Poly(ethyl methacrylate) Composite Coatings Containing Halogen-Free Inorganic Additives with Flame-Retardant Properties
Abstract
:1. Introduction
2. Materials and Methods
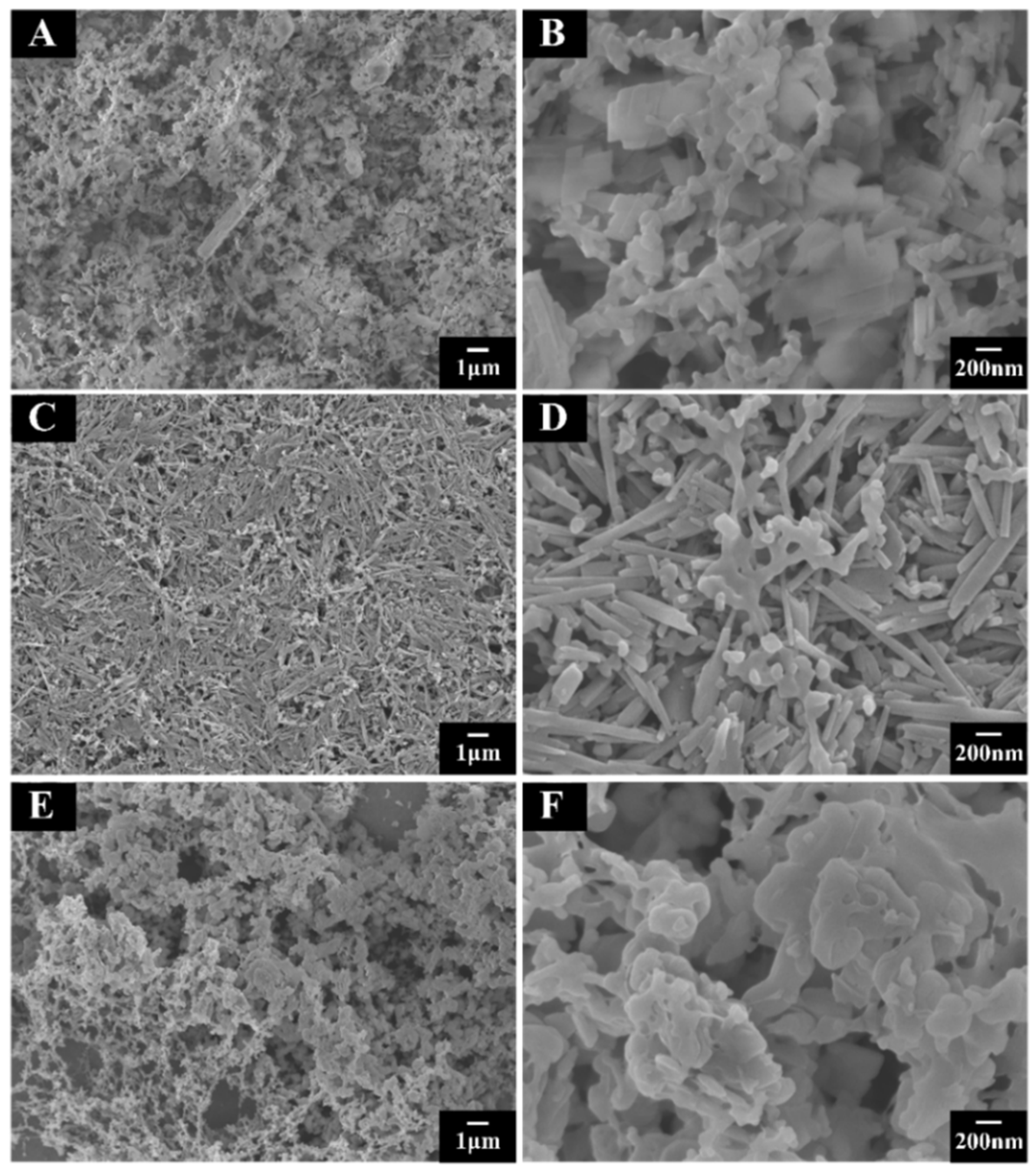
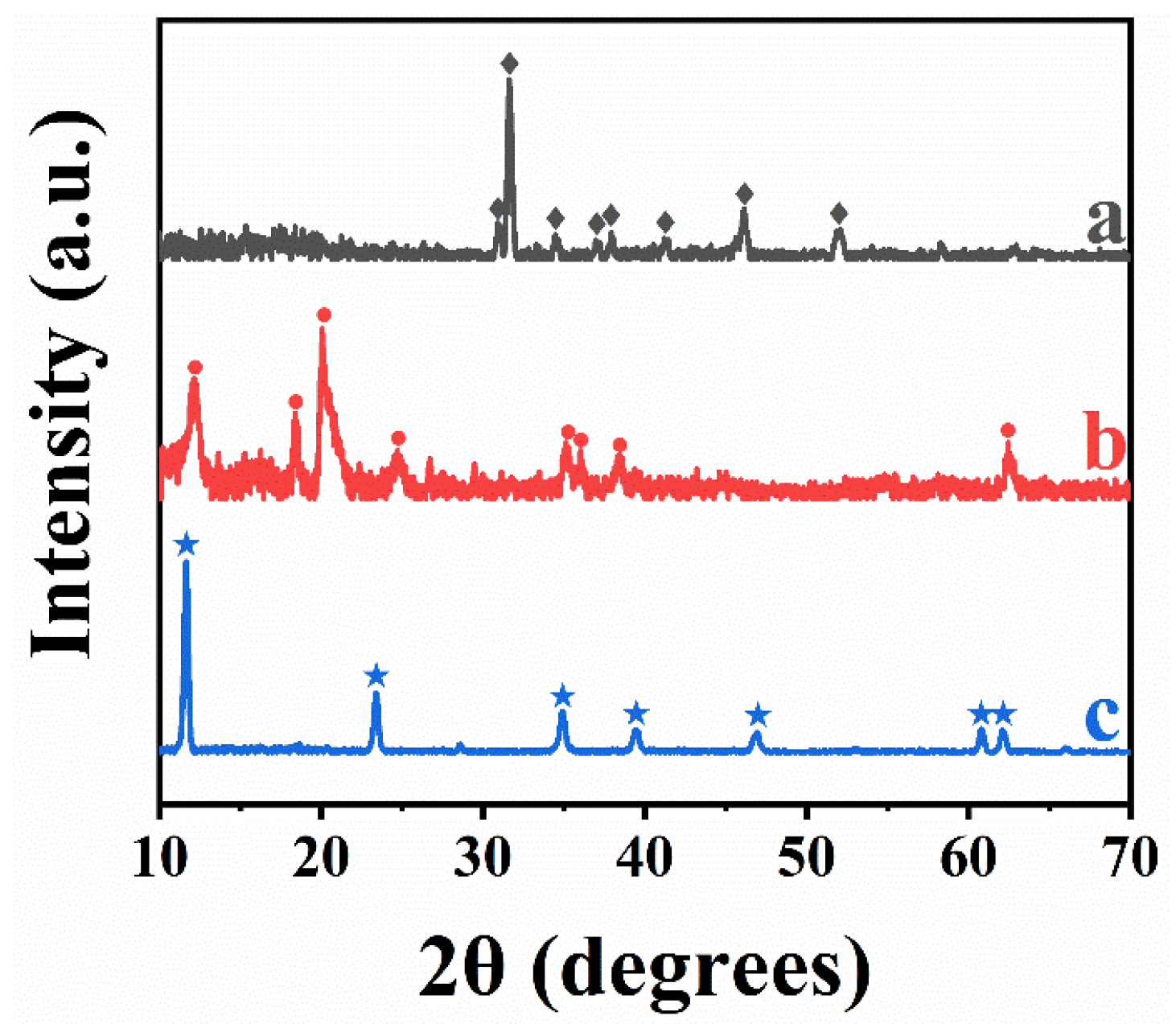
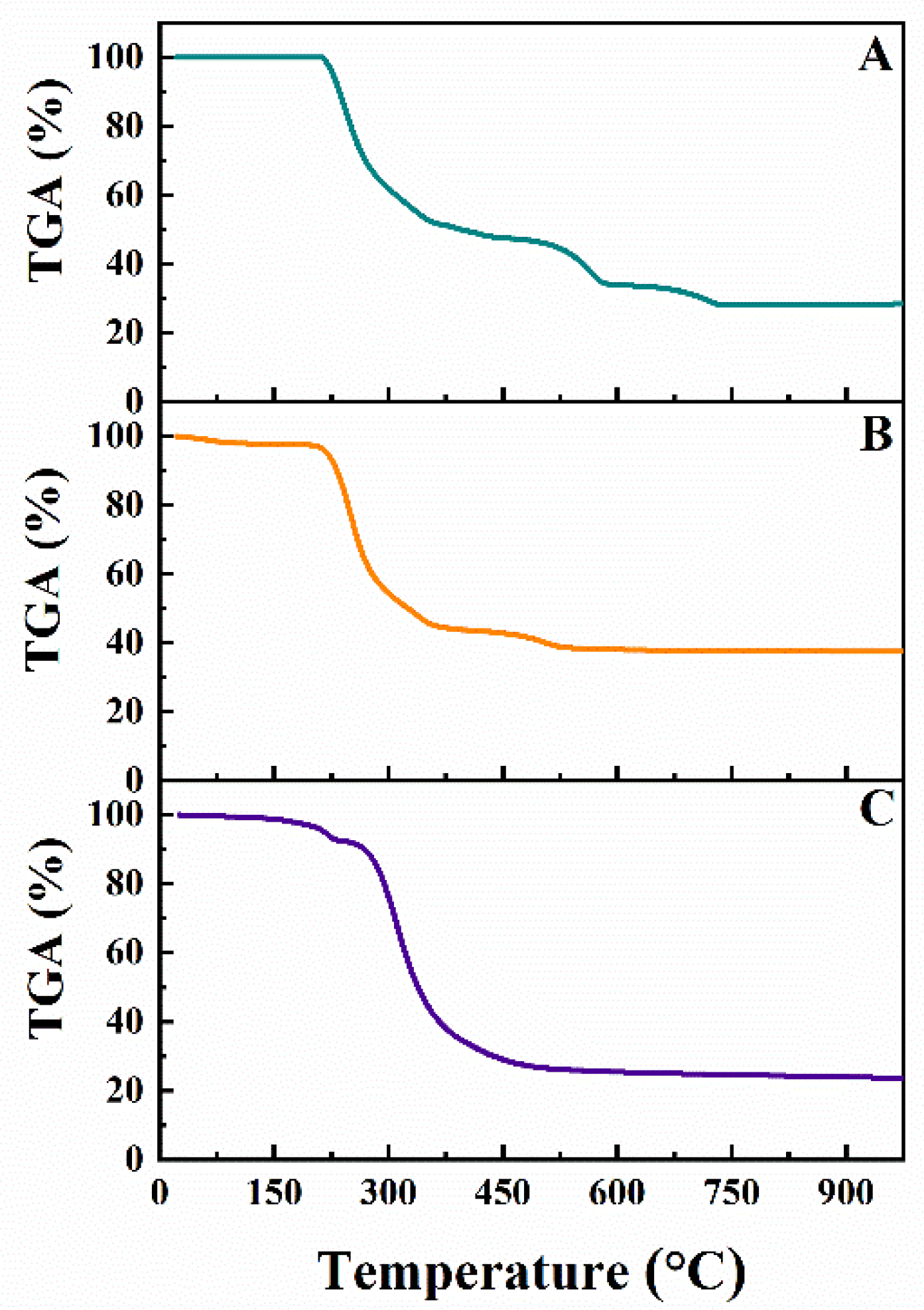
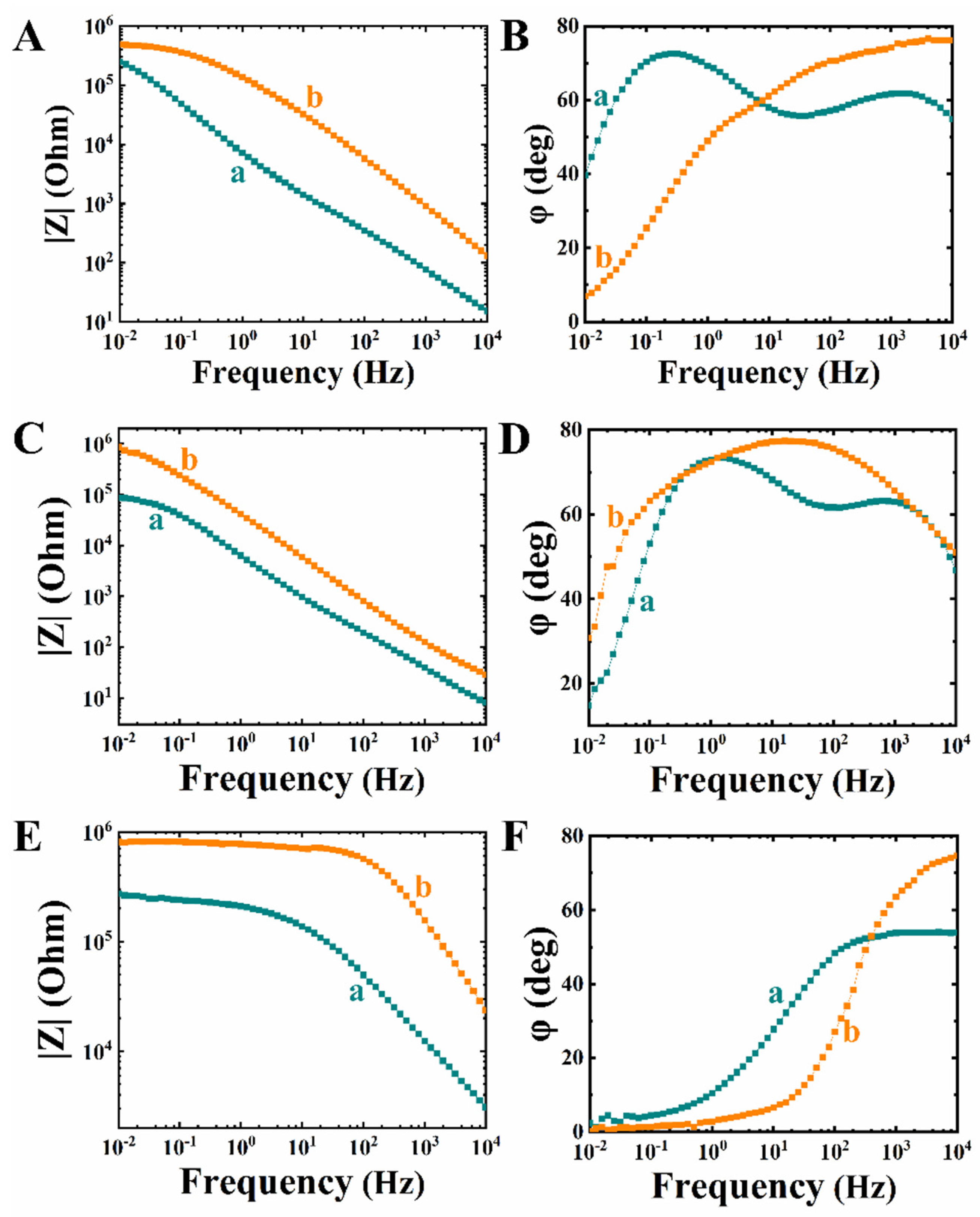
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Wang, Y.Z. A review on flame retardant technology in China. Part I: Development of flame retardants. Polym. Adv. Technol. 2010, 21, 1–26. [Google Scholar] [CrossRef]
- Lu, H.; Song, L.; Hu, Y. A review on flame retardant technology in China. Part II: Flame retardant polymeric nanocomposites and coatings. Polym. Adv. Technol. 2011, 22, 379–394. [Google Scholar] [CrossRef]
- Gordon, K.L.; Thompson, C.M.; Lyon, R.E. Flame retardant epoxy resins containing aromatic poly(phosphonamides). High Perform. Polym. 2010, 22, 945–958. [Google Scholar] [CrossRef]
- Weil, E.D. Fire-Protective and Flame-Retardant Coatings-A State-of-the-Art Review. J. Fire Sci. 2011, 29, 259–296. [Google Scholar] [CrossRef]
- Wang, Z.T.; Luo, Y.K.; Ding, X.; Xi, G.F. The Research on Flame Retardant Wind Turbines Nacelle Made through VARTM Process. Adv. Mater. Res. 2012, 450, 508–512. [Google Scholar] [CrossRef]
- Xu, T.; Huang, X. Combustion properties of asphalt binder containing flame retardant. Fire Mater. 2012, 36, 97–106. [Google Scholar] [CrossRef]
- Wang, S.; Huang, J.; Chen, F. Study on mg-al hydrotalcites in flame retardant paper preparation. BioResources 2012, 7, 997–1007. [Google Scholar]
- Nam, N.D.; Park, I.J.; Kim, J.G.; Kim, H.S. Effect of flame-retarding additives on surface chemistry in Li-ion batteries. Mater. Res. Bull. 2012, 47, 2811–2814. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Z.; Zhao, H.; Qiao, H.; Luan, H.; Chen, L. A novel flame retardant and film-forming electrolyte additive for lithium ion batteries. J. Power Sources 2009, 187, 229–232. [Google Scholar] [CrossRef]
