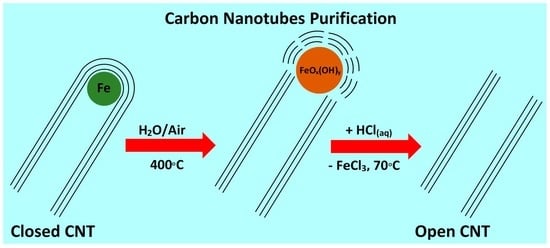
The Importance of Water for Purification of Longer Carbon Nanotubes for Nanocomposite Applications
Abstract
:1. Introduction
2. Materials and Methods
3. Results
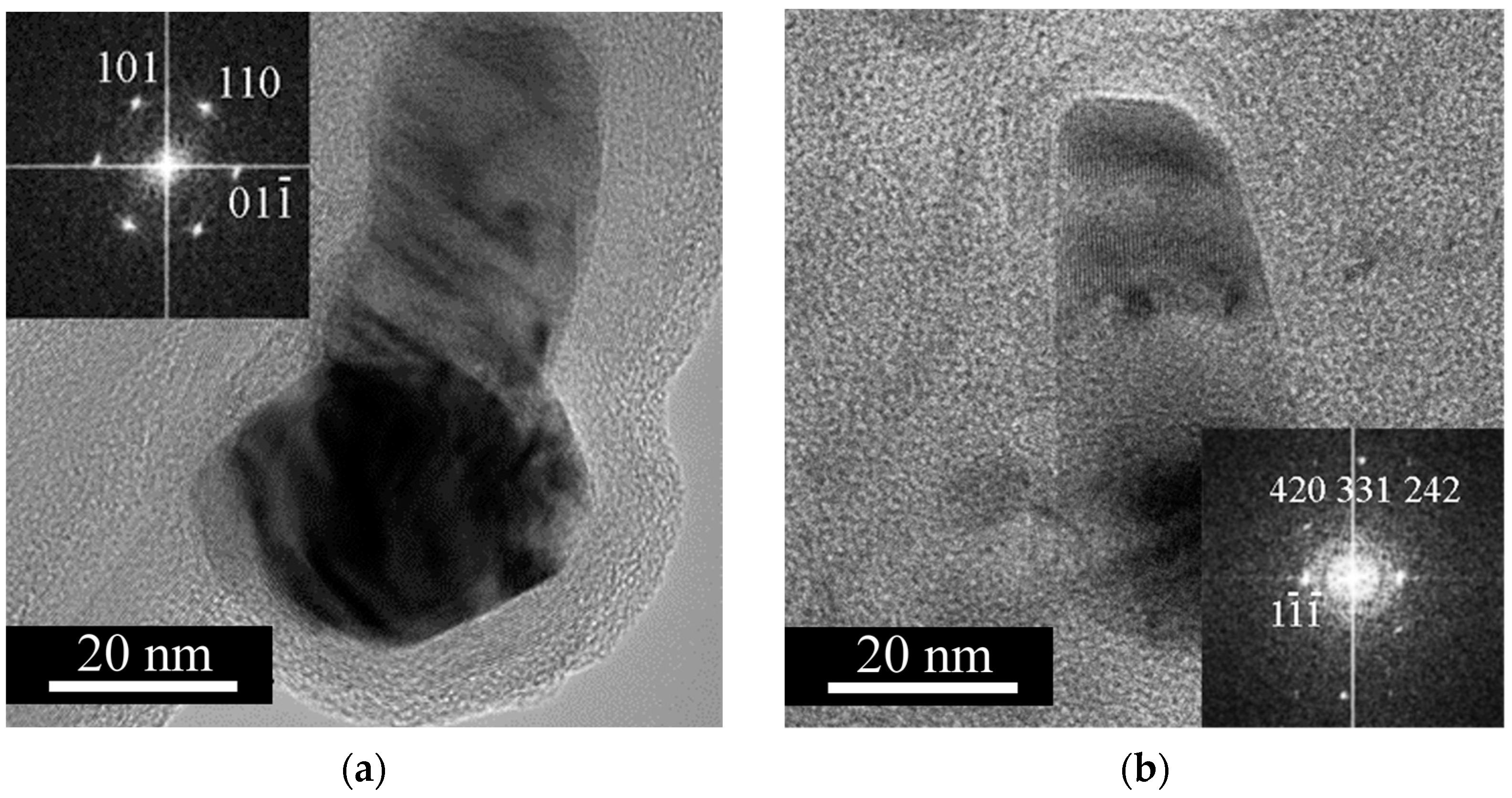
3.1. Free or Unbound Iron
3.2. Water Influence at the Stage of Mild Oxidation
3.3. Influence of Intensity of Mild Oxidation with Water
3.4. Oxidative Heat Treatment after Mild Oxidation
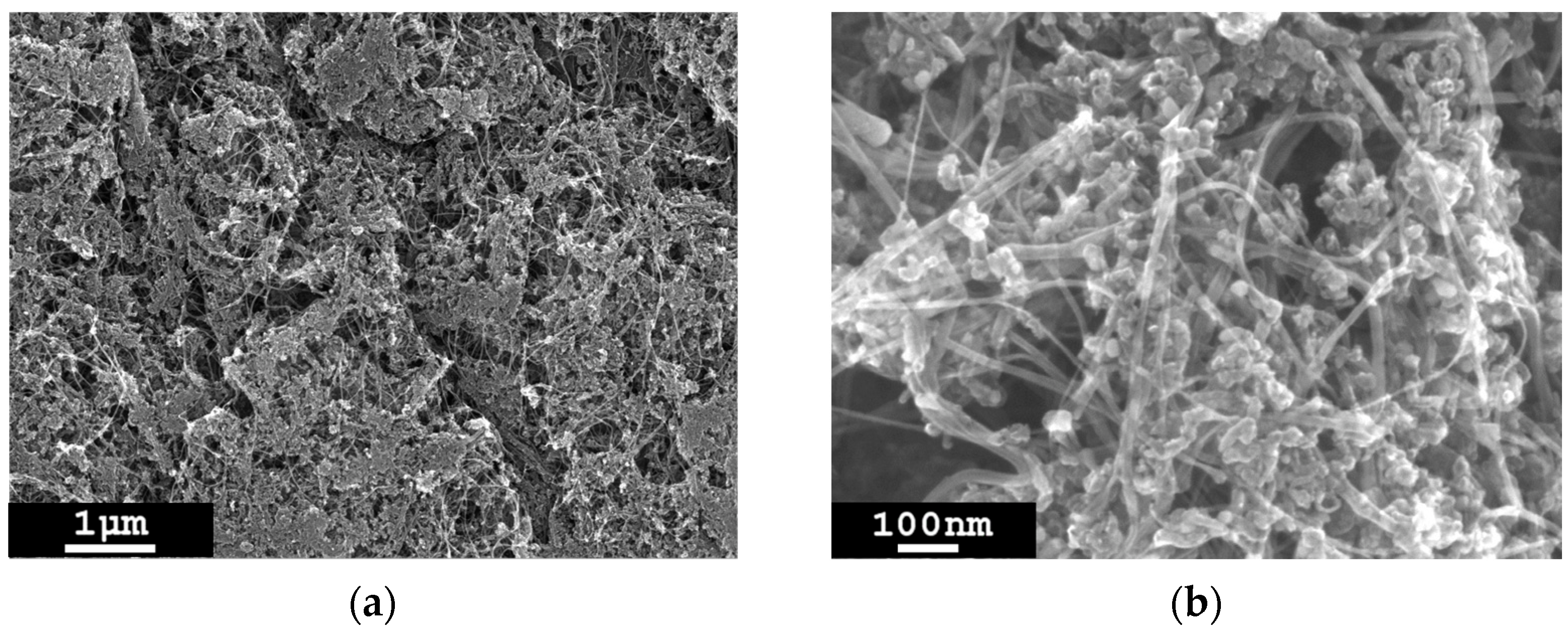
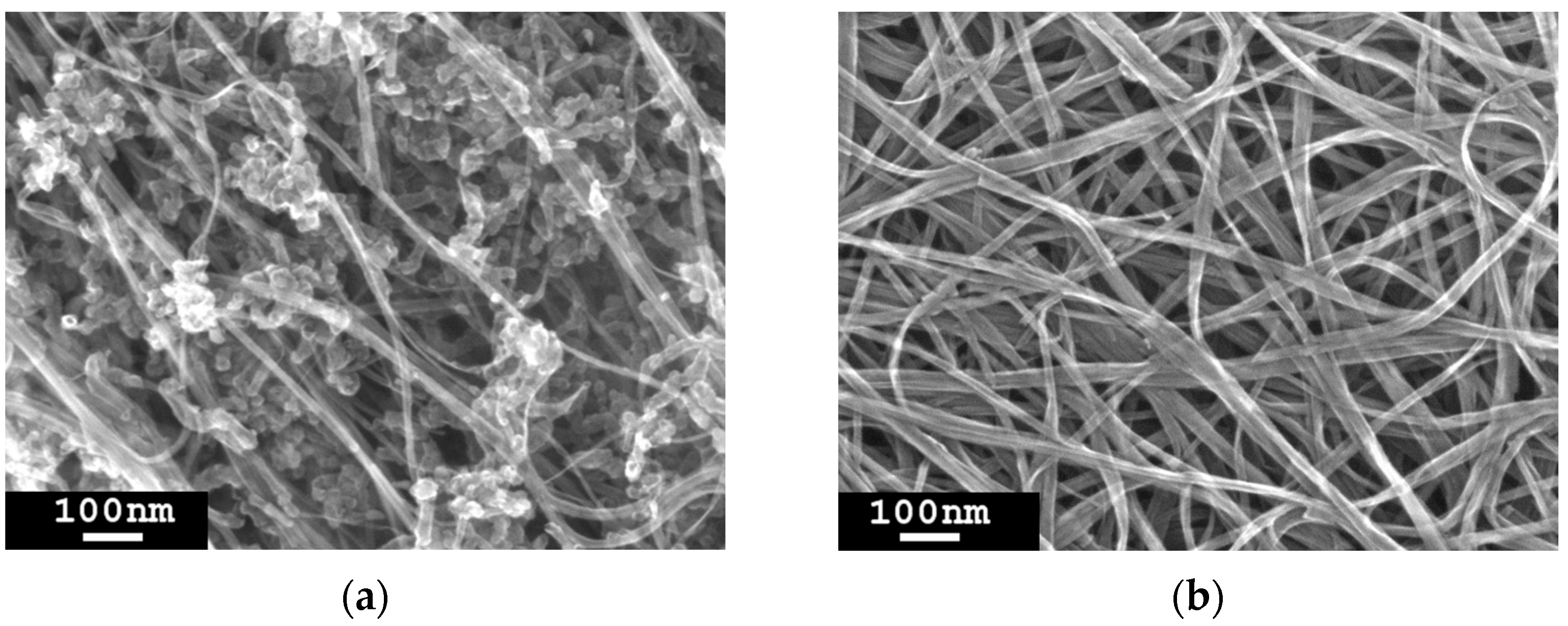
3.5. Removal of Non-CNT Particles from the Sample
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ates, M.; Eker, A.A.; Eker, B. Carbon nanotube-based nanocomposites and their applications. J. Adhes. Sci. Technol. 2017, 31, 1977–1997. [Google Scholar] [CrossRef]
- Peng, H.; Sun, X.; Chen, T. Polymer Composites with Carbon Nanotubes in Alignment. In Carbon Nanotubes—Polymer Nanocomposites, 1st ed.; Yellampalli, S., Ed.; InTech: Rijeka, Croatia, 2011; Volume 12, pp. 231–250. [Google Scholar] [CrossRef] [Green Version]
- Inam, F.; Vo, T.; Jones, J.P.; Lee, X. Effect of carbon nanotube lengths on the mechanical properties of epoxy resin: An experimental study. J. Compos. Mater. 2013, 47, 2321–2330. [Google Scholar] [CrossRef]
- Tinh, T.X.; Chuc, N.V.; Jourdain, V.; Paillet, M.; Kim, D.-Y.; Sauvajol, J.-L.; Tam, N.T.T.; Minh, P.N. Synthesis of individual ultra-long carbon nanotubes and transfer to other substrates. J. Exp. Nanosci. 2011, 6, 547–556. [Google Scholar] [CrossRef]
