Assessment of the Attenuation Properties of Commercial Lead-Free Radiation-Shielding Composite Materials Against Medical X-rays
Abstract
:1. Introduction
- Coherent (elastic) scattering occurs when the incident X-rays change their direction upon interaction with the outer electrons of the atoms in the shielding material, but still remain with the same energy. This elastic scattering is dependent on the metal atomic number, Z, and usually occurs at X-ray photon energies of less than 10 keV.
- Photoelectric effect occurs when the X-rays are colliding with an electron closer to the nucleus in the atoms of the shielding material, the electron is being ejected from its shell, and then its “spot” is filled with an electron from an outer shell, a process that is accompanied by an emission of fluorescent X-rays characteristic for that element. The fluorescent X-rays are usually of lower photon energy than the incident X-rays and are highly dependent on the Z-number of the element(s) in the shielding material.
- Compton effect occurs when the incident X-rays are deflected from their original direction by the loosely bound outer electrons of the atoms in the shielding material, which is associated with a partial loss of the incident photon energy. The photons continue to move throughout the material in a different direction with diminished energy until they undergo other interactions or leave the medium at a different angle than the incident X-rays. The energy shift depends mostly on the angle of scattering and not on the nature of the material. This is a type of incoherent scattering since the X-ray photon energy change is not always orderly and is not consistent; therefore, it is hard to predict. The probability for the Compton effect is directly proportional to the electron density and the physical density of the material, but does not depend on the Z-number, unlike the photoelectron effect.
2. Materials and Methods
2.1. Materials
2.2. Materials Characterization
3. Results and Discussion
3.1. Qualitative Assessment
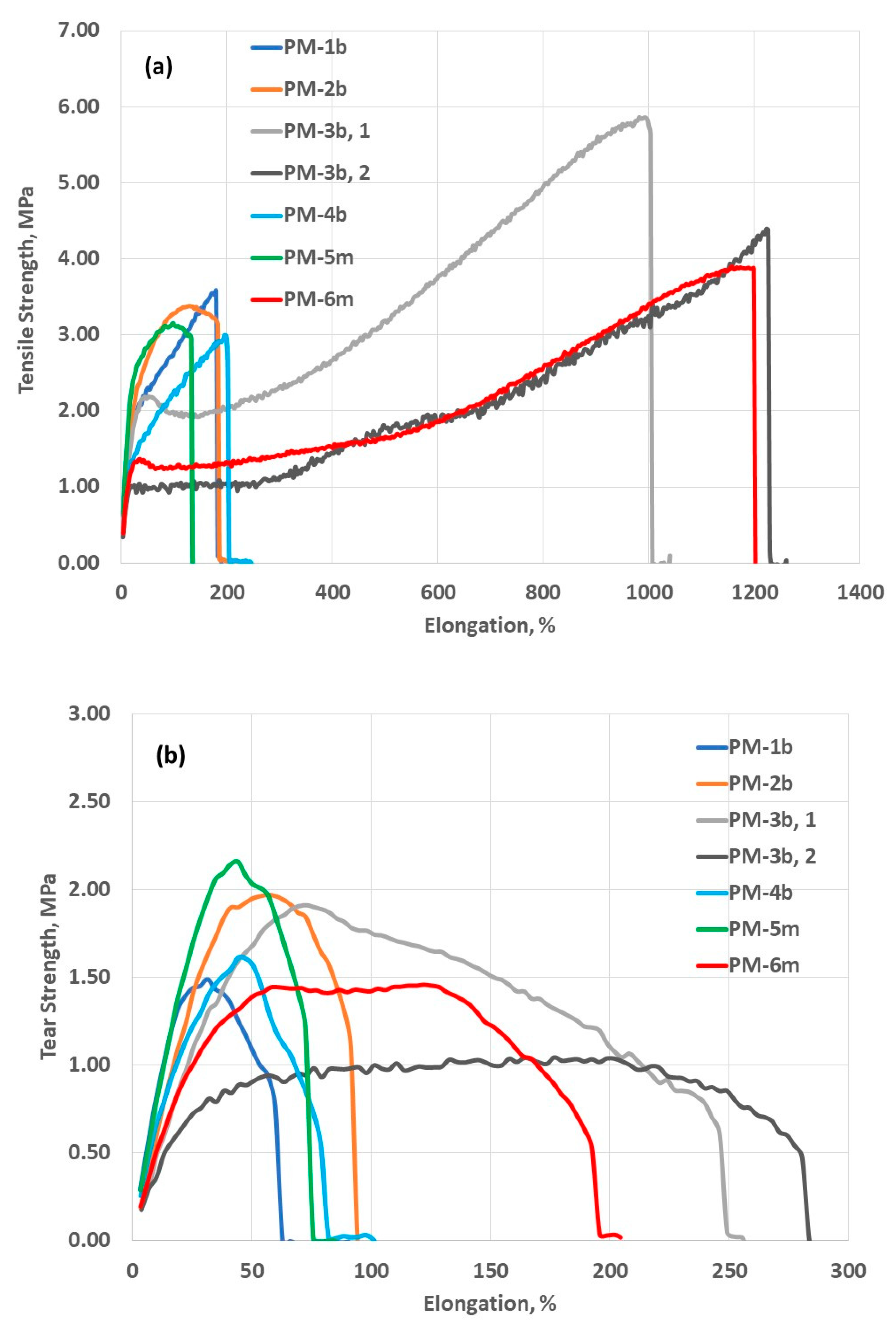
3.2. Mechanical Properties
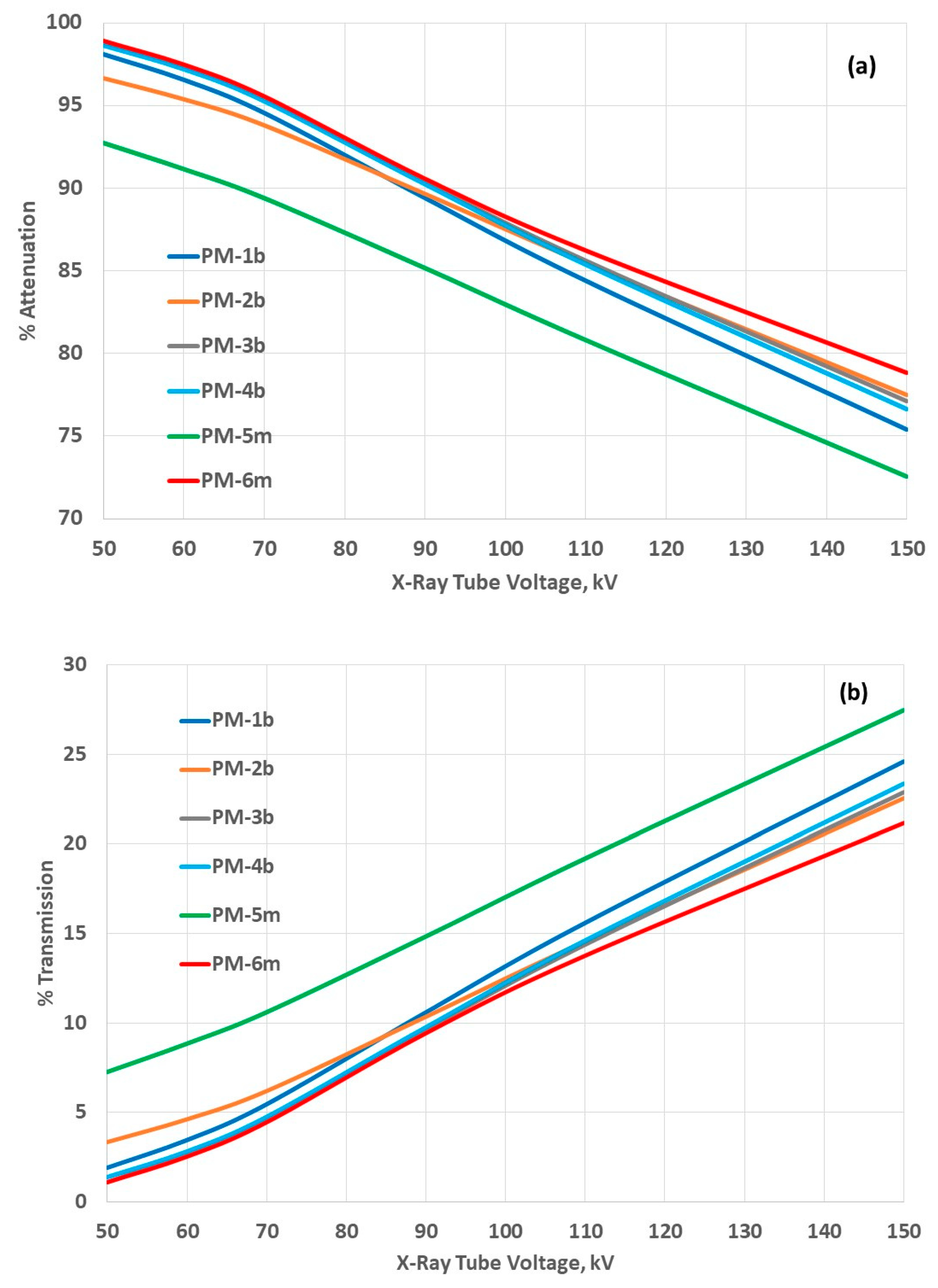
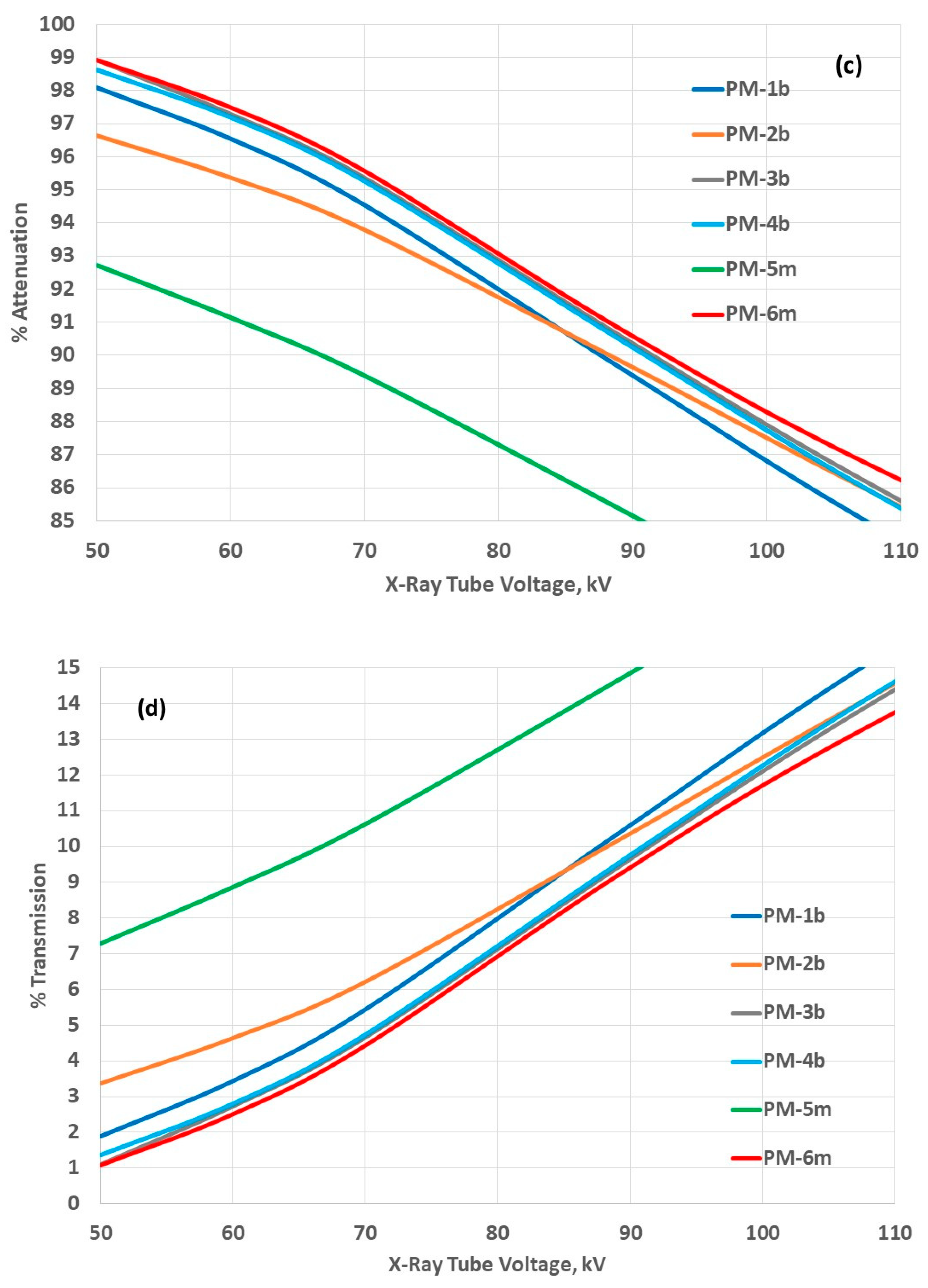
3.3. Attenuation Properties
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Beyer, T.; Bailey, D.L.; Birk, U.J.; Buvat, I.; Catana, C.; Cheng, Z.; Fang, Q.; Giove, F.; Kuntner, C.; Laistler, E.; et al. Medical Physics and Imaging—A Timely Perspective. Front. Phys. 2021, 9, 634693. [Google Scholar] [CrossRef]
- Hussain, S.; Mubeen, I.; Ullah, N.; Shah, S.S.U.D.; Khan, B.A.; Zahoor, M.; Ullah, R.; Khan, F.A.; Sultan, M.A. Modern Diagnostic Imaging Technique Applications and Risk Factors in the Medical Field: A Review. Hindawi BioMed Res. Int. 2022, 2022, 5164970. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Yang, Q.; Maier, A. X Ray Imaging. In Medical Imaging Systems: An Introductory Guide; Maier, A., Steidl, S., Horneggel, J., Eds.; Springer: Cham, Switzerland, 2018; pp. 119–145. [Google Scholar] [CrossRef]
