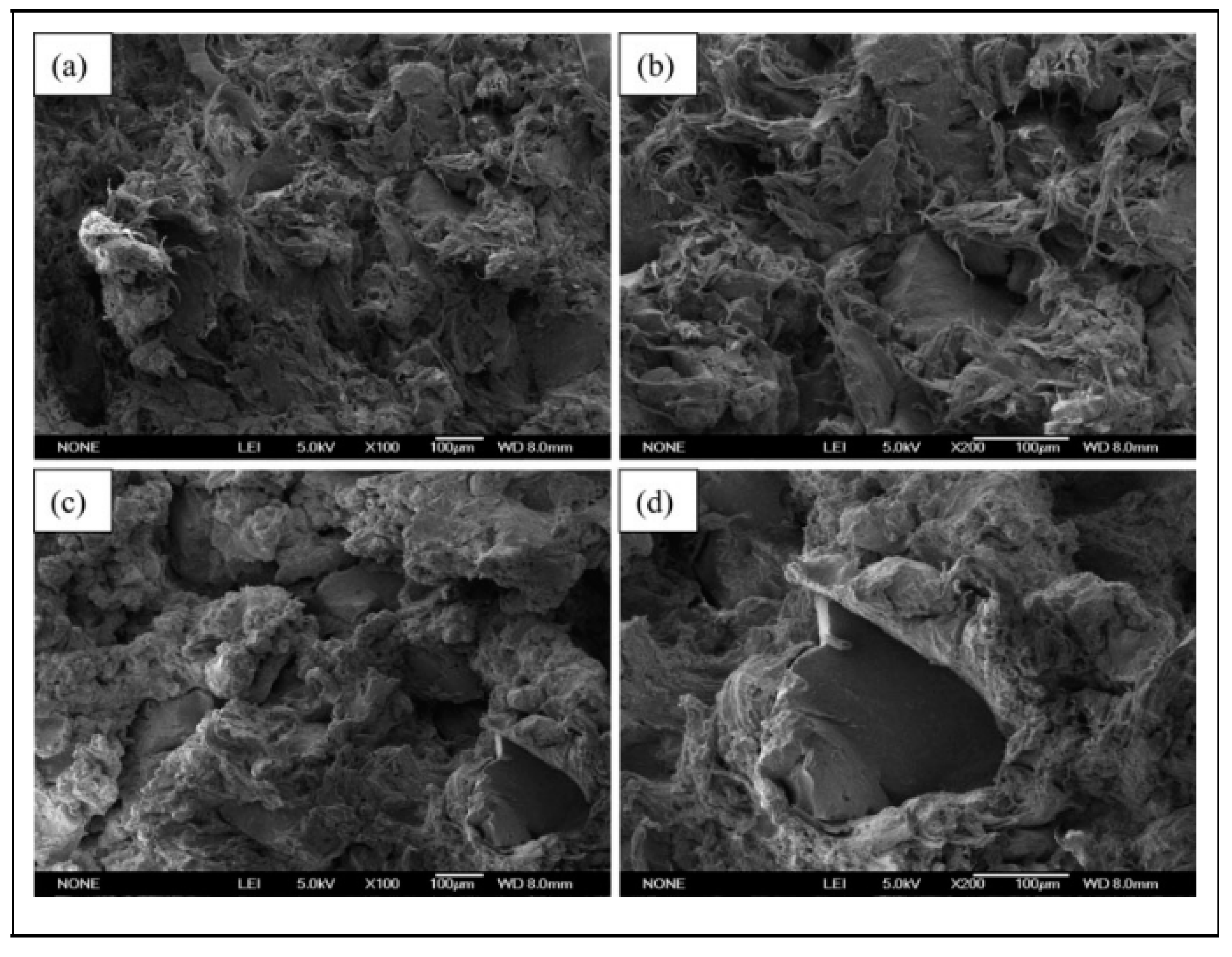
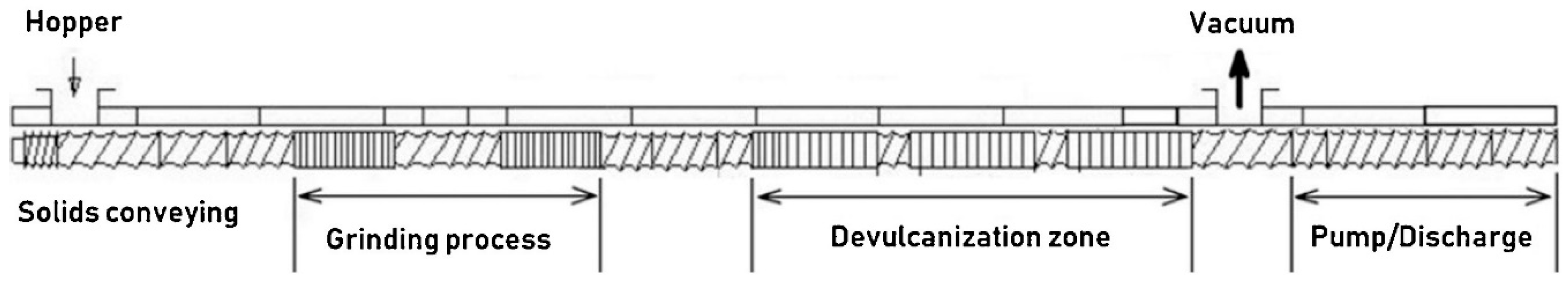
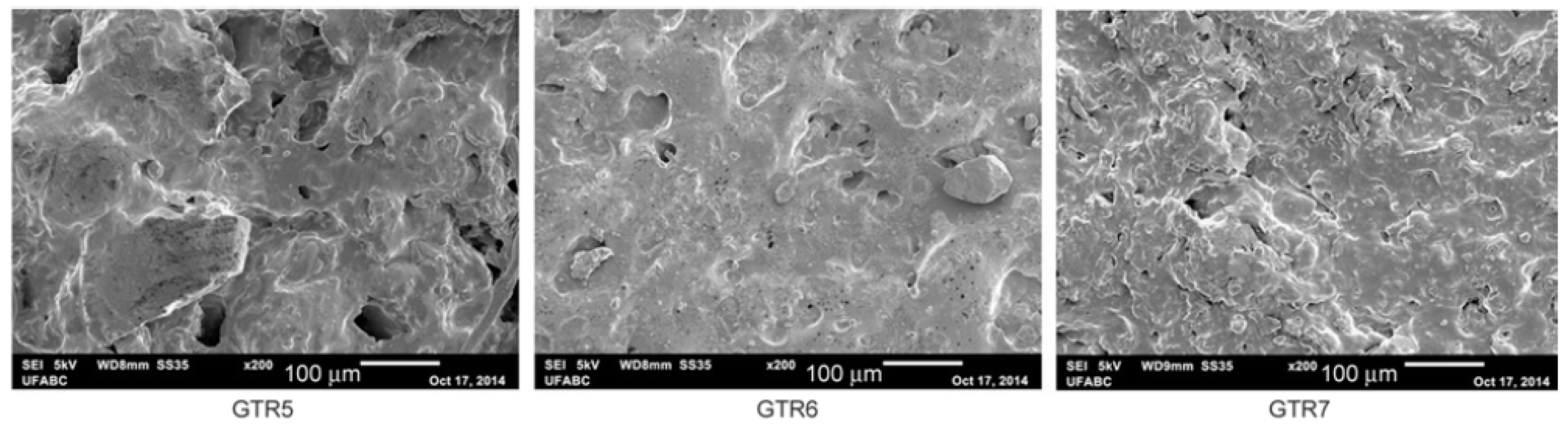








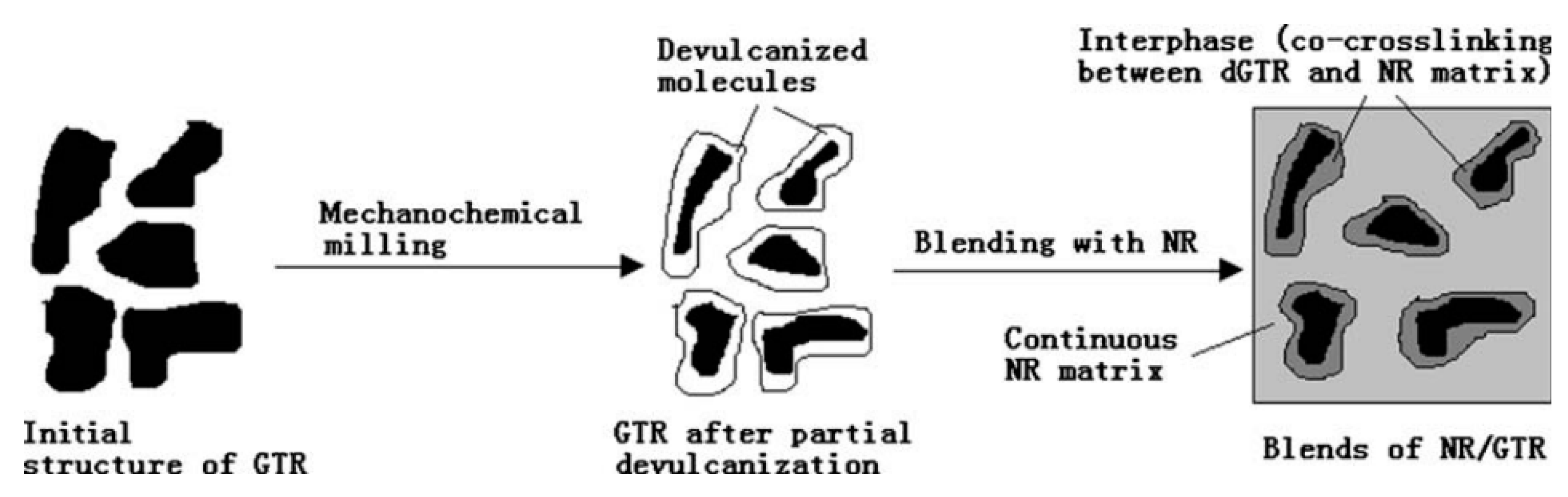
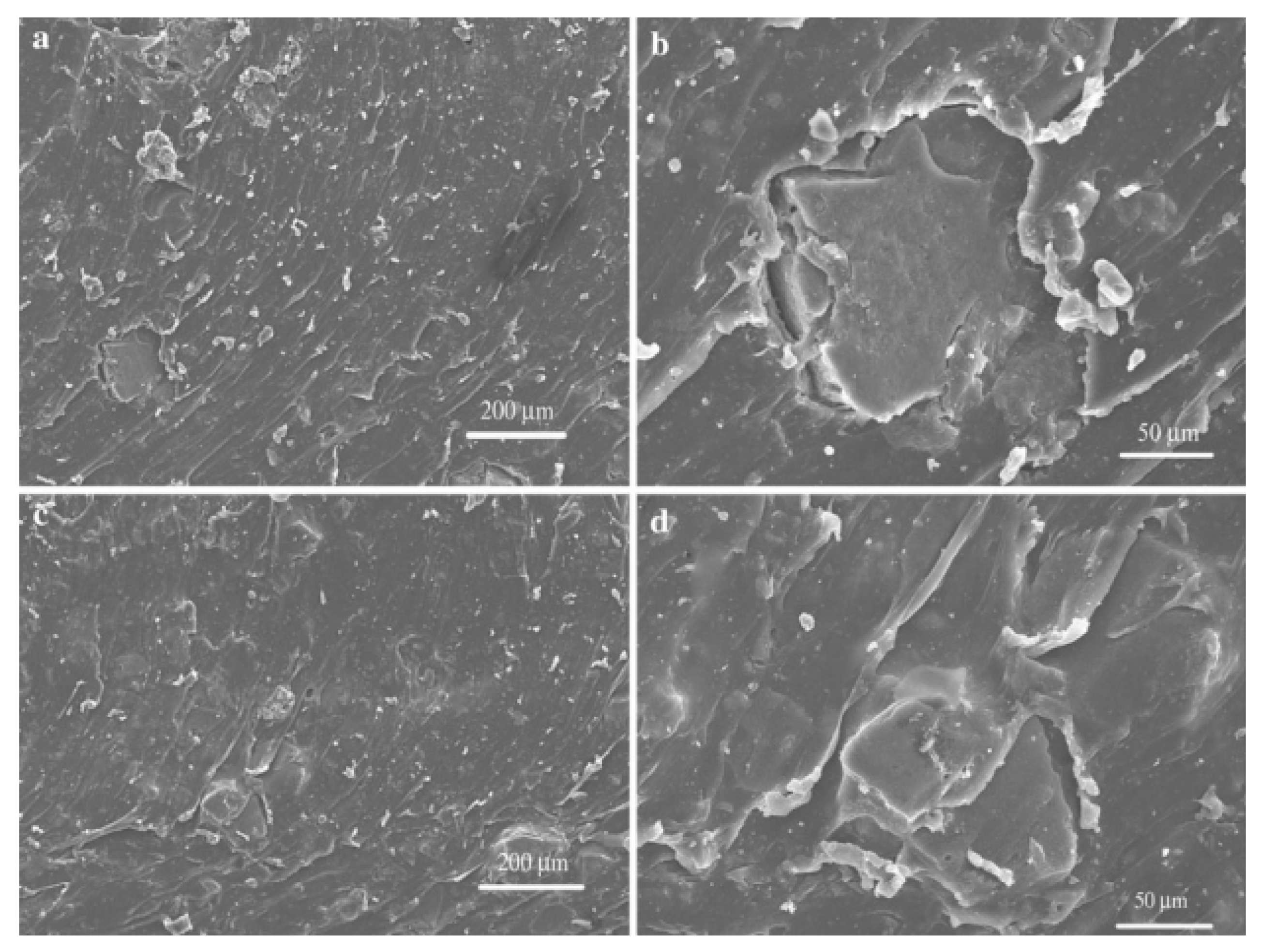
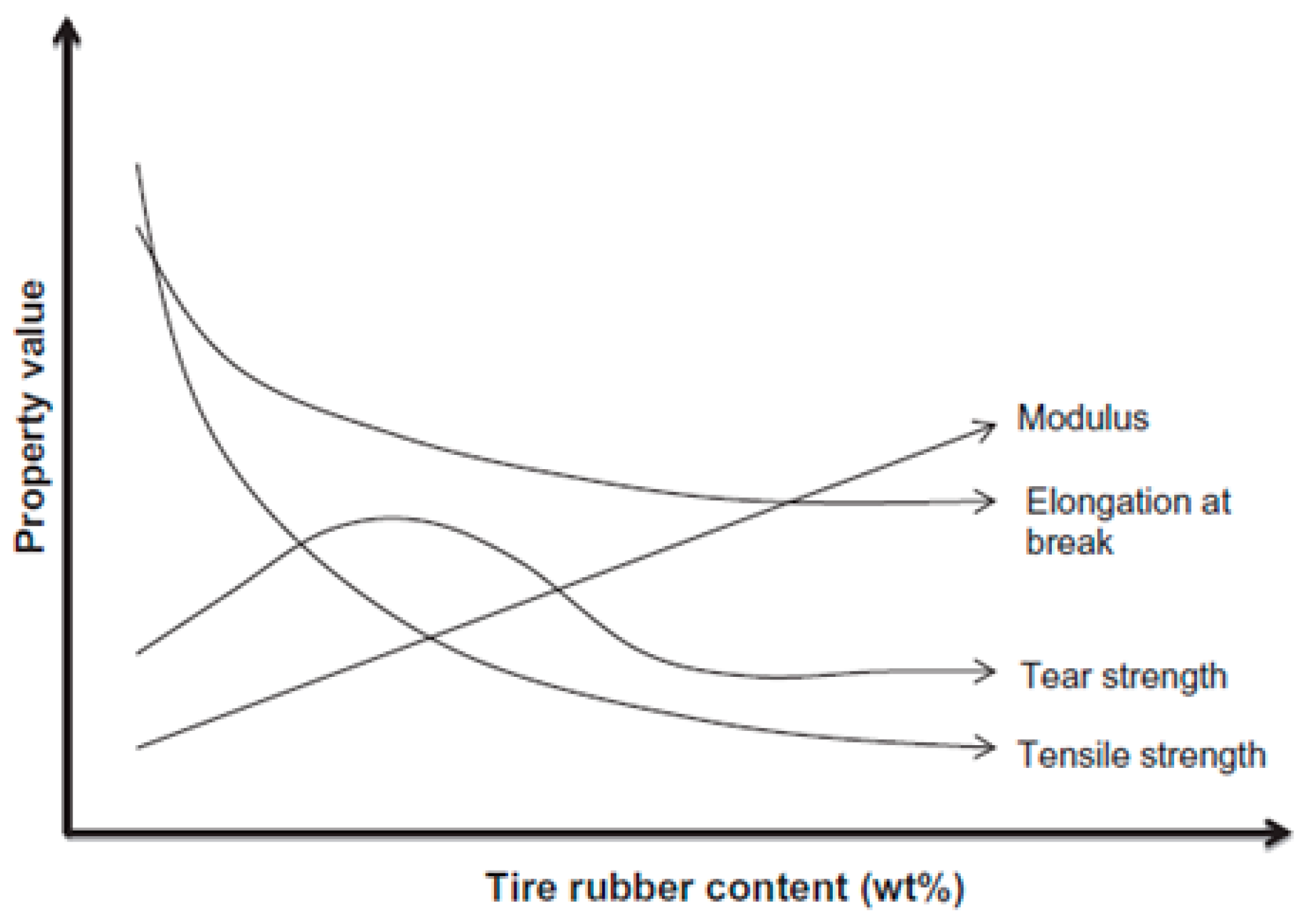
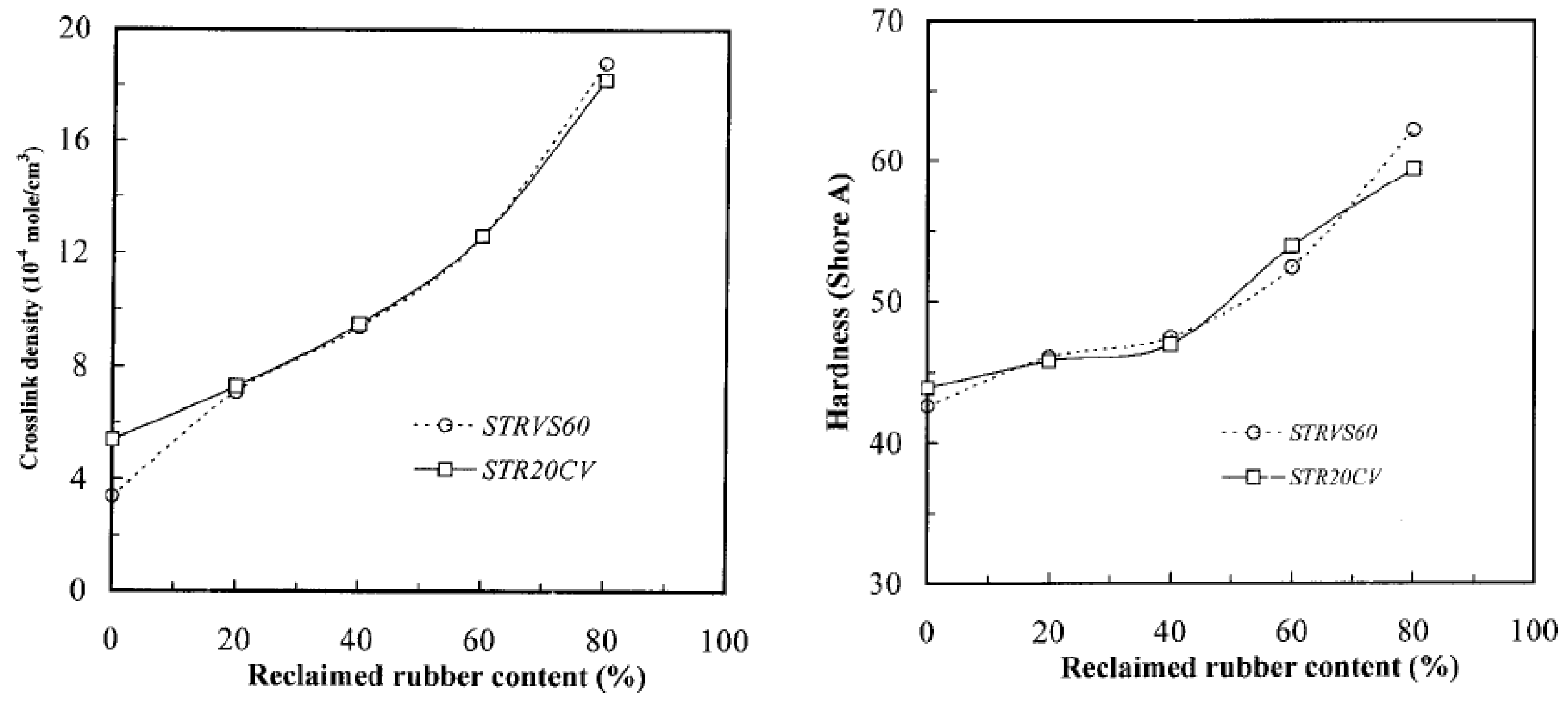




| Material | Car Tire | Truck Tire | Off-the-Road Tire |
|---|---|---|---|
| Rubbers/Elastomers (wt.%) | 47 | 45 | 47 |
| Carbon black and silica (wt.%) | 22.5 | 21 | 22 |
| Metals (wt.%) | 14 | 23.5 | 12 |
| Textiles (wt.%) | 5.5 | 1 | 10 |
| Vulcanization agents (wt.%) | 2.5 | 3 | 3 |
| Additives (wt.%) | 8.5 | 6.5 | 6 |
| Sample | Relative Concentration (%) | ||
|---|---|---|---|
| C | O | S | |
| Without milling | 96.46 | 3.17 | 0.37 |
| Milled for 15 cycles | 94.82 | 4.74 | 0.44 |
| Milled for 25 cycles | 94.43 | 5.10 | 0.47 |
| Sample | Temperature (°C) | Gel Content (%) |
|---|---|---|
| SBR-r | - | 93 ± 2 |
| rSBR 1 min | 45 ± 2 | 90 ± 1 |
| rSBR 2 min | 60 ± 2 | 43 ± 2 |
| rSBR 3 min | 80 ± 4 | 33 ± 1 |
| Blends | GTR/RTR Loading | Tensile Strength (MPa) | Elongation at Break (%) | Tear Strength (N/mm) | Abrasion Resistance (cc/h) | Compression Set (%) | Resilience (%) |
|---|---|---|---|---|---|---|---|
| NR/RTR [112] | 20 | 15 | 610 | 27 | 10.6 | 18 | 58 |
| 40 | 12.5 | 580 | 29 | 10.7 | 25 | 52 | |
| 60 | 12 | 60 | 30 | 10.7 | 25.2 | 50 | |
| NR/GTR [114] | 20 | 15.9 | 500 | Not reported | |||
| 40 | 13.4 | 417 | |||||
| 60 | 11.6 | 260 | |||||