- Hamdani, S.; Longuet, C.; Perrin, D.; Lopez-cuesta, J.-M.; Ganachaud, F.O. Flame retardancy of silicone-based materials. Polym. Degrad. Stab. 2009, 94, 465–495. [Google Scholar] [CrossRef]
- Mouritz, A.P. Review of smoke toxicity of fiber-polymer composites used in aircraft. J. Aircr. 2009, 46, 735–745. [Google Scholar] [CrossRef]
- Holbery, J.; Houston, D. Natural-fiber-reinforced polymer composites in automotive applications. JOM 2006, 58, 80–86. [Google Scholar] [CrossRef]
- Ding, P.; Tang, S.; Yang, H.; Shi, L. PP/LDH nanocomposites via melt-intercalation: Synergistic flame retardant effects, properties, and applications in automobile industries. Adv. Mater. Res. 2010, 87–88, 427–432. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.L.; Alexandre, M.L.; Lopez-Cuesta, J.-M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Camino, G.; Costa, L.; Martinasso, G. Intumescent fire-retardant systems. Polym. Degrad. Stab. 1989, 23, 359–376. [Google Scholar] [CrossRef]
- Camino, G.; Costa, L.; Luda di Cortemiglia, M. Overview of fire retardant mechanisms. Polym. Degrad. Stab. 1991, 33, 131–154. [Google Scholar] [CrossRef]
- Wilford, B.H.; Shoeib, M.; Harner, T.; Zhu, J.; Jones, K.C. Polybrominated diphenyl ethers in indoor dust in Ottawa, Canada: Implications for sources and exposure. Environ. Sci. Technol. 2005, 39, 7027–7035. [Google Scholar] [CrossRef]
- Porter, D.; Metcalfe, E.; Thomas, M. Nanocomposite fire retardants—A review. Fire Mater. 2000, 24, 45–52. [Google Scholar] [CrossRef]
