The Effect of Ca, Sr, and Ba Chloride Complexes with Dibenzo-18-Crown-6 Ether as Catalysts on the Process Criteria for the Efficiency of Cumene Oxidation (the First Stage in the Chain of Polymer Composite Production)
Abstract
:1. Introduction
- (1)
- carrying out the experimental kinetic studies;
- (2)
- based on the experimental and theoretical prerequisites, a kinetic scheme of the process was proposed where the kinetic model is created using the mass action law;
- (3)
- the kinetic model is verified using experimental data (at this stage, the kinetic scheme and values of the model parameters can be revised); the kinetic model is basic, but often it is enough for preliminary calculations (the kinetic model can be made into a more complicated macrokinetic model to take into account the hydrodynamics of the reactor or can be built-in into the chemical process model which is implemented in a universal modeling program such as Aspen Hysys);
- (4)
- the computational experiments are carried out using the model, reproducing the industrial conditions of the process; according to the results of computational experiments, the catalyst can be either recommended or not recommended for use in industrial conditions.
2. Materials and Methods
2.1. Materials
- (1)
- cumene produced by PJSC Kazanorgsintez;
- (2)
- cumene hydroperoxide with the content of the main substance being 99.4% wt.;
- (3)
- air oxygen as an oxidizing agent;
- (4)
- Ca, Sr, and Ba chloride complexes with dibenzo-18-crown-6 ether are the catalysts for cumene oxidation and cumene hydroperoxide decomposition: they were synthesized by reacting a solution of Ca (or Ba) chloride in n-butanol or a solution of Sr chloride in ethanol with a solution dibenzo-18-crown-6 ether in acetone (stirring in a flask under reflux at a temperature of 343–348 K for 1–1.5 h); the resulting complexes in the form of white crystalline substances were filtered off, washed with n-butanol (Ca, Ba) or ethanol (Sr), and dried in air (the formation of complexes with a composition of 1:1 was proved by the molar mass of the complex, determined by cryoscopy: M(Ca) = 482.98 g/mol, M(Sr) = 527.55 g/mol, M(Ba) = 571.61 g/mol);
- (5)
- chlorobenzene is a solvent in the decomposition of cumene hydroperoxide.
2.2. Cumene Oxidation
2.3. Cumene Hydroperoxide Decomposition
3. Results
- (1)
- reaction (5) is written by analogy with reaction (1);
- (2)
- reaction (46), which describes the decomposition of the intermediate ROH·Cat adduct to acetophenone (the formation of acetophenone also runs without the participation of a catalyst, which was experimentally shown in [24]; in the mechanism, the non-catalytic pathway for the formation of acetophenone is represented by reactions (21) and (44); however, much more acetophenone is formed in catalytic processes than in non-catalytic processes, which also indicates the catalytic pathway of its formation);
- (3)
- reaction (47) is shown by analogy with [25];
- (4)
- (5)
- reaction (49) is shown by analogy with [27];
- (6)
- reaction (50) probably runs due to the ability of unsaturated hydrocarbons to be hydrated under the action of catalysts into the corresponding alcohols [28].