| NBR/RTR [116] | 20 | 2.5 | 320 | 27.9 | 2.5 | 18 | 30 |
| 40 | 3.0 | 380 | 27.5 | 5 | 21 | 29 | |
| 60 | 5.5 | 500 | 27.3 | 8 | 26.5 | 28 | |
| NBR/GTR [124] | 20 | 4 | 325 | 22 | 4 | 15.5 | 30.5 |
| 40 | 6 | 310 | 27 | 7 | 17.5 | 27 | |
| SBR/RTR [117] | 20 | 2.5 | 350 | 13 | 14.5 | 4.5 | 46 |
| 40 | 3.8 | 525 | 17 | 19 | 5.0 | 45 | |
| 60 | 5.5 | 600 | 21 | 20 | 6.2 | 38 | |
| SBR/RTR [114] | 20 | 17.5 | 350 | Not reported | |||
| 40 | 14.8 | 317 | |||||
| 60 | 8.7 | 260 | |||||
| Sample | Tensile Strength (MPa) | Retention (%) | |
|---|---|---|---|
| Before Aging | After Aging | ||
| NBR | 1.8 | 2.2 | 120 |
| NBR/RTR20 | 2.6 | 2.3 | 89 |
| NBR/RTR40 | 2.6 | 2.5 | 92 |
| NBR/RTR60 | 5.0 | 4.7 | 94 |
| NBR/RTR80 | 6.3 | 5.7 | 91 |
| Composition | Passenger Car Tire | Truck Tire |
|---|---|---|
| Natural rubber | 25% | 35% |
| Synthetic rubber | 32% | 25% |
| Carbon black | 33% | 30% |
| SiO2 | 5% | 6% |
| Other additives (curing system, processing aids, etc.) | 5% | 4% |
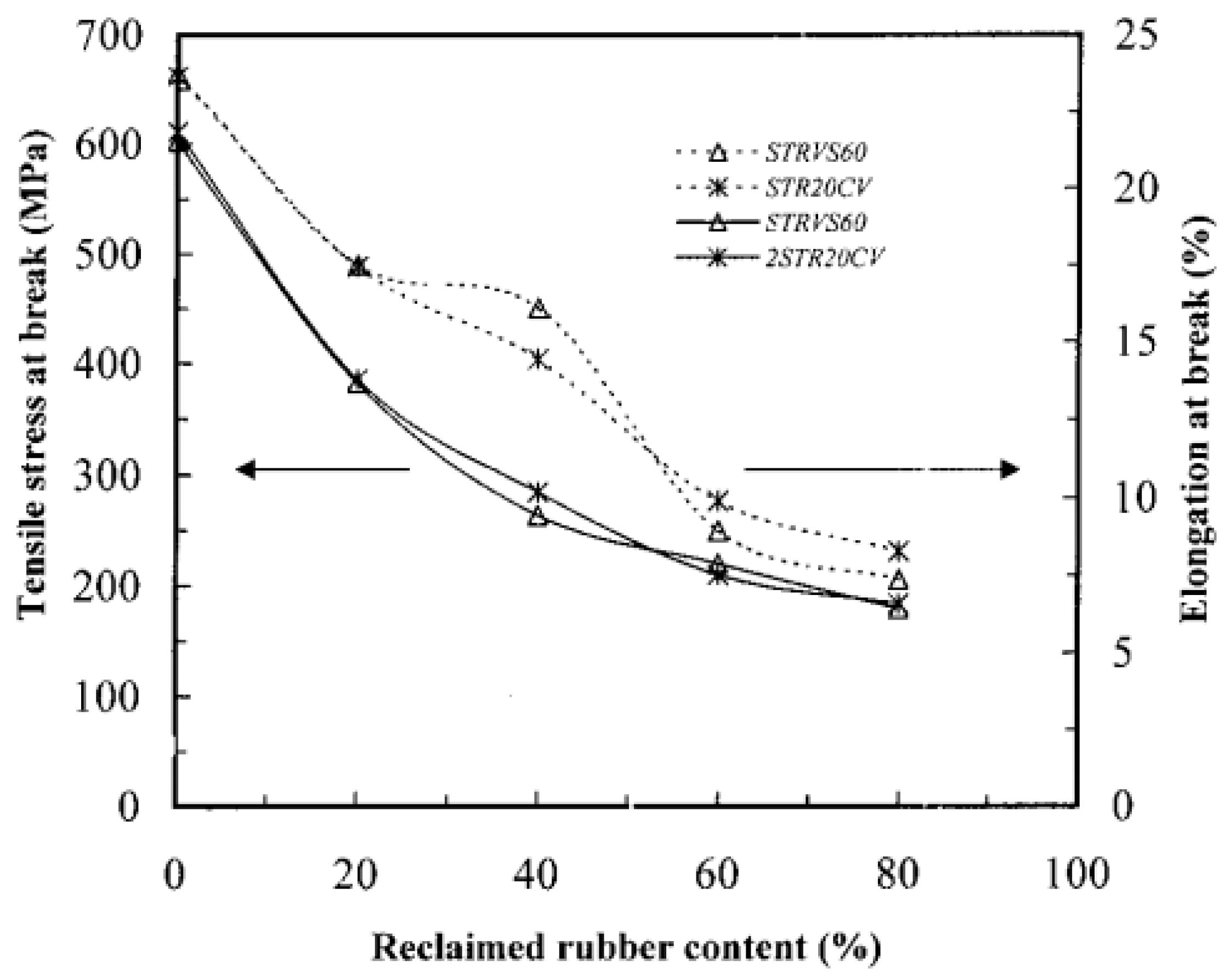
| RTR Content (%) | STRVS60 | STR20CV | ||
|---|---|---|---|---|
| n | K × 102 (g/g minn) | n | K × 102 (g/g minn) | |
| 0 | 0.40 | 1.44 | 0.39 | 1.39 |
| 20 | 0.34 | 1.58 | 0.38 | 1.47 |
| 40 | 0.35 | 1.65 | 0.34 | 1.52 |
| 60 | 0.32 | 1.81 | 0.32 | 1.63 |
| 80 | 0.27 | 2.07 | 0.28 | 1.93 |
| Compound | GTR/RTR Content | Mixing | Results | Ref. |
|---|---|---|---|---|