- Sugime, H.; Sato, T.; Nakagawa, R.; Hayashi, T.; Inoue, Y.; Noda, S. Ultra-long carbon nanotube forest via in situ supplements of iron and aluminum vapor sources. Carbon 2021, 172, 772–780. [Google Scholar] [CrossRef]
- Issman, L.; Kloza, P.A.; Portas, J.T.; Collins, B.; Pendashteh, A.; Pick, M.; Vilatela, J.J.; Elliott, J.A.; Boies, A. Highly Oriented Direct-Spun Carbon Nanotube Textiles Aligned by In Situ Radio-Frequency Fields. ACS Nano 2022, 16, 9583–9597. [Google Scholar] [CrossRef]
- Karaeva, A.R.; Khaskov, M.A.; Mitberg, E.B.; Kulnitskiy, B.A.; Perezhogin, I.A.; Ivanov, L.A.; Denisov, V.N.; Kirichenko, A.N.; Mordkovich, V.Z. Longer Carbon Nanotubes by Controlled Catalytic Growth in the Presence of Water Vapor. Fuller. Nanotub. Carbon Nanostruct. 2012, 20, 411–418. [Google Scholar] [CrossRef]
- Yaya, A.; Dodoo-Arhin, D.; Onwona-Agyeman, B.; Konadu, D.S.; Mensah Brown, H.; Sinayobye, E. Effects of Purity on the Mechanical Properties of Single-Walled Carbon Nanotubes-Polymer Nanocomposites. Br. J. Appl. Sci. Technol. 2013, 3, 884–897. [Google Scholar] [CrossRef]
- Chen, J.; Han, J.; Xu, D. Thermal and electrical properties of the epoxy nanocomposites reinforced with purified carbon nanotubes. Mater. Lett. 2019, 246, 20–23. [Google Scholar] [CrossRef]
- Hou, P.-X.; Liu, C.; Cheng, H.-M. Purification of carbon nanotubes. Carbon 2008, 46, 2003–2025. [Google Scholar] [CrossRef]
- Ageeva, E.A.; Zhukova, E.A.; Karaeva, A.R.; Mordkovich, V.Z. Changes in physical properties of super long carbon nanotubes after different methods of purification. Izv. Vyssh. Uchebn. Zaved. Khim. Khim. Tekhnol. 2016, 59, 74–79. [Google Scholar] [CrossRef]