4. Discussion
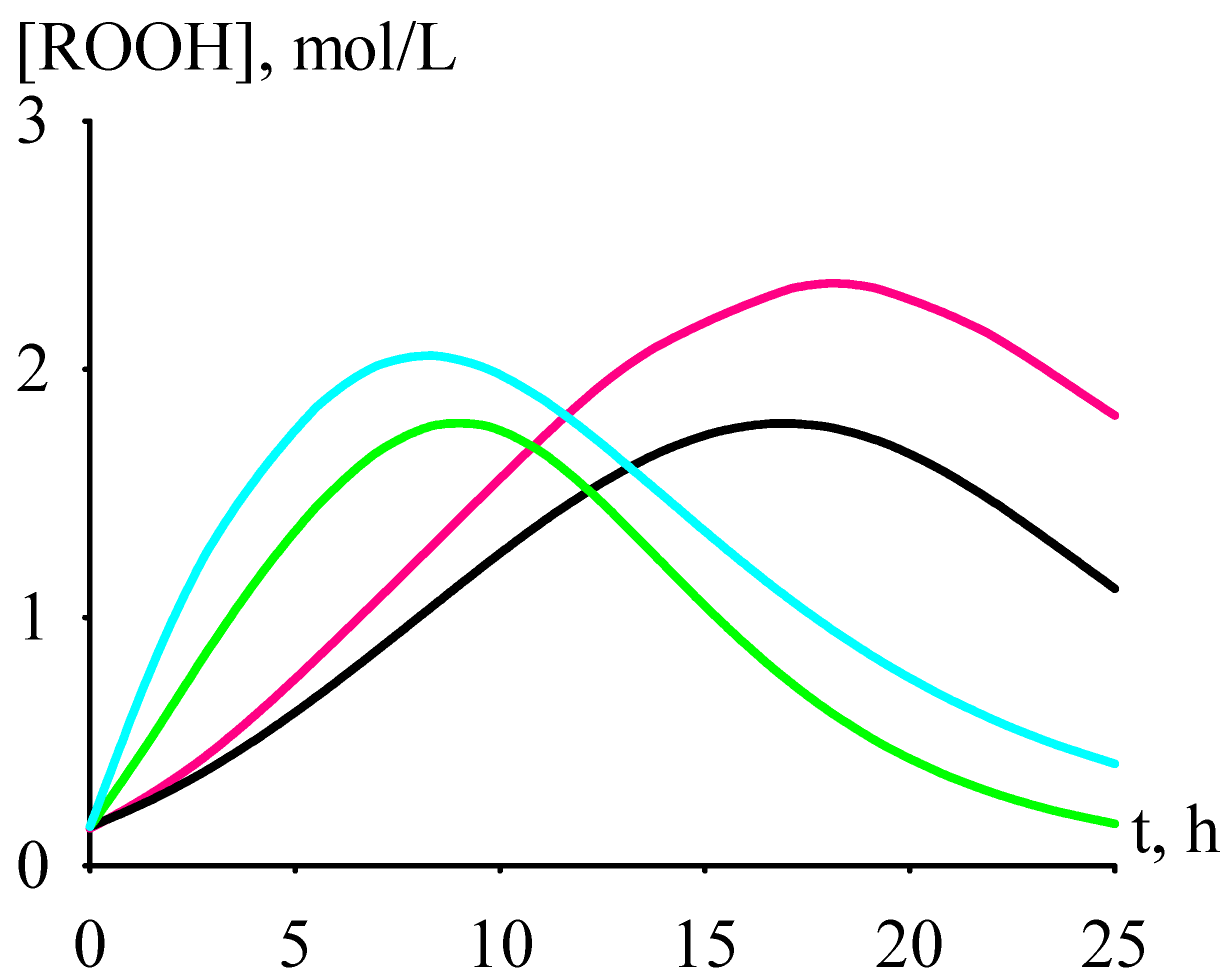
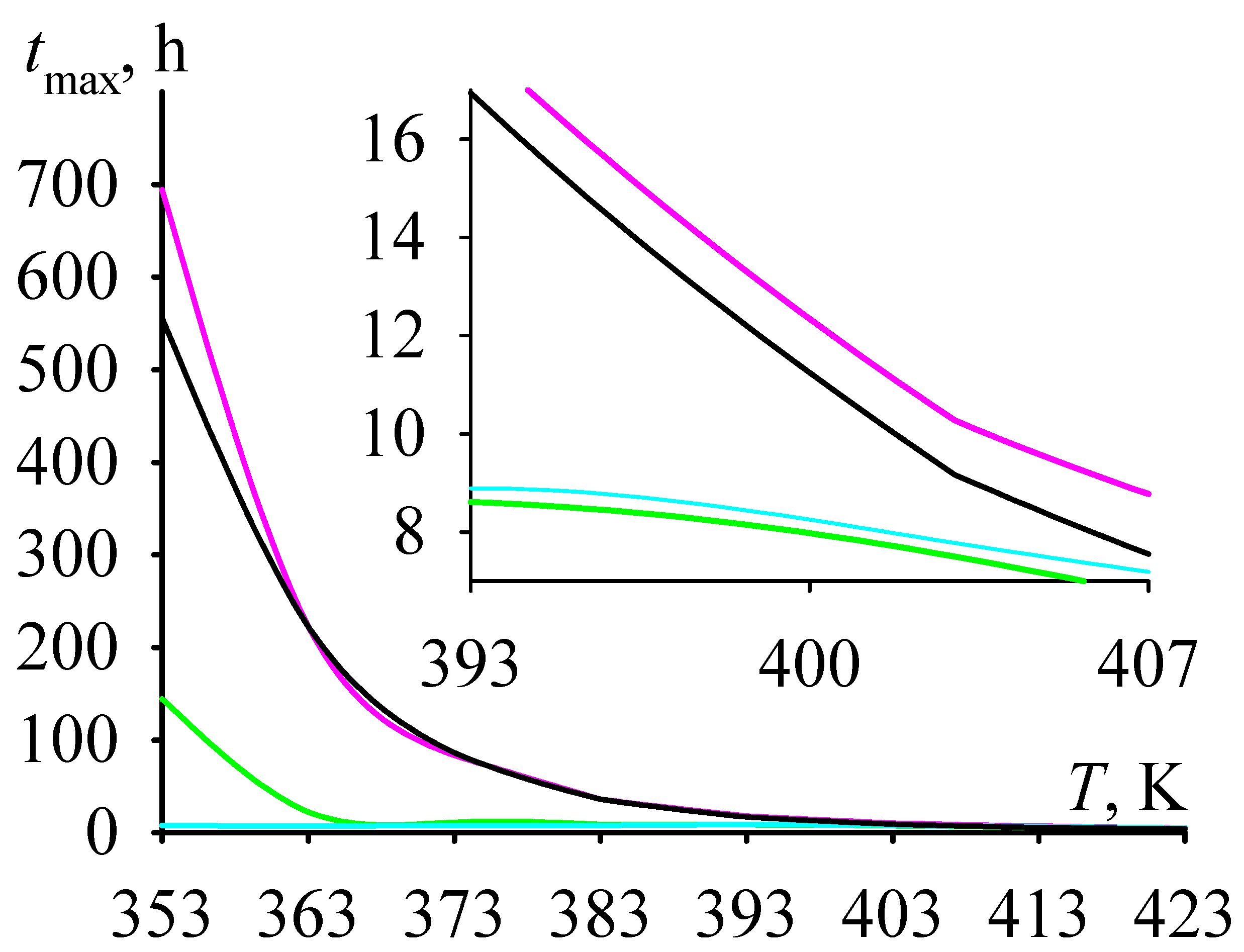
- (1)
- criterion C for Sr has a minimum in the range [Cat]0 = 2–4 mmol/L, and at [Cat]0 > 4 mmol/L it reaches a plateau, the value of which is comparable to the value of criterion C at [Cat]0 < 2 mmol/L;
- (2)
- criterion C for Ca and Ba falls with growing [Cat]0 and reaches a plateau at [Cat]0 = 7 mmol/L;
- (3)
- criterion C falls in the row Sr >> Ca >> Ba.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Global Cumene Market Size study, By Production (Zeolite, Solid phosphoric acid, Aluminum chloride), By Application (Phenol, Acetone, Others), and Regional Forecasts. Available online: https://www.giiresearch.com/report/bzc1090047-global-cumene-market-size-study-by-production.html (accessed on 9 June 2022).