- Datta, A.; Zhong, Z.; Motakef, S. A new generation of direct X-ray detectors for medical and synchrotron imaging applications. Sci. Rep. 2020, 10, 20097. [Google Scholar] [CrossRef] [PubMed]
- Kovács, A.; Bischoff, P.; Haddad, H.; Kovács, G.; Schaefer, A.; Zhou, W.; Pinkawa, M. Personalized Image-Guided Therapies for Local Malignencies: Interdisciplinary Options for Interventional Radiology and Interventional Radiotherapy. Front. Oncol. 2021, 11, 616058. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, P.; Ginjaume, M.; Hupe, O.; O’Connor, U.; Vanhavere, F.; Bakhanova, E.; Becker, F.; Campani, L.; Carinou, E.; Clairand, I.; et al. What Is Worth Knowing in Interventional Practices about Medical Staff Radiation Exposure Monitoring: A Review of Recent Outcomes of EURADOS Working Group 12. Environments 2022, 9, 53. [Google Scholar] [CrossRef]
- Nowak, M.; Carbonez, P.; Krauss, M.; Verdun, F.R.; Damet, J. Characterisation and mapping of scattered radiation fields in interventional radiology theatres. Sci. Rep. 2020, 10, 18754. [Google Scholar] [CrossRef]
- Eder, H.; Seidenbusch, M.; Oechler, L.S. Tertiary X-Radiation—A problem for Staff Protection? Radiat. Prot. Dosim. 2020, 189, 304–311. [Google Scholar] [CrossRef]
- Pavlicek, W.; Sensakovic, W.F.; Zhou, Y.; Paden, R.G.; Panda, A.; Hines, J.; Naidu, S.G.; Oklu, R. Sample content of kinesthetic educational training: Reducing scattered X-ray exposures to interventional physician operators of fluoroscopy. J. Appl. Clin. Med. Phys. 2019, 21, 196–208. [Google Scholar] [CrossRef]
- Khafaji, M.; Albadawi, G.H. Assessment of Scattered Dose to the Eye in Dentistry: A Systematic Review. Cureus 2023, 15, e43113. [Google Scholar] [CrossRef]
- Low, I.M.; Azman, N.Z.N. Polymer Composites and Nanocomposites for X-Rays Shielding; Springer Nature Singapore Pte Ltd.: Singapore, 2020; ISBN 978-981-13-9809-4. [Google Scholar] [CrossRef]
- Osanai, M.; Kudo, K.; Hosoda, M.; Tazoe, H.; Akata, N.; Kitajima, M.; Tsushima, M.; Komiya, N.; Kudo, M.; Tsujiguchi, T.; et al. The impact on the eye lens of radiation emitted by natural radionuclides (Lead-210) present in radiation protection glasses. Radiat. Prot. Dosim. 2019, 188, 13–21. [Google Scholar] [CrossRef]
- Garg, T.; Shrigiriwar, A. Radiation Protection in Interventional Radiology. Indian J. Radiol. Imaging 2021, 31, 939–945. [Google Scholar] [CrossRef]