- Clement, R.E.; Reiner, E.J.; Bhavsar, S.P. Organohalogen contaminants of emerging concern in Great Lakes fish: A review. Anal. Bioanal. Chem. 2012, 404, 2639–2658. [Google Scholar] [CrossRef]
- Shoeib, M.; Harner, T.; Webster, G.M.; Sverko, E.; Cheng, Y. Legacy and current-use flame retardants in house dust from Vancouver, Canada. Environ. Pollut. 2012, 169, 175–182. [Google Scholar] [CrossRef]
- Gentes, M.-L.; Letcher, R.J.; Caron-Beaudoin, E.; Verreault, J. Novel flame retardants in urban-feeding ring-billed gulls from the St. Lawrence River, Canada. Environ. Sci. Technol. 2012, 46, 9735–9744. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Letcher, R.J.; Burgess, N.M.; Champoux, L.; Elliott, J.E.; Hebert, C.E.; Martin, P.; Wayland, M.; Chip Weseloh, D.V.; Wilson, L. Flame retardants in eggs of four gull species (Laridae) from breeding sites spanning Atlantic to Pacific Canada. Environ. Pollut. 2012, 168, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Alava, J.J.; Lambourn, D.; Olesiuk, P.; Lance, M.; Jeffries, S.J.; Gobas, F.A.P.C.; Ross, P.S. PBDE flame retardants and PCBs in migrating Steller sea lions (Eumetopias jubatus) in the Strait of Georgia, British Columbia, Canada. Chemosphere 2012, 88, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.; Kurt-Karakus, P.; Bidleman, T.F. Fate of brominated flame retardants and organochlorine pesticides in urban soil: Volatility and degradation. Environ. Sci. Technol. 2012, 46, 2668–2674. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Shen, L.; Su, Y.; Barresi, E.; Dejong, M.; Hung, H.; Lei, Y.-D.; Wania, F.; Reiner, E.J.; Sverko, E.; et al. Atmospheric concentrations of halogenated flame retardants at two remote locations: The Canadian High Arctic and the Tibetan Plateau. Environ. Pollut. 2012, 161, 154–161. [Google Scholar] [CrossRef]
