The Influence of Pristine and Aminoacetic Acid-Treated Aluminum Nitride on the Structure, Curing Processes, and Properties of Epoxy Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
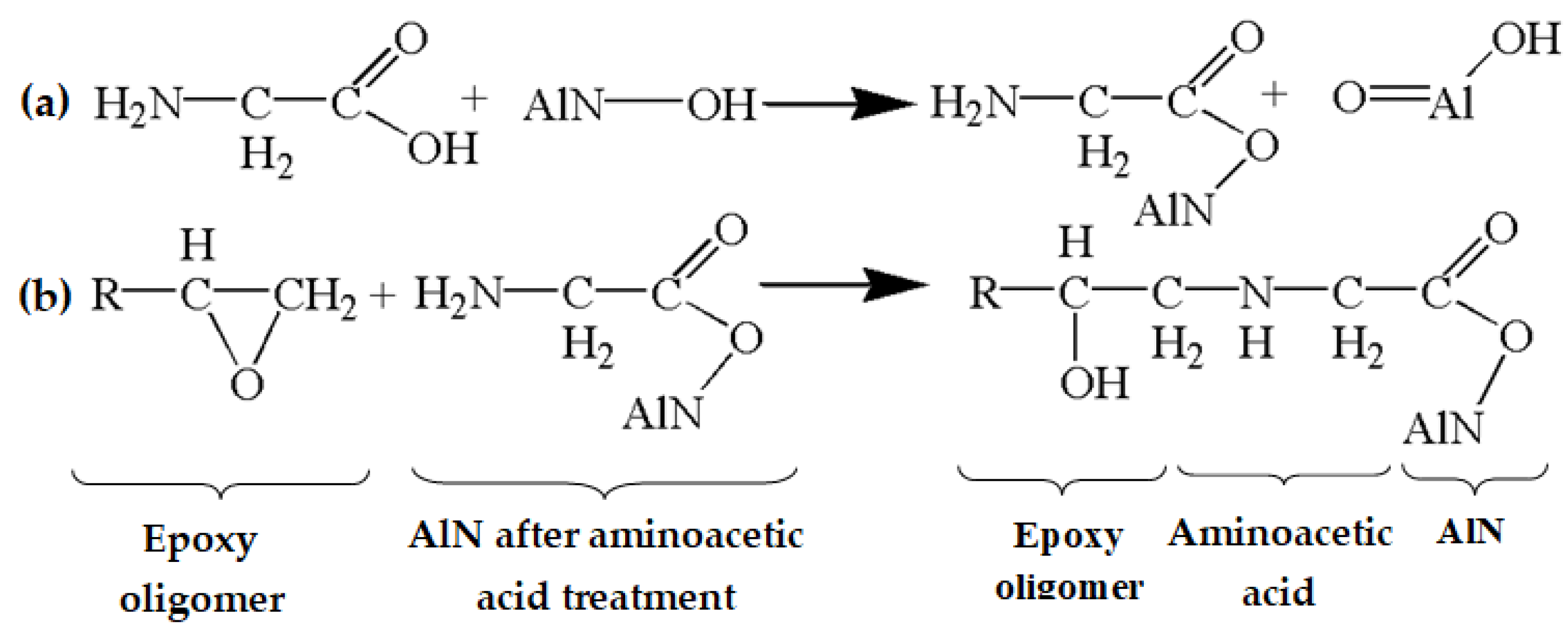
2.2. Functionalization of the AlN Surface
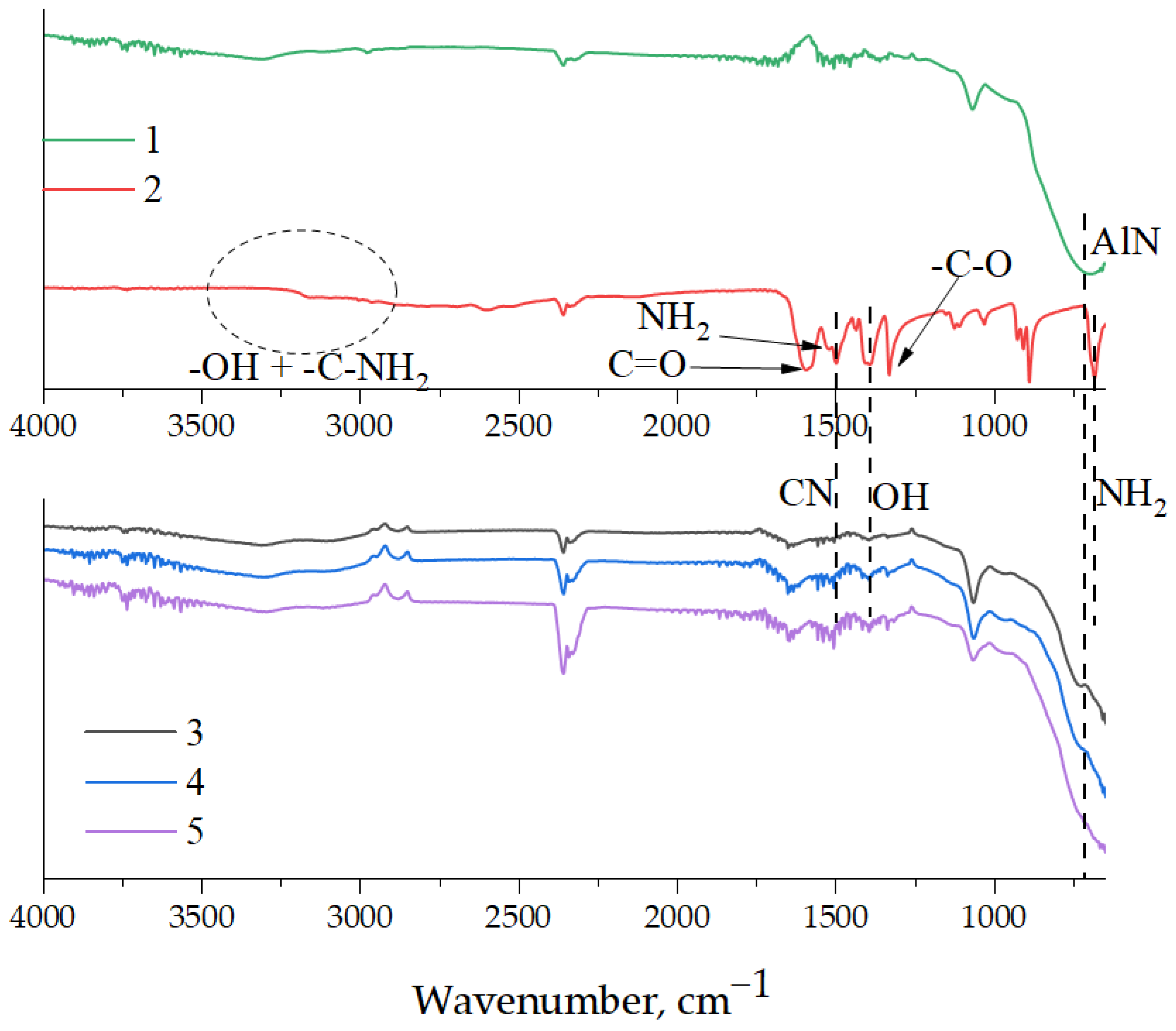
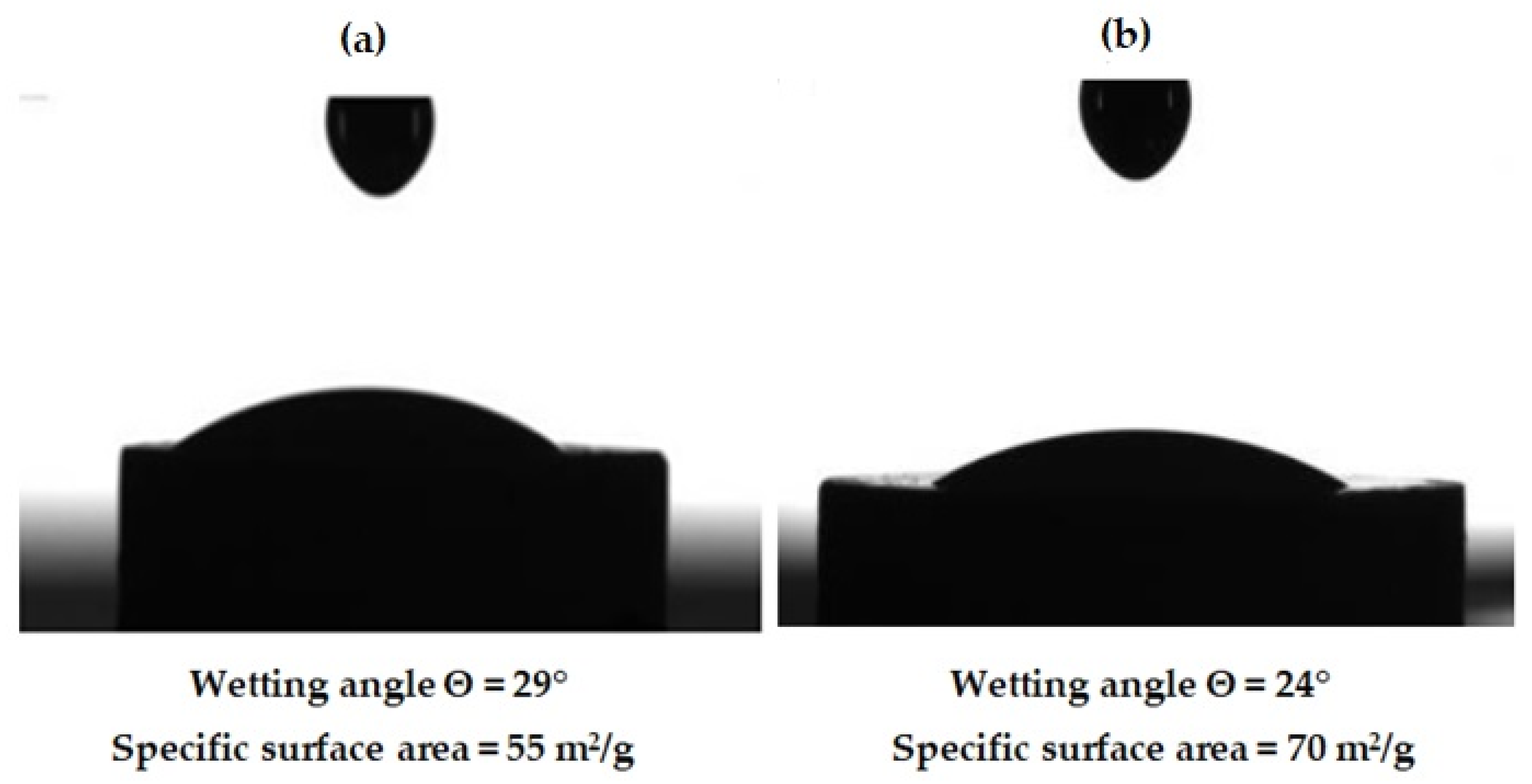
2.3. Characterization of AlN
2.4. Preparation of Epoxy Composites
2.5. Testing of the Composites
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hou, W.; Xiao, Y.; Han, G.; Lin, J.-Y. The Applications of Polymers in Solar Cells: A Review. Polymers 2019, 11, 143. [Google Scholar] [CrossRef]
- Janani, R.; Majumder, D.; Scrimshire, A.; Stone, A.; Wakelin, E.; Jones, A.H.; Wheeler, N.V.; Brooks, W.; Bingham, P.A. From Acrylates to Silicones: A Review of Common Optical Fibre Coatings Used for Normal to Harsh Environments. Prog. Org. Coat. 2023, 180, 107557. [Google Scholar] [CrossRef]
- Namsheer, K.; Rout, C.S. Conducting Polymers: A Comprehensive Review on Recent Advances in Synthesis, Properties and Applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar] [CrossRef]
- Bansal, S.A.; Khanna, V.; Twinkle; Singh, A.P.; Kumar, S. Small Percentage Reinforcement of Carbon Nanotubes (CNTs) in Epoxy(Bisphenol-A) for Enhanced Mechanical Performance. Mater. Today Proc. 2022, 61, 275–279. [Google Scholar] [CrossRef]
- Shcherbakov, A.S.; Mostovoy, A.S.; Yakovlev, N.A.; Arzamastsev, S.V. Effect of Carbon Nanotube Functionalization on the Physicochemical and Mechanical Properties of Modified Fiber-Reinforced Composites Based on an Epoxy Resin. Russ. J. Appl. Chem. 2021, 94, 1080–1087. [Google Scholar] [CrossRef]
- Akkalamattam Maitheenkunju, R.; Gopalakrishnan, J. Reduced Graphene Oxide/Epoxy Nanocomposites with Enhanced Dielectric, Mechanical, Thermomechanical and Thermal Properties. J. Elastomers Plast. 2022, 54, 407–428. [Google Scholar] [CrossRef]
- Du, X.; Zhou, H.; Sun, W.; Liu, H.-Y.; Zhou, G.; Zhou, H.; Mai, Y.-W. Graphene/Epoxy Interleaves for Delamination Toughening and Monitoring of Crack Damage in Carbon Fibre/Epoxy Composite Laminates. Compos. Sci. Technol. 2017, 140, 123–133. [Google Scholar] [CrossRef]
