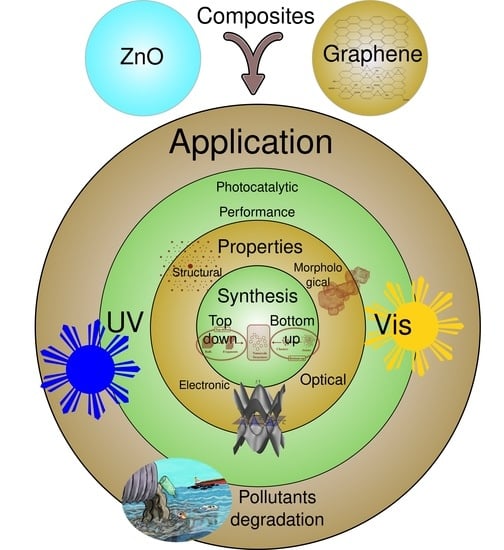
Synthesis, Characterization, and Photocatalytic Performance of ZnO–Graphene Nanocomposites: A Review
Abstract
:1. Introduction
2. Synthesis Methods of ZnO–Graphene Nanocomposites
2.1. Main Methods to Obtain ZnO–Graphene
2.1.1. Hydrothermal and Solvothermal
2.1.2. Co-Precipitation
2.1.3. Sol–Gel
2.1.4. Microwave-Assisted
2.1.5. Electrochemical
2.1.6. Photochemical
2.2. Type of Carbonaceous Support
2.3. Zinc Precursor
2.4. Incorporation of the ZnO
2.5. Surfactants
2.6. Morphologies and Sizes of ZnO
3. Characterization and Physicochemical Properties
3.1. Morphology
3.1.1. Scanning Electron Microscopy (SEM)
3.1.2. Transmission Electron Microscopy (TEM)
3.1.3. Scanning Tunneling Microscopy (STM)
3.1.4. Atomic Force Microscopy (AFM)
3.2. Structure
3.2.1. X-Ray Diffraction (XDR)
3.2.2. Energy Dispersive X-Ray (EDX)
3.2.3. Infrared (IR) Spectroscopy
3.2.4. Raman Spectroscopy
3.2.5. X-ray Photoelectron Spectroscopy (XPS)
3.3. Texture
Analysis BET
3.4. Thermal Properties
Thermogravimetric Analysis/Differential Thermal Analyzer (TG/DTA)
3.5. Optical Properties
Ultraviolet-Visible Spectroscopy (UV-vis)
3.6. Electrical Propierties
Electrochemical Impedance Spectroscopy (EIS)
3.7. Active Species Determination
3.7.1. Free-Radical-Capturing Experiments (Radical Scavengers)
3.7.2. Electron Paramagnetic Resonance Spectroscopy (EPR)
4. Photocatalytic Performance and Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, J.; Zhang, S.; Sun, M.; Yang, L.; Luo, S.; Crittenden, J.C. A Critical Review on Energy Conversion and Environmental Remediation of Photocatalysts with Remodeling Crystal Lattice, Surface, and Interface. ACS Nano 2019, 13, 9811–9840. [Google Scholar] [CrossRef]
- Li, C.; Xu, Y.; Tu, W.; Chen, G.; Xu, R. Metal-Free Photocatalysts for Various Applications in Energy Conversion and Environmental Purification. Green Chem. 2017, 19, 882–899. [Google Scholar] [CrossRef]
- Singla, S.; Sharma, S.; Basu, S.; Shetti, N.P.; Reddy, K.R. Graphene/Graphitic Carbon Nitride-Based Ternary Nanohybrids: Synthesis Methods, Properties, and Applications for Photocatalytic Hydrogen Production. FlatChem 2020, 24, 100200. [Google Scholar] [CrossRef]
- Tahir, M.; Tasleem, S.; Tahir, B. Recent Development in Band Engineering of Binary Semiconductor Materials for Solar Driven Photocatalytic Hydrogen Production. Int. J. Hydrog. Energy 2020, 45, 15985–16038. [Google Scholar] [CrossRef]
- Long, Z.; Li, Q.; Wei, T.; Zhang, G.; Ren, Z. Historical Development and Prospects of Photocatalysts for Pollutant Removal in Water. J. Hazard. Mater. 2020, 395, 122599. [Google Scholar] [CrossRef]
- Janković, N.Z.; Plata, D.L. Engineered Nanomaterials in the Context of Global Element Cycles. Environ. Sci. Nano 2019, 6, 2697–2711. [Google Scholar] [CrossRef] [Green Version]
- Moezzi, A.; McDonagh, A.M.; Cortie, M.B. Zinc Oxide Particles: Synthesis, Properties and Applications. Chem. Eng. J. 2012, 185–186, 1–22. [Google Scholar] [CrossRef]
- Bhati, V.S.; Hojamberdiev, M.; Kumar, M. Enhanced Sensing Performance of ZnO Nanostructures-Based Gas Sensors: A Review. Energy Rep. 2020, 6, 46–62. [Google Scholar] [CrossRef]
- Rackauskas, S.; Mustonen, K.; Järvinen, T.; Mattila, M.; Klimova, O.; Jiang, H.; Tolochko, O.; Lipsanen, H.; Kauppinen, E.I.; Nasibulin, A.G. Synthesis of ZnO Tetrapods for Flexible and Transparent UV Sensors. Nanotechnology 2012, 23, 095502. [Google Scholar] [CrossRef] [Green Version]
- Carofiglio, M.; Barui, S.; Cauda, V.; Laurenti, M. Doped Zinc Oxide Nanoparticles: Synthesis, Characterization and Potential Use in Nanomedicine. Appl. Sci. 2020, 10, 5194. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.; Liu, Q.; Duan, H. Recent Progress in Zn-Based Anodes for Advanced Lithium Ion Batteries. Mater. Chem. Front. 2018, 2, 1414–1435. [Google Scholar] [CrossRef]
- Consonni, V.; Briscoe, J.; Kärber, E.; Li, X.; Cossuet, T. ZnO Nanowires for Solar Cells: A Comprehensive Review. Nanotechnology 2019, 30, 362001. [Google Scholar] [CrossRef] [PubMed]
- Arrabito, G.; Aleeva, Y.; Pezzilli, R.; Ferrara, V.; Medaglia, P.G.; Pignataro, B.; Prestopino, G. Printing ZnO Inks: From Principles to Devices. Crystals 2020, 10, 449. [Google Scholar] [CrossRef]
- Singh, A.; Singh, N.B.; Afzal, S.; Singh, T.; Hussain, I. Zinc Oxide Nanoparticles: A Review of Their Biological Synthesis, Antimicrobial Activity, Uptake, Translocation and Biotransformation in Plants. J. Mater. Sci. 2018, 53, 185–201. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A Review of ZnO Nanoparticles as Solar Photocatalysts: Synthesis, Mechanisms and Applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Kegel, J.; Povey, I.M.; Pemble, M.E. Zinc Oxide for Solar Water Splitting: A Brief Review of the Material’s Challenges and Associated Opportunities. Nano Energy 2018, 54, 409–428. [Google Scholar] [CrossRef]
- Desai, M.A.; Vyas, A.N.; Saratale, G.D.; Sartale, S.D. Zinc Oxide Superstructures: Recent Synthesis Approaches and Application for Hydrogen Production via Photoelectrochemical Water Splitting. Int. J. Hydrog. Energy 2019, 44, 2091–2127. [Google Scholar] [CrossRef]
- Das, A.; Nikhil, S.K.; Nair, R.G. Influence of Surface Morphology on Photocatalytic Performance of Zinc Oxide: A Review. Nano-Struct. Nano-Objects 2019, 19, 100353. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Lojkowski, W. A Review of Microwave Synthesis of Zinc Oxide Nanomaterials: Reactants, Process Parameters and Morphologies. Nanomaterials 2020, 10, 1086. [Google Scholar] [CrossRef]