| NR/RTR | RTR (25, 40, 50 and 60 phr) | NR regeneration by diallyl disulfide (DADS) at 60 °C for 35 min on an open two-roll mixing mill. Compounding in a laboratory size two-roll mill based on ASTM D 15-54T (1954). | Increasing RTR content resulted in decreased scorch time and optimum cure time. Addition of N-cyclohexylthiophthalimide as PVI increased the scorch time of NR/RTR (50/50) blend. | [120] |
| NBR/RTR | RTR (20, 40, 60 and 80 phr) | MA grafting on RTR at 150 °C in a Brabender Plasticorder at 30 rpm for 3 min. Blends preparation on a laboratory size two-roll mill based on ASTM D 3182. | MA modification of RTR led to longer cure time, scorch time and lower cure rate in all blends since anhydrides grafted on the RTR surface as cure retarders delay the onset of cure reaction resulting in higher cure times. | [115] |
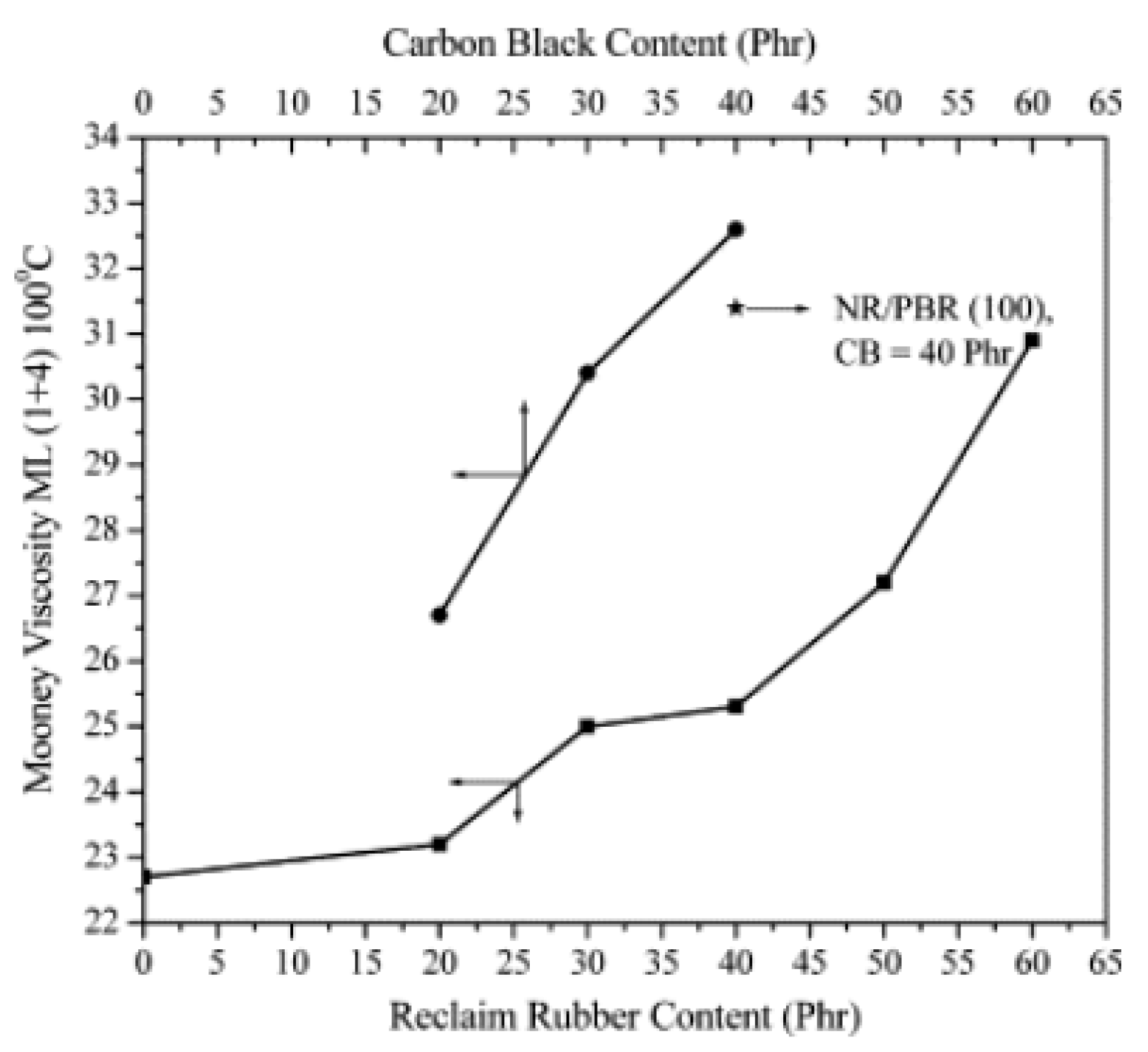
| NR/PBR/RTR | RTR (20, 30, 40, 50 and 60 phr) | Regeneration of GRT by tetramethyl thiuram disulfide (TMTD) in the presence of spindle oil. Mixing of NR/PBR/RTR compounds for 15 min via laboratory size two-roll mill at room temperature. | Increase in minimum torque and Mooney viscosity of the compounds by RTR addition due to higher carbon black content resulting in higher stiffness and chain mobility restriction, as well as lower optimum cure time related to the presence of active crosslinking sites in RTR. Increase in tensile modulus with increasing RTR content attributed to the higher gel fraction and crosslink density of the compounds. Also, increase in 200% moduli retention of NR/PBR/RTR with increasing RTR loading after aging attributed to higher crosslink density and the formation of new crosslinks due to the presence of active sites in RTR. | [121] |