- Shahzad, K.; Kausar, A.; Manzoor, S.; Rakha, S.A.; Uzair, A.; Sajid, M.; Arif, A.; Khan, A.F.; Diallo, A.; Ahmad, I. Views on Radiation Shielding Efficiency of Polymeric Composites/Nanocomposites and Multi-Layered Materials: Current State and Advancements. Radiation 2022, 3, 1–20. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, W.; Zhang, X.; Gao, Y.; Guo, S. High-efficiency, flexibility and lead-free X-ray shielding multilayered polymer composites: Layered structure design and shielding mechanism. Sci. Rep. 2021, 11, 4384. [Google Scholar] [CrossRef] [PubMed]
- Asadpour, N.; Malekzadeh, R.; Rajabpour, S.; Refahi, S.; Mehnati, P.; Shanei, A. Shielding performance of multi-metal nanoparticle composites for diagnostic radiology: An MCNPX and Geant4 study. Radiol. Phys. Technol. 2022, 16, 57–68. [Google Scholar] [CrossRef] [PubMed]
- More, C.V.; Alsayed, Z.; Badawi, M.S.; Thabet, A.A.; Pawar, P.P. Polymeric composite materials for radiation shielding: A review. Environ. Chem. Lett. 2021, 19, 2057–2090. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wei, Q.; Zheng, W.; Zheng, Y.; Okosi, N.; Wang, Z.; Su, M. Enhanced Radiation Shielding with Conformal Light-Weight Nanoparticle–Polymer Composite. ACS Appl. Mater. Interfaces 2018, 10, 35510–35515. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Choi, J.R.; Jeon, B.K. Physical analysis of the shielding capacity for a lightweight apron designed for shielding low intensity scattering X-rays. Sci. Rep. 2016, 6, 27721. [Google Scholar] [CrossRef]
- Almurayshid, M.; Alssalim, Y.; Aksouh, F.; Almsalam, R.; Alqahtani, M.; Sayyed, M.I.; Almasoud, F. Development of New Lead-Free Composite Materials as Potential Radiation Shields. Materials 2021, 14, 4957. [Google Scholar] [CrossRef]
- Kim, S.-C. Comparison of Shielding Material Dispersion Characteristics and Shielding Efficiency for Manufacturing Medical X-ray Shielding Barriers. Materials 2022, 15, 6075. [Google Scholar] [CrossRef]
- Eder, H.; Schlattl, H. Shielding effectiveness of X-ray protective garment. Phys. Medica 2021, 82, 343–350. [Google Scholar] [CrossRef]
- Nambiar, S.; Osei, E.K.; Yeow, J.T.W. Polymer Nanocomposite-based Shielding Against Diagnostic X-rays. J. Appl. Polym. Sci. 2013, 127, 4939–4946. [Google Scholar] [CrossRef]
- Thumwong, A.; Darachai, J.; Saenboonruang, K. Comparative X-ray Shielding Properties of Single-Layered and Multi-Layered Bi2O3/NR Composites: Simulation and Numerical Studies. Polymers 2022, 14, 1788. [Google Scholar] [CrossRef] [PubMed]
- McCaffrey, J.P.; Mainegra-Hing, E.; Shen, H. Optimizing non-Pb radiation shielding materials using bilayers. Med. Phys. 2009, 36, 5586–5594. [Google Scholar] [CrossRef] [PubMed]