- Ivol, F.; Porcher, M.; Ghosh, A.; Jacquemin, J.; Ghamouss, F. Phenylacetonitrile (C6H5CH2CN) Ionic Liquid Blends as Alternative Electrolytes for Safe and High-Performance Supercapacitors. Molecules 2020, 25, 2697. [Google Scholar] [CrossRef]
- McDonough, C.A.; Puggioni, G.; Helm, P.A.; Muir, D.; Lohmann, R. Spatial Distribution and Air–Water Exchange of Organic Flame Retardants in the Lower Great Lakes. Environ. Sci. Technol. 2016, 50, 9133–9141. [Google Scholar] [CrossRef]
- Venier, M.; Dove, A.; Romanak, K.; Backus, S.; Hites, R. Flame retardants and legacy chemicals in Great Lakes’ water. Environ. Sci. Technol. 2014, 48, 9563–9572. [Google Scholar] [CrossRef]
- Yang, J.; Liang, J.Z.; Tang, C.Y. Studies on melt flow properties during capillary extrusion of PP/Al(OH)3/Mg(OH)2 flame retardant composites. Polym. Test. 2009, 28, 907–911. [Google Scholar] [CrossRef]
- Jizhao, L.; Yingjie, Z. A study of the flame-retardant properties of polypropylene/Al(OH)3/Mg(OH)2 composites. Polym. Int. 2010, 59, 539–542. [Google Scholar]
- Armstrong, R.; Wright, J. Impedance studies of poly ethylmethacrylate coatings formed upon tin-free steel. Corros. Sci. 1992, 33, 1529–1539. [Google Scholar] [CrossRef]
- Sari, A.; Karlı, A.; Alkan, C.; Karaipekli, A. Polyethyl methacrylate (PEMA)/fatty acids blends as novel phase change materials for thermal energy storage. Energy Sources Part A Recovery Util. Environ. Eff. 2013, 35, 1813–1819. [Google Scholar] [CrossRef]
- Ulaganathan, M.; Mathew, C.M.; Rajendran, S. Highly porous lithium-ion conducting solvent-free poly (vinylidene fluoride-co-hexafluoropropylene)/poly (ethyl methacrylate) based polymer blend electrolytes for Li battery applications. Electrochim. Acta 2013, 93, 230–235. [Google Scholar] [CrossRef]