- Fang, F.; Ran, S.; Fang, Z.; Song, P.; Wang, H. Improved Flame Resistance and Thermo-Mechanical Properties of Epoxy Resin Nanocomposites from Functionalized Graphene Oxide via Self-Assembly in Water. Compos. Part B Eng. 2019, 165, 406–416. [Google Scholar] [CrossRef]
- Feng, A.; Hou, T.; Jia, Z.; Zhang, Y.; Zhang, F.; Wu, G. Preparation and Characterization of Epoxy Resin Filled with Ti3C2Tx MXene Nanosheets with Excellent Electric Conductivity. Nanomaterials 2020, 10, 162. [Google Scholar] [CrossRef]
- Song, P.; Qiu, H.; Wang, L.; Liu, X.; Zhang, Y.; Zhang, J.; Kong, J.; Gu, J. Honeycomb Structural rGO-MXene/Epoxy Nanocomposites for Superior Electromagnetic Interference Shielding Performance. Sustain. Mater. Technol. 2020, 24, e00153. [Google Scholar] [CrossRef]
- Mostovoi, A.S.; Plakunova, E.V.; Panova, L.G. New Epoxy Composites Based on Potassium Polytitanates. Int. Polym. Sci. Technol. 2013, 40, 49–51. [Google Scholar] [CrossRef]
- Mostovoy, A.S.; Kadykova, Y.A.; Bekeshev, A.Z.; Tastanova, L.K. Epoxy Composites Modified with Microfibers of Potassium Polytitanates. J. Appl. Polym. Sci. 2018, 135, 46651. [Google Scholar] [CrossRef]
- Mostovoi, A.S.; Yakovlev, E.A.; Burmistrov, I.N.; Panova, L.G. Use of Modified Nanoparticles of Potassium Polytitanate and Physical Methods of Modification of Epoxy Compositions for Improving Their Operational Properties. Russ. J. Appl. Chem. 2015, 88, 129–137. [Google Scholar] [CrossRef]
- Gao, W.; Yu, Y.; Chen, T.; Zhang, Q.; Chen, Z.; Chen, Z.; Jiang, J. Enhanced Flame Retardancy of Unsaturated Polyester Resin Composites Containing Ammonium Polyphosphate and Metal Oxides. J. Appl. Polym. Sci. 2020, 137, 49148. [Google Scholar] [CrossRef]
- Prabhu, S.; Bubbly, S.G.; Gudennavar, S.B. Bismuth (III) Oxide Decorated Graphene Oxide Filled Epoxy Nanocomposites: Thermo-Mechanical and Photon Attenuation Properties. Adv. Compos. Mater. 2023, 32, 602–628. [Google Scholar] [CrossRef]