- Gilys, L.; Griškonis, E.; Griškevičius, P.; Adlienė, D. Lead Free Multilayered Polymer Composites for Radiation Shielding. Polymers 2022, 14, 1696. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, S.; Seo, Y. Enhanced X-ray Shielding Ability of Polymer–Nonleaded Metal Composites by Multilayer Structuring. Ind. Eng. Chem. Res. 2015, 54, 5968–5973. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.; Kim, Y.; Kim, E.; Seo, Y. Multilayer-Structured Non-leaded Metal/Polymer Composites for Enhanced X-ray Shielding. MRS Adv. 2018, 3, 1789–1797. [Google Scholar] [CrossRef]
- Kazempour, M.; Saeedimoghadam, M.; Shooli, F.S.; Shokrpour, N. Assessment of the Radiation Attenuation Properties of Several Lead Free Composites by Monte Carlo Simulation. J. Biomed. Phys. Eng. 2015, 5, 67–76. [Google Scholar]
- Nikeghbal, K.; Zamanian, Z.; Shahidi, S.; Spagnuolo, G.; Soltani, P. Designing and Fabricating Nano-Structured and Micro-Structured Radiation Shields for Protection against CBCT Exposure. Materials 2020, 13, 4371. [Google Scholar] [CrossRef]
- Yücel, H.; Güllüoğlu, E.; Çubukçu, S.; Ali Üncü, Y. Measurement of the Attenuation Properties of the Protective Materials Used as a Thyroid Guard and Apron for Personnel Protection against Diagnostic Medical X-rays. J. Phys. Sci. 2016, 27, 111–128. [Google Scholar]
- Kim, S.-C. Analysis of Shielding Performance of Radiation-Shielding Materials According to Particle Size and Clustering Effects. Appl. Sci. 2021, 11, 4010. [Google Scholar] [CrossRef]
- Fakhoury, E.; Provencher, J.-A.; Subramaniam, R.; Finlay, D.J. Not all lightweight lead aprons and thyroid shields are alike. J. Vasc. Surg. 2018, 70, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Hertault, A. Shields Up! How Much Should You Rely on Your Lightweight Garments? Eur. J. Vasc. Endovasc. Surg. 2019, 57, 740. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Boyd, C.; Dawson, J. Lightweight Lead Aprons: The Emperor’s New Clothes in the Angiography Suite? Eur. J. Vasc. Endovasc. Surg. 2019, 57, 730–739. [Google Scholar] [CrossRef]
- Kim, S.-C.; Cho, S.-H. Analysis of the Correlation between Shielding Material Blending Characteristics and Porosity for Radiation Shielding Films. Appl. Sci. 2019, 9, 1765. [Google Scholar] [CrossRef]
- Pianpanit, T.; Saenboonruang, K. High-Energy Photon Attenuation Properties of Lead-Free and Self-Healing Poly (Vinyl Alcohol) (PVA) Hydrogels: Numerical Determination and Simulation. Gels 2022, 8, 197. [Google Scholar] [CrossRef] [PubMed]
- Elsafi, M.; El-Nahal, M.A.; Sayyed, M.I.; Saleh, I.H.; Abbas, M.I. Novel 3-D printed radiation shielding materials embedded with bulk and nanoparticles of bismuth. Sci. Rep. 2022, 12, 12467. [Google Scholar] [CrossRef]
- Kaewpirom, S.; Chousangsuntorn, K.; Boonsang, S. Evaluation of Micro- and Nano-Bismuth(III) Oxide Coated fabric for Environmentally Friendly X-Ray Shielding Materials. ACS Omega 2022, 7, 28248–28257. [Google Scholar] [CrossRef]