- Hock, H.; Lang, S. Autoxydation von Kohlenwasserstoffen, IX. Mitteil.: Über Peroxyde von Benzol-Derivaten. Ber. Dtsch. Chem. Ges. 1944, 77, 257–264. [Google Scholar] [CrossRef]
- Michelena, A.H.; Summerscales, J.; Graham-Jones, J.; Hall, W. Sustainable manufacture of natural fibre reinforced epoxy resin composites with coupling agent in the hardener. J. Compos. Sci. 2022, 6, 97. [Google Scholar] [CrossRef]
- Purse, M.; Holmes, B.; Sacchi, M.; Howlin, B. Simulating the complete pyrolysis and charring process of phenol–formaldehyde resins using reactive molecular dynamics. J. Mater. Sci. 2022, 57, 7600–7620. [Google Scholar] [CrossRef]
- Brunella, V.; Rossatto, B.G.; Mastropasqua, C.; Cesano, F.; Scarano, D. Thermal/Electrical properties and texture of carbon black PC polymer composites near the electrical percolation threshold. J. Compos. Sci. 2021, 5, 212. [Google Scholar] [CrossRef]
- Khvatov, A.V.; Brevnov, P.N.; Shilkina, N.G.; Lomakin, S.M. Thermal and physical and mechanical properties of polysulfone composites with carbon nanotubes. Russ. J. Phys. Chem. B 2019, 13, 519–524. [Google Scholar] [CrossRef]
- Kim, J.; Cho, D. Effects of alkali-treatment and feeding route of henequen fiber on the heat deflection temperature, mechanical, and impact properties of novel henequen Fiber/Polyamide 6 composites. J. Compos. Sci. 2022, 6, 89. [Google Scholar] [CrossRef]
- Mehrdoost, A.; Yengejeh, R.J.; Mohammadi, M.K.; Haghighatzadeh, A.; Babaei, A.A. Adsorption removal and photocatalytic degradation of azithromycin from aqueous solution using PAC/Fe/Ag/Zn nanocomposite. Environ. Sci. Pollut. Res. 2022, 29, 33514–33527. [Google Scholar] [CrossRef]
- Mehrdoost, A.; Yengejeh, R.J.; Mohammadi, M.K.; Babaei, A.A.; Haghighatzadeh, A. Comparative analysis of UV-assisted removal of azithromycin and cefixime from aqueous solution using PAC/Fe/Si/Zn nanocomposite. J. Health Sci. Surveill. Syst. 2021, 9, 39–49. [Google Scholar] [CrossRef]
- Weber, M.; Daldrup, J.-B.G.; Weber, M. Noncatalyzed radical chain oxidation: Cumene hydroperoxide. In Liquid Phase Aerobic Oxidation Catalysis; Stahl, S.S., Alsters, P.L., Eds.; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2016; pp. 15–31. [Google Scholar] [CrossRef]
- Kuznetsova, N.I.; Babushkin, D.E.; Zudin, V.N.; Koscheeva, O.S.; Kuznetsova, L.I. Low-temperature oxidation of isopropylbenzene mediated by the system of NHPI, Fe(acac)3 and 1,10-phenanthroline. Catal. Commun. 2021, 149, 106218. [Google Scholar] [CrossRef]
- Turovskij, N.; Raksha, E.; Berestneva, Y.; Eresko, A. Anion effect on the cumene hydroperoxide decomposition in the presence of Cu(II) 1,10-phenanthrolinates. J. Organomet. Chem. 2020, 922, 121371. [Google Scholar] [CrossRef]
- Nowacka, A.; Briantais, P.; Prestipino, C.; Llabrés i Xamena, F.X. Selective aerobic oxidation of cumene to cumene hydroperoxide over mono- and bimetallic trimesate metal-organic frameworks prepared by a facile “green” aqueous synthesis. ACS Sustain. Chem. Eng. 2019, 7, 7708–7715. [Google Scholar] [CrossRef]