| NR/RTR | RTR (20, 40, 60 and 80 phr) | NR mastication on a laboratory two-roll mill for 10, 20 or 30 min followed by blending with RTR for 10 min at 25 °C. | Shear viscosity increased with RTR content, but decreased with mastication time. Increase in tensile modulus with RTR loading due to the variation of crosslink density and chain mobility of RTR, as well as better blends homogeneity. | [122] |
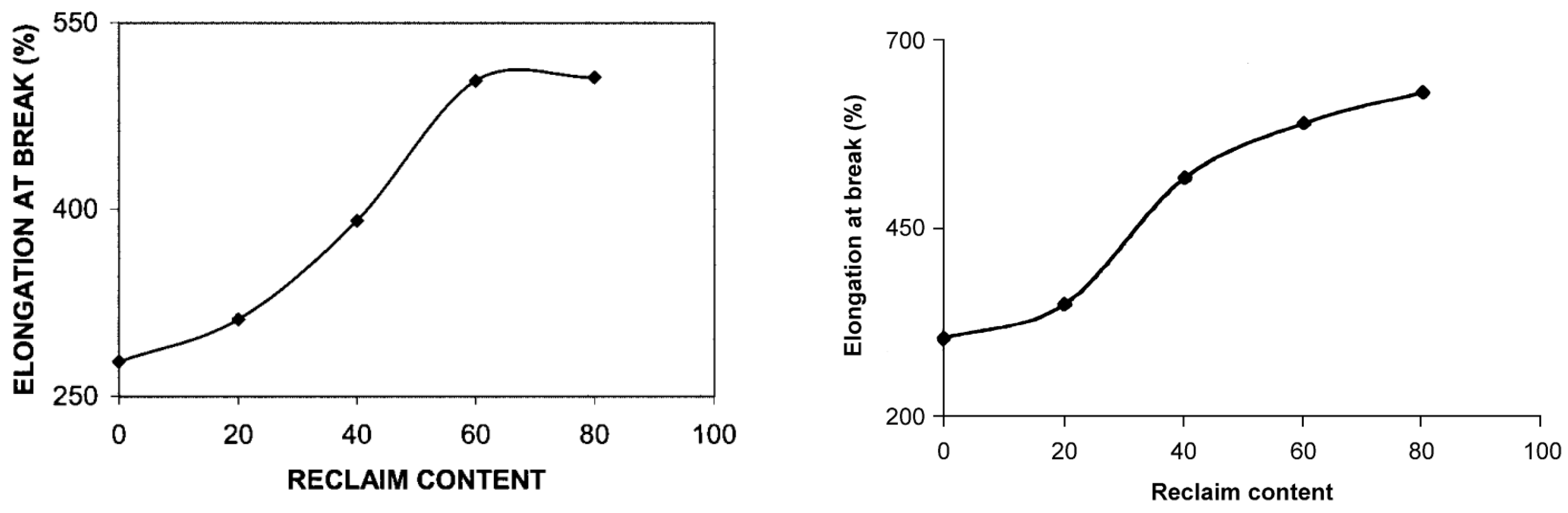
| NBR/RTR | RTR (20, 40, 60 and 80 phr) | Compound mixing according to ASTM D 3182 on a two-roll laboratory size mixing mill. | Increasing trend of elongation at break with RTR incorporation attributed to a plasticization effect of the processing oil in RTR. Decrease in aging resistance of the blends after RTR addition as it contains NR which is more prone to degradation during mixing. | [116] |
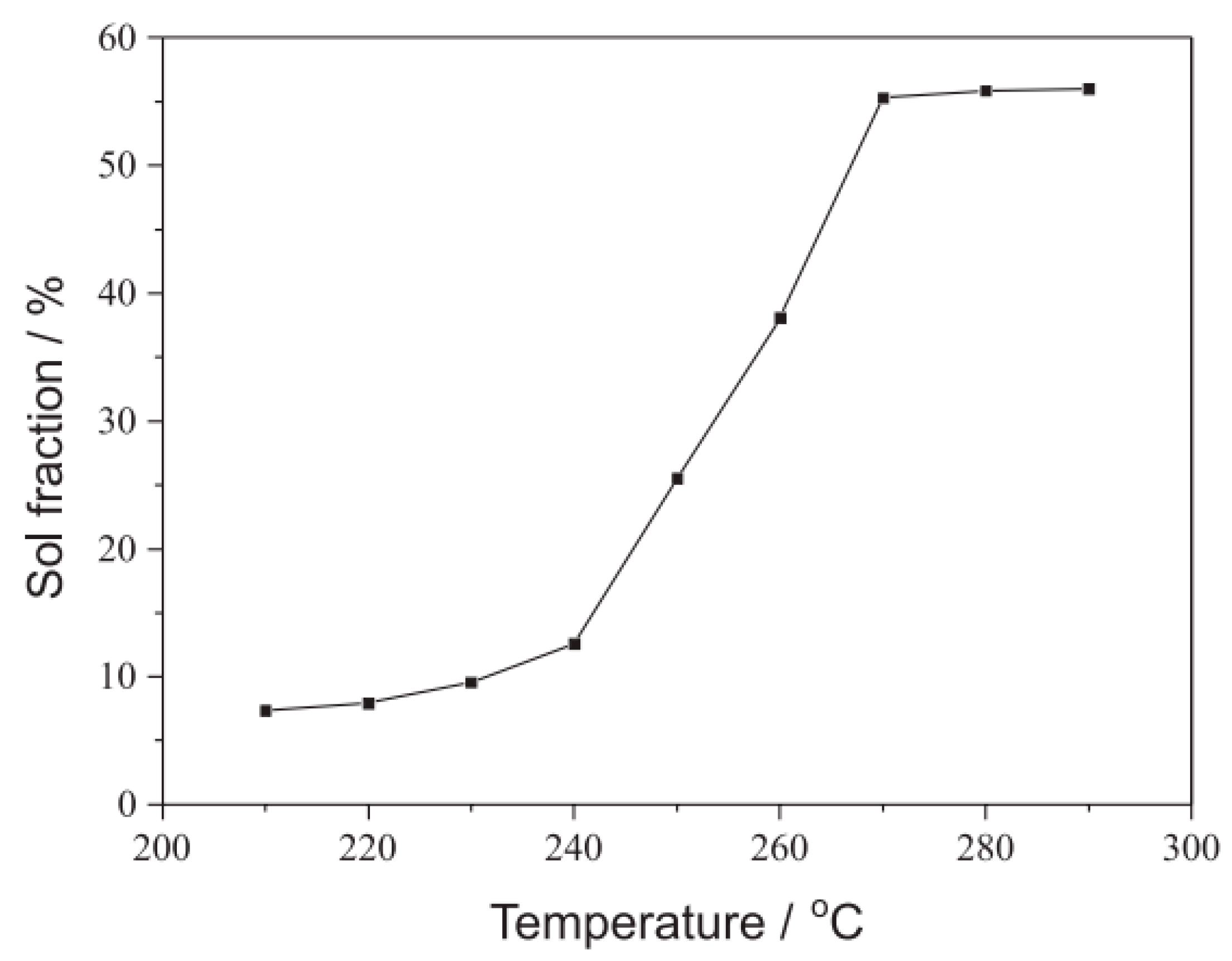
| SBR/GTR and SBR/RTR | GTR and RTR (10, 20, and 30) phr) | SBR mastication on a two-roll mill and blending with various contents of GTR or RTR for 10 min. | Microbial desulfurization of the GTR led to increased GTR sol fraction from 4.69-8.68%. Higher storage modulus and rigidity of SBR/GTR compounds than SBR/RTR compounds attributed to the higher crosslink density, carbon black