The Effect of Pulse Current on Electrolytically Plating Nickel as a Catalyst for Grafting Carbon Nanotubes onto Carbon Fibers via the Chemical Vapor Deposition Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sizing Agent Removal Method
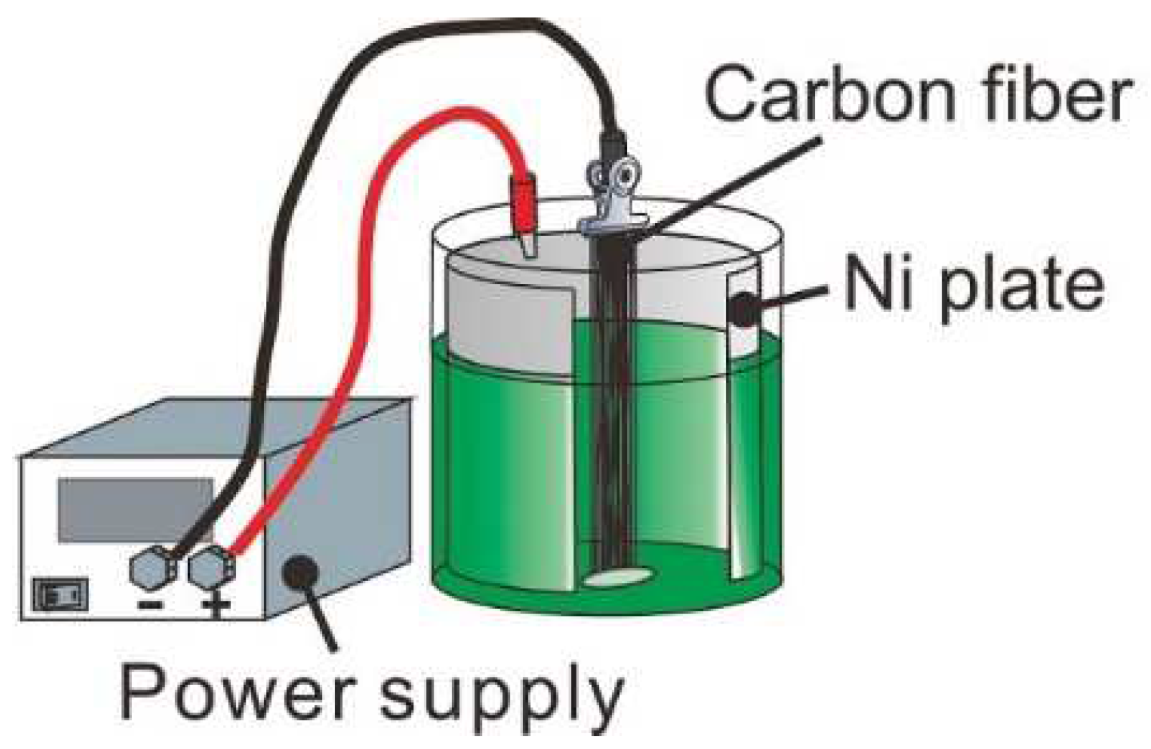
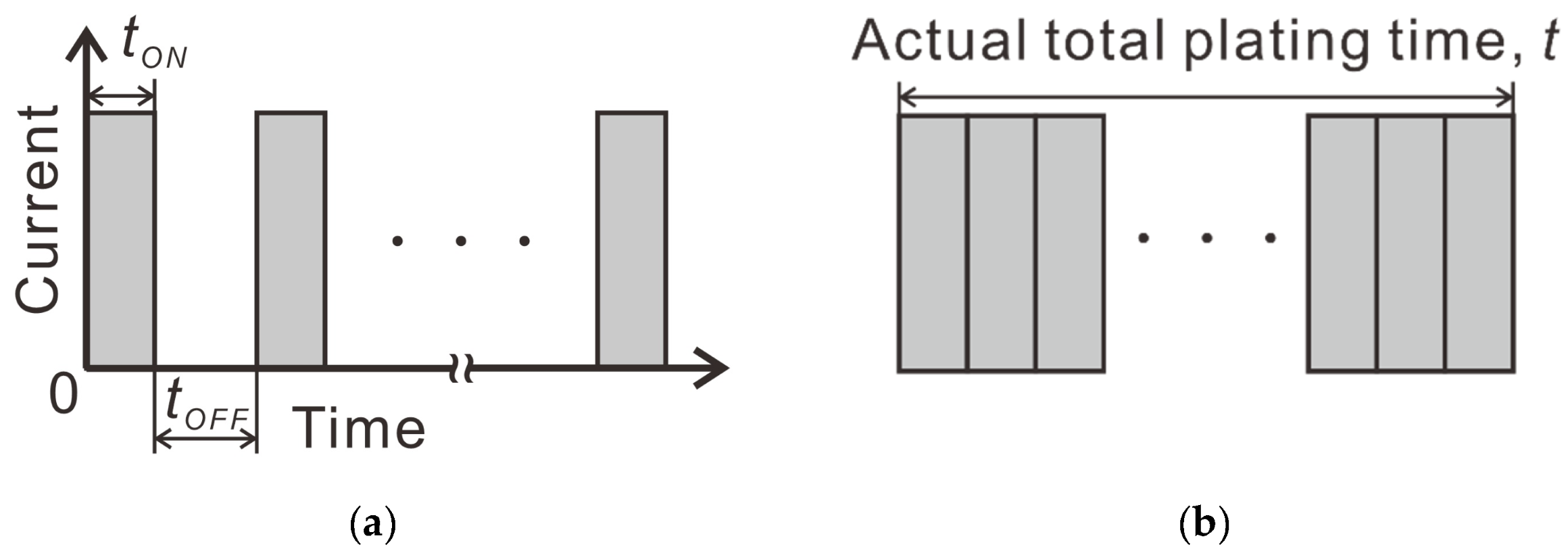
2.2. Electrolytic Ni Plating onto Carbon Fibers