- El-Khatib, A.M.; Shalaby, T.I.; Antar, A.; Elsafi, M. Experimental Study of Polypropylene with Additives of Bi2O3 Nanoparticles as Radiation-Shielding Materials. Polymer 2022, 14, 2253. [Google Scholar] [CrossRef]
- Tiamduangtawan, P.; Kamkaew, C.; Kuntonwatchara, S.; Wimolmala, E.; Saenboonruang, K. Comparative mechanical, self-healing, and gamma attenuation properties of PVA hydrogels containing either nano- or micro-sized Bi2O3 for use as gamma-shielding materials. Radiat. Phys. Chem. 2020, 177, 109164. [Google Scholar] [CrossRef]
- Winter, H.; Brown, A.L.; Goforth, A.M. Bismuth-Based Nano- and Microparticles in X-Ray Contrast, Radiation Therapy, and Radiation Shielding Applications. In Bismuth—Advanced Applications and Defects Characterization; Zhou, Y., Dong, F., Jin, S., Eds.; InterOpen: London, UK, 2018; Chapter 4; ISBN 978-1-78923-263-9. [Google Scholar] [CrossRef]
- Wang, B.; Ting, C.-Y.; Lai, C.-S.; Tsai, Y.-S. Bismuth Pelvic X-Ray Shielding Reduces Radiation Dose Exposure in Pediatric Radiography. Hindawi BioMed Res. Int. 2021, 2021, 9985714. [Google Scholar] [CrossRef]
- Cadavid, D.A.R.; Layman, R.R.; Nishino, T.; Slutzky, J.L.; Li, Z.; Cornish, K. Guayule Natural Rubber Latex and Bi2O3 Films for X-ray Attenuating Medical Gloves. Materials 2022, 15, 1184. [Google Scholar] [CrossRef] [PubMed]
- Alshahri, S.; Alsuhybani, M.; Alosime, E.; Almurayshid, M.; Alrwais, A.; Alotaibi, S. LDPE/Bismuth Oxide Nanocomposite: Preparation, Characterization and Application in X-ray Shielding. Polymers 2021, 13, 3081. [Google Scholar] [CrossRef] [PubMed]
- Poltabtim, W.; Wimolmala, E.; Markpin, T.; Sombatsompop, N.; Rosarpitak, V.; Saenboonruang, K. X-ray Shielding, Mechanical, Physical, and Water Absorption Properties of Wood/PVC Composites Containing Bismuth Oxide. Polymers 2021, 13, 2212. [Google Scholar] [CrossRef] [PubMed]
- Thumwong, A.; Chinnawet, M.; Intarasena, P.; Rattanapongs, C.; Tokonami, S.; Ishikawa, T.; Saenboonruang, K. A Comparative Study on X-ray Shielding and Mechanical Properties of Natural Rubber Latex Nanocomposites Containing Bi2O3 or BaSO4: Experimental and Numerical Determination. Polymers 2022, 14, 3654. [Google Scholar] [CrossRef] [PubMed]
- Seenappa, L.; Manjunatha, H.; Chandrika, B.; Chikka, H. A Study of Shielding Properties of X-ray and Gamma in Barium Compounds. J. Radiat. Prot. Res. 2017, 42, 26–32. [Google Scholar] [CrossRef]
- Kim, S.-C.; Dong, K.-R.; Chung, W.-K. Medical radiation shielding effect by composition of barium compounds. Ann. Nucl. Energy 2012, 47, 1–5. [Google Scholar] [CrossRef]
- Issa, S.A.; Zakaly, H.M.; Pyshkina, M.; Mostafa, M.Y.; Rashad, M.; Soliman, T. Structure, optical, and radiation shielding properties of PVA–BaTiO3 nanocomposite films: An experimental investigation. Radiat. Phys. Chem. 2020, 180, 109281. [Google Scholar] [CrossRef]