- Matienko, L.I.; Binyukov, V.I.; Mil, E.M.; Zaikov, G.E. Supramolecular macrostructures in the mechanisms of catalysis with nickel or iron heteroligand complexes. Curr. Organocatalysis. 2019, 6, 36–43. [Google Scholar] [CrossRef]
- Mu, C.H.; Cao, Y.; Wang, H.; Yu, H.; Peng, F. A kinetics study on cumene oxidation catalyzed by carbon nanotubes: Effect of N-doping. Chem. Eng. Sci. 2018, 177, 391–398. [Google Scholar] [CrossRef]
- Lu, Q.; Shi, G.; Zhou, H.; Yuan, E.; Chen, C.H.; Ji, l. A highly efficient transformation from cumene to cumyl hydroperoxide via catalytic aerobic oxidation at room temperature and investigations into solvent effects, reaction networks and mechanisms. Appl. Catal. A Cen. 2022, 630, 118441. [Google Scholar] [CrossRef]
- Lu, Y.; Sheng, X.; Zhang, J.; Wang, Y.; Du, L.; Zhu, J. Cumene autooxidation to cumene hydroperoxide based on a gas-liquid microdispersion strategy. Chem. Eng. Process. 2022, 174, 108861. [Google Scholar] [CrossRef]
- Matienko, L.I.; Mosolova, L.A.; Zaikov, G.E. Selective catalytic oxidation of hydrocarbons. New prospects. Russ. Chem. Rev. 2009, 78, 211–230. [Google Scholar] [CrossRef]
- Ulitin, N.V.; Kharlampidi, K.E.; Tereshchenko, K.A.; Novikov, N.A.; Shiyan, D.A.; Nurmurodov, T.S.; Nurullina, N.M.; Ziyatdinov, N.N.; Miroshkin, N.P. The cumene oxidation and cumene hydroperoxide decomposition in the presence of Zn, Cd or Hg 2-ethylhexanoate: Kinetic model and analysis of its sensitivity. Mol. Catal. 2021, 515, 111886. [Google Scholar] [CrossRef]
- Liao, S.; Peng, F.; Hao, Y.; Wang, H. Carbon nanotubes as catalyst for the aerobic oxidation of cumene to cumene hydroperoxide. Appl. Catal. A Gen. 2014, 478, 1–8. [Google Scholar] [CrossRef]
- Nowacka, A.; Vismara, R.; Mercuri, G.; Moroni, M.; Palomino, M.; Domasevitch, K.V.; Nicola, C.; Di Pettinari, C.; Giambastiani, G.; Llabrés i Xamena, F.X.; et al. Cobalt(II) Bipyrazolate Metal—Organic Frameworks as Heterogeneous Catalysts in Cumene Aerobic Oxidation: A Tag-Dependent Selectivity. Inorg. Chem. 2020, 59, 8161–8172. [Google Scholar] [CrossRef]
- Denisov, E.T.; Afanas’ev, I.B. Oxidation and Antioxidants in Organic Chemistry and Biology; Taylor & Francis Group: Boca Raton, FL, USA, 2005; 1024p. [Google Scholar]
- Masters, C. Homogeneous Transition-Metal Catalysis; Chapman and Hall: London, UK, 1981. [Google Scholar]
- Kharlampidi, K.E.; Tereshchenko, K.A.; Nurmurodov, T.S.; Shiyan, D.A.; Miroshkin, N.P.; Ziyatdinov, N.N.; Ziganshina, A.S.; Nurullina, N.M.; Khursan, S.L.; Ulitin, N.V. The kinetic modeling of cumene oxidation taking into account oxygen mass transfer. Chem. Eng. J. 2020, 392, 123811. [Google Scholar] [CrossRef]
- Dumbre, D.K.; Choudhary, V.R.; Patil, N.S.; Uphade, B.S.; Bhargava, S.K. Calcium oxide supported gold nanoparticles as catalysts for the selective epoxidation of styrene by t-butyl hydroperoxide. J. Colloid Interface Sci. 2014, 415, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wei, C.; Chai, Y.; Zhang, J.; Ji, H. Dibenzo-18-crown-6-functionalized organic nanotubes for the synergistic adsorption of dyes and phenols from aqueous solutions. J. Water Process Eng. 2022, 50, 103213. [Google Scholar] [CrossRef]