2.3. CNT Grafting onto Ni-CF via the CVD Method
3. Results and Discussion
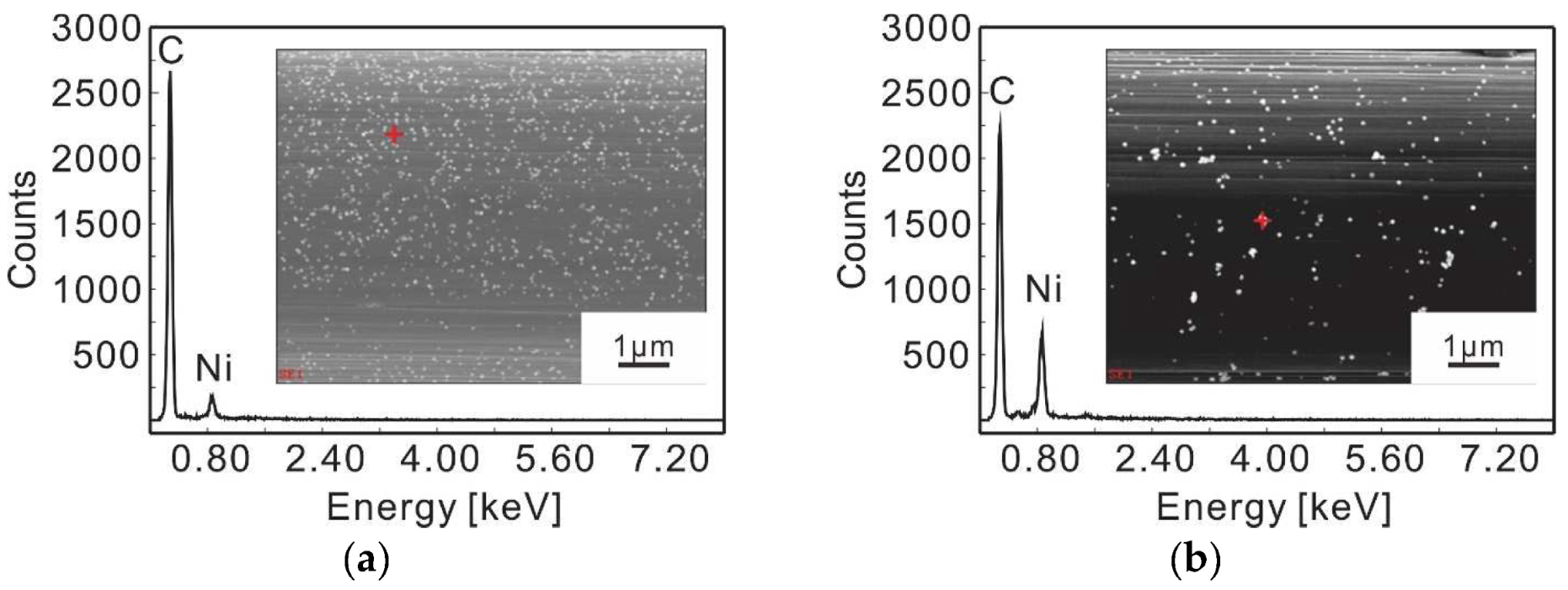