- Pan, D.; Zhang, X.; Yang, G.; Shang, Y.; Su, F.; Hu, Q.; Patil, R.R.; Liu, H.; Liu, C.; Guo, Z. Thermally Conductive Anticorrosive Epoxy Nanocomposites with Tannic Acid-Modified Boron Nitride Nanosheets. Ind. Eng. Chem. Res. 2020, 59, 20371–20381. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Negi, A.; He, J.; Hu, G.; Tian, S.; Liu, J. Synergistic Effects of Boron Nitride (BN) Nanosheets and Silver (Ag) Nanoparticles on Thermal Conductivity and Electrical Properties of Epoxy Nanocomposites. Polymers 2020, 12, 426. [Google Scholar] [CrossRef]
- Liang, X.; Dai, F. Epoxy Nanocomposites with Reduced Graphene Oxide-Constructed Three-Dimensional Networks of Single Wall Carbon Nanotube for Enhanced Thermal Management Capability with Low Filler Loading. ACS Appl. Mater. Interfaces 2020, 12, 3051–3058. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, M.; Wu, K.; Yao, S.; Du, X.; Chen, G.; Zhang, Q.; Liang, L.; Lu, M. Anisotropic Thermal Conductivity and Electromagnetic Interference Shielding of Epoxy Nanocomposites Based on Magnetic Driving Reduced Graphene oxide@Fe3O4. Compos. Sci. Technol. 2019, 174, 1–10. [Google Scholar] [CrossRef]
- Li, X.; Wang, Q.; Cui, X.; Feng, X.; Teng, F.; Xu, M.; Su, W.; He, J. Study on the Mechanical and Toughness Behavior of Epoxy Nano-Composites with Zero-Dimensional and Two-Dimensional Nano-Fillers. Polymers 2022, 14, 3618. [Google Scholar] [CrossRef]
- Lee Sanchez, W.A.; Li, J.-W.; Chiu, H.-T.; Cheng, C.-C.; Chiou, K.-C.; Lee, T.-M.; Chiu, C.-W. Highly Thermally Conductive Epoxy Composites with AlN/BN Hybrid Filler as Underfill Encapsulation Material for Electronic Packaging. Polymers 2022, 14, 2950. [Google Scholar] [CrossRef]
- Malekshahinezhad, K.; Ahmadi-khaneghah, A.; Behniafar, H. Amine-Functionalized TiO2 Nanoparticles Covalently Loaded into Epoxy Networks via Thermal and Microwave Curing Processes. Macromol. Res. 2020, 28, 567–572. [Google Scholar] [CrossRef]
- Mozaffarinasab, H.; Jamshidi, M. Surface Modification of Carbon Nanotubes by a Bifunctional Amine Silane; Effects on Physical/Mechanical/Thermal Properties of Epoxy Nanocomposite. Prog. Org. Coat. 2023, 179, 107521. [Google Scholar] [CrossRef]
- Jung, H.; Choi, H.K.; Oh, Y.; Hong, H.; Yu, J. Enhancement of Thermo-Mechanical Stability for Nanocomposites Containing Plasma Treated Carbon Nanotubes with an Experimental Study and Molecular Dynamics Simulations. Sci. Rep. 2020, 10, 405. [Google Scholar] [CrossRef] [PubMed]