- Ramesh, S.; Uma, O.; Shanti, R.; Yi, L.J.; Ramesh, K. Preparation and characterization of poly (ethyl methacrylate) based polymer electrolytes doped with 1-butyl-3-methylimidazolium trifluoromethanesulfonate. Measurement 2014, 48, 263–273. [Google Scholar] [CrossRef]
- Mohanty, F.; Swain, S.K. Silver Nanoparticles Decorated Polyethylmethacrylate/Graphene Oxide Composite: As Packaging Material. Polym. Compos. 2019, 40, E1199–E1207. [Google Scholar] [CrossRef]
- Mohanty, F.; Swain, S.K. Nano silver embedded starch hybrid graphene oxide sandwiched poly (ethylmethacrylate) for packaging application. Nano-Struct. Nano-Objects 2019, 18, 100300. [Google Scholar] [CrossRef]
- Arnold, J.C.; Venditti, N.P. Prediction of the long-term creep behaviour of hydroxyapatite-filled polyethylmethacrylate bone cements. J. Mater. Sci. Mater. Med. 2007, 18, 1849–1858. [Google Scholar] [CrossRef]
- Peng, Y.; Musah, M.; Via, B.; Wang, X. Calcium Carbonate Particles Filled Homopolymer Polypropylene at Different Loading Levels: Mechanical Properties Characterization and Materials Failure Analysis. J. Compos. Sci. 2021, 5, 302. [Google Scholar] [CrossRef]
- Li, X.; Zhitomirsky, I. Deposition of poly (methyl methacrylate) and composites containing bioceramics and bioglass by dip coating using isopropanol-water co-solvent. Prog. Org. Coat. 2020, 148, 105883. [Google Scholar] [CrossRef]
- Jankovic, I.A.; Saponjic, Z.V.; Comor, M.I.; Nedeljkovic, J.M. Surface modification of colloidal TiO2 nanoparticles with bidentate benzene derivatives. J. Phys. Chem. C 2009, 113, 12645–12652. [Google Scholar] [CrossRef]
- Zhang, T.; Luo, D.; Wojtal, P.; Zhitomirsky, I. Electrophoretic deposition of flame retardant polymer–Huntite coatings. Mater. Lett. 2015, 159, 106–109. [Google Scholar] [CrossRef]
- Şen, F.; Madakbaş, S.; Kahraman, M.V. Preparation and characterization of polyaniline/Turkish Huntite–Hydromagnesite composites. Polym. Compos. 2014, 35, 456–460. [Google Scholar] [CrossRef]