- Samadi, M.; Zirak, M.; Naseri, A.; Kheirabadi, M.; Ebrahimi, M.; Moshfegh, A.Z. Design and Tailoring of One-Dimensional ZnO Nanomaterials for Photocatalytic Degradation of Organic Dyes: A Review. Res. Chem. Intermed. 2019, 45, 2197–2254. [Google Scholar] [CrossRef]
- Ma, M.; Huang, Y.; Liu, J.; Liu, K.; Wang, Z.; Zhao, C.; Qu, S.; Wang, Z. Engineering the Photoelectrochemical Behaviors of ZnO for Efficient Solar Water Splitting. J. Semicond. 2020, 41, 091702. [Google Scholar] [CrossRef]
- Chang, J.; Waclawik, E.R. Facet-Controlled Self-Assembly of ZnO Nanocrystals by Non-Hydrolytic Aminolysis and Their Photodegradation Activities. CrystEngComm 2012, 14, 4041–4048. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.-K.; Tsang, S.C.E. Facet-Dependent Photocatalysis of Nanosize Semiconductive Metal Oxides and Progress of Their Characterization. Nano Today 2018, 18, 15–34. [Google Scholar] [CrossRef]
- Kumar, S.G.; Rao, K.S.R.K. Zinc Oxide Based Photocatalysis: Tailoring Surface-Bulk Structure and Related Interfacial Charge Carrier Dynamics for Better Environmental Applications. RSC Adv. 2015, 5, 3306–3351. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, A.; Kumar, A.; Krishnan, V. Nanoscale Zinc Oxide Based Heterojunctions as Visible Light Active Photocatalysts for Hydrogen Energy and Environmental Remediation. Catal. Rev. 2020, 62, 346–405. [Google Scholar] [CrossRef]
- Albiter, E.; Barrera-Andrade, J.M.; Rojas-García, E.; Valenzuela, M.A. Recent advances of nanocarbon-inorganic hybrids in photocatalysis. In Nanocarbon and Its Composites; Khan, A., Jawaid, M., Inamuddin Asiri, A.M., Eds.; Woodhead Publishing Series in Composites Science and Engineering; Woodhead Publishing: Sawston, UK, 2019; pp. 521–588. ISBN 978-0-08-102509-3. [Google Scholar]
- Mamba, G.; Gangashe, G.; Moss, L.; Hariganesh, S.; Thakur, S.; Vadivel, S.; Mishra, A.K.; Vilakati, G.D.; Muthuraj, V.; Nkambule, T.T.I. State of the Art on the Photocatalytic Applications of Graphene Based Nanostructures: From Elimination of Hazardous Pollutants to Disinfection and Fuel Generation. J. Environ. Chem. Eng. 2020, 8, 103505. [Google Scholar] [CrossRef]
- Grajek, H.; Jonik, J.; Witkiewicz, Z.; Wawer, T.; Purchała, M. Applications of Graphene and Its Derivatives in Chemical Analysis. Crit. Rev. Anal. Chem. 2020, 50, 445–471. [Google Scholar] [CrossRef]
- Li, D.; Wang, T.; Li, Z.; Xu, X.; Wang, C.; Duan, Y. Application of Graphene-Based Materials for Detection of Nitrate and Nitrite in Water—A Review. Sensors 2020, 20, 54. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Wang, S. Research Advances in the Synthesis of Nanocarbon-Based Photocatalysts and Their Applications for Photocatalytic Conversion of Carbon Dioxide to Hydrocarbon Fuels. Energy Fuels 2014, 28, 22–36. [Google Scholar] [CrossRef]
- Ashouri, R.; Ghasemipoor, P.; Rasekh, B.; Yazdian, F.; Mofradnia, S.R. The Effect of ZnO-Based Carbonaceous Materials for Degradation of Benzoic Pollutants: A Review. Int. J. Environ. Sci. Technol. 2019, 16, 1729–1740. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Serrà, A.; Ibrahim, M.N.M. Advances and Challenges in Developing Efficient Graphene Oxide-Based ZnO Photocatalysts for Dye Photo-Oxidation. Nanomaterials 2020, 10, 932. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Xie, J. Graphene in Photocatalysis: A Review. Small 2016, 12, 6640–6696. [Google Scholar] [CrossRef] [PubMed]
- Mohd, A.M.A.; Julkapli, N.M.; Abd, H.S.B. Review on ZnO Hybrid Photocatalyst: Impact on Photocatalytic Activities of Water Pollutant Degradation. Rev. Inorg. Chem. 2016, 36, 77–104. [Google Scholar] [CrossRef]
- Sushma, C.; Girish Kumar, S. Advancements in the Zinc Oxide Nanomaterials for Efficient Photocatalysis. Chem. Pap. 2017, 71, 2023–2042. [Google Scholar] [CrossRef]
- Hong, X.; Wang, X.; Li, Y.; Fu, J.; Liang, B. Progress in Graphene/Metal Oxide Composite Photocatalysts for Degradation of Organic Pollutants. Catalysts 2020, 10, 921. [Google Scholar] [CrossRef]
- Prasad, C.; Liu, Q.; Tang, H.; Yuvaraja, G.; Long, J.; Rammohan, A.; Zyryanov, G.V. An Overview of Graphene Oxide Supported Semiconductors Based Photocatalysts: Properties, Synthesis and Photocatalytic Applications. J. Mol. Liq. 2020, 297, 111826. [Google Scholar] [CrossRef]
- Gautam, S.; Agrawal, H.; Thakur, M.; Akbari, A.; Sharda, H.; Kaur, R.; Amini, M. Metal Oxides and Metal Organic Frameworks for the Photocatalytic Degradation: A Review. J. Environ. Chem. Eng. 2020, 8, 103726. [Google Scholar] [CrossRef]
- Adam, R.E.; Chalangar, E.; Pirhashemi, M.; Pozina, G.; Liu, X.; Palisaitis, J.; Pettersson, H.; Willander, M.; Nur, O. Graphene-Based Plasmonic Nanocomposites for Highly Enhanced Solar-Driven Photocatalytic Activities. RSC Adv. 2019, 9, 30585–30598. [Google Scholar] [CrossRef] [Green Version]
- Kindalkar, V.S.; Sandeep, K.M.; Kumara, K.; Dharmaprakash, S.M. Sol-Gel Synthesized Spin Coated GO: ZnO Composite Thin Films: Optical, Structural and Electrical Studies. Mater. Res. Express 2019, 6, 096435. [Google Scholar] [CrossRef]
- Yein, W.T.; Wang, Q.; Feng, X.; Li, Y.; Wu, X. Enhancement of Photocatalytic Performance in Sonochemical Synthesized ZnO–RGO Nanocomposites Owing to Effective Interfacial Interaction. Environ. Chem. Lett. 2018, 16, 251–264. [Google Scholar] [CrossRef]
- Samal, A.; Das, D.P. Transfiguring UV Light Active “Metal Oxides” to Visible Light Active Photocatayst by Reduced Graphene Oxide Hypostatization. Catal. Today 2018, 300, 124–135. [Google Scholar] [CrossRef]