- Seghini, M.C.; Touchard, F.; Sarasini, F.; Cech, V.; Chocinski-Arnault, L.; Mellier, D.; Tirillò, J.; Bracciale, M.P.; Zvonek, M. Engineering the Interfacial Adhesion in Basalt/Epoxy Composites by Plasma Polymerization. Compos. Part A Appl. Sci. Manuf. 2019, 122, 67–76. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, P.; Hu, J.; Liu, B.; Yang, J.; Liang, S.; Xiao, K.; Hou, H. A Review on Microwave Irradiation to the Properties of Geopolymers: Mechanisms and Challenges. Constr. Build. Mater. 2021, 294, 123491. [Google Scholar] [CrossRef]
- Shcherbakov, A.; Mostovoy, A.; Bekeshev, A.; Burmistrov, I.; Arzamastsev, S.; Lopukhova, M. Effect of Microwave Irradiation at Different Stages of Manufacturing Unsaturated Polyester Nanocomposite. Polymers 2022, 14, 4594. [Google Scholar] [CrossRef] [PubMed]
- Domingues, D.; Logakis, E.; Skordos, A.A. The Use of an Electric Field in the Preparation of Glass Fibre/Epoxy Composites Containing Carbon Nanotubes. Carbon 2012, 50, 2493–2503. [Google Scholar] [CrossRef]
- Miękoś, E.; Cichomski, M.; Zieliński, M.; Klepka, T.; Sroczyński, D.; Fenyk, A. Tests of Physicochemical and Mechanical Strength Properties of Polymer Composites on an Epoxy Resin Matrix, Modified by a Constant Magnetic Field. Materials 2022, 15, 6730. [Google Scholar] [CrossRef]
- Hetemi, D.; Pinson, J. Surface Functionalisation of Polymers. Chem. Soc. Rev. 2017, 46, 5701–5713. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, F.; Wang, S.; Yan, M.; Hu, X.; Xu, M. D-GQDs Modified Epoxy Resin Enhances the Thermal Conductivity of AlN/Epoxy Resin Thermally Conductive Composites. Polymers 2021, 13, 4074. [Google Scholar] [CrossRef]
- Wang, H.; Qi, Q.; Zhang, Y.; Chen, S.; Dong, B.; Zhu, S.; Hu, Q.; Guo, Z. Anticorrosive Epoxy Nanocomposite Coatings Filled with Polyaniline-Functionalized Silicon Nitride Particles. Ind. Eng. Chem. Res. 2020, 59, 16649–16659. [Google Scholar] [CrossRef]
- Jung, J.; Sodano, H.A. High Strength Epoxy Nanocomposites Reinforced by Epoxy Functionalized Aramid Nanofibers. Polymer 2020, 195, 122438. [Google Scholar] [CrossRef]
