The Accumulation of Metal Ions by a Soy Protein–Inorganic Composite Material
Abstract
:1. Introduction
2. Materials and Methods
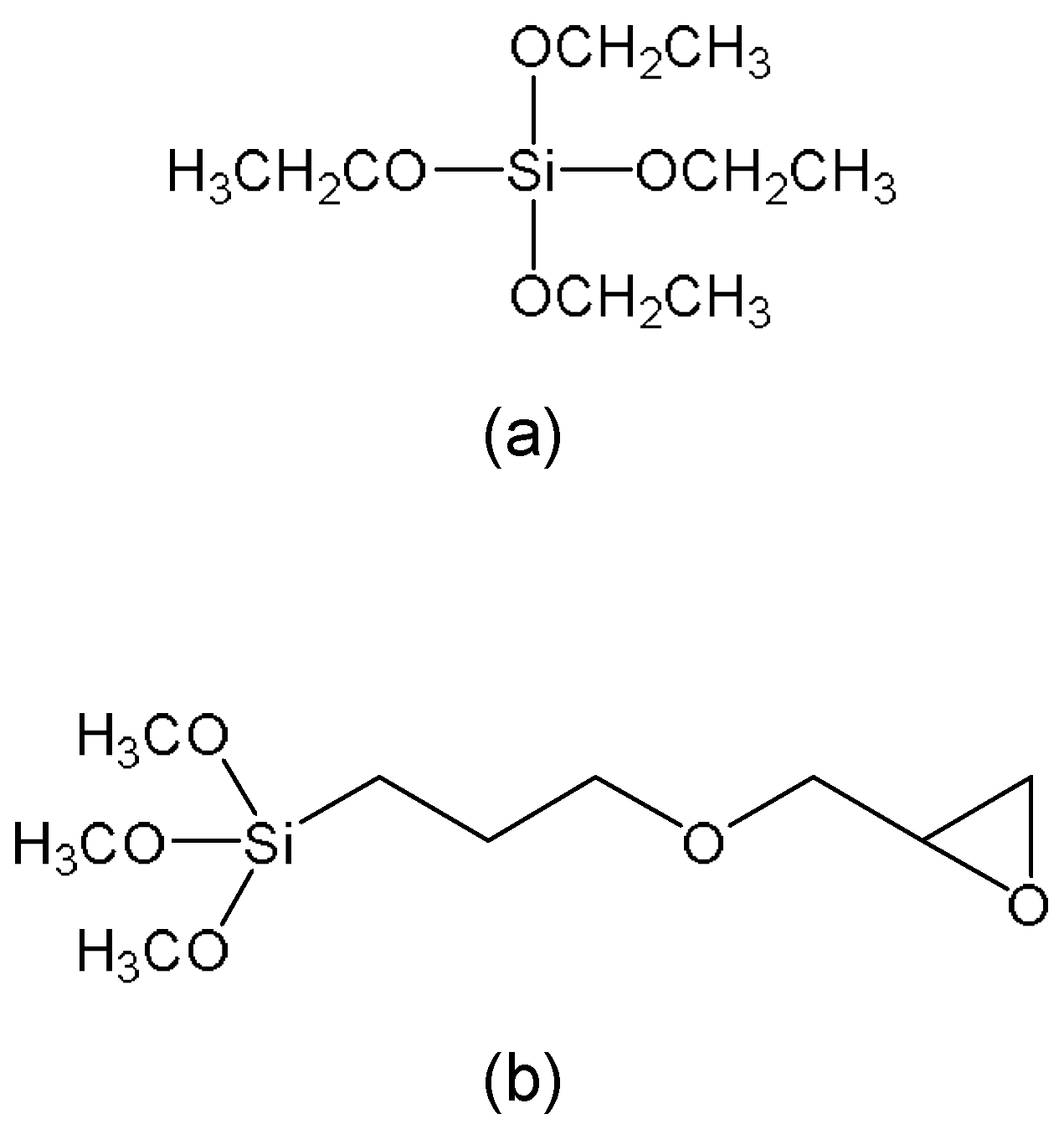
2.1. Material
2.2. Preparation of the SP−GPTMS Composite Material
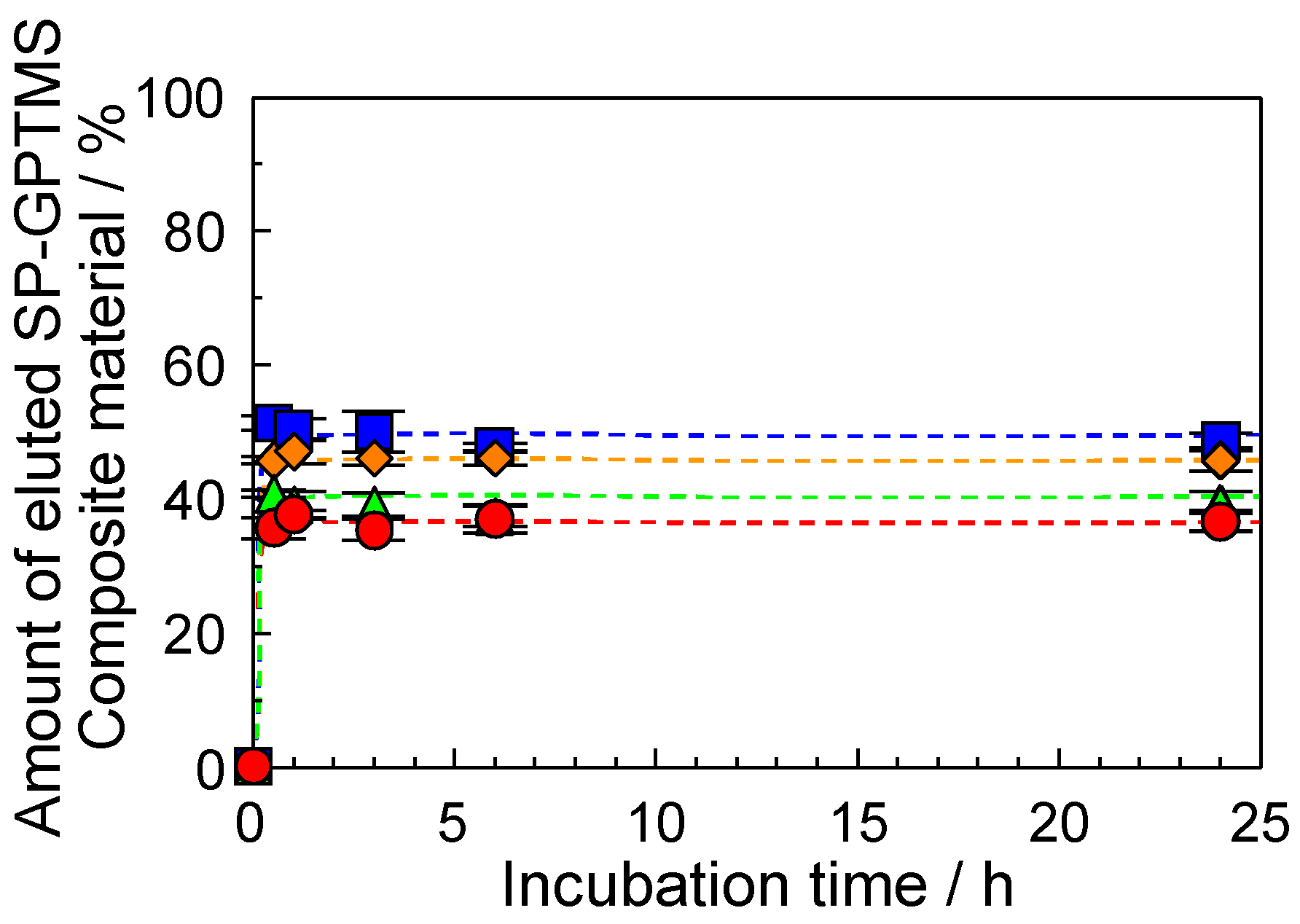