- Yu, W.; Chen, X.; Mei, W.; Chen, C.; Tsang, Y. Photocatalytic and Electrochemical Performance of Three-Dimensional Reduced Graphene Oxide/WS2/Mg-Doped ZnO Composites. Appl. Surf. Sci. 2017, 400, 129–138. [Google Scholar] [CrossRef]
- Moqbel, R.A.; Gondal, M.A.; Qahtan, T.F.; Dastageer, M.A. Pulsed Laser Synthesis in Liquid of Efficient Visible-Light-Active ZnO/RGO Nanocomposites for Improved Photo-Catalytic Activity. Mater. Res. Express 2018, 5, 035050. [Google Scholar] [CrossRef]
- Jin, X.; Ma, Z.; Liu, G.; Hu, D.; Song, C.; Huang, Q. In-Situ Ionothermal Precipitation of Well-Dispersed ZnO Nanoparticles onto 2-Dimension Neat Graphene Sheets with Excellent Photocatalytic Activity. J. Environ. Chem. Eng. 2020, 8, 104030. [Google Scholar] [CrossRef]
- Shwetharani, R.; Chandan, H.R.; Sakar, M.; Balakrishna, G.R.; Reddy, K.R.; Raghu, A.V. Photocatalytic Semiconductor Thin Films for Hydrogen Production and Environmental Applications. Int. J. Hydrog. Energy 2020, 45, 18289–18308. [Google Scholar] [CrossRef]
- Neelgund, G.M.; Oki, A. ZnO Conjugated Graphene: An Efficient Sunlight Driven Photocatalyst for Degradation of Organic Dyes. Mater. Res. Bull. 2020, 129, 110911. [Google Scholar] [CrossRef]
- Van Tuan, P.; Phuong, T.T.; Tan, V.T.; Nguyen, S.X.; Khiem, T.N. In-Situ Hydrothermal Fabrication and Photocatalytic Behavior of ZnO/Reduced Graphene Oxide Nanocomposites with Varying Graphene Oxide Concentrations. Mater. Sci. Semicond. Process. 2020, 115, 105114. [Google Scholar] [CrossRef]
- Boukhoubza, I.; Khenfouch, M.; Achehboune, M.; Leontie, L.; Carlescu, A.; Doroftei, C.; Mothudi, B.M.; Zorkani, I.; Jorio, A. Graphene Oxide Coated Flower-Shaped ZnO Nanorods: Optoelectronic Properties. J. Alloy. Compd. 2020, 831, 154874. [Google Scholar] [CrossRef]
- Mahmud, R.A.; Shafawi, A.N.; Ahmed Ali, K.; Putri, L.K.; Md Rosli, N.I.; Mohamed, A.R. Graphene Nanoplatelets with Low Defect Density as a Synergetic Adsorbent and Electron Sink for ZnO in the Photocatalytic Degradation of Methylene Blue under UV–Vis Irradiation. Mater. Res. Bull. 2020, 128, 110876. [Google Scholar] [CrossRef]
- Lee, K.S.; Park, Y.J.; Shim, J.; Lim, G.-H.; Yim, S.-Y.; Seo, J.W.; Ryu, J.H.; Son, D.I. Inhibition of Photoconversion Activity in Self-Assembled ZnO-Graphene Quantum Dots Aggregated by 4-Aminophenol Used as a Linker. Molecules 2020, 25, 2802. [Google Scholar] [CrossRef]
- Yu, X.; Li, Z.; Dang, K.; Zhang, Z.; Gao, L.; Duan, L.; Jiang, Z.; Fan, J.; Zhao, P. Enhanced Photocatalytic Activity of Ag–ZnO/RGO Nanocomposites for Removal of Methylene Blue. J. Mater. Sci. Mater. Electron. 2018, 29, 8729–8737. [Google Scholar] [CrossRef]
- Varanda, L.C.; de Souza, C.G.S.; Perecin, C.J.; de Moraes, D.A.; de Queiróz, D.F.; Neves, H.R.; Souza Junior, J.B.; da Silva, M.F.; Albers, R.F.; da Silva, T.L. Inorganic and organic–inorganic composite nanoparticles with potential biomedical applications: Synthesis challenges for enhanced performance. In Materials for Biomedical Engineering; Grumezescu, V., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 47–99. ISBN 978-0-12-818431-8. [Google Scholar]
- Rane, A.V.; Kanny, K.; Abitha, V.K.; Thomas, S. Methods for Synthesis of Nanoparticles and Fabrication of Nanocomposites. In Synthesis of Inorganic Nanomaterials; Mohan Bhagyaraj, S., Oluwafemi, O.S., Kalarikkal, N., Thomas, S., Eds.; Micro and Nano Technologies; Woodhead Publishing: Sawston, UK, 2018; pp. 121–139. ISBN 978-0-08-101975-7. [Google Scholar]
- Prasad, S.; Kumar, V.; Kirubanandam, S.; Barhoum, A. Engineered nanomaterials: Nanofabrication and surface functionalization. In Emerging Applications of Nanoparticles and Architecture Nanostructures; Barhoum, A., Makhlouf, A.S.H., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2018; pp. 305–340. ISBN 978-0-323-51254-1. [Google Scholar]
- Yao, C.; Zhu, J.; Li, H.; Zheng, B.; Wei, Y. Synthesis of ZnO Photocatalysts Using Various Surfactants. Russ. J. Phys. Chem. 2017, 91, 2680–2685. [Google Scholar] [CrossRef]
- Oppong, S.O.B.; Anku, W.W.; Shukla, S.K.; Govender, P.P. Synthesis and Characterisation of Neodymium Doped-Zinc Oxide–Graphene Oxide Nanocomposite as a Highly Efficient Photocatalyst for Enhanced Degradation of Indigo Carmine in Water under Simulated Solar Light. Res. Chem. Intermed. 2017, 43, 481–501. [Google Scholar] [CrossRef]
- Ahmad, I.; Akhtar, M.S.; Ahmed, E.; Ahmad, M. Highly Efficient Visible Light Driven Photocatalytic Activity of Graphene and CNTs Based Mg Doped ZnO Photocatalysts: A Comparative Study. Sep. Purif. Technol. 2020, 245, 116892. [Google Scholar] [CrossRef]
- Pogacean, F.; Ştefan, M.; Toloman, D.; Popa, A.; Leostean, C.; Turza, A.; Coros, M.; Pana, O.; Pruneanu, S. Photocatalytic and Electrocatalytic Properties of NGr-ZnO Hybrid Materials. Nanomaterials 2020, 10, 1473. [Google Scholar] [CrossRef]
- Yadav, R.; Kumar, V.; Saxena, V.; Singh, P.; Singh, V.K. Preparation of Controlled Lotus like Structured ZnO Decorated Reduced Graphene Oxide Nanocomposites to Obtain Enhanced Photocatalytic Properties. Ceram. Int. 2019, 45, 24999–25009. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Jiang, J.; Wan, Z.; Su, Y.; Tang, H. Insight into Charge Carrier Separation and Solar-Light Utilization: RGO Decorated 3D ZnO Hollow Microspheres for Enhanced Photocatalytic Hydrogen Evolution. J. Colloid Interface Sci. 2020, 564, 322–332. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.K.; Singh, D.P.; Savu, R.; Moshkalev, S.A. Microwave Heating Time Dependent Synthesis of Various Dimensional Graphene Oxide Supported Hierarchical ZnO Nanostructures and Its Photoluminescence Studies. Mater. Des. 2016, 111, 291–300. [Google Scholar] [CrossRef]