- Madakbaş, S.; Celik, Z.; Dumludağ, F.; Kahraman, M.V. Preparation, characterization and electrical properties of polyacrylonitrile/huntite composites. Polym. Bull. 2014, 71, 1471–1481. [Google Scholar] [CrossRef]
- Seki, Y.; Sever, K.; Sarikanat, M.; Sakarya, A.; Elik, E. Effect of huntite mineral on mechanical, thermal and morphological properties of polyester matrix. Compos. Part B Eng. 2013, 45, 1534–1540. [Google Scholar] [CrossRef]
- Zainuddin, S.; Fahim, A.; Shoieb, S.; Syed, F.; Hosur, M.V.; Li, D.; Hicks, C.; Jeelani, S. Morphological and mechanical behavior of chemically treated jute-PHBV bio-nanocomposites reinforced with silane grafted halloysite nanotubes. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Li, C.; Zhao, Y.; Zhu, T.; Ruan, J.; Li, G. Effective solvent-free oxidation of cyclohexene to allylic products with oxygen by mesoporous etched halloysite nanotube supported Co2+. RSC Adv. 2018, 8, 14870–14878. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, Z.; Sakib, S.; Mathews, R.; Zhitomirsky, I. Poly (Methyl Methacrylate) Coatings Containing Flame Retardant Additives from Suspensions in Water-2-Propanol. Molecules 2021, 26, 1974. [Google Scholar] [CrossRef]
- González, M.A.; Trócoli, R.; Pavlovic, I.; Barriga, C.; La Mantia, F. Capturing Cd (II) and Pb (II) from contaminated water sources by electro-deposition on hydrotalcite-like compounds. Phys. Chem. Chem. Phys. 2016, 18, 1838–1845. [Google Scholar] [CrossRef]
- Tian, X.; Wang, J.; Zhang, H.; Cao, Z.; Zhao, M.; Guan, Y.; Zhang, Y. Establishment of transport channels with carriers for water in reverse osmosis membrane by incorporating hydrotalcite into the polyamide layer. RSC Adv. 2018, 8, 12439–12448. [Google Scholar] [CrossRef] [Green Version]