2.3. Water Stability of the SP−GPTMS Composite Material
2.4. Structural Analysis of the SP−GPTMS Composite Material
2.5. Thermal Analysis of the SP−GPTMS Composite Material
2.6. Accumulation of Metal Ions by the SP−GPTMS Composite Material
2.7. IR Measurements of Metal Ion-Accumulated SP−GPTMS Composite Material
3. Results and Discussion
3.1. Preparation of the SP−GPTMS Composite Material
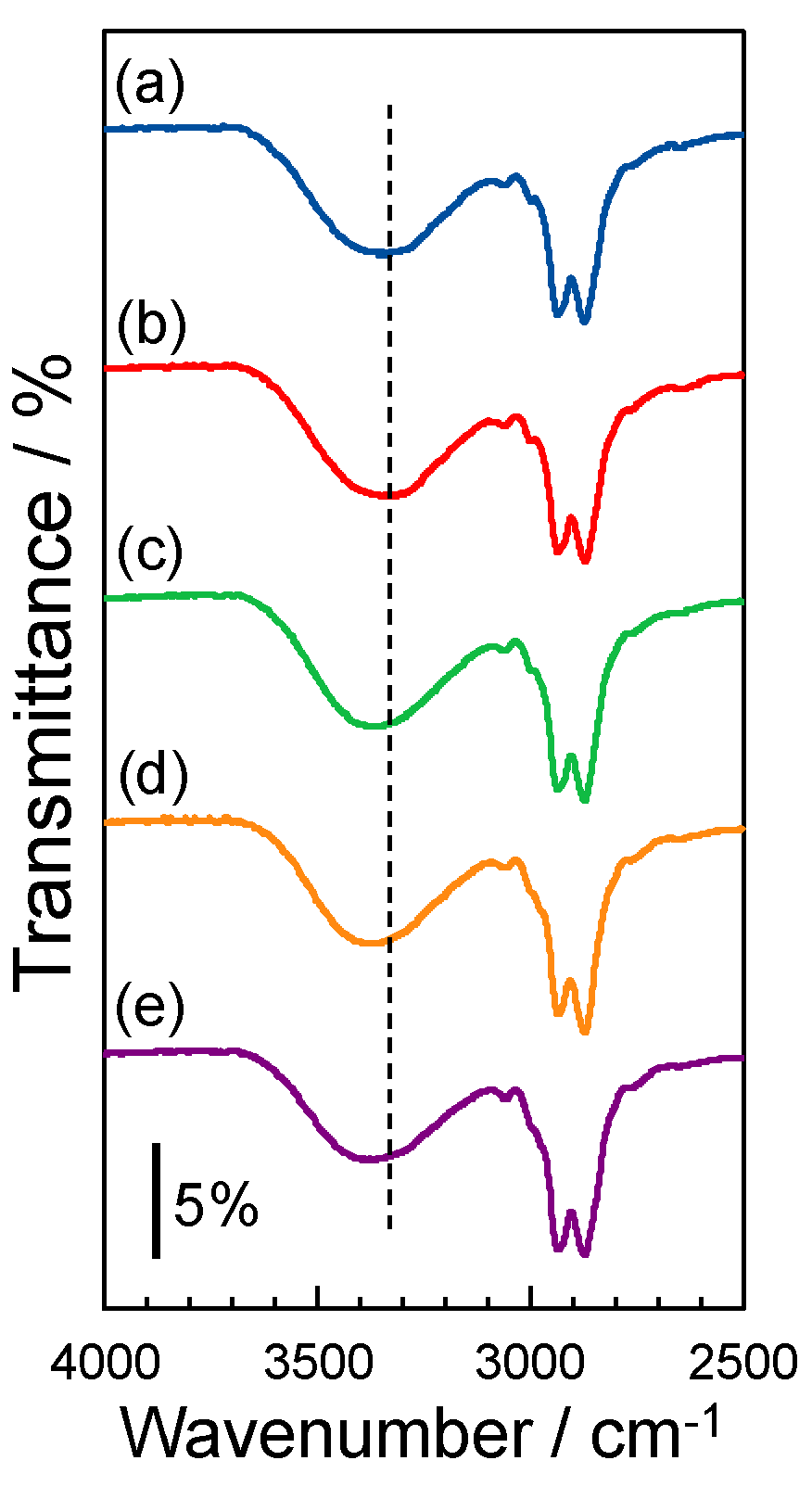
3.2. Molecular Structure of the SP−GPTMS Composite Material