- Bensebaa, F. Wet Production Methods. In Interface Science and Technology; Bensebaa, F., Ed.; Nanoparticle Technologies; Elsevier: Amsterdam, The Netherlands, 2013; Volume 19, pp. 85–146. [Google Scholar]
- Török, B.; Dransfield, T. (Eds.) Green Chemistry, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 978-0-12-809270-5. [Google Scholar]
- Abdel-Karim, R. Electrochemical Synthesis of Nanocomposites. Electrodepos. Compos. Mater. 2016. [Google Scholar] [CrossRef] [Green Version]
- Teh, S.J.; Lai, C.W.; Hamid, S.B.A. Novel Layer-by-Layer Assembly of RGO-Hybridised ZnO Sandwich Thin Films for the Improvement of Photo-Catalysed Hydrogen Production. J. Energy Chem. 2016, 25, 336–344. [Google Scholar] [CrossRef]
- Wang, P.; Wu, D.; Ao, Y.; Wang, C.; Hou, J. ZnO Nanorod Arrays Co-Loaded with Au Nanoparticles and Reduced Graphene Oxide: Synthesis, Characterization and Photocatalytic Application. Colloids Surf. A Physicochem. Eng. Asp. 2016, 492, 71–78. [Google Scholar] [CrossRef]
- Teh, S.J.; Yeoh, S.L.; Lee, K.M.; Lai, C.W.; Abdul Hamid, S.B.; Thong, K.L. Effect of Reduced Graphene Oxide-Hybridized ZnO Thin Films on the Photoinactivation of Staphylococcus Aureus and Salmonella Enterica Serovar Typhi. J. Photochem. Photobiol. B Biol. 2016, 161, 25–33. [Google Scholar] [CrossRef]
- Nanakkal, A.R.; Alexander, L.K. Photocatalytic Activity of Graphene/ZnO Nanocomposite Fabricated by Two-Step Electrochemical Route. J. Chem. Sci. 2017, 129, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.-S. Inorganic Photochemical Synthesis. In Modern Inorganic Synthetic Chemistry, 2nd ed.; Xu, R., Xu, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 143–165. ISBN 978-0-444-63591-4. [Google Scholar]
- Chen, C.; Zhou, P.; Wang, N.; Ma, Y.; San, H. UV-Assisted Photochemical Synthesis of Reduced Graphene Oxide/ZnO Nanowires Composite for Photoresponse Enhancement in UV Photodetectors. Nanomaterials 2018, 8, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdolhosseinzadeh, S.; Asgharzadeh, H.; Sadighikia, S.; Khataee, A. UV-Assisted Synthesis of Reduced Graphene Oxide–ZnO Nanorod Composites Immobilized on Zn Foil with Enhanced Photocatalytic Performance. Res. Chem. Intermed. 2016, 42, 4479–4496. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Ding, Y.; Zhu, Y. ZnO/Graphene/Ag Composite as Recyclable Surface-Enhanced Raman Scattering Substrates. Appl. Opt. AO 2016, 55, 9105–9112. [Google Scholar] [CrossRef]
- Jabeen, M.; Ishaq, M.; Song, W.; Xu, L.; Maqsood, I.; Deng, Q. UV-Assisted Photocatalytic Synthesis of ZnO-Reduced Graphene Oxide Nanocomposites with Enhanced Photocatalytic Performance in Degradation of Methylene Blue. ECS J. Solid State Sci. Technol. 2017, 6, M36. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Jaroniec, M.; Chen, X. Cocatalysts for Selective Photoreduction of CO2 into Solar Fuels. Chem. Rev. 2019, 119, 3962–4179. [Google Scholar] [CrossRef]
- Hu, D.; Song, C.; Jin, X.; Huang, Q. Polymer Solution-Assisted Assembly of Hierarchically Nanostructured ZnO onto 2D Neat Graphene Sheets with Excellent Photocatalytic Performance. J. Alloy. Compd. 2020, 843, 156030. [Google Scholar] [CrossRef]
- Moradi, S.; Sobhgol, S.A.; Hayati, F.; Isari, A.A.; Kakavandi, B.; Bashardoust, P.; Anvaripour, B. Performance and Reaction Mechanism of MgO/ZnO/Graphene Ternary Nanocomposite in Coupling with LED and Ultrasound Waves for the Degradation of Sulfamethoxazole and Pharmaceutical Wastewater. Sep. Purif. Technol. 2020, 251, 117373. [Google Scholar] [CrossRef]
- Labhane, P.K.; Sonawane, S.H.; Sonawane, G.H.; Patil, S.P.; Huse, V.R. Influence of Mg Doping on ZnO Nanoparticles Decorated on Graphene Oxide (GO) Crumpled Paper like Sheet and Its High Photo Catalytic Performance under Sunlight. J. Phys. Chem. Solids 2018, 114, 71–82. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, J.; Cui, G.; Deng, N.; Wang, W.; Wang, Q.; Tang, B. Photocatalytic H2 Evolution Improvement for H Free-Radical Stabilization by Electrostatic Interaction of a Cu-BTC MOF with ZnO/GO. Nano Res. 2018, 11, 979–987. [Google Scholar] [CrossRef]
- Yuan, M.; Xiong, C.; Jiang, L.; Li, H.; Yuan, M. The Preparation, Characterization, Mechanical and Antibacterial Properties of GO-ZnO Nanocomposites with a Poly(l-Lactide)-Modified Surface. Materials 2018, 11, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, T.; Hu, J.; Yang, Y.; Que, W.; Yin, X.; Wu, H.; Chen, L. Solid-State Synthesis of ZnO Nanorods Coupled with Reduced Graphene Oxide for Photocatalytic Application. J. Mater. Sci. Mater. Electron. 2018, 29, 4888–4894. [Google Scholar] [CrossRef]
- Mohamed, H.H. Sonochemical Synthesis of ZnO Hollow Microstructure/Reduced Graphene Oxide for Enhanced Sunlight Photocatalytic Degradation of Organic Pollutants. J. Photochem. Photobiol. A Chem. 2018, 353, 401–408. [Google Scholar] [CrossRef]
- Yousefi, R.; Beheshtian, J.; Seyed-Talebi, S.M.; Azimi, H.R.; Jamali-Sheini, F. Experimental and Theoretical Study of Enhanced Photocatalytic Activity of Mg-Doped ZnO NPs and ZnO/RGO Nanocomposites. Chem. Asian J. 2018, 13, 194–203. [Google Scholar] [CrossRef]
- Guo, Q.; Ghadiri, R.; Weigel, T.; Aumann, A.; Gurevich, E.L.; Esen, C.; Medenbach, O.; Cheng, W.; Chichkov, B.; Ostendorf, A. Comparison of in Situ and Ex Situ Methods for Synthesis of Two-Photon Polymerization Polymer Nanocomposites. Polymers 2014, 6, 2037–2050. [Google Scholar] [CrossRef]