- Abdelrazek, E.; Elashmawi, I. Characterization and physical properties of CoCl2 filled polyethyl-methacrylate films. Polym. Compos. 2008, 29, 1036–1043. [Google Scholar] [CrossRef]
- Gradwell, M.; Hourston, D.; Pabunruang, T.; Schafer, F.U.; Reading, M. High-resolution thermogravimetric analysis of polyurethane/poly (ethyl methacrylate) interpenetrating polymer networks. J. Appl. Polym. Sci. 1998, 70, 287–295. [Google Scholar] [CrossRef]
- Agrawal, M.; Zafeiropoulos, N.E.; Gupta, S.; Svetushkina, E.; Pionteck, J.; Pich, A.; Stamm, M. A novel approach for mixing ZnO nanoparticles into poly (ethyl methacrylate). Macromol. Rapid Commun. 2010, 31, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, S.; Prabhu, M.R.; Rani, M.U. Characterization of PVC/PEMA based polymer blend electrolytes. Int. J. Electrochem. Sci. 2008, 3, 282–290. [Google Scholar]
- Sim, L.; Majid, S.; Arof, A. Effects of 1–butyl–3–methyl imidazolium trifluoromethanesulfonate ionic liquid in poly (ethyl methacrylate)/poly (vinylidenefluoride–co–hexafluoropropylene) blend based polymer electrolyte system. Electrochim. Acta 2014, 123, 190–197. [Google Scholar] [CrossRef]
- Sun, Y.; Ata, M.; Zhitomirsky, I. Electrophoretic deposition of linear polyethylenimine and composite films. Surf. Eng. 2013, 29, 495–499. [Google Scholar] [CrossRef]
- Nicolini, K.P.; Fukamachi, C.R.B.; Wypych, F.; Mangrich, A.S. Dehydrated halloysite intercalated mechanochemically with urea: Thermal behavior and structural aspects. J. Colloid Interface Sci. 2009, 338, 474–479. [Google Scholar] [CrossRef]
- Peng, Y.; Burtovyy, R.; Bordia, R.; Luzinov, I. Fabrication of Porous Carbon Films and Their Impact on Carbon/Polypropylene Interfacial Bonding. J. Compos. Sci. 2021, 5, 108. [Google Scholar] [CrossRef]