3.3. Thermal Stability of the SP−GPTMS Composite Material
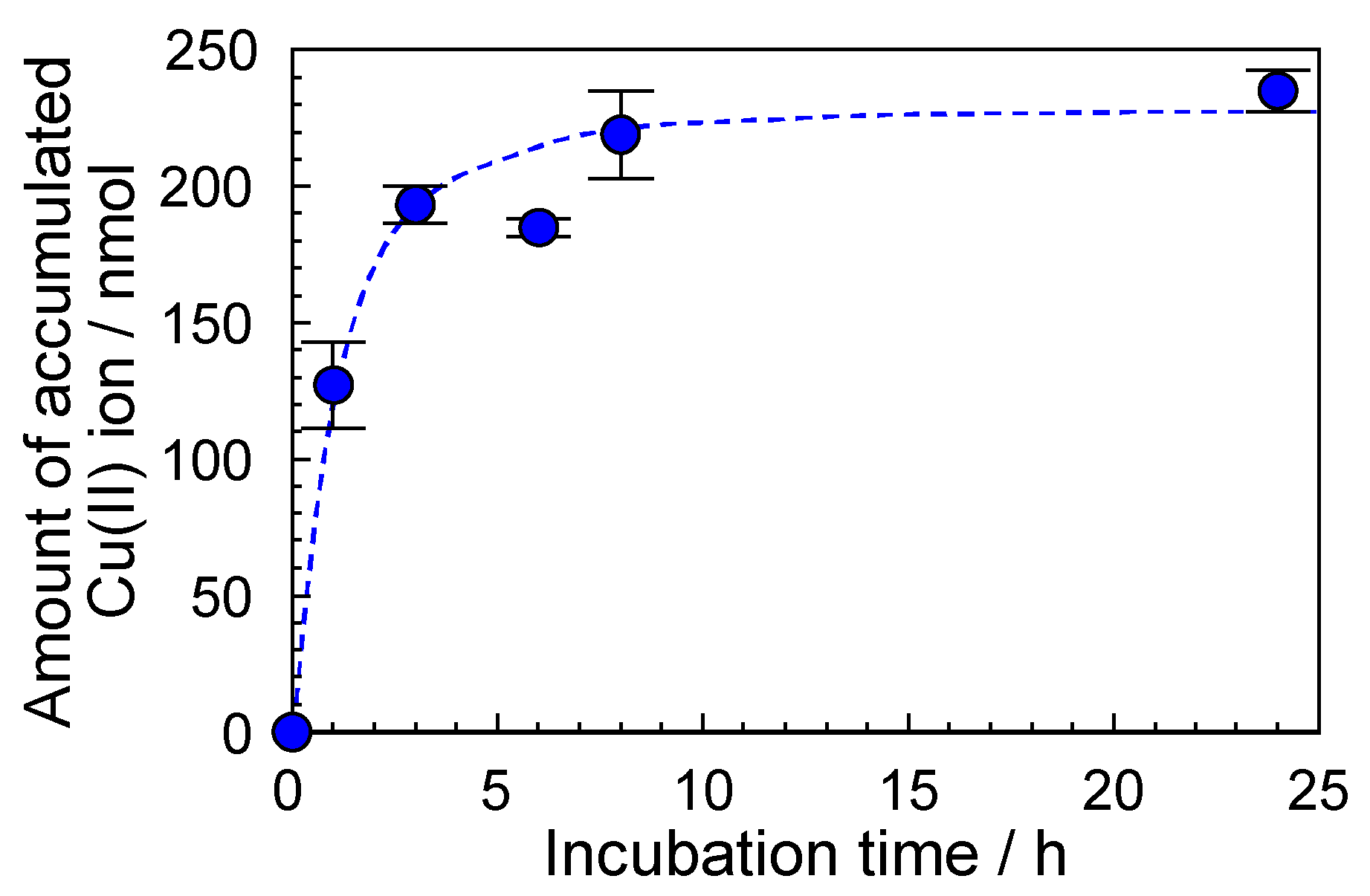
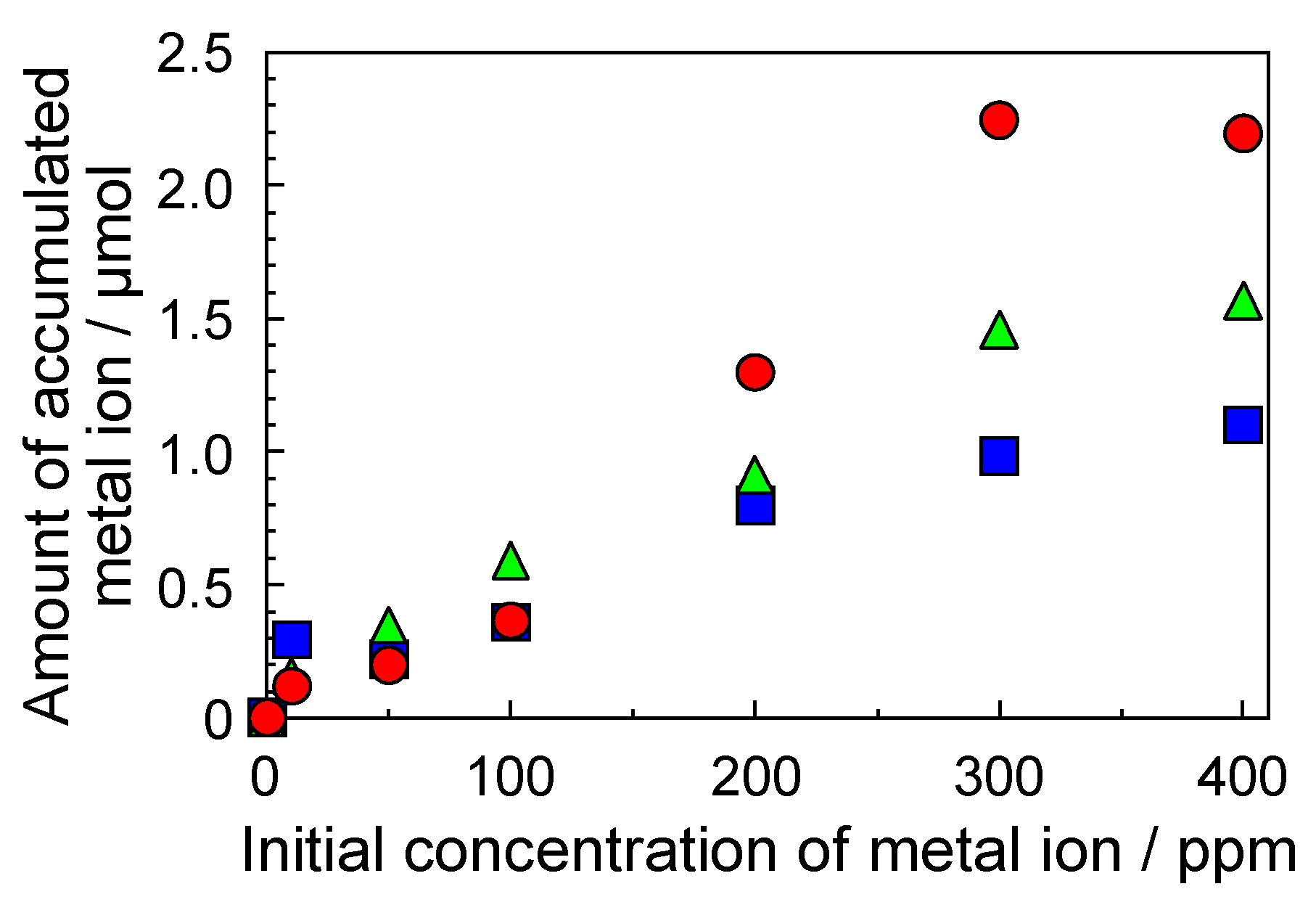
3.4. Accumulation of Metal Ions by the SP−GPTMS Composite Material
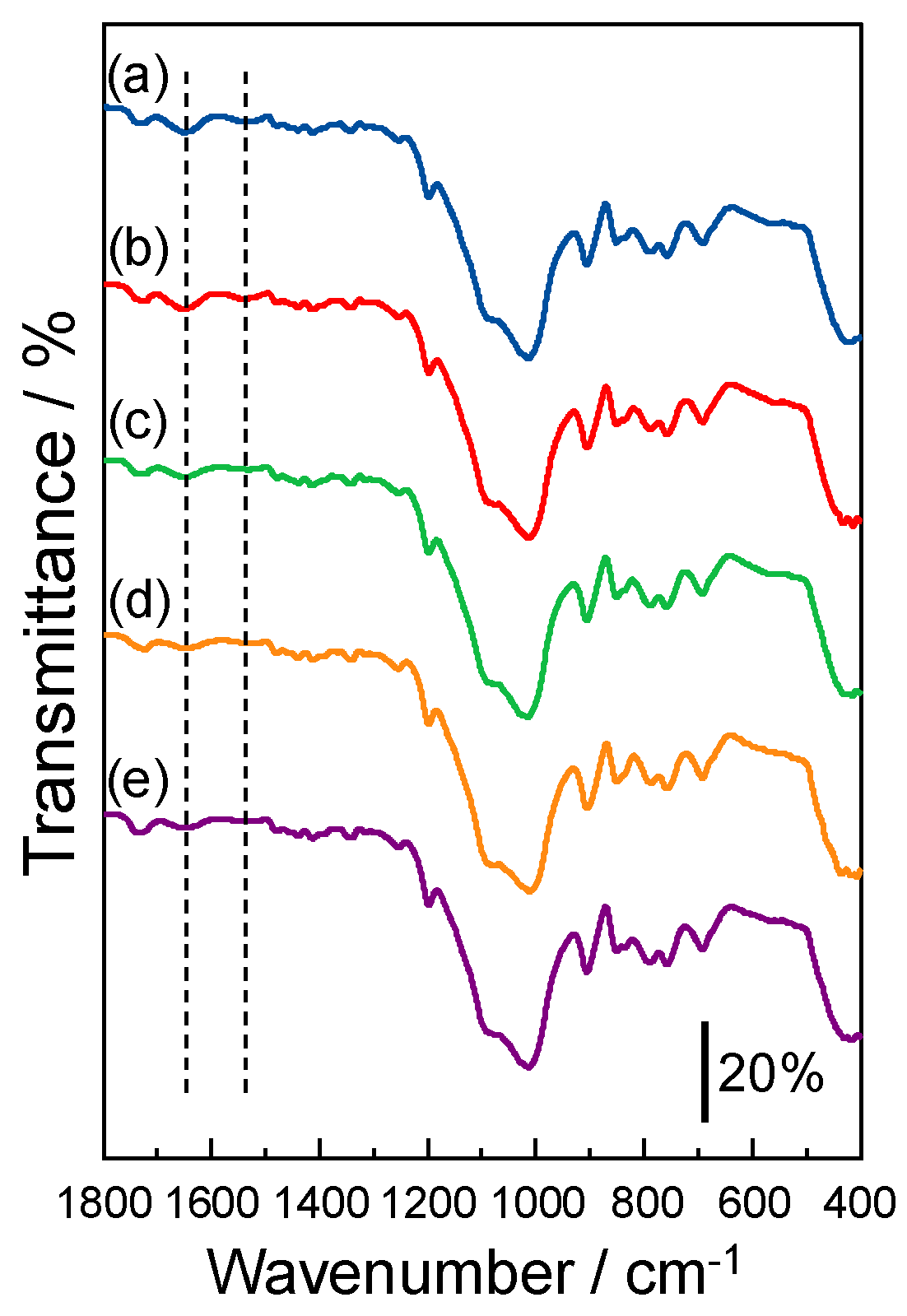
3.5. IR Spectra of the Ca(II) Ion-Accumulated SP−GPTMS Composite Material
3.6. Selective Mechanism of Metal Ions by the SP−GPTMS Composite Material
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Visakh, P.M.; Nazarenko, O. Soy Protein-Based Blends, Composites and Nanocomposites; John Wiley & Sons: Hoboken, NJ, USA, 1998. [Google Scholar]
- El-Shemy, H. Soybean Bio-Active Compounds; IntechOpen Limited: London, UK, 2013. [Google Scholar]
- Japan Oilseed Processors Association. Available online: https://www.oil.or.jp/ (accessed on 1 August 2023).