- Jafarisani, M.; Cheshme Khavar, A.H.; Mahjoub, A.R.; Luque, R.; Rodríguez-Padrón, D.; Satari, M.; Gharravi, A.M.; Khastar, H.; Kazemi, S.S.; Masoumikarimi, M. Enhanced Visible-Light-Driven Photocatalytic Degradation of Emerging Water Contaminants by a Modified Zinc Oxide-Based Photocatalyst; In-Vivo and in-Vitro Toxicity Evaluation of Wastewater and PCO-Treated Water. Sep. Purif. Technol. 2020, 243, 116430. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, N.; Chauhan, R.; Singh, V.; Srivastava, V.C.; Bhatnagar, R. Growth of Hierarchical ZnO Nano Flower on Large Functionalized RGO Sheet for Superior Photocatalytic Mineralization of Antibiotic. Chem. Eng. J. 2020, 392, 123746. [Google Scholar] [CrossRef]
- Hu, L.; Xing, X.; Zhang, W.; Hu, C.; Li, J. A Facile Chemical Solution Route to Synthesize ZnO/Graphene Composite and the Improved Photocatalytic Property. J. Nanosci. Nanotechnol. 2020, 20, 4480–4484. [Google Scholar] [CrossRef]
- Ramos, P.G.; Luyo, C.; Sánchez, L.A.; Gomez, E.D.; Rodriguez, J.M. The Spinning Voltage Influence on the Growth of ZnO-RGO Nanorods for Photocatalytic Degradation of Methyl Orange Dye. Catalysts 2020, 10, 660. [Google Scholar] [CrossRef]
- El-Fawal, E.M.; Younis, S.A.; Moustafa, Y.M.; Serp, P. Preparation of Solar-Enhanced AlZnO@carbon Nano-Substrates for Remediation of Textile Wastewaters. J. Environ. Sci. 2020, 92, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, H.; Bagheri, H. Two Novel Sets of UiO-66@ Metal Oxide/Graphene Oxide Z-Scheme Heterojunction: Insight into Tetracycline and Malathion Photodegradation. J. Environ. Sci. 2020, 91, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Ding, J.; Yu, P.; He, G.; Chen, Y.; Chen, H. Low-Temperature Preparation of Magnetically Separable Fe3O4@ZnO-RGO for High-Performance Removal of Methylene Blue in Visible Light. J. Alloy. Compd. 2020, 821, 153366. [Google Scholar] [CrossRef]
- Murali, A.; Sarswat, P.K.; Free, M.L. Minimizing Electron-Hole Pair Recombination through Band-Gap Engineering in Novel ZnO-CeO2-RGO Ternary Nanocomposite for Photoelectrochemical and Photocatalytic Applications. Environ. Sci. Pollut. Res. 2020, 27, 25042–25056. [Google Scholar] [CrossRef]
- Yin, P.T.; Shah, S.; Chhowalla, M.; Lee, K.-B. Design, Synthesis, and Characterization of Graphene–Nanoparticle Hybrid Materials for Bioapplications. Chem. Rev. 2015, 115, 2483–2531. [Google Scholar] [CrossRef] [Green Version]
- Ghanem, A.F.; Badawy, A.A.; Mohram, M.E.; Rehim, M.H.A. Synergistic Effect of Zinc Oxide Nanorods on the Photocatalytic Performance and the Biological Activity of Graphene Nano Sheets. Heliyon 2020, 6, e03283. [Google Scholar] [CrossRef]
- Shamsuri, A.A.; Jamil, S.N.A. A Short Review on the Effect of Surfactants on the Mechanico-Thermal Properties of Polymer Nanocomposites. Appl. Sci. 2020, 10, 4867. [Google Scholar] [CrossRef]
- Muthukrishnaraj, A.; Kalaivani, S.S.; Manikandan, A.; Kavitha, H.P.; Srinivasan, R.; Balasubramanian, N. Sonochemical Synthesis and Visible Light Induced Photocatalytic Property of Reduced Graphene Oxide@ZnO Hexagonal Hollow Rod Nanocomposite. J. Alloy. Compd. 2020, 836, 155377. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Song, L.; Su, Y.; Guo, Y.; Wu, L.; Zhang, T. Illustration of Charge Transfer in Graphene-Coated Hexagonal ZnO Photocatalysts Using Kelvin Probe Force Microscopy. RSC Adv. 2018, 8, 885–894. [Google Scholar] [CrossRef] [Green Version]
- Louis, J.; Kavitha, M.K.; Anjana, V.; Jayaraj, M.K.; John, H. A Facile Surfactant Assisted Hydrothermal Synthesis of ZnO and Graphene Loaded ZnO for Efficient Photocatalytic Self-Cleaning. Mater. Res. Express 2020, 6, 1250i4. [Google Scholar] [CrossRef]
- Li, Y.-M.; Chen, X.; Liu, X.-J.; Li, W.-Y.; He, Y.-Q. Electrochemical Reduction of Graphene Oxide on ZnO Substrate and Its Photoelectric Properties. Acta Phys. Chim. Sin. 2017, 33, 554–562. [Google Scholar] [CrossRef]
- She, P.; Yin, S.; He, Q.; Zhang, X.; Xu, K.; Shang, Y.; Men, X.; Zeng, S.; Sun, H.; Liu, Z. A Self-Standing Macroporous Au/ZnO/Reduced Graphene Oxide Foam for Recyclable Photocatalysis and Photocurrent Generation. Electrochim. Acta 2017, 246, 35–42. [Google Scholar] [CrossRef]
- Bera, S.; Pal, M.; Naskar, A.; Jana, S. Hierarchically Structured ZnO-Graphene Hollow Microspheres towards Effective Reusable Adsorbent for Organic Pollutant via Photodegradation Process. J. Alloy. Compd. 2016, 669, 177–186. [Google Scholar] [CrossRef]
- Rokhsat, E.; Akhavan, O. Improving the Photocatalytic Activity of Graphene Oxide/ZnO Nanorod Films by UV Irradiation. Appl. Surf. Sci. 2016, 371, 590–595. [Google Scholar] [CrossRef]
- Hao, C.; Yang, Y.; Shen, Y.; Feng, F.; Wang, X.; Zhao, Y.; Ge, C. Liquid Phase-Based Ultrasonic-Assisted Synthesis of G–ZnO Nanocomposites and Its Sunlight Photocatalytic Activity. Mater. Des. 2016, 89, 864–871. [Google Scholar] [CrossRef]
- Bera, S.; Naskar, A.; Pal, M.; Jana, S. Low Temperature Synthesis of Graphene Hybridized Surface Defective Hierarchical Core–Shell Structured ZnO Hollow Microspheres with Long-Term Stable and Enhanced Photoelectrochemical Activity. RSC Adv. 2016, 6, 36058–36068. [Google Scholar] [CrossRef]
- Tie, W.; Bhattacharyya, S.S.; Wang, Y.; He, W.; Lee, S.H. Facile In-Situ Synthesis of a Zinc Oxide Crystals/Few-Layered Graphene Flake Composite for Enhanced Photocatalytic Performance. J. Photochem. Photobiol. A Chem. 2017, 348, 89–95. [Google Scholar] [CrossRef]
- Hu, W.-C.; Chen, Y.-A.; Hsieh, P.-Y.; Tsao, C.-W.; Chiu, Y.-H.; Chang, T.-F.M.; Chen, C.-Y.; Sone, M.; Hsu, Y.-J. Reduced Graphene Oxides-Wrapped ZnO with Notable Photocatalytic Property. J. Taiwan Inst. Chem. Eng. 2020, 112, 337–344. [Google Scholar] [CrossRef]