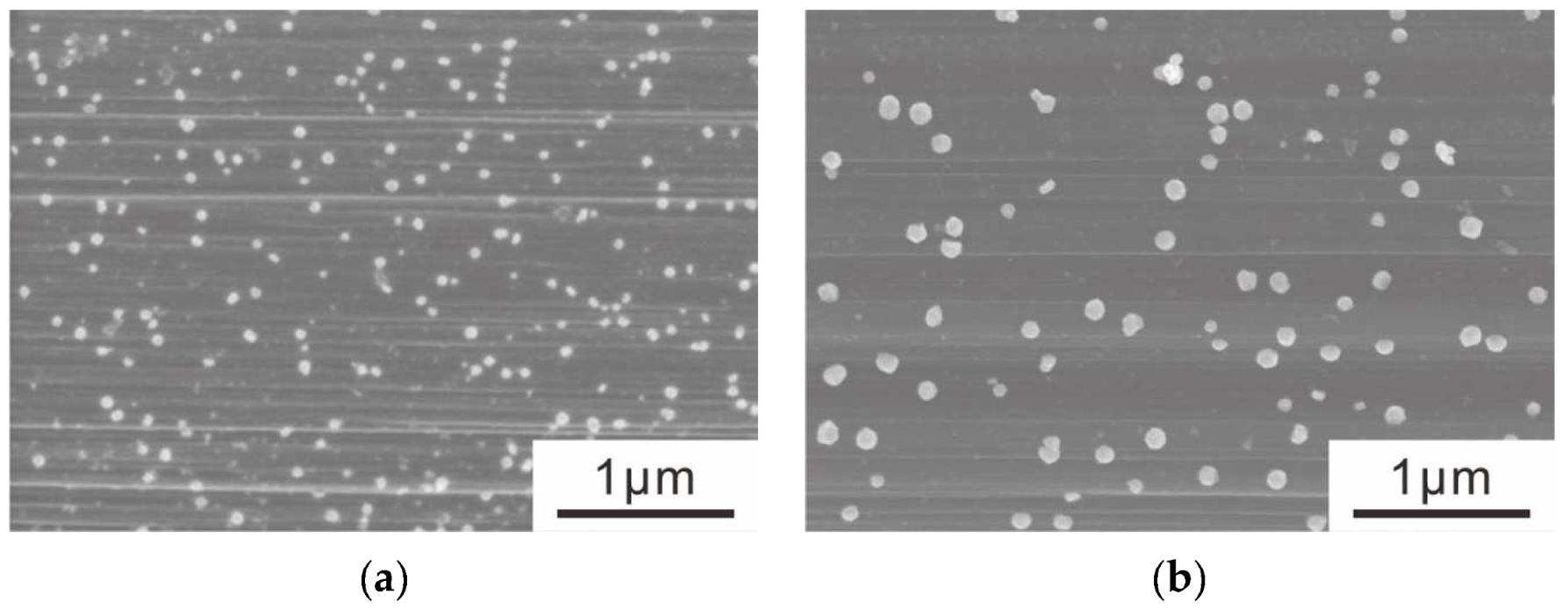
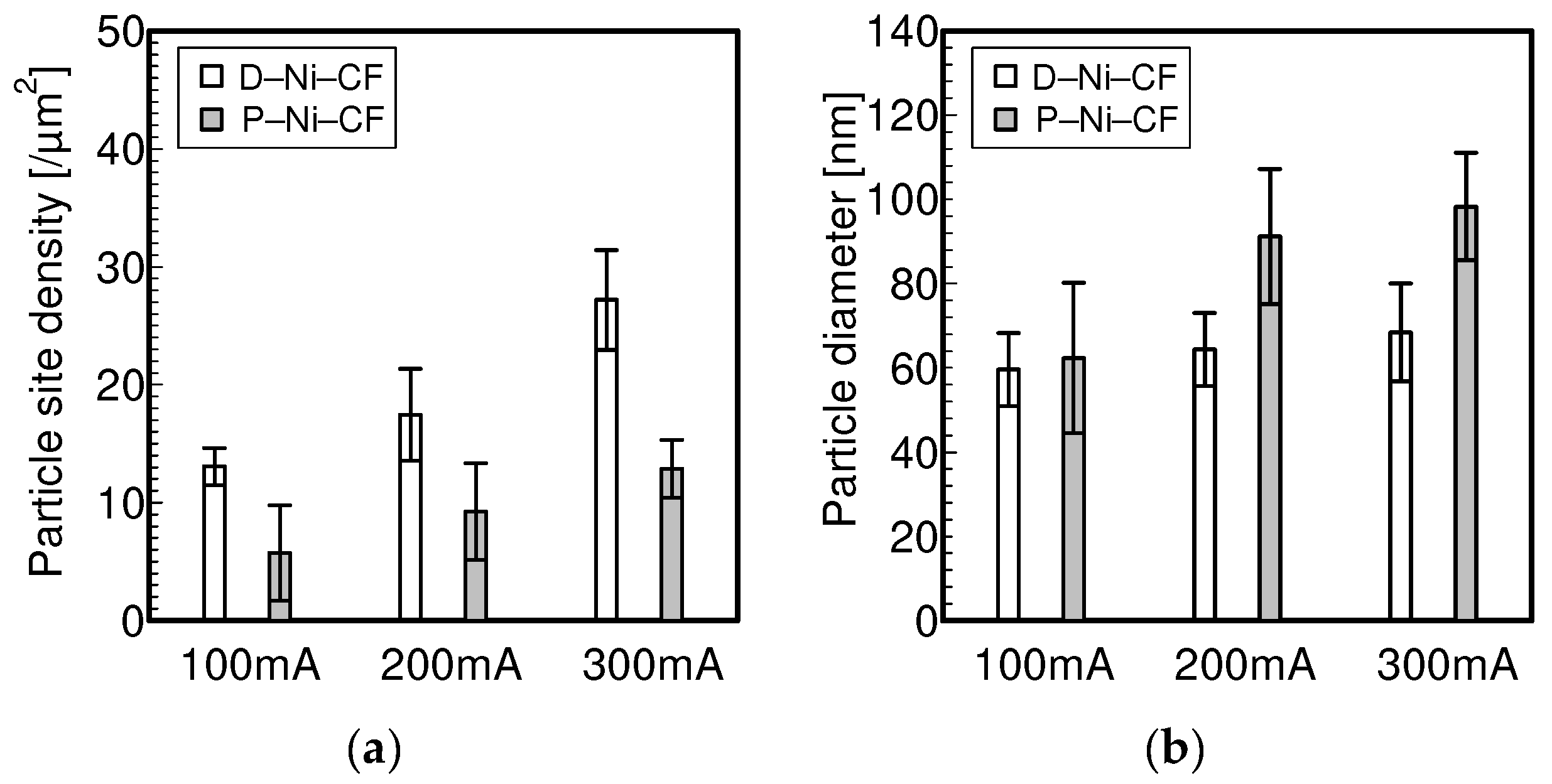
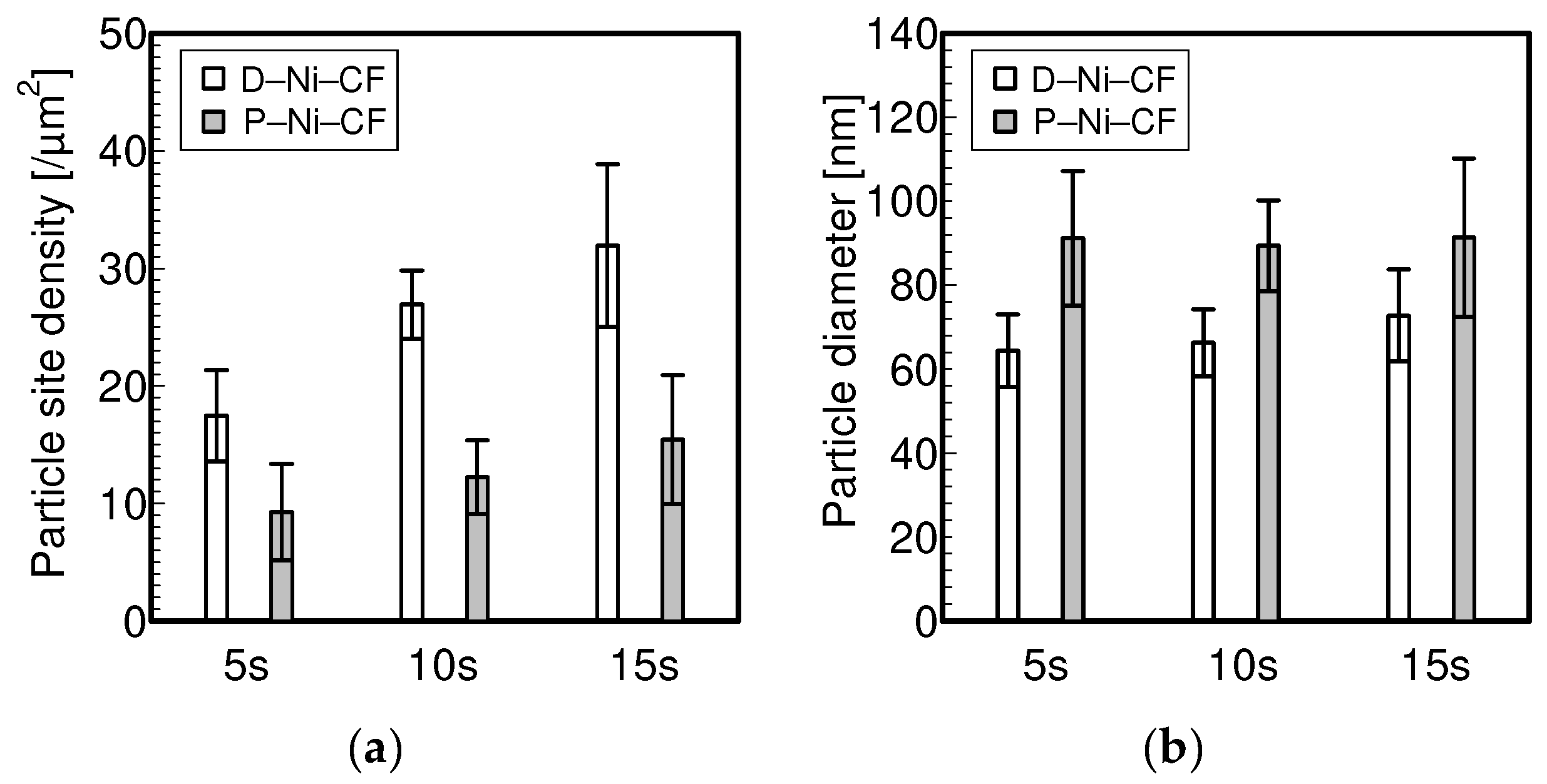