- Guo, B.; Sun, L.; Jiang, S.; Ren, H.; Sun, R.; Wei, Z.; Hong, H.; Luan, X.; Wang, J.; Wang, X.; et al. Soybean genetic resources contributing to sustainable protein production. Theor. Appl. Genet. 2022, 135, 4095–4121. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, S.K.; Boga, K.; Arukula, R.; Gaddam, S.K. Natural fibre reinforced vegetable-oil based polyurethane composites: A review. J. Polym. Res. 2023, 30, 325. [Google Scholar] [CrossRef]
- Mori, R. Replacing all petroleum-based chemical products with natural biomass-based chemical products: A tutorial review. RSC Sustain. 2023, 1, 179–212. [Google Scholar] [CrossRef]
- Vaz, C.M.; van Doeveren, P.F.N.M.; Reis, R.L.; Cunha, A.M. Soy matrix drug delivery systems obtained by melt-processing techniques. Biomacromolecules 2003, 4, 1520–1529. [Google Scholar] [CrossRef] [PubMed]
- Vaz, C.M.; Fossen, M.; van Tuil, R.F.; de Graaf, L.A.; Reis, R.L.; Cunha, A.M. Casein and soybean protein-based thermoplastics and composites as alternative biodegradable polymers for biomedical applications. J. Biomed. Mater. Res. A 2003, 65, 60–70. [Google Scholar] [CrossRef]
- Rani, S.; Kumar, R. A review on material and antimicrobial properties of soy protein isolate film. J. Polym. Environ. 2019, 27, 1613–1628. [Google Scholar] [CrossRef]
- Yamada, M.; Morimitsu, S.; Hosono, E.; Yamada, T. Preparation of bioplastic using soy protein. Int. J. Biol. Macromol. 2020, 149, 1077–1083. [Google Scholar] [CrossRef]
- Yamada, M.; Nagano, Y.; Yamada, T. Anhydrous proton conduction of soy protein. Int. J. Electrochem. Sci. 2021, 16, 151046. [Google Scholar] [CrossRef]
- Bayuo, J.; Rwiza, M.J.; Sillanpää, M.; Mtei, K.M. Removal of heavy metals from binary and multicomponent adsorption systems using various adsorbents—A systematic review. RSC Adv. 2023, 13, 13052–13093. [Google Scholar] [CrossRef]
- Aziz, K.H.H.; Mustafa, F.S.; Omer, K.M.; Hama, S.; Hamarawf, R.F.; Rahman, K.O. Heavy metal pollution in the aquatic environment: Efficient and low-cost removal approaches to eliminate their toxicity: A review. RSC Adv. 2023, 13, 17595–17610. [Google Scholar] [CrossRef]
- Elbshary, R.E.; Gouda, A.A.; Sheikh, R.E.; Alqahtani, M.S.; Hanfi, M.Y.; Atia, B.M.; Sakr, A.K.; Gado, M.A. Recovery of W(VI) from wolframite ore using new synthetic Schiff base derivative. Int. J. Mol. Sci. 2023, 24, 7423. [Google Scholar] [CrossRef] [PubMed]
- Dhir, B. Potential of biological materials for removing heavy metals from wastewater. Environ. Sci. Pollut. Res. 2014, 21, 1614–1627. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef]
- Liu, C.; Liu, H.; Xiong, T.; Xu, A.; Pan, B.; Tang, K. Graphene oxide reinforced alginate/PVA double network hydrogels for efficient dye removal. Polymers 2018, 10, 835. [Google Scholar] [CrossRef] [PubMed]
- Sakr, A.K.; Aal, M.M.A.; El-Rahem, K.A.A.; Allam, E.M.; Dayem, S.M.A.; Elshehy, E.A.; Hanfi, M.Y.; Alqahtani, M.S.; Cheira, M.F. Characteristic aspects of uranium(VI) adsorption utilizing nano-silica/chitosan from wastewater solution. Nanomaterials 2022, 12, 3866. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.D.; Yamada, M.; Matsunaga, M.; Nishi, N. Functional materials derived from DNA. Adv. Polym. Sci. 2007, 209, 149–178. [Google Scholar]
- Yamada, M.; Kametani, Y. Preparation of gellan gum-inorganic composite film and its metal ion accumulation property. J. Compos. Sci. 2022, 6, 42. [Google Scholar] [CrossRef]