- Raja, A.; Rajasekaran, P.; Selvakumar, K.; Arunpandian, M.; Kaviyarasu, K.; Asath Bahadur, S.; Swaminathan, M. Visible Active Reduced Graphene Oxide-BiVO4-ZnO Ternary Photocatalyst for Efficient Removal of Ciprofloxacin. Sep. Purif. Technol. 2020, 233, 115996. [Google Scholar] [CrossRef]
- Mandal, S.K.; Dutta, K.; Pal, S.; Mandal, S.; Naskar, A.; Pal, P.K.; Bhattacharya, T.S.; Singha, A.; Saikh, R.; De, S.; et al. Engineering of ZnO/RGO Nanocomposite Photocatalyst towards Rapid Degradation of Toxic Dyes. Mater. Chem. Phys. 2019, 223, 456–465. [Google Scholar] [CrossRef]
- Neelgund, G.M.; Oki, A. Photothermal Effect: An Important Aspect for the Enhancement of Photocatalytic Activity under Illumination by NIR Radiation. Mater. Chem. Front. 2017, 2, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Kim, T.W.; Guo, T.; Li, F. Unique Visible-Light-Assisted Field Emission of Tetrapod-Shaped ZnO/Reduced Graphene-Oxide Core/Coating Nanocomposites. Sci. Rep. 2016, 6, 38613. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Megala, S.; Harish, S.; Navaneethan, M.; Maadeswaran, P.; Sohila, S.; Ramesh, R. Enhanced Photocatalytic Activities of ZnO Dumbbell/Reduced Graphene Oxide Nanocomposites for Degradation of Organic Pollutants via Efficient Charge Separation Pathway. Appl. Surf. Sci. 2019, 487, 1279–1288. [Google Scholar] [CrossRef]
- Kumaresan, N.; Ramamurthi, K. Synthesis of ZnO/RGO Nanocomposites by Wet Impregnation Method for Photocatalytic Performance against RhB Dye and 4-Chlorophenol under UV Light Irradiation. J. Mater. Sci. Mater. Electron. 2020, 31, 3361–3374. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, D.; Yang, L.; Zhao, Z.; Li, D.; Luo, Q.; Zhang, Y.; Cao, F. Influences of Graphene Nanosheets Coating on Selectively Exposed Crystal Facet ZnO. ChemistrySelect 2018, 3, 4495–4500. [Google Scholar] [CrossRef]
- Pruna, A.; Cembrero, J.; Pullini, D.; Mocioiu, A.M.; Busquets-Mataix, D. Effect of Reduced Graphene Oxide on Photocatalytic Properties of Electrodeposited ZnO. Appl. Phys. A 2017, 123, 792. [Google Scholar] [CrossRef]
- Merlano, A.S.; Hoyos, L.M.; Gutiérrez, G.J.; Valenzuela, M.A.; Salazar, Á. Effect of Zn Precursor Concentration in the Synthesis of RGO/ZnO Composites and Their Photocatalytic Activity. New J. Chem. 2020, 44, 19858–19867. [Google Scholar] [CrossRef]
- Zhu, Z.; Guo, F.; Xu, Z.; Di, X.; Zhang, Q. Photocatalytic Degradation of an Organophosphorus Pesticide Using a ZnO/RGO Composite. RSC Adv. 2020, 10, 11929–11938. [Google Scholar] [CrossRef] [Green Version]
- Padmalaya, G.; Sreeja, B.S.; Shoba, S.; Rajavel, R.; Radha, S.; Arivanandan, M.; Shrestha, S. Synthesis of Micro-Dumbbell Shaped RGO/ZnO Composite Rods and Its Application Towards as Electrochemical Sensor for the Simultaneous Determination of Ammonia and Formaldehyde Using Hexamine and Its Structural Analysis. J. Inorg. Organomet. Polym. 2020, 30, 943–954. [Google Scholar] [CrossRef]
- Wang, C.-C.; Shieu, F.-S.; Shih, H.C. Enhanced Photodegradation by RGO/ZnO Core-Shell Nanostructures. J. Environ. Chem. Eng. 2020, 8, 103589. [Google Scholar] [CrossRef]
- Williams, D.B.; Carter, C.B. Transmission Electron Microscopy: A Textbook for Materials Science, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 978-0-387-76500-6. [Google Scholar]
- Zhong, J.; Yan, J. Seeing Is Believing: Atomic Force Microscopy Imaging for Nanomaterial Research. RSC Adv. 2015, 6, 1103–1121. [Google Scholar] [CrossRef]
- Jabbar, A.; Yasin, G.; Khan, W.Q.; Anwar, M.Y.; Korai, R.M.; Nizam, M.N.; Muhyodin, G. Electrochemical Deposition of Nickel Graphene Composite Coatings: Effect of Deposition Temperature on Its Surface Morphology and Corrosion Resistance. RSC Adv. 2017, 7, 31100–31109. [Google Scholar] [CrossRef] [Green Version]
- Sengunthar, P.; Bhavsar, K.H.; Balasubramanian, C.; Joshi, U.S. Physical Properties and Enhanced Photocatalytic Activity of ZnO-RGO Nanocomposites. Appl. Phys. A 2020, 126, 567. [Google Scholar] [CrossRef]
- Sharma, M.; Sondhi, H.; Krishna, R.; Srivastava, S.K.; Rajput, P.; Nigam, S.; Joshi, M. Assessment of GO/ZnO Nanocomposite for Solar-Assisted Photocatalytic Degradation of Industrial Dye and Textile Effluent. Environ. Sci. Pollut. Res. 2020, 27, 32076–32087. [Google Scholar] [CrossRef]
- Chidhambaram, N.; Valanarasu, S.; Ganesh, V.; Gobalakrishnan, S. Unraveling the Enhanced Photocatalytic Decomposition Efficacy of the Al-Doped ZnO Nanoparticles@graphene Sheets. J. Phys. D Appl. Phys. 2020, 53, 465111. [Google Scholar] [CrossRef]
- Kumaresan, N.; Sinthiya, M.M.A.; Ramamurthi, K.; Ramesh Babu, R.; Sethuraman, K. Visible Light Driven Photocatalytic Activity of ZnO/CuO Nanocomposites Coupled with RGO Heterostructures Synthesized by Solid-State Method for RhB Dye Degradation. Arab. J. Chem. 2020, 13, 3910–3928. [Google Scholar] [CrossRef]
- Naseem, T.; Waseem, M.; Hafeez, M.; Din, S.U.; Haq, S. Mahfoz-ur-Rehman Reduced Graphene Oxide/Zinc Oxide Nanocomposite: From Synthesis to Its Application for Wastewater Purification and Antibacterial Activity. J. Inorg. Organomet. Polym. 2020, 30, 3907–3919. [Google Scholar] [CrossRef]
- Aqad, K.M.A.; Basheer, C. Photocatalytic Degradation of Basic Blue Dye Using Zinc Nanoparticles Decorated Graphene Oxide Nanosheet. J. Phys. Org. Chem. 2020, 34, e4117. [Google Scholar] [CrossRef]