- Hong, J.W.; Kim, D.H.; Kim, S.W.; Choi, S.H.; Lee, G.-E.; Seo, H.-K.; Kim, S.-H.; Lee, Y. Effectiveness evaluation of self-produced micro- and nanosized tungsten materials for radiation shielding with diagnostic X-ray imaging system. Optik 2018, 172, 760–765. [Google Scholar] [CrossRef]
- Al-Ghamdi, H.; Hemily, H.M.; Saleh, I.H.; Ghataas, Z.F.; Abdel-Halim, A.A.; Sayyed, M.I.; Yasmin, S.; Almuqrin, A.H.; Elsafi, M. Impact of WO3-Nanoparticles on Silicone Rubber for Radiation Protection Efficiency. Materials 2022, 15, 5706. [Google Scholar] [CrossRef]
- Aghaz, A.; Faghihi, R.; Mortazavi, S.; Haghparast, A.; Mehdizadeh, S.; Sina, S. Radiation Attenuation Properties of Shielding Materials Containing Micro and Nano-sized WO3 in Diagnostic X-ray Energy Range. Int. J. Radiat. Res. 2016, 14, 127–131. [Google Scholar] [CrossRef]
- Azman, N.N.; Siddiqui, S.; Hart, R.; Low, I. Effect of particle size, filler loadings and x-ray tube voltage on the transmitted x-ray transmission in tungsten oxide—Epoxy composites. Appl. Radiat. Isot. 2012, 71, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.A.; Mousavi, S.M.; Faghihi, R.; Arjmand, M.; Rahsepar, M.; Bahrani, S.; Ramakrishna, S.; Lai, C.W. Superior X-ray Radiation Shielding Effectiveness of Biocompatible Polyaniline Reinforced with Hybrid Graphene Oxide-Iron Tungsten Nitride Flakes. Polymers 2020, 12, 1407. [Google Scholar] [CrossRef] [PubMed]
- Kijima, K.; Monzen, H.; Matsumoto, K.; Tamura, M.; Nishimura, Y. The Shielding Ability of Novel Tungsten Rubber Against the Electron Beam for Clinical Use in Radiation Therapy. Anticancer. Res. 2018, 38, 3919–3927. [Google Scholar] [CrossRef] [PubMed]
- Dejangah, M.; Ghojavand, M.; Poursalehi, R.; Gholipour, P.R. X-ray attenuation and mechanical properties of tungsten-silicone rubber nanocomposites. Mater. Res. Express 2019, 6, 085045. [Google Scholar] [CrossRef]
- Yonphan, S.; Chaiphaksa, W.; Kalkornsurapranee, E.; Tuljittraporn, A.; Kothan, S.; Kaewjaeng, S.; Intachai, N.; Wongdamnern, N.; Kedkaew, C.; Kim, H.; et al. Development of flexible radiation shielding materials from natural Rubber/Sb2O3 composites. Radiat. Phys. Chem. 2022, 200, 110379. [Google Scholar] [CrossRef]
- Zarei, M.; Sina, S.; Hashemi, S.A. Superior X-ray radiation shielding of biocompatible platform based on reinforced polyaniline by decorated graphene oxide with interconnected tungsten–bismuth–tin complex. Radiat. Phys. Chem. 2021, 188, 109588. [Google Scholar] [CrossRef]
- Uthoff, H.; Benenati, M.J.; Katzen, B.T.; Peña, C.; Gandhi, R.; Staub, D.; Schernthaner, M. Lightweight Bilayer Barium Sulfate–Bismuth Oxide Composite Thyroid Collars for Superior Radiation Protection in Fluoroscopy-guided Interventions: A Prospective Randomized Controlled Trial. Radiology 2014, 270, 601–606. [Google Scholar] [CrossRef]