- Garcia Filho, F.C.; Luz, F.S.; Oliveira, M.S.; Bezerra, W.B.A.; Barbosa, J.D.V.; Monteiro, S.N. Influence of Rigid Brazilian Natural Fiber Arrangements in Polymer Composites: Energy Absorption and Ballistic Efficiency. J. Compos. Sci. 2021, 5, 201. [Google Scholar] [CrossRef]
- Tsukamoto, H. Tribological Characterization of Carbon Nanotube/Aluminum Functionally Graded Materials Fabricated by Centrifugal Slurry Methods. J. Compos. Sci. 2021, 5, 254. [Google Scholar] [CrossRef]
- Su, Y.; Zhitomirsky, I. Electrophoretic deposition of graphene, carbon nanotubes and composite films using methyl violet dye as a dispersing agent. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 97–103. [Google Scholar] [CrossRef]
- Wu, K.; Wang, Y.; Zhitomirsky, I. Electrophoretic deposition of TiO2 and composite TiO2–MnO2 films using benzoic acid and phenolic molecules as charging additives. J. Colloid Interface Sci. 2010, 352, 371–378. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Veldhuis, S.; Mathews, R.; Zhitomirsky, I. Poly(ethyl methacrylate) Composite Coatings Containing Halogen-Free Inorganic Additives with Flame-Retardant Properties. J. Compos. Sci. 2022, 6, 104. https://doi.org/10.3390/jcs6040104
Liu X, Veldhuis S, Mathews R, Zhitomirsky I. Poly(ethyl methacrylate) Composite Coatings Containing Halogen-Free Inorganic Additives with Flame-Retardant Properties. Journal of Composites Science. 2022; 6(4):104. https://doi.org/10.3390/jcs6040104
Chicago/Turabian StyleLiu, Xinqian, Stephen Veldhuis, Ritch Mathews, and Igor Zhitomirsky. 2022. "Poly(ethyl methacrylate) Composite Coatings Containing Halogen-Free Inorganic Additives with Flame-Retardant Properties" Journal of Composites Science 6, no. 4: 104. https://doi.org/10.3390/jcs6040104