content and chain mobility restriction of GTR. Higher elasticity of RTR compounds determined by the lower tan δ peak height of RTR. Also, better wettability of RTR due to more compatibility and good interfacial interactions between RTR and SBR. | [126] |
| NR/RTR NR/GTR | GTR and RTR (10, 30 and 50 phr) | GTR regeneration via a pan-mill type mechano-chemical reactor for 25 cycles. Blending with NR using a two-roll mill. | Lower scorch time and optimum cure time of NR/RTR blends compared to NR/GTR blends due to more unsaturated rubber in the RTR. Higher tensile strength and elongation at break of NR/RTR blends because of better compatibility and homogeneity of the blends. Also, RTR induced a higher degree of swelling compared to GTR attributed to the lower crosslink density of the partially regenerated RTR. | [124] |
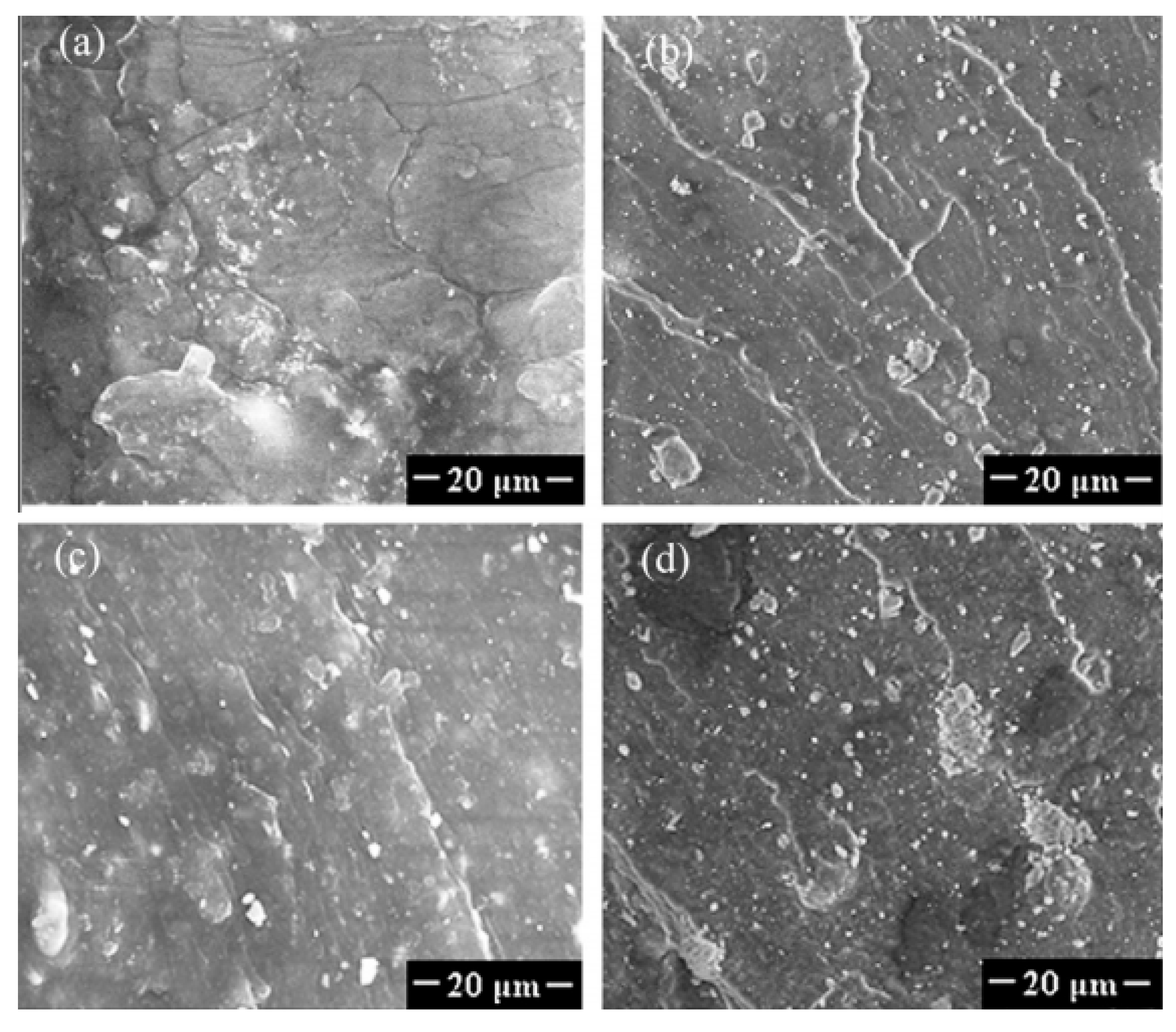
| NR/GTR | GTR (50 phr) | GTRcar and GTRtruck were exposed to microwave irradiation for 0-10 min. Preparation of NR/GTR blends at 70 °C using an internal batch mixer with rotational speed of 100 rpm. | Better thermal stability of NR/GTRcar than NR/GTRtruck attributed to the higher content of synthetic rubbers in GTRcar and also the evaporation of low MW volatile products formed during the thermal degradation of GTR. Improvement of tensile properties of modified GTR containing blends due to improved interfacial interaction confirmed by SEM images. | [131] |