- Rouf, S.A.; Usman, Z.; Masood, H.T.; Majeed, A.M.; Sarwar, M.; Abbas, W. Synthesis and Purifiation of Carbon Nanotubes. In Book Carbon Nanotubes—Redefining the World of Electronics, 2nd ed.; Ghosh, P.K., Datta, K., Rushi, A.D., Eds.; IntechOpen: London, UK, 2022; Volume 3, pp. 415–479. [Google Scholar] [CrossRef]
- Chiang, I.W.; Brinson, B.E.; Huang, A.Y.; Willis, P.A.; Bronikowski, M.J.; Margrave, J.L.; Smalley, R.E.; Hauge, R.H. Purification and Characterization of Single-Wall Carbon Nanotubes (SWNTs) Obtained from the Gas-Phase Decomposition of CO (HiPco Process). J. Phys. Chem. B 2001, 105, 8297–8301. [Google Scholar] [CrossRef] [Green Version]
- Kuziel, A.W.; Dzido, G.; Turczyn, R.; Jędrysiak, R.G.; Kolanowska, A.; Tracz, A.; Zięba, W.; Cyganiuk, A.; Terzyk, A.P.; Boncel, S. Ultra-long carbon nanotube-paraffin composites of record thermal conductivity and high phase change enthalpy among paraffin-based heat storage materials. J. Energy Storage 2021, 36, 102396. [Google Scholar] [CrossRef]
- Li, L.; Wu, Z.; Yuan, S.; Zhang, X.-B. Advances and challenges for flexible energy storage and conversion devices and systems. Energy Environ. Sci. 2014, 7, 2101–2122. [Google Scholar] [CrossRef]
- Choi, Y.C.; Min, K.-I.; Jeong, M.S. Novel Method of Evaluating the Purity of Multiwall Carbon Nanotubes Using Raman Spectroscopy. J. Nanomater. 2013, 2013, 615915. [Google Scholar] [CrossRef] [Green Version]