- Bekeshev, A.; Mostovoy, A.; Shcherbakov, A.; Zhumabekova, A.; Serikbayeva, G.; Vikulova, M.; Svitkina, V. Effect of Phosphorus and Chlorine Containing Plasticizers on the Physicochemical and Mechanical Properties of Epoxy Composites. J. Compos. Sci. 2023, 7, 178. [Google Scholar] [CrossRef]
- Bekeshev, A.; Mostovoy, A.; Tastanova, L.; Kadykova, Y.; Kalganova, S.; Lopukhova, M. Reinforcement of Epoxy Composites with Application of Finely-Ground Ochre and Electrophysical Method of the Composition Modification. Polymers 2020, 12, 1437. [Google Scholar] [CrossRef] [PubMed]
- Demir, K.; Gavgali, E.; Yetim, A.F.; Akpinar, S. The Effects of Nanostructure Additive on Fracture Strength in Adhesively Bonded Joints Subjected to Fully Reversed Four-Point Bending Fatigue Load. Int. J. Adhes. Adhes. 2021, 110, 102943. [Google Scholar] [CrossRef]
- Rodríguez, H.A.; Kriven, W.M.; Casanova, H. Development of Mechanical Properties in Dental Resin Composite: Effect of Filler Size and Filler Aggregation State. Mater. Sci. Eng. C 2019, 101, 274–282. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, D.; He, Z.; Wang, D.; Zheng, Y. Enhanced Mechanical and Damping Properties of Epoxy Using Aggregated Nanoparticles Organic-Inorganic Hybrid as a Filler. Compos. Interfaces 2022, 29, 523–536. [Google Scholar] [CrossRef]
- Baek, K.; Shin, H.; Cho, M. Multiscale Modeling of Mechanical Behaviors of Nano-SiC/Epoxy Nanocomposites with Modified Interphase Model: Effect of Nanoparticle Clustering. Compos. Sci. Technol. 2021, 203, 108572. [Google Scholar] [CrossRef]
- Prasad, T.; Halder, S.; Dhar, S.S. Imidazole-Supported Silica One-Pot Processed Nanoparticles to Enhance Toughness of Epoxy Based Nanocomposites. Mater. Chem. Phys. 2019, 231, 75–86. [Google Scholar] [CrossRef]
- Feichtenschlager, B.; Pabisch, S.; Svehla, J.; Peterlik, H.; Sajjad, M.; Koch, T.; Kickelbick, G. Epoxy Resin Nanocomposites: The Influence of Interface Modification on the Dispersion Structure—A Small-Angle-X-Ray-Scattering Study. Surfaces 2020, 3, 664–682. [Google Scholar] [CrossRef]
- Bao, T.; Wang, Z.; Zhao, Y.; Wang, Y.; Yi, X. Improving Tribological Performance of Epoxy Composite by Reinforcing with Polyetheramine-Functionalized Graphene Oxide. J. Mater. Res. Technol. 2021, 12, 1516–1529. [Google Scholar] [CrossRef]