- Maruthupandy, M.; Qin, P.; Muneeswaran, T.; Rajivgandhi, G.; Quero, F.; Song, J.-M. Graphene-Zinc Oxide Nanocomposites (G-ZnO NCs): Synthesis, Characterization and Their Photocatalytic Degradation of Dye Molecules. Mater. Sci. Eng. B 2020, 254, 114516. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, D.; Yang, L.; Li, C.; Liu, Y.; Lu, J.; Wang, Y. Preparation of 3D Urchin-like RGO/ZnO and Its Photocatalytic Activity. J. Mater. Sci. Mater. Electron. 2017, 28, 7935–7942. [Google Scholar] [CrossRef]
- Sreejesh, M.; Dhanush, S.; Rossignol, F.; Nagaraja, H.S. Microwave Assisted Synthesis of RGO/ZnO Composites for Non-Enzymatic Glucose Sensing and Supercapacitor Applications. Ceram. Int. 2017, 43, 4895–4903. [Google Scholar] [CrossRef]
- King, A.A.K.; Davies, B.R.; Noorbehesht, N.; Newman, P.; Church, T.L.; Harris, A.T.; Razal, J.M.; Minett, A.I. A New Raman Metric for the Characterisation of Graphene Oxide and Its Derivatives. Sci. Rep. 2016, 6, 19491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moorthy, S.K.; Viswanathan, C.; Ponpandian, N. Facile Approach for Synthesis of GO/ZnO Nanocomposite for Highly Efficient Photocatalytic Degradation of Organic Dyes under Visible Light. Nano Hybrids Compos. 2017, 17, 121–126. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, X.; Yang, C.; Cao, M.; Ma, M.; Liu, R. ZnO Microspheres-Reduced Graphene Oxide Nanocomposite for Photocatalytic Degradation of Methylene Blue Dye. Appl. Surf. Sci. 2017, 392, 196–203. [Google Scholar] [CrossRef]
- Ong, C.B.; Mohammad, A.W.; Ng, L.Y.; Mahmoudi, E.; Azizkhani, S.; Hayati Hairom, N.H. Solar Photocatalytic and Surface Enhancement of ZnO/RGO Nanocomposite: Degradation of Perfluorooctanoic Acid and Dye. Process Saf. Environ. Prot. 2017, 112, 298–307. [Google Scholar] [CrossRef]
- Bardestani, R.; Patience, G.S.; Kaliaguine, S. Experimental Methods in Chemical Engineering: Specific Surface Area and Pore Size Distribution Measurements—BET, BJH, and DFT. Can. J. Chem. Eng. 2019, 97, 2781–2791. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Amador, R.; Alvarado, J.; Flores-Carrasco, G.; Morales-de la Garza, L.; Alcántara-Iniesta, S.; Luna-Flores, A.; Bernal, Y.P.; Méndez Rojas, M.Á.; Gervacio-Arciniega, J.J.; Martínez Hernández, H.P.; et al. The Influence of Deposition Time on the Structural, Morphological, Optical and Electrical Properties of ZnO-RGO Nanocomposite Thin Films Grown in a Single Step by USP. Crystals 2020, 10, 73. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Qiu, J.; Liu, M.; Ming, H.; Zhang, H.; Li, M.; Zhang, S.; Zhang, T. A Surface Multiple Effect on the ZnO Anode Induced by Graphene for a High Energy Lithium-Ion Full Battery. J. Alloy. Compd. 2020, 824, 153945. [Google Scholar] [CrossRef]
- Zhu, P.; Duan, M.; Wang, R.; Xu, J.; Zou, P.; Jia, H. Facile Synthesis of ZnO/GO/Ag3PO4 Heterojunction Photocatalyst with Excellent Photodegradation Activity for Tetracycline Hydrochloride under Visible Light. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125118. [Google Scholar] [CrossRef]
- Hamid, S.B.A.; Teh, S.J.; Lai, C.W. Photocatalytic Water Oxidation on ZnO: A Review. Catalysts 2017, 7, 93. [Google Scholar] [CrossRef]
- Fung, C.-M.; Tang, J.-Y.; Tan, L.-L.; Mohamed, A.R.; Chai, S.-P. Recent Progress in Two-Dimensional Nanomaterials for Photocatalytic Carbon Dioxide Transformation into Solar Fuels. Mater. Today Sustain. 2020, 9, 100037. [Google Scholar] [CrossRef]
- Kang, W.; Jimeng, X.; Xitao, W. The Effects of ZnO Morphology on Photocatalytic Efficiency of ZnO/RGO Nanocomposites. Appl. Surf. Sci. 2016, 360, 270–275. [Google Scholar] [CrossRef]
- Jia, W.; Yuan, X. Concurrent and Dual N-Doping of Graphene/ZnO Nanocomposites for Enhanced Cr(VI) Photoreduction Activity under Visible-Light Irradiation. RSC Adv. 2020, 10, 30832–30839. [Google Scholar] [CrossRef]
- Kumar, K.V.A.; Lakshminarayana, B.; Suryakala, D.; Subrahmanyam, C. Reduced Graphene Oxide Supported ZnO Quantum Dots for Visible Light-Induced Simultaneous Removal of Tetracycline and Hexavalent Chromium. RSC Adv. 2020, 10, 20494–20503. [Google Scholar] [CrossRef]
- Srirattanapibul, S.; Tang, I.-M.; Thongmee, S. Photo Catalytic Reduction of Cr6+ by ZnO Decorated on Reduced Graphene Oxide (RGO) Nanocomposites. Mater. Res. Bull. 2020, 122, 110705. [Google Scholar] [CrossRef]
- Yang, L.; Xu, C.; Wan, F.; He, H.; Gu, H.; Xiong, J. Synthesis of RGO/BiOI/ZnO Composites with Efficient Photocatalytic Reduction of Aqueous Cr(VI) under Visible-Light Irradiation. Mater. Res. Bull. 2019, 112, 154–158. [Google Scholar] [CrossRef]
- Kumar, S.; Pandit, V.; Bhattacharyya, K.; Krishnan, V. Sunlight Driven Photocatalytic Reduction of 4-Nitrophenol on Pt Decorated ZnO-RGO Nanoheterostructures. Mater. Chem. Phys. 2018, 214, 364–376. [Google Scholar] [CrossRef]
- Van Thuan, D.; Nguyen, T.K.; Kim, S.-W.; Chung, J.S.; Hur, S.H.; Kim, E.J.; Hahn, S.H.; Wang, M. Chemical-Hydrothermal Synthesis of Oval-Shaped Graphene/ZnO Quantum Hybrids and Their Photocatalytic Performances. Catal. Commun. 2017, 101, 102–106. [Google Scholar] [CrossRef]
- Ong, W.-J.; Putri, L.K.; Mohamed, A.R. Rational Design of Carbon-Based 2D Nanostructures for Enhanced Photocatalytic CO2 Reduction: A Dimensionality Perspective. Chem. A Eur. J. 2020, 26, 9710–9748. [Google Scholar] [CrossRef] [PubMed]
- Gusain, R.; Gupta, K.; Joshi, P.; Khatri, O.P. Adsorptive Removal and Photocatalytic Degradation of Organic Pollutants Using Metal Oxides and Their Composites: A Comprehensive Review. Adv. Colloid Interface Sci. 2019, 272, 102009. [Google Scholar] [CrossRef] [PubMed]
- Talaiekhozani, A.; Rezania, S.; Kim, K.-H.; Sanaye, R.; Amani, A.M. Recent Advances in Photocatalytic Removal of Organic and Inorganic Pollutants in Air. J. Clean. Prod. 2021, 278, 123895. [Google Scholar] [CrossRef]