- Jorio, A.; Saito, R. Raman spectroscopy for carbon nanotube applications. J. Appl. Phys. 2021, 129, 021102. [Google Scholar] [CrossRef]
- Kulnitskiy, B.; Karaeva, A.; Mordkovich, V.; Urvanov, S.; Bredikhina, A. TEM studies of conical scroll carbon nanotubes formed by aerosol synthesis. IOP Conf. Ser. Mater. Sci. Eng. 2019, 693, 012017. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-C.; Chen, C.-C.; Hung, W.-H.; Hsu, I.-K.; Pimenta, M.A.; Cronin, S.B. Strain-Induced D Band Observed in Carbon Nanotubes. Nano Res. 2012, 5, 854–862. [Google Scholar] [CrossRef]
- Karim, W.; Kleibert, A.; Hartfelder, U.; Balan, A.; Gobrecht, J.; Bokhoven, J.A.v.; Ekinci, Y. Size-dependent redox behavior of iron observed by in-situ single nanoparticle spectro-microscopy on well-defined model systems. Sci. Rep. 2016, 6, 18818. [Google Scholar] [CrossRef]
- Fiquet, G.; Badro, J.; Gregoryanz, E.; Fei, Y.; Occelli, F. Sound velocity in iron carbide (Fe3C) at high pressure: Implications for the carbon content of the Earth’s inner core. Phys. Earth Planet. Inter. 2009, 172, 125–129. [Google Scholar] [CrossRef] [Green Version]
- Freel, J.; Wheeler, B.R.; Galwey, A.K. Kinetic study of the oxidation of the carbides of iron. Trans. Faraday Soc. 1970, 66, 1015–1024. [Google Scholar] [CrossRef]
- Bertrand, N.; Desgranges, C.; Poquillon, D.; Lafont, M.C.; Monceau, D. Iron Oxidation at Low Temperature (260–500 °C) in Air and the Effect of Water Vapor. Oxid. Met. 2009, 73, 139–162. [Google Scholar] [CrossRef] [Green Version]