| NBR/NR/GTR and NBR/NR/RTR | GTR (50 phr) | GTR regeneration in a microwave reactor for 3, 5 and 10 min with a power of 700 W. Blends preparation at 70 °C using an internal batch mixer with a rotational speed of 100 rpm. The curing system was based on zinc oxide, stearic acid, TBBS, TMTD and sulphur. | Microwave modification of GTR enhanced the tensile properties due to good interaction between the RTR and the matrix. GTR microwaves treatment resulted in an improvement of the crosslinking and hence better thermal stability of the blends after revulcanization, but microwave radiation for more than 5 min led to the degradation of the GTR main chains. | [70] |
| SBR/RTR | RTR (20, 30, 40, 50 and 60 phr) | GRT regeneration by tetramethyl thiuram disulfide (TMTD) in the presence of spindle oil in an open two-roll mill. Mixing of fresh SBR and RTR on an open two-roll mill at room temperature for 15 min. | Increase in minimum torque and Mooney viscosity of the SBR/RTR compounds with increasing RTR content, while the scorch time remained unchanged. RTR addition led to increase in tensile strength by about 19% for 20 parts filler and 115% for 60 parts RTR in compounds. Enhanced thermal stability of SBR/RTR compounds with RTR incorporation as char residue of SBR increased from 5.3% to 22.6% with the addition of 60 parts RTR. | [128] |
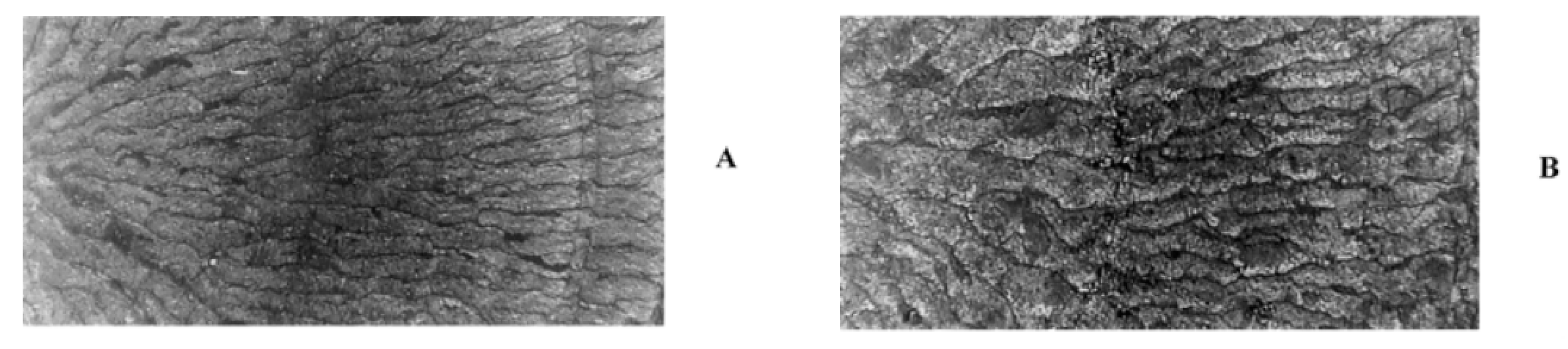