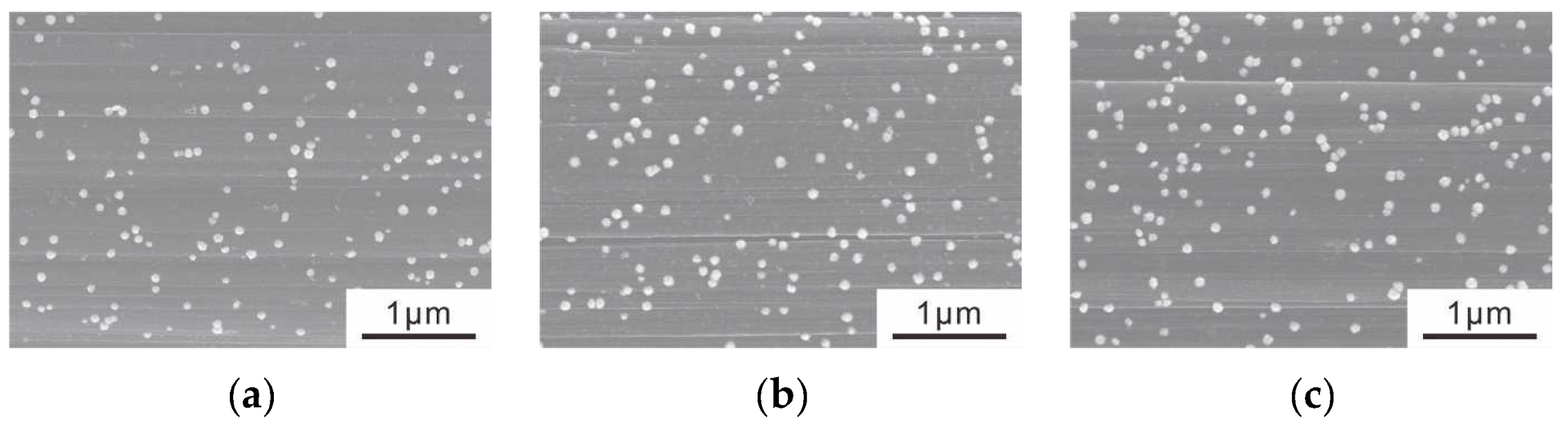
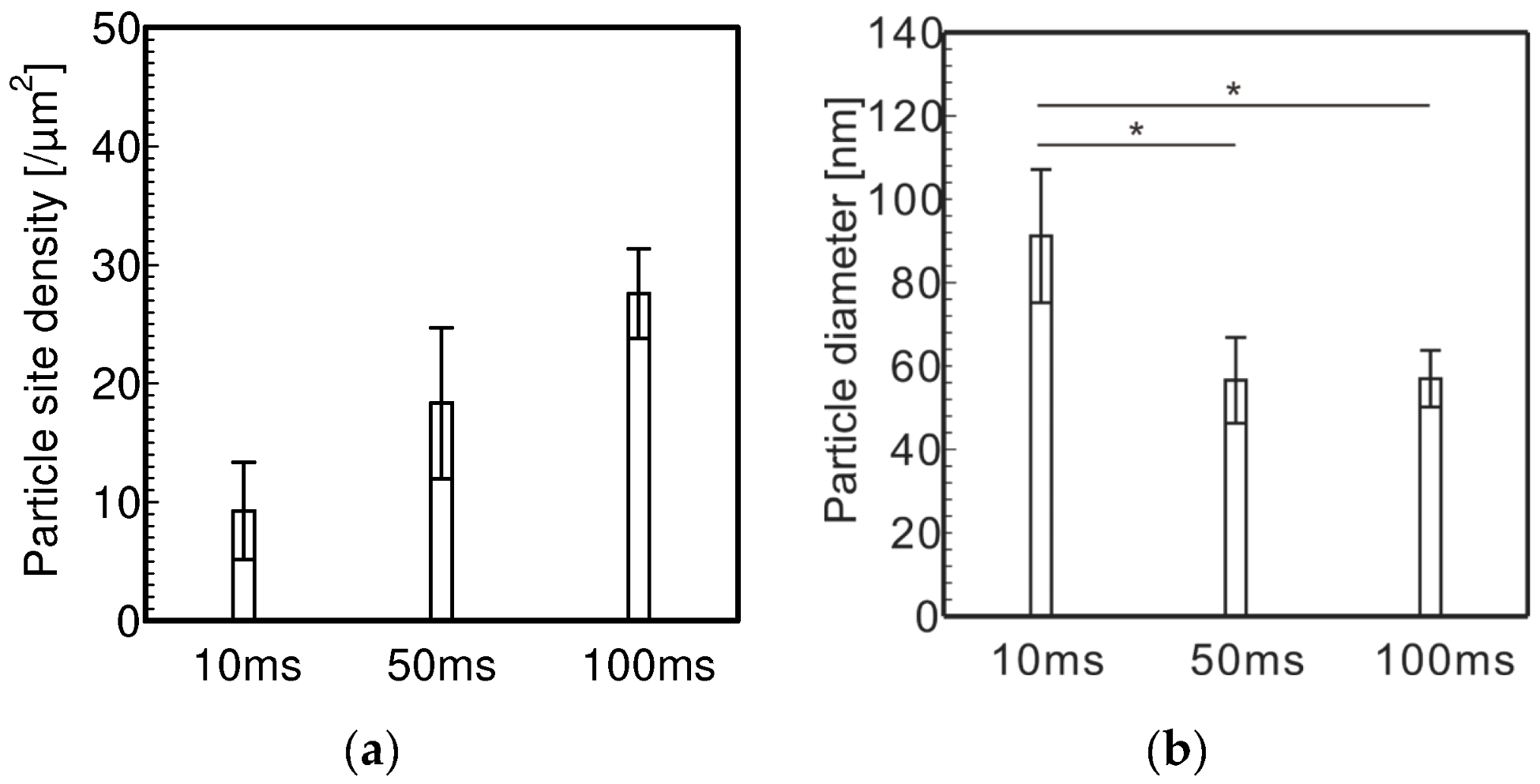
3.1. Electrolytic Ni Plating onto Carbon Fibers