- Koga, N.; Takemoto, S.; Okada, S.; Tanaka, H. A kinetic study of the thermal decomposition of iron(III) hydroxide oxides. Part 1. α-FeO(OH) in banded iron formations. Thermochim. Acta 1995, 254, 193–207. [Google Scholar] [CrossRef]
- Karaeva, A.R.; Zhukova, E.A.; Urvanov, S.A.; Senatulin, B.R.; Skryleva, E.A.; Mordkovich, V.Z. Modification of surface of double wall carbon nanotubes by fullerene C60. Izv. Vyssh. Uchebn. Zaved. Khim. Khim. Tekhnol. 2016, 59, 12–20. [Google Scholar] [CrossRef]



| Sample | Description |
|---|---|
| INI | Initial CNTs synthesis product |
| AT | INI after acid treatment |
| MO_0 | INI after mild oxidation without water at TCNT * = 400 °C and tMO ** = 6 h and acid treatment |
| MO_W1 | INI after mild oxidation with water at TCNT * = 400 °C, TH2O *** = 50 °C, tMO ** = 6 h and acid treatment |
| MO_W2 | INI after mild oxidation with water at TCNT * = 365 °C, TH2O *** = 50 °C, tMO ** = 6 h and acid treatment |
| MO_W3 | INI after mild oxidation with water at TCNT * = 400 °C, TH2O *** = 80 °C, tMO ** = 6 h and acid treatment |
| MO_W4 | INI after mild oxidation with water at TCNT * = 400 °C, TH2O *** = 80 °C, tMO ** = 12 h and acid treatment |
| Sample | TONSET 1, °C | TENDSET 2, °C | m(Fe), wt.% | D/G 3 |
|---|---|---|---|---|
| INI | 534 | 679 | 11.5 | 0.66 |
| AT | 528 | 682 | 10.7 | 0.77 |
| MO_0 | 524 | 678 | 11.2 | 0.82 |
| MO_W1 | 523 | 684 | 8.1 4 | 0.87 |
| MO_W2 | 531 | 703 | 9.1 4 | 0.82 |
| MO_W3 | 550 | 726 | 3.8 | 0.95 |
| MO_W4 | 550 | 767 | 3.8 | 1.18 |