- Tiwari, N.; Shaikh, A.A.; Malek, N.I. Modification of the Multiphase Shape Memory Composites with Functionalized Graphene Nanoplatelets: Enhancement of Thermomechanical and Interfacial Properties. Mater. Today Chem. 2022, 24, 100826. [Google Scholar] [CrossRef]
- Mostovoy, A.; Shcherbakov, A.; Yakovlev, A.; Arzamastsev, S.; Lopukhova, M. Reinforced Epoxy Composites Modified with Functionalized Graphene Oxide. Polymers 2022, 14, 338. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.-C.; Ma, C.-C.M.; Chiou, K.-C.; Lee, T.-M. Synergetic Effect of Thermal Conductive Properties of Epoxy Composites Containing Functionalized Multi-Walled Carbon Nanotubes and Aluminum Nitride. Compos. Part B Eng. 2012, 43, 265–271. [Google Scholar] [CrossRef]
- Zhang, Y.; Park, M.; Park, S.-J. Implication of Thermally Conductive Nanodiamond-Interspersed Graphite Nanoplatelet Hybrids in Thermoset Composites with Superior Thermal Management Capability. Sci. Rep. 2019, 9, 2893. [Google Scholar] [CrossRef]
- Zappalorto, M.; Pontefisso, A.; Fabrizi, A.; Quaresimin, M. Mechanical Behaviour of Epoxy/Silica Nanocomposites: Experiments and Modelling. Compos. Part A Appl. Sci. Manuf. 2015, 72, 58–64. [Google Scholar] [CrossRef]
- Esmaeili, A.; Ma, D.; Manes, A.; Oggioni, T.; Jiménez-Suárez, A.; Ureña, A.; Hamouda, A.M.S.; Sbarufatti, C. An Experimental and Numerical Investigation of Highly Strong and Tough Epoxy Based Nanocomposite by Addition of MWCNTs: Tensile and Mode I Fracture Tests. Compos. Struct. 2020, 252, 112692. [Google Scholar] [CrossRef]
- Goyat, M.S.; Hooda, A.; Gupta, T.K.; Kumar, K.; Halder, S.; Ghosh, P.K.; Dehiya, B.S. Role of Non-Functionalized Oxide Nanoparticles on Mechanical Properties and Toughening Mechanisms of Epoxy Nanocomposites. Ceram. Int. 2021, 47, 22316–22344. [Google Scholar] [CrossRef]
- Zhang, Q.; Bai, G.; Xiao, W.; Sui, G.; Yang, X. Effect of Amine Functionalized MWCNT-Epoxy Interfacial Interaction on MWCNT Dispersion and Mechanical Properties of Epoxy-Amine Composites. Polym. Compos. 2018, 39, E2552–E2561. [Google Scholar] [CrossRef]
- Sari, M.G.; Ramezanzadeh, B. Epoxy Composite Coating Corrosion Protection Properties Reinforcement through the Addition of Hydroxyl-Terminated Hyperbranched Polyamide Non-Covalently Assembled Graphene Oxide Platforms. Constr. Build. Mater. 2020, 234, 117421. [Google Scholar] [CrossRef]