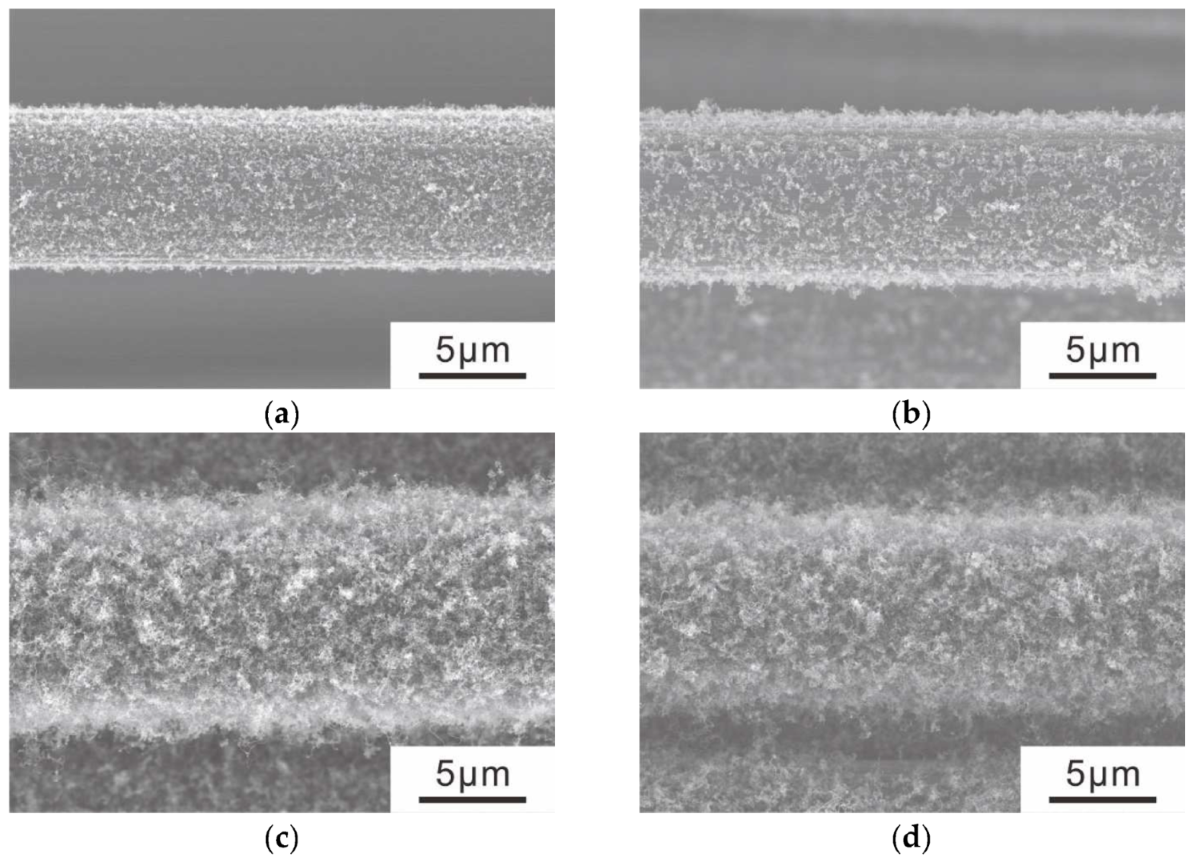
3.2. CNT Grafting onto Ni-CF via the CVD Method
4. Conclusions