- Zawadiak, J.; Gilner, D.; Mazurkiewicz, R. Copper salt—Crown ether systems as catalysts for the oxidation of cumene with 1-methyl-1-phenylethylhydroperoxide to bis(1-methyl-1-phenylethyl)peroxide. Tetrahedron Lett. 1999, 40, 4059–4062. [Google Scholar] [CrossRef]
- Bianchini, E.; Pietrobon, L.; Ronchin, L.; Tortato, C.; Vavasori, A. Trifluoroacetic acid promoted hydration of styrene catalyzed by sulfonic resins: Comparison of the reactivity of styrene, n-hexene and cyclohexene. Appl. Catal. A Gen. 2019, 570, 130–138. [Google Scholar] [CrossRef]
- Kharlampidi, K.E.; Nurmurodov, T.S.; Ulitin, N.V.; Tereshchenko, K.A.; Miroshkin, N.P.; Shiyan, D.A.; Novikov, N.A.; Stoyanov, O.V.; Ziyatdinov, N.N.; Lapteva, T.V.; et al. Design of cumene oxidation process. Chem. Eng. Process. 2021, 161, 108314. [Google Scholar] [CrossRef]
- Dolan, E.D.; Lewis, R.M.; Torczon, V. On the local convergence of pattern search. SIAM J. Optim. 2003, 14, 567–583. [Google Scholar] [CrossRef]






| Catalyst | T, K | Oxidation Time, h | Cumene Conversion, % | Selectivity of the Process According to CHP, % |
|---|---|---|---|---|
| Acetylacetonate Fe Fe(acac)3/NHPI/Phen [9] | 333 | 2.5 | 8.5–58 | 50–95 |
| Carbon nanotubes [20] | 353 | 8 | 24.1 | 88.4 |
| Ni metal–organic frameworks [21] | 363 | 7 | 6 | 91 |
| Co metal–organic frameworks [21] | 363 | 7 | 49 | 69 |
| Zn metal–organic frameworks [21] | 363 | 7 | 3 | 95 |
| Ca, Sr, and Ba complexes with dibenzo-18-crown-6 ether (results of this work) | 393 | 6 | 60–70 | 40–60 |
| k | A, 1/s is for Monomolecular Reactions, L/(mol × s) is for Bimolecular Reactions, L2/(mol2 × s) is for Trimolecular Reactions | E, J/mol | ||||
|---|---|---|---|---|---|---|
| Ca | Sr | Ba | Ca | Sr | Ba | |
| k1 | 4.63 × 104 | 3.57 × 106 | 7.64 × 1014 | 55,000 | 58,900 | 130,000 |
| k1’ | 3.97 × 104 | 2.20 × 105 | 1.65 × 105 | 70,100 | 74,700 | 69,000 |
| k2 | 1.37 × 103 | 1.18 × 103 | 1.73 × 1018 | 29,000 | 23,000 | 139,500 |
| k2’ | 1.35 × 1020 | 4.01 × 1012 | 1.68 × 1015 | 123,800 | 63,300 | 89,400 |
| k3 | 6.07 × 1014 | 1.72 × 103 | 1.25 × 1018 | 136,400 | 39,000 | 132,300 |
| k3’ | 8.36 × 1019 | 2.21 × 1016 | 1.05 × 103 | 107,600 | 79,000 | 35,000 |
| k4 | 8.49 × 103 | 2.36 × 103 | 4.25 × 107 | 19,000 | 24,400 | 78,200 |
| k5 | 1.30 × 105 | 1.10 × 103 | 1.91 × 103 | 38,500 | 22,200 | 10,400 |
| k5’ | 1.25 × 103 | 2.57 × 103 | 1.55 × 104 | 24,300 | 24,900 | 49,400 |
| k6 | 6.14 × 106 | 106,500 | ||||
| k7 | 5.23 × 107 | 1.16 × 1010 | 1.02 × 1017 | 2900 | 15,000 | 103,100 |
| k8 | 9.27 × 106 | 95,700 | ||||
| k9 | 9.97 × 105 | 6.27 × 1014 | 8.87 × 106 | 27,000 | 93,200 | 37,800 |
| k10 | 1.28 × 106 | 94,700 | ||||
| k11 | 1.54 × 109 | 8.36 × 107 | 1.94 × 109 | 46,100 | 36,600 | 46,700 |
| k12 | 9.60 × 1011 | 21,700 | ||||
| k13 | 1.43 × 1014 | 95,900 | ||||
| k14 | 3.35 × 1010 | 1.91 × 1013 | 2.66 × 1013 | 23,100 | 40,400 | 68,800 |
| k15 | 6.34 × 1010 | 42,000 | ||||
| k16 | 2.58 × 1014 | 2.78 × 1013 | 1.44 × 1012 | 2500 | 600 | 32,500 |
| k17 | 5.96 × 1012 | 24,400 | ||||
| k18 | 7.64 × 108 | 4.38 × 1010 | 2.82 × 1018 | 16,600 | 27,300 | 89,300 |