- Lucas, M.S.; Dias, A.A.; Sampaio, A.; Amaral, C.; Peres, J.A. Degradation of a Textile Reactive Azo Dye by a Combined Chemical–Biological Process: Fenton’s Reagent-Yeast. Water Res. 2007, 41, 1103–1109. [Google Scholar] [CrossRef]
- Samadi, M.; Zirak, M.; Naseri, A.; Khorashadizade, E.; Moshfegh, A.Z. Recent Progress on Doped ZnO Nanostructures for Visible-Light Photocatalysis. Thin Solid Films 2016, 605, 2–19. [Google Scholar] [CrossRef] [Green Version]
- Mclaren, A.; Valdes-Solis, T.; Li, G.; Tsang, S.C. Shape and Size Effects of ZnO Nanocrystals on Photocatalytic Activity. J. Am. Chem. Soc. 2009, 131, 12540–12541. [Google Scholar] [CrossRef]
- Neelgund, G.M.; Oki, A. Graphene-Coupled ZnO: A Robust NIR-Induced Catalyst for Rapid Photo-Oxidation of Cyanide. ACS Omega 2017, 2, 9095–9102. [Google Scholar] [CrossRef]
- Rakkesh, R.A.; Durgalakshmi, D.; Balakumar, S. Beyond Chemical Bonding Interaction: An Insight into the Growth Process of 1D ZnO on Few-Layer Graphene for Excellent Photocatalytic and Room Temperature Gas Sensing Applications. ChemistrySelect 2018, 3, 7302–7309. [Google Scholar] [CrossRef]
- Neena, D.; Kondamareddy, K.K.; Humayun, M.; Mohan, V.B.; Lu, D.; Fu, D.; Gao, W. Fabrication of ZnO/N-RGO Composite as Highly Efficient Visible-Light Photocatalyst for 2,4-DCP Degradation and H2 Evolution. Appl. Surf. Sci. 2019, 488, 611–619. [Google Scholar] [CrossRef]
- Nguyen, V.Q.; Baynosa, M.L.; Nguyen, V.H.; Tuma, D.; Lee, Y.R.; Shim, J.-J. Solvent-Driven Morphology-Controlled Synthesis of Highly Efficient Long-Life ZnO/Graphene Nanocomposite Photocatalysts for the Practical Degradation of Organic Wastewater under Solar Light. Appl. Surf. Sci. 2019, 486, 37–51. [Google Scholar] [CrossRef]
- Tayebi, M.; Tayyebi, A.; Masoumi, Z.; Lee, B.-K. Photocorrosion Suppression and Photoelectrochemical (PEC) Enhancement of ZnO via Hybridization with Graphene Nanosheets. Appl. Surf. Sci. 2020, 502, 144189. [Google Scholar] [CrossRef]
- Flores, K.; Valdes, C.; Ramirez, D.; Eubanks, T.M.; Lopez, J.; Hernandez, C.; Alcoutlabi, M.; Parsons, J.G. The Effect of Hybrid Zinc Oxide/Graphene Oxide (ZnO/GO) Nano-Catalysts on the Photocatalytic Degradation of Simazine. Chemosphere 2020, 259, 127414. [Google Scholar] [CrossRef] [PubMed]
- Amna, T. Shape-Controlled Synthesis of Three-Dimensional Zinc Oxide Nanoflowers for Disinfection of Food Pathogens. Z. Nat. C 2018, 73, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ahmad, M.; Sun, H. Three-Dimensional ZnO Hierarchical Nanostructures: Solution Phase Synthesis and Applications. Materials 2017, 10, 1304. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Hu, Y.-L.; Pelligra, C.; Chen, C.-H.; Jin, L.; Huang, H.; Sithambaram, S.; Aindow, M.; Joesten, R.; Suib, S.L. ZnO with Different Morphologies Synthesized by Solvothermal Methods for Enhanced Photocatalytic Activity. Chem. Mater. 2009, 21, 2875–2885. [Google Scholar] [CrossRef]
- Johar, M.A.; Afzal, R.A.; Alazba, A.A.; Manzoor, U. Photocatalysis and Bandgap Engineering Using ZnO Nanocomposites. Adv. Mater. Sci. Eng. 2015, 2015, 934587. [Google Scholar] [CrossRef] [Green Version]
- Opoku, F.; Govender, K.K.; van Sittert, C.G.C.E.; Govender, P.P. Understanding the Mechanism of Enhanced Charge Separation and Visible Light Photocatalytic Activity of Modified Wurtzite ZnO with Nanoclusters of ZnS and Graphene Oxide: From a Hybrid Density Functional Study. New J. Chem. 2017, 41, 8140–8155. [Google Scholar] [CrossRef]

































| Title | Published Year | Reference |
|---|---|---|
| Graphene in photocatalysis: a review | 2016 | [33] |
| Review on ZnO hybrid photocatalyst: impact on photocatalytic activities of water pollutant degradation | 2016 | [34] |
| Advancements in the zinc oxide nanomaterials for efficient photocatalysis | 2017 | [35] |
| The effect of ZnO-based carbonaceous materials for degradation of benzoic pollutants: a review | 2019 | [31] |
| Progress in Graphene/Metal Oxide Composite Photocatalysts for Degradation of Organic Pollutants | 2020 | [36] |
| Nanoscale zinc oxide-based heterojunctions as visible light active photocatalysts for hydrogen energy and environmental remediation | 2020 | [25] |
| Advances and Challenges in Developing Efficient Graphene Oxide-Based ZnO Photocatalysts for Dye Photo-Oxidation | 2020 | [32] |
| State of the art on the photocatalytic applications of graphene-based nanostructures: From elimination of hazardous pollutants to disinfection and fuel generation | 2020 | [27] |
| An overview of graphene oxide supported semiconductors based photocatalysts: Properties, synthesis, and photocatalytic applications | 2020 | [37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albiter, E.; Merlano, A.S.; Rojas, E.; Barrera-Andrade, J.M.; Salazar, Á.; Valenzuela, M.A. Synthesis, Characterization, and Photocatalytic Performance of ZnO–Graphene Nanocomposites: A Review. J. Compos. Sci. 2021, 5, 4. https://doi.org/10.3390/jcs5010004
Albiter E, Merlano AS, Rojas E, Barrera-Andrade JM, Salazar Á, Valenzuela MA. Synthesis, Characterization, and Photocatalytic Performance of ZnO–Graphene Nanocomposites: A Review. Journal of Composites Science. 2021; 5(1):4. https://doi.org/10.3390/jcs5010004
Chicago/Turabian StyleAlbiter, Elim, Aura S. Merlano, Elizabeth Rojas, José M. Barrera-Andrade, Ángel Salazar, and Miguel A. Valenzuela. 2021. "Synthesis, Characterization, and Photocatalytic Performance of ZnO–Graphene Nanocomposites: A Review" Journal of Composites Science 5, no. 1: 4. https://doi.org/10.3390/jcs5010004