| NBR/GTR | GTR (5, 10, 15 and 20 phr) | The plasma treatment modification of GTR was carried out before mixing with NBR for 2 min with a plasma discharge power in range of 30 to 80 W. NBR/GTR compounding was performed on a two-roll mill for 10 min. | The water contact angle of the modified GTR decreased from 116° to 0° after 10 s of irritation inducing a hydrophilic nature to the modified GTR. The TS and tear strength of NBR/GTR improved by 42% and 21% respectively, due to increased interfacial bonding between the plasma modified GTR (20 wt.%) and NBR matrix. | [19] |
| SBR/RTR | RTR (20, 40, 60 and 80 phr) | The SBR/RTR blends were prepared in a laboratory size two-roll mill and vulcanization was carried out at 150 °C and 180 kg/cm2 using an electrically heated hydraulic press. | The tensile strength of SBR/RTR compounds increased from 1.9 MPa to 5.1 MPa with the addition of 80 parts RTR. Also, the elongation at break of SBR increased from 506% to 629% at 80 parts of RTR due to a plasticization effect caused by the presence of the processing oil in RTR. | [117] |
| NR/RTR | RTR (20, 40, 60 and 80 phr) | NR masticating was performed on a laboratory two-roll mill for 10 min followed by the addition of RTR and further mastication for 2 min. NR compounding with RTR was performed on a two-roll mill for 8 min at 25 °C. | The swelling degree of the compounds at the equilibrium state decreased with RTR content due to the increased crosslink density and the polymer–solvent interaction. Also, increasing the RTR content led to a higher elastic behavior (reduced tan δmax) and Tg attributed to the increase in crosslink density and the presence of carbon black in RTR. | [133] |