| Sample | TONSET, °C | TENDSET, °C | ∆mTO *, % | m(Fe), wt.% |
|---|---|---|---|---|
| INI_HT | 547 | 715 | −34.5 | 4.6 |
| MO_0_HT | 542 | 674 | −30.9 | 3.1 |
| MO_W1_HT | 549 | 710 | −19.4 | 2.2 |
| MO_W2_HT | 547 | 731 | −20.7 | 2.0 |
| MO_W3_HT | 557 | 749 | −4.5 | 1.8 |
| MO_W4_HT | 550 | 746 | −4.6 | 1.9 |
| Sample | C/O Molar Ratio | Fe/O Molar Ratio |
|---|---|---|
| INI | 87.8 | 3.1 |
| MO_0 | 72.6 | 2.8 |
| MO_W1 | 19.0 | 0.7 |
| MO_W3 | 15.9 | 0.4 |
| MO_W4 | 13.2 | 0.3 |
| Sample | m(Fe), wt.% | ∆m, % |
|---|---|---|
| MO_400-6 * | 3.8 | −12.9 |
| HT_480-2 ** | 4.6 | −34.5 |
| HT_480-6 *** | 3.1 | −83.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mordkovich, V.Z.; Khaskov, M.A.; Naumova, V.A.; De, V.V.; Kulnitskiy, B.A.; Karaeva, A.R. The Importance of Water for Purification of Longer Carbon Nanotubes for Nanocomposite Applications. J. Compos. Sci. 2023, 7, 79. https://doi.org/10.3390/jcs7020079
Mordkovich VZ, Khaskov MA, Naumova VA, De VV, Kulnitskiy BA, Karaeva AR. The Importance of Water for Purification of Longer Carbon Nanotubes for Nanocomposite Applications. Journal of Composites Science. 2023; 7(2):79. https://doi.org/10.3390/jcs7020079
Chicago/Turabian StyleMordkovich, Vladimir Z., Maxim A. Khaskov, Veronika A. Naumova, Victor V. De, Boris A. Kulnitskiy, and Aida R. Karaeva. 2023. "The Importance of Water for Purification of Longer Carbon Nanotubes for Nanocomposite Applications" Journal of Composites Science 7, no. 2: 79. https://doi.org/10.3390/jcs7020079