| k19 | 1.20 × 107 | 3700 | ||||
| k2 | 2.31 × 1010 | 6.77 × 109 | 1.33 × 1010 | 2700 | 1600 | 12,200 |
| k21 | 5.83 × 1013 | 60,200 | ||||
| k22 | 5.04 × 107 | 6700 | ||||
| k23 | 8.82 × 109 | 8700 | ||||
| k24 | 4.50 × 106 | 62,100 | ||||
| k25 | 5.47 × 1019 | 8.32 × 1016 | 9.02 × 108 | 107,600 | 86,700 | 35,500 |
| k26 | 3.58 × 1012 | 81,900 | ||||
| k27 | 2.33 × 1011 | 7700 | ||||
| k28 | 4.99 × 1010 | 420 | ||||
| k29 | 7.60 × 1012 | 21,100 | ||||
| k30 | 2.26 × 1013 | 59,300 | ||||
| k31 | 7.41 × 1011 | 36,200 | ||||
| k32 | 1.65 × 1011 | 63,400 | ||||
| k33 | 4.89 × 1012 | 99,100 | ||||
| k34 | 4.39 × 108 | 2020 | ||||
| k35 | 1.12 × 107 | 89,100 | ||||
| k36 | 1.81 × 109 | 1.93 × 1014 | 1.17 × 109 | 85,600 | 119,000 | 86,300 |
| k37 | 3.88 × 1019 | 1.11 × 105 | 2.66 × 1018 | 145,300 | 42,600 | 85,000 |
| k38 | 1.21 × 105 | 83,600 | ||||
| k39 | 1.73 × 105 | 1.98 × 1010 | 1.05 × 104 | 70,400 | 109,000 | 16,300 |
| k40 | 6.38 × 108 | 102,200 | ||||
| k41 | 5.38 × 109 | 1.20 × 103 | 1.83 × 1019 | 24,700 | 9200 | 72,800 |
| k42 | 5.99 × 106 | 75,100 | ||||
| k42’ | 2.34 × 1012 | 113,600 | ||||
| k43 | 7.10 × 107 | 88,900 | ||||
| k44 | 2.94 × 106 | 58,500 | ||||
| k45 | 1.32 × 103 | 1.20 × 1013 | 8.85 × 1011 | 62,000 | 130,000 | 116,400 |
| k46 | 3.53 × 109 | 1.87 × 1010 | 3.83 × 104 | 63,100 | 21,200 | 68,800 |
| k47 | 9.36 × 108 | 9.63 × 1012 | 2.56 × 104 | 63,200 | 91,700 | 68,800 |
| k48 | 1.26 × 1012 | 8.39 × 1016 | 2.87 × 106 | 117,200 | 147,500 | 58,000 |
| k49 | 1.25 × 1010 | 1.91 × 1010 | 2.26 × 1011 | 85,100 | 86,200 | 88,800 |
| k50 | 2.17 × 1010 | 8.19 × 103 | 1.24 × 103 | 76,700 | 28,500 | 12,900 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulitin, N.V.; Novikov, N.A.; Lyulinskaya, Y.L.; Shiyan, D.A.; Tereshchenko, K.A.; Nurullina, N.M.; Denisova, M.N.; Mezhuev, Y.O.; Kharlampidi, K.E. The Effect of Ca, Sr, and Ba Chloride Complexes with Dibenzo-18-Crown-6 Ether as Catalysts on the Process Criteria for the Efficiency of Cumene Oxidation (the First Stage in the Chain of Polymer Composite Production). J. Compos. Sci. 2023, 7, 60. https://doi.org/10.3390/jcs7020060
Ulitin NV, Novikov NA, Lyulinskaya YL, Shiyan DA, Tereshchenko KA, Nurullina NM, Denisova MN, Mezhuev YO, Kharlampidi KE. The Effect of Ca, Sr, and Ba Chloride Complexes with Dibenzo-18-Crown-6 Ether as Catalysts on the Process Criteria for the Efficiency of Cumene Oxidation (the First Stage in the Chain of Polymer Composite Production). Journal of Composites Science. 2023; 7(2):60. https://doi.org/10.3390/jcs7020060
Chicago/Turabian StyleUlitin, Nikolai V., Nikolay A. Novikov, Yana L. Lyulinskaya, Daria A. Shiyan, Konstantin A. Tereshchenko, Natalia M. Nurullina, Marina N. Denisova, Yaroslav O. Mezhuev, and Kharlampii E. Kharlampidi. 2023. "The Effect of Ca, Sr, and Ba Chloride Complexes with Dibenzo-18-Crown-6 Ether as Catalysts on the Process Criteria for the Efficiency of Cumene Oxidation (the First Stage in the Chain of Polymer Composite Production)" Journal of Composites Science 7, no. 2: 60. https://doi.org/10.3390/jcs7020060