- Yamada, M.; Shimanouchi, Y. Selective accumulation of rare-earth and heavy metal ions by a fucoidan-inorganic composite material. Separations 2022, 9, 219–229. [Google Scholar] [CrossRef]
- Samiey, B.; Cheng, C.H.; Wu, J. Organic-inorganic hybrid polymers as adsorbents for removal of heavy metal ions from solutions: A review. Materials 2014, 7, 673–726. [Google Scholar] [CrossRef]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of hybrid organic–inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Pandey, S.; Mishra, S.B. Sol–gel derived organic–inorganic hybrid materials: Synthesis, characterizations and applications. J. Sol-Gel Sci. Technol. 2011, 59, 73–94. [Google Scholar] [CrossRef]
- Ferreira, V.R.A.; Azenha, M.A.; Bustamante, A.G.; Mêna, M.T.; Moura, C.; Pereira, C.M.; Silva, A.F. Metal cation sorption ability of immobilized and reticulated chondroitin sulfate or fucoidan through a sol-gel crosslinking scheme. Mater. Today Commun. 2016, 8, 172–182. [Google Scholar] [CrossRef]
- Plueddemann, E.P. Silane Coupling Agents, 2nd ed.; Plenum Press: New York, NY, USA, 1991. [Google Scholar]
- Unnikrishnan, K.P.; Thachil, E.T. Toughening of epoxy resins. Des. Monomers Polym. 2006, 9, 129–152. [Google Scholar] [CrossRef]
- Theophanides, T. Infrared Spectroscopy—Materials Science, Engineering and Technology; IntechOpen: London, UK, 2012. [Google Scholar]
- Ma, S.; Liu, W.; Wei, Z.; Li, H. Mechanical and thermal properties and morphology of epoxy resins modified by a silicon compound. J. Macromol. Sci. A 2010, 47, 1084–1090. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X. Spectrometric Identification of Organic Compounds; John Wiley & Sons: New York, NY, USA, 1998. [Google Scholar]
- Yajima, H.; Miyamoto, T.; Endo, R.; Furuya, S. Susceptibility of cobalt(II)-alginate complex films to moisture. Kobunshi Ronbunshu 1989, 46, 577–581. [Google Scholar] [CrossRef]
- Boltinghouse, F.; Abel, K. Development of an optical relative humidity sensor. Cobalt chloride optical absorbency sensor study. Anal. Chem. 1989, 61, 1863–1866. [Google Scholar] [CrossRef]
- Ptak, S.H.; Sanchez, L.; Fretté, X.; Kurouski, D. Complementarity of Raman and infrared spectroscopy for rapid characterization of fucoidan extracts. Plant Methods 2021, 17, 130. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Gunasekaran, S. Preparation of pectin–ZnO nanocomposite. Nanoscale Res. Lett. 2008, 3, 491–495. [Google Scholar] [CrossRef]
- Ho, T.L. Hard soft acids bases (HSAB) principle and organic chemistry. Chem. Rev. 1975, 75, 1–20. [Google Scholar] [CrossRef]
- Cotton, F.A.; Wilkinson, G.; Gaus, P.L. Basic Inorganic Chemistry; John Wiley & Sons: New York, NY, USA, 1991. [Google Scholar]
- Ayers, P.W. The physical basis of the hard/soft acid/base principle. Faraday Discuss. 2007, 135, 161–190. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, M.; Ujihara, M.; Yamada, T. The Accumulation of Metal Ions by a Soy Protein–Inorganic Composite Material. J. Compos. Sci. 2023, 7, 419. https://doi.org/10.3390/jcs7100419
Yamada M, Ujihara M, Yamada T. The Accumulation of Metal Ions by a Soy Protein–Inorganic Composite Material. Journal of Composites Science. 2023; 7(10):419. https://doi.org/10.3390/jcs7100419
Chicago/Turabian StyleYamada, Masanori, Maika Ujihara, and Tetsuya Yamada. 2023. "The Accumulation of Metal Ions by a Soy Protein–Inorganic Composite Material" Journal of Composites Science 7, no. 10: 419. https://doi.org/10.3390/jcs7100419