- Ali, F.; Ishfaq, N.; Said, A.; Nawaz, Z.; Ali, Z.; Ali, N.; Afzal, A.; Bilal, M. Fabrication, Characterization, Morphological and Thermal Investigations of Functionalized Multi-Walled Carbon Nanotubes Reinforced Epoxy Nanocomposites. Prog. Org. Coat. 2021, 150, 105962. [Google Scholar] [CrossRef]
- Tikhani, F.; Moghari, S.; Jouyandeh, M.; Laoutid, F.; Vahabi, H.; Saeb, M.R.; Dubois, P. Curing Kinetics and Thermal Stability of Epoxy Composites Containing Newly Obtained Nano-Scale Aluminum Hypophosphite (AlPO2). Polymers 2020, 12, 644. [Google Scholar] [CrossRef]
- Sul, J.-H.; Prusty, B.G.; Crosky, A. Effect of the Addition of Multi-Walled Carbon Nanotubes on the Thermomechanical Properties of Epoxy Resin. Polym. Compos. 2017, 38, 1873–1880. [Google Scholar] [CrossRef]
- Hameed, A.; Islam, M.; ahmad, I.; Mahmood, N.; Saeed, S.; Javed, H. Thermal and Mechanical Properties of Carbon Nanotube/Epoxy Nanocomposites Reinforced with Pristine and Functionalized Multiwalled Carbon Nanotubes. Polym. Compos. 2015, 36, 1891–1898. [Google Scholar] [CrossRef]
- Shahrestanaki, A.A.K.; Mehrshad, M.; Akhlaghi, S.H. Preparation and Non-Isothermal Cure Kinetics Study of Epoxy Resin Nanocomposites with Amine and Epoxy Functionalized Magnetic Nanoparticles. High Perform. Polym. 2021, 33, 1025–1034. [Google Scholar] [CrossRef]
- Konuray, O.; Fernández-Francos, X.; Ramis, X.; Serra, À. State of the Art in Dual-Curing Acrylate Systems. Polymers 2018, 10, 178. [Google Scholar] [CrossRef] [PubMed]
- Ribas-Massonis, A.; Cicujano, M.; Duran, J.; Besalú, E.; Poater, A. Free-Radical Photopolymerization for Curing Products for Refinish Coatings Market. Polymers 2022, 14, 2856. [Google Scholar] [CrossRef] [PubMed]

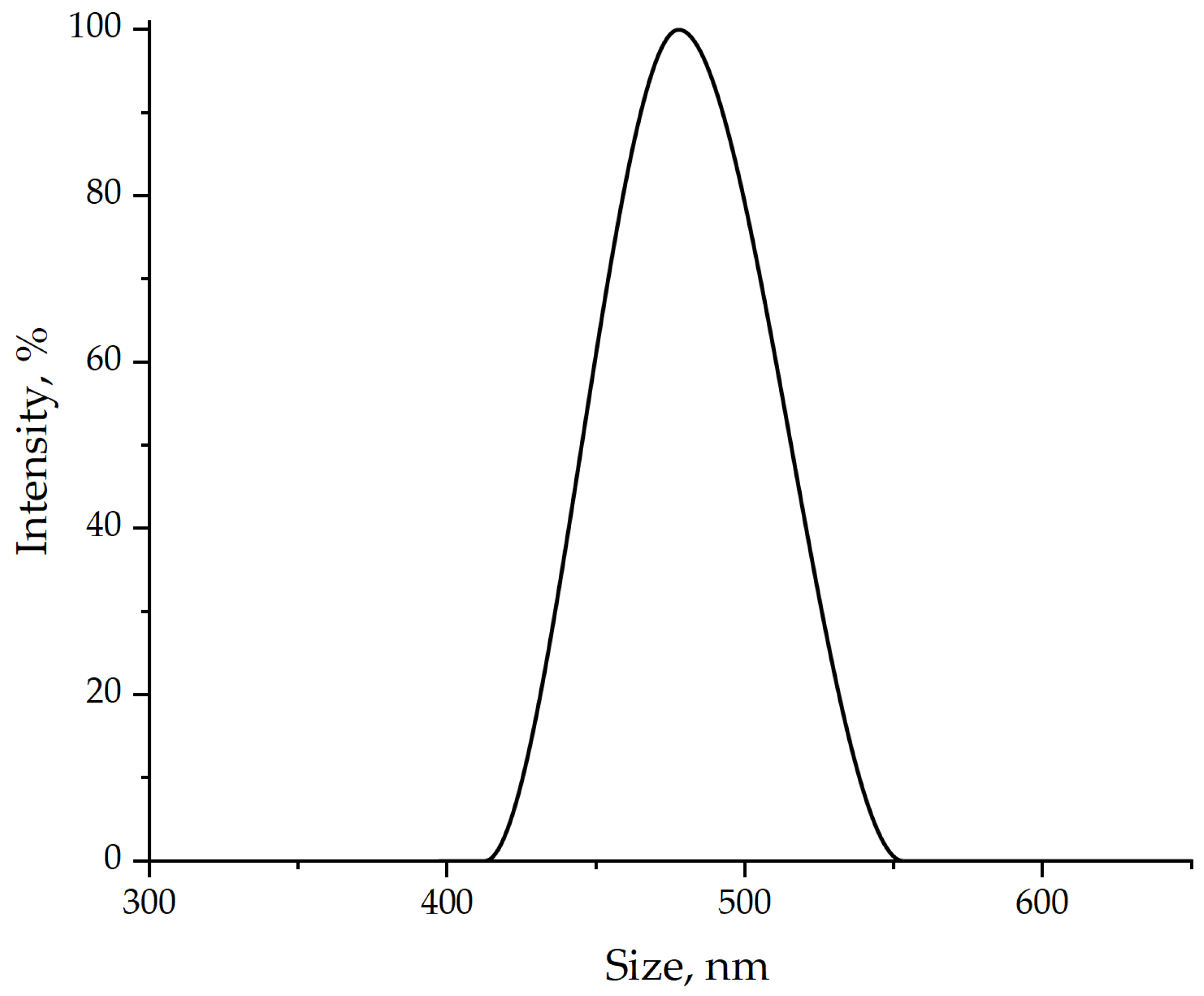
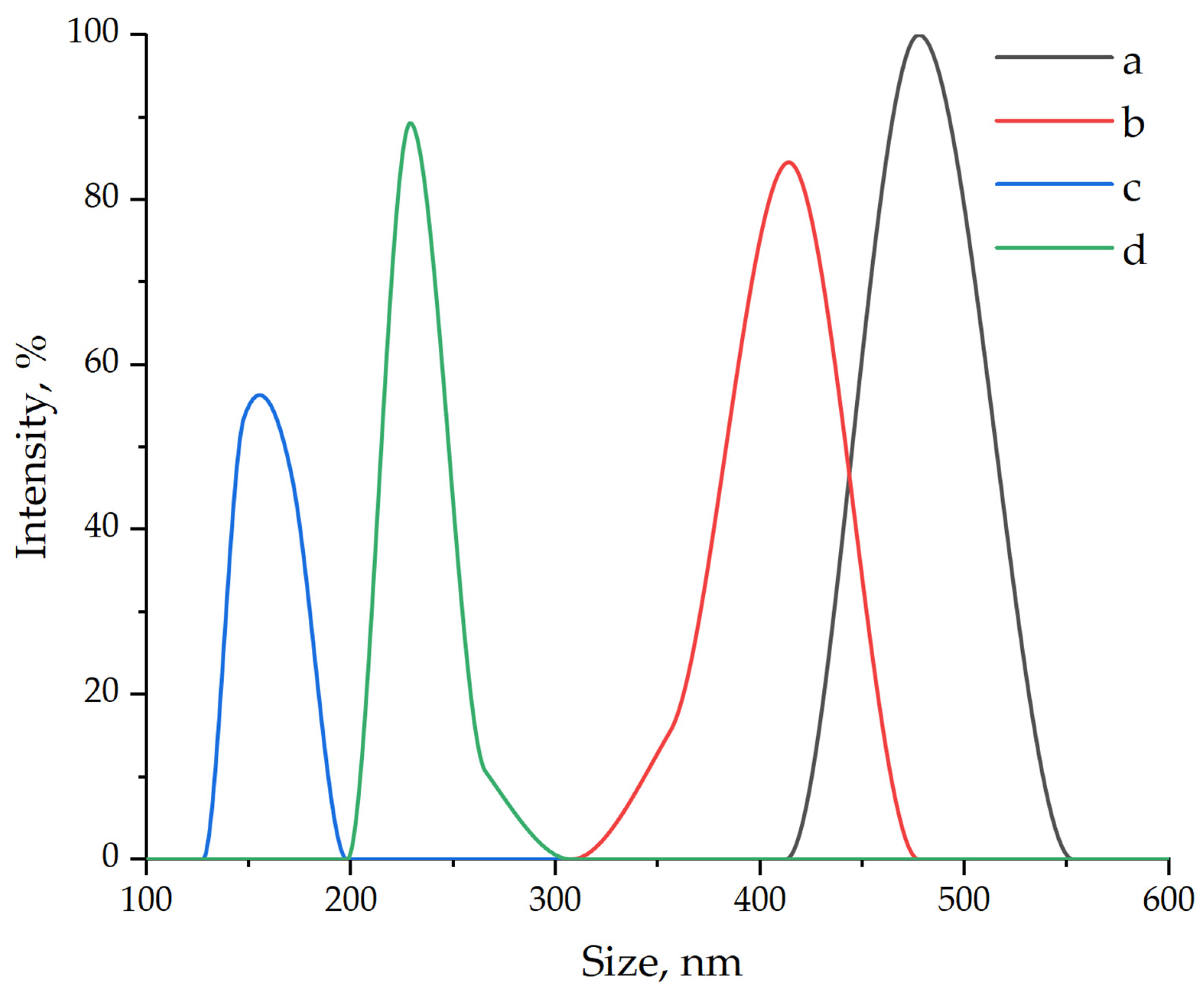





| Samples | σben, MPa | Eben, MPa | σten, MPa | Eten, MPa | aim, kJ/m2 |
|---|---|---|---|---|---|
| 100 ED-20 + 15 PEPA + 40 TCPP | 85 ± 2.8 | 2077 ± 62 | 34 ± 1.7 | 1634 ± 50 | 9.0 ± 0.35 |
| 100 ED-20 + 15 PEPA + 40 TCPP + 0.01 AlN | 91 ± 2.5 | 2503 ± 75 | 42 ± 2.0 | 1980 ± 79 | 13.0 ± 0.52 |