- Johansen, S.; Hauge, I.H.R.; Hogg, P.; England, A.; Lança, L.; Gunn, C.; Sanderud, A. Are Antimony-Bismuth Aprons as Efficient as Lead Rubber Aprons in Providing Shielding against Scattered Radiation? J. Med. Imaging Radiat. Sci. 2018, 49, 201–206. [Google Scholar] [CrossRef]
- Kim, S.-C. Development of a Lightweight Tungsten Shielding Fiber That Can Be Used for Improving the Performance of Medical Radiation Shields. Appl. Sci. 2021, 11, 6475. [Google Scholar] [CrossRef]
- Kim, S.-C. Construction of a Medical Radiation-Shielding Environment by Analyzing the Weaving Characteristics and Shielding Performance of Shielding Fibers Using X-ray-Impermeable Materials. Appl. Sci. 2021, 11, 1705. [Google Scholar] [CrossRef]
- Kim, S.-C.; Son, J.-S. Manufacturing and performance evaluation of medical radiation shielding fiber with plasma thermal spray coating technology. Sci. Rep. 2021, 11, 22418. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, M.; Zarrebini, M.; Shirani, A.; Shanbeh, M.; Borhani, S. X-ray shielding behavior of garment woven with melt-spun polypropylene monofilament. Powder Technol. 2018, 345, 15–25. [Google Scholar] [CrossRef]
- Mirzaei, M.; Zarrebini, M.; Shirani, A.; Shanbeh, M.; Borhani, S. X-ray shielding by a novel garment woven with melt-spun monofilament weft yarn containing lead and tin particles. Text. Res. J. 2017, 89, 63–75. [Google Scholar] [CrossRef]
- Liang, D.; Shen, F.; Bao, Z.; Liu, Y.; Li, H. Research on textile materials for X-ray shielding. E3S Web Conf. 2021, 290, 01013. [Google Scholar] [CrossRef]
- Aral, N.; Nergis, F.B.; Candan, C. An alternative X-ray shielding material based on coated textiles. Text. Res. J. 2015, 86, 803–811. [Google Scholar] [CrossRef]
- Verma, S.; Mili, M.; Sharma, C.; Bajpai, H.; Pal, K.; Qureshi, D.; Hashmi, S.A.R.; Srivastava, A.K. Advanced X-ray shielding and antibacterial smart multipurpose fabric impregnated with polygonal shaped bismuth oxide nanoparticles in carbon nanotubes via green synthesis. Green Chem. Lett. Rev. 2021, 14, 272–285. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, P.; Xu, H.; Li, Q.; Guo, J.; Liao, X.; Shi, B. Advanced X-ray Shielding Materials Enabled by the Coordination of Well-Dispersed High Atomic Number Elements in Natural Leather. ACS Appl. Mater. Interfaces 2020, 12, 19916–19926. [Google Scholar] [CrossRef]
- Dehghan, N.; Movahedi, M.; Abdi, A.; Mehdizadeh, A.; Heidari, E.; Masumi, Y.; Abbaszadeh, M. Novel paint design based on nanopowder to protection against X and gamma rays. Indian J. Nucl. Med. 2014, 29, 18–21. [Google Scholar] [CrossRef]
- Schmid, E.; Panzer, W.; Schlattl, H.; Eder, H. Emission of fluorescent x-radiation from non-lead based shielding materials of protective clothing: A radiobiological problem? J. Radiol. Prot. 2012, 32, N129–N139. [Google Scholar] [CrossRef]