| NR/GTR and NR/RTR | GTR and RTR (0, 10, 20, 30 and 40 phr) | GTR surface regeneration was performed using a biological treatment by Thiobacillus sp. NR was masticated on a two-roll mill and then blended with GTR or RTR for 10 min followed by vulcanization in a press at 15 MPa and 150 °C according to ASTM D 2084. | GTR regeneration resulted in 30% increase of oxygen content on the GTR surface and a reduction of the GTR contact angle from 120.5° to 93.5° (RTR) after regeneration. NR/RTR compounds showed better compatibility and co-crosslinking at the interphase between RTR and virgin NR leading to better tensile properties. Addition of 10 phr RTR in the compounds retained 91% and 92% of their original TS and EB, respectively. | [127] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fazli, A.; Rodrigue, D. Recycling Waste Tires into Ground Tire Rubber (GTR)/Rubber Compounds: A Review. J. Compos. Sci. 2020, 4, 103. https://doi.org/10.3390/jcs4030103
Fazli A, Rodrigue D. Recycling Waste Tires into Ground Tire Rubber (GTR)/Rubber Compounds: A Review. Journal of Composites Science. 2020; 4(3):103. https://doi.org/10.3390/jcs4030103
Chicago/Turabian StyleFazli, Ali, and Denis Rodrigue. 2020. "Recycling Waste Tires into Ground Tire Rubber (GTR)/Rubber Compounds: A Review" Journal of Composites Science 4, no. 3: 103. https://doi.org/10.3390/jcs4030103