| 100 ED-20 + 15 PEPA + 40 TCPP + 0.05 AlN | 96 ± 2.8 | 3286 ± 91 | 53 ± 2.3 | 2091 ± 82 | 14.0 ± 0.56 |
| 100 ED-20 + 15 PEPA + 40 TCPP + 0.10 AlN | 94 ± 2.6 | 3422 ± 99 | 51 ± 2.2 | 2096 ± 83 | 12.6 ± 0.50 |
| 100 ED-20 + 15 PEPA + 40 TCPP + 0.50 AlN | 92 ± 2.5 | 3537 ± 105 | 40 ± 2.0 | 2102 ± 84 | 10.8 ± 0.43 |
| Composition | τgel, min | τcur, min | Tmax, °C |
|---|---|---|---|
| ED-20 + TCPP + PEPA | 104 | 146 | 88 |
| ED-20 + TCPP + AlN + PEPA | 75 | 105 | 105 |
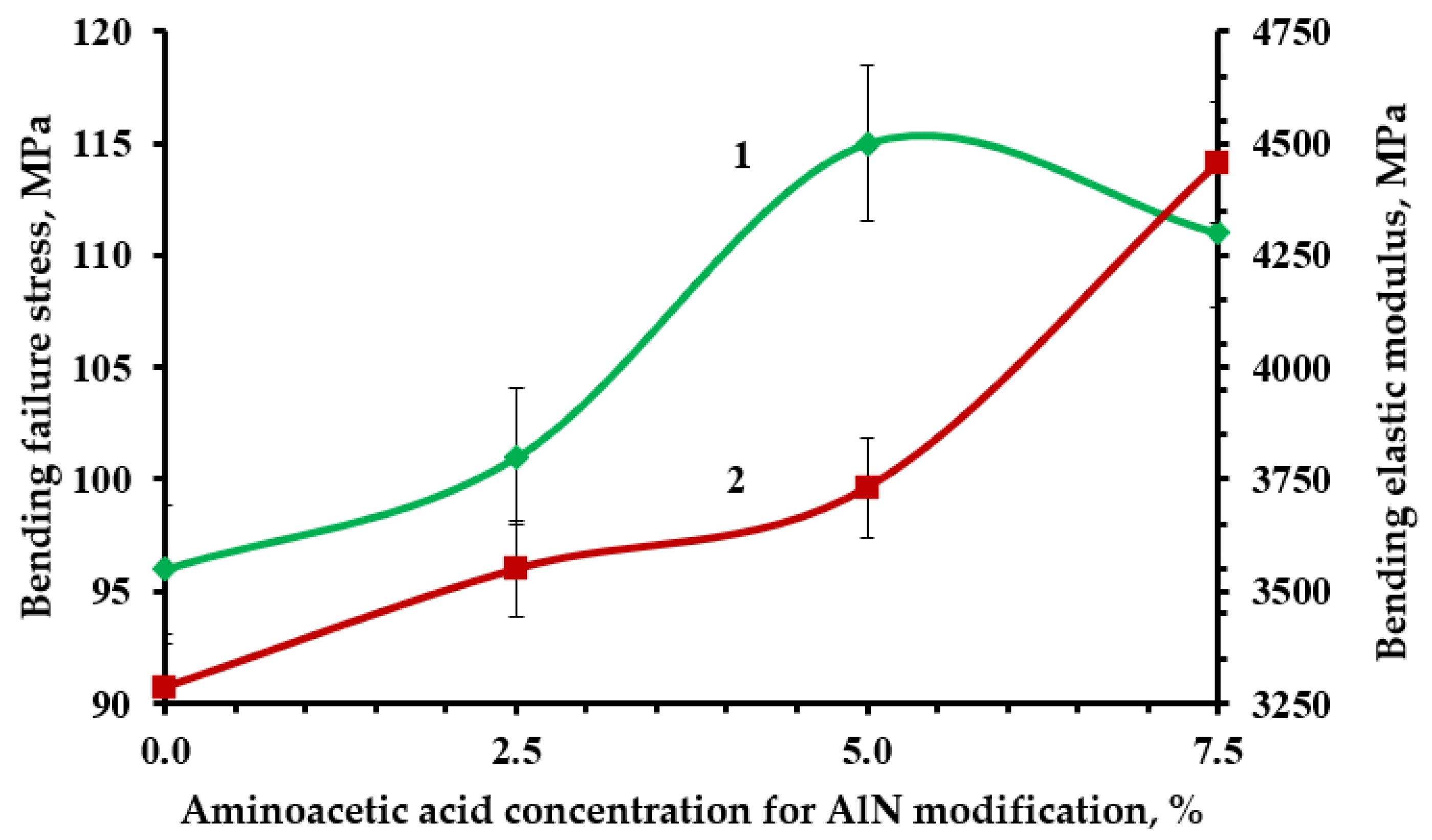
| ED-20 + TCPP + AlN(2.5% aminoacetic acid) + PEPA | 70 | 97 | 120 |
| ED-20 + TCPP + AlN(5.0% aminoacetic acid) + PEPA | 66 | 91 | 122 |
| ED-20 + TCPP + AlN(7.5% aminoacetic acid) + PEPA | 53 | 79 | 128 |
| Composition | Tstart–Tend Tmax °C | H, J/g |
|---|---|---|
| ED-20 + TCPP + AlN + PEPA | 57–176 108 | 660 |
| ED-20 + TCPP + AlN (2.5% aminoacetic acid) + PEPA | 52–175 108 | 671 |
| ED-20 + TCPP + AlN (5.0% aminoacetic acid) + PEPA | 50–176 108 | 683 |
| ED-20 + TCPP + AlN (7.5% aminoacetic acid) + PEPA | 43–173 108 | 700 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bekeshev, A.; Mostovoy, A.; Shcherbakov, A.; Tastanova, L.; Akhmetova, M.; Apendina, A.; Orynbassar, R.; Lopukhova, M. The Influence of Pristine and Aminoacetic Acid-Treated Aluminum Nitride on the Structure, Curing Processes, and Properties of Epoxy Nanocomposites. J. Compos. Sci. 2023, 7, 482. https://doi.org/10.3390/jcs7120482
Bekeshev A, Mostovoy A, Shcherbakov A, Tastanova L, Akhmetova M, Apendina A, Orynbassar R, Lopukhova M. The Influence of Pristine and Aminoacetic Acid-Treated Aluminum Nitride on the Structure, Curing Processes, and Properties of Epoxy Nanocomposites. Journal of Composites Science. 2023; 7(12):482. https://doi.org/10.3390/jcs7120482
Chicago/Turabian StyleBekeshev, Amirbek, Anton Mostovoy, Andrey Shcherbakov, Lyazzat Tastanova, Marzhan Akhmetova, Ainagul Apendina, Raigul Orynbassar, and Marina Lopukhova. 2023. "The Influence of Pristine and Aminoacetic Acid-Treated Aluminum Nitride on the Structure, Curing Processes, and Properties of Epoxy Nanocomposites" Journal of Composites Science 7, no. 12: 482. https://doi.org/10.3390/jcs7120482