- Schlattl, H.; Zankl, M.; Eder, H.; Hoeschen, C. Shielding properties of lead-free protective clothing and their impact on radiation doses. Med. Phys. 2007, 34, 4270–4280. [Google Scholar] [CrossRef]
- Eder, H.; Schlattl, H. IEC 61331-1: A new setup for testing lead free X-ray protective clothing. Phys. Medica 2018, 45, 6–11. [Google Scholar] [CrossRef]
- Eder, H.; Panzer, W.; Schöfer, H. Is the lead equivalent suitable for assessing the protective effect of lead-free X-ray protective clothing? Rofo 2005, 177, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Schöpf, T.; Pichler, T. Radiation Protection Clothing in X-Ray Diagnostics—Influence of the Different Methods of Measurement on the Lead Equivalent and the Required Mass. Rofo 2016, 188, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.K.; Wagner, L.K. On the (f)utility of measuring the lead equivalence of protective garments. Med. Phys. 2013, 40, 063902. [Google Scholar] [CrossRef]
- Büermann, L. Determination of lead equivalent values according to IEC 61331-1:2014—Report and short guidelines for testing laboratories. J. Instrum. 2016, 11, T09002. [Google Scholar] [CrossRef]
- McCaffrey, J.P.; Shen, H.; Downton, B.; Mainegra-Hing, E. Radiation attenuation by lead and nonlead materials used in radiation shielding garments. Med. Phys. 2007, 34, 530–537. [Google Scholar] [CrossRef] [PubMed]

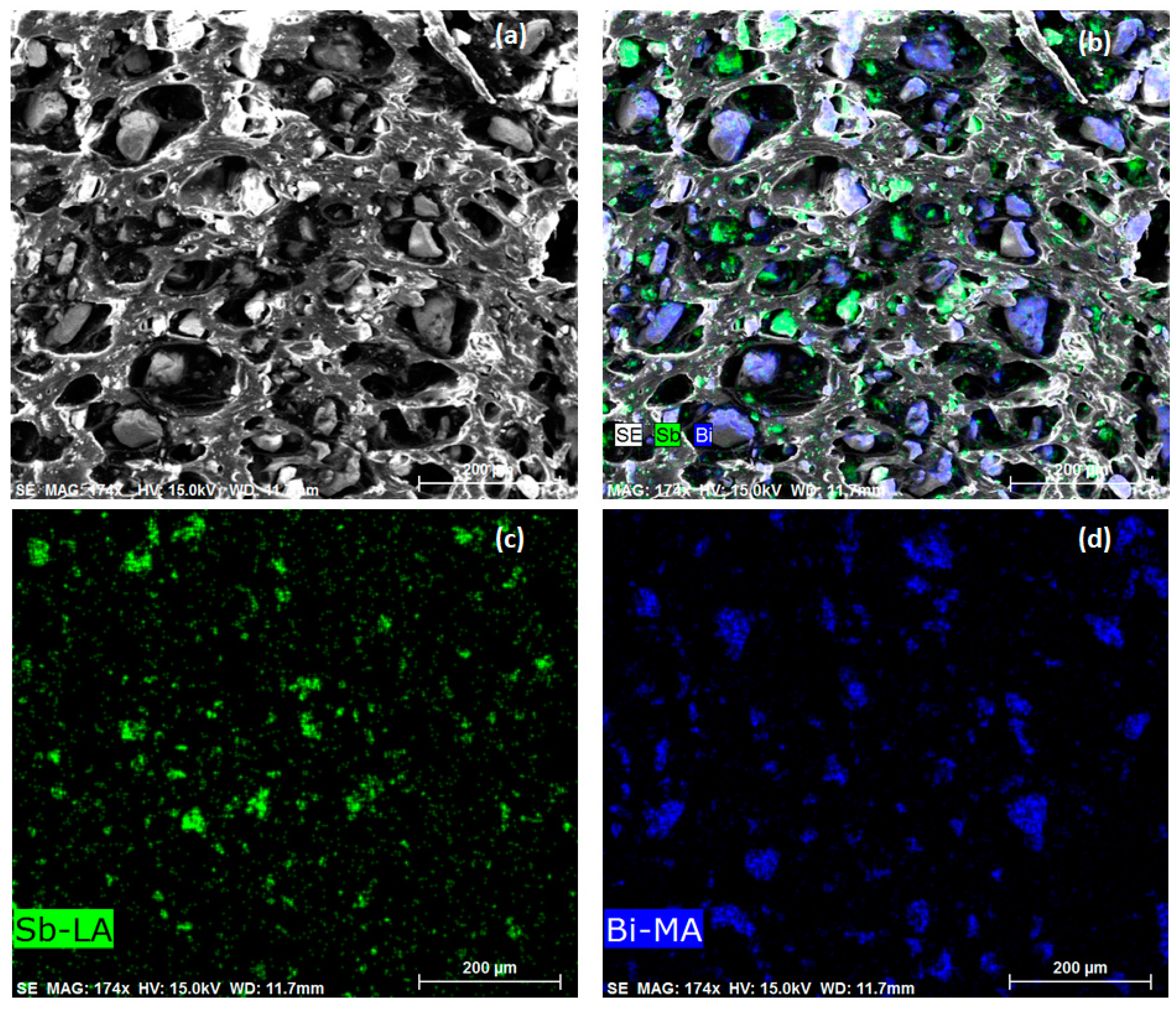
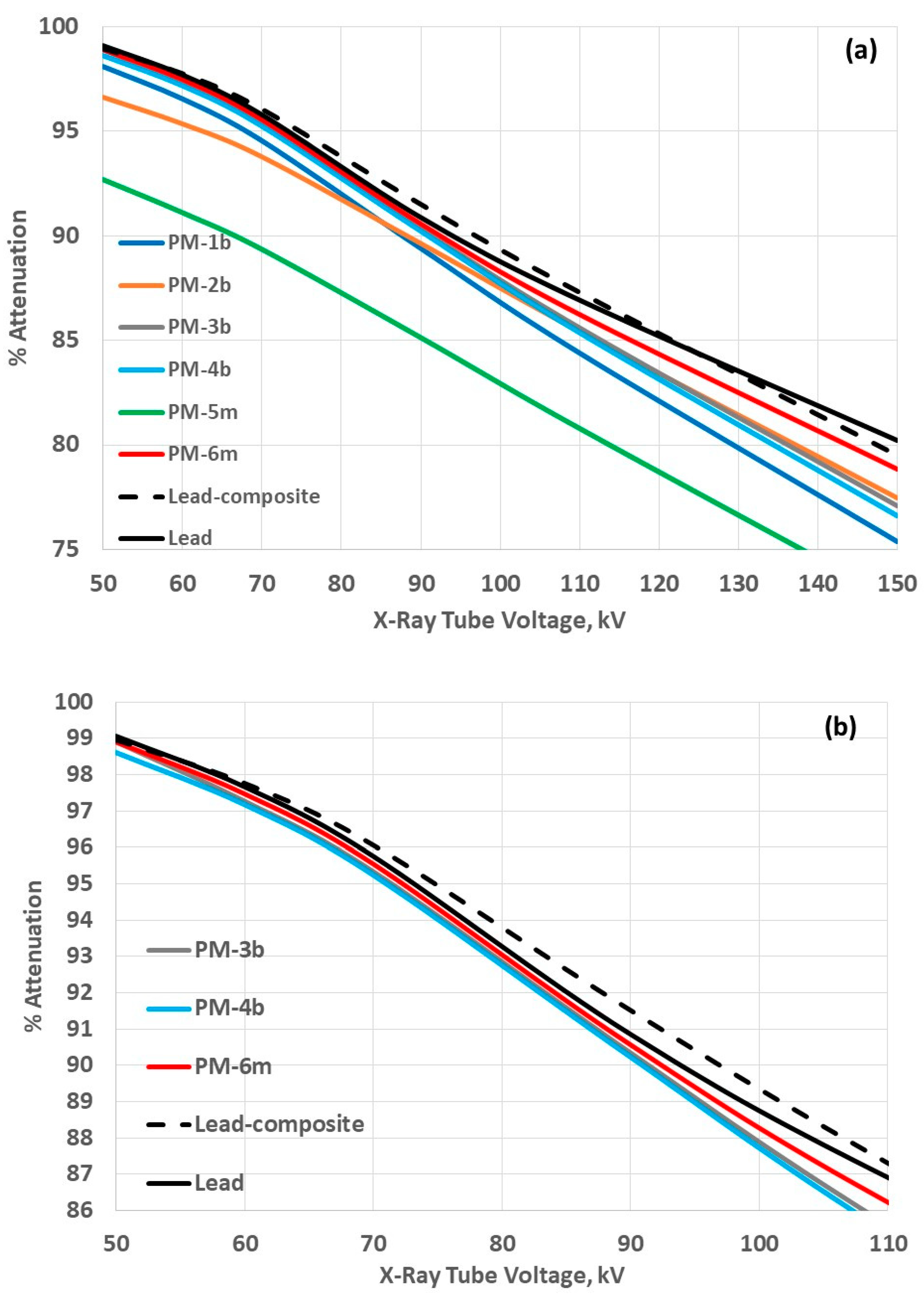
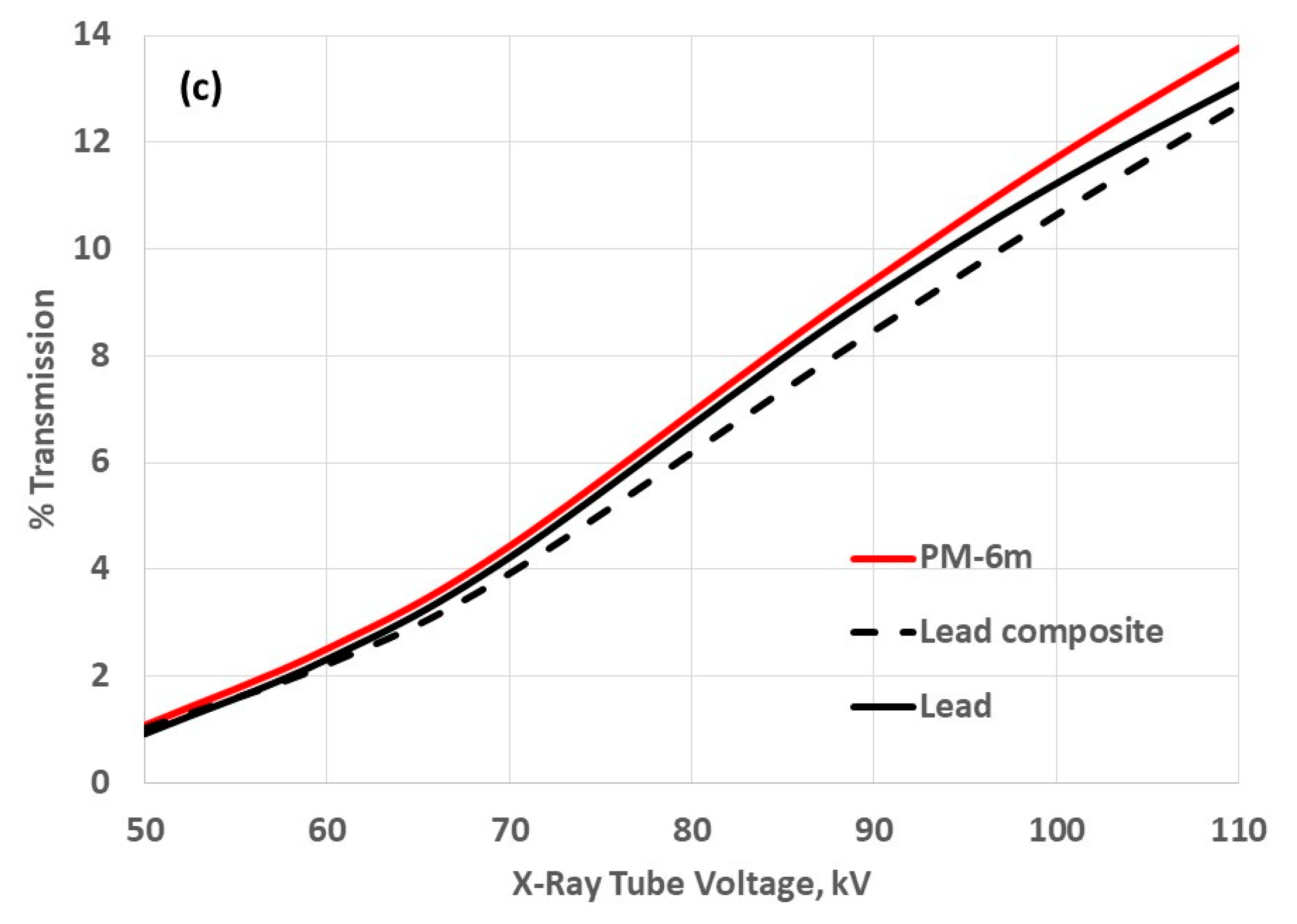
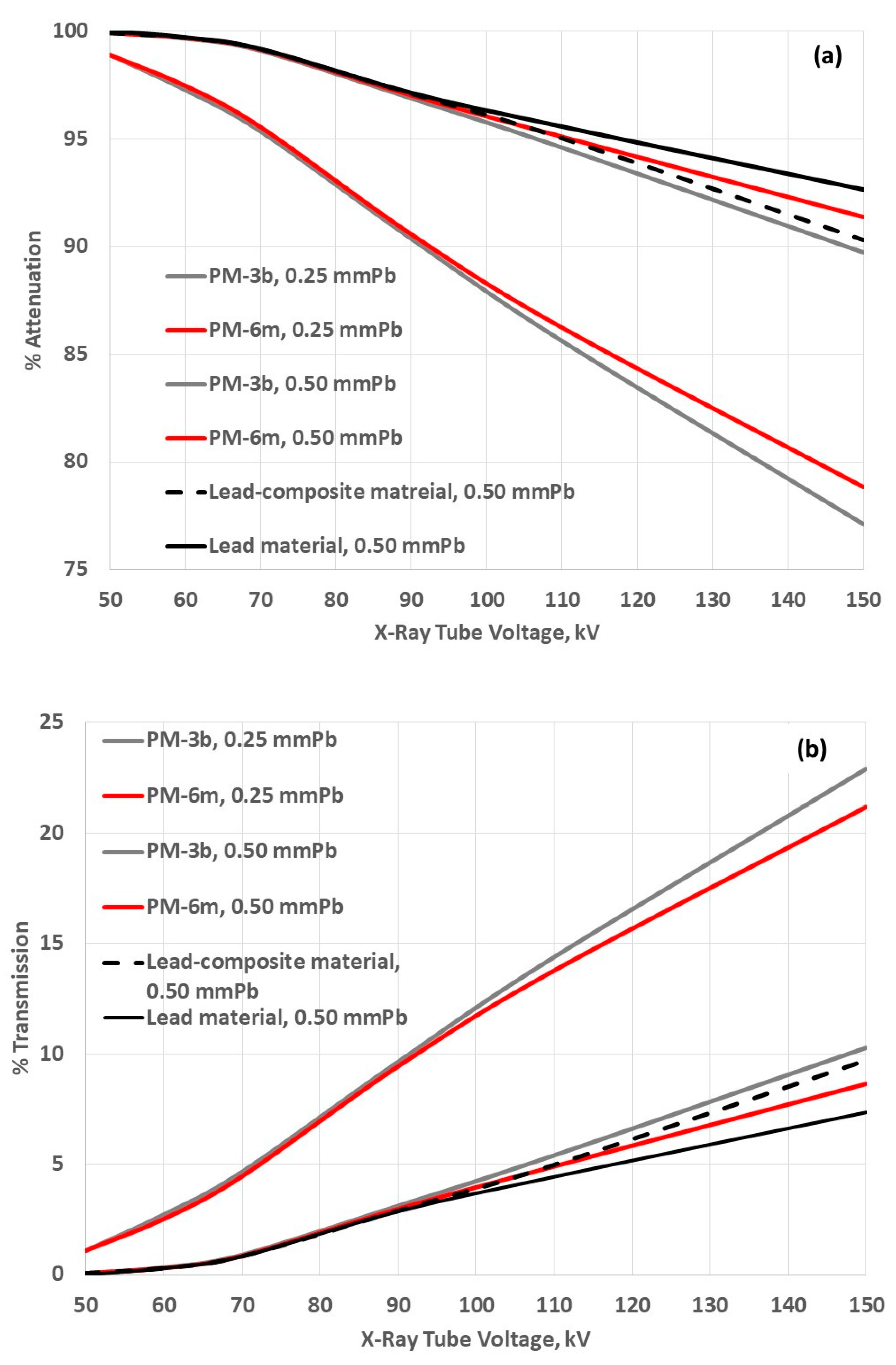

| Medicinal X-Rays | Tube Accelerating Voltage, kV |
|---|---|
| Mammography | 20–30 |
| Dental diagnostic | 60–70 |
| General diagnostic | 40–140 |
| Interventional radiology | 60–120 |
| Computed tomography (CT) | 80–140 |
| Protective Material (PM) | Weight [g] |
|---|---|
| PM-1b | 25.44 |
| PM-2b | 28.10 |
| PM-3b | 28.10 |
| PM-4b | 29.08 |
| PM-5m | 30.27 |
| PM-6m | 30.90 |
| Lead-composite PM | 33.30 |
| Lead PM | 36.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trajkovska Petkoska, A. Assessment of the Attenuation Properties of Commercial Lead-Free Radiation-Shielding Composite Materials Against Medical X-rays. J. Compos. Sci. 2023, 7, 424. https://doi.org/10.3390/jcs7100424
Trajkovska Petkoska A. Assessment of the Attenuation Properties of Commercial Lead-Free Radiation-Shielding Composite Materials Against Medical X-rays. Journal of Composites Science. 2023; 7(10):424. https://doi.org/10.3390/jcs7100424
Chicago/Turabian StyleTrajkovska Petkoska, Anka. 2023. "Assessment of the Attenuation Properties of Commercial Lead-Free Radiation-Shielding Composite Materials Against Medical X-rays" Journal of Composites Science 7, no. 10: 424. https://doi.org/10.3390/jcs7100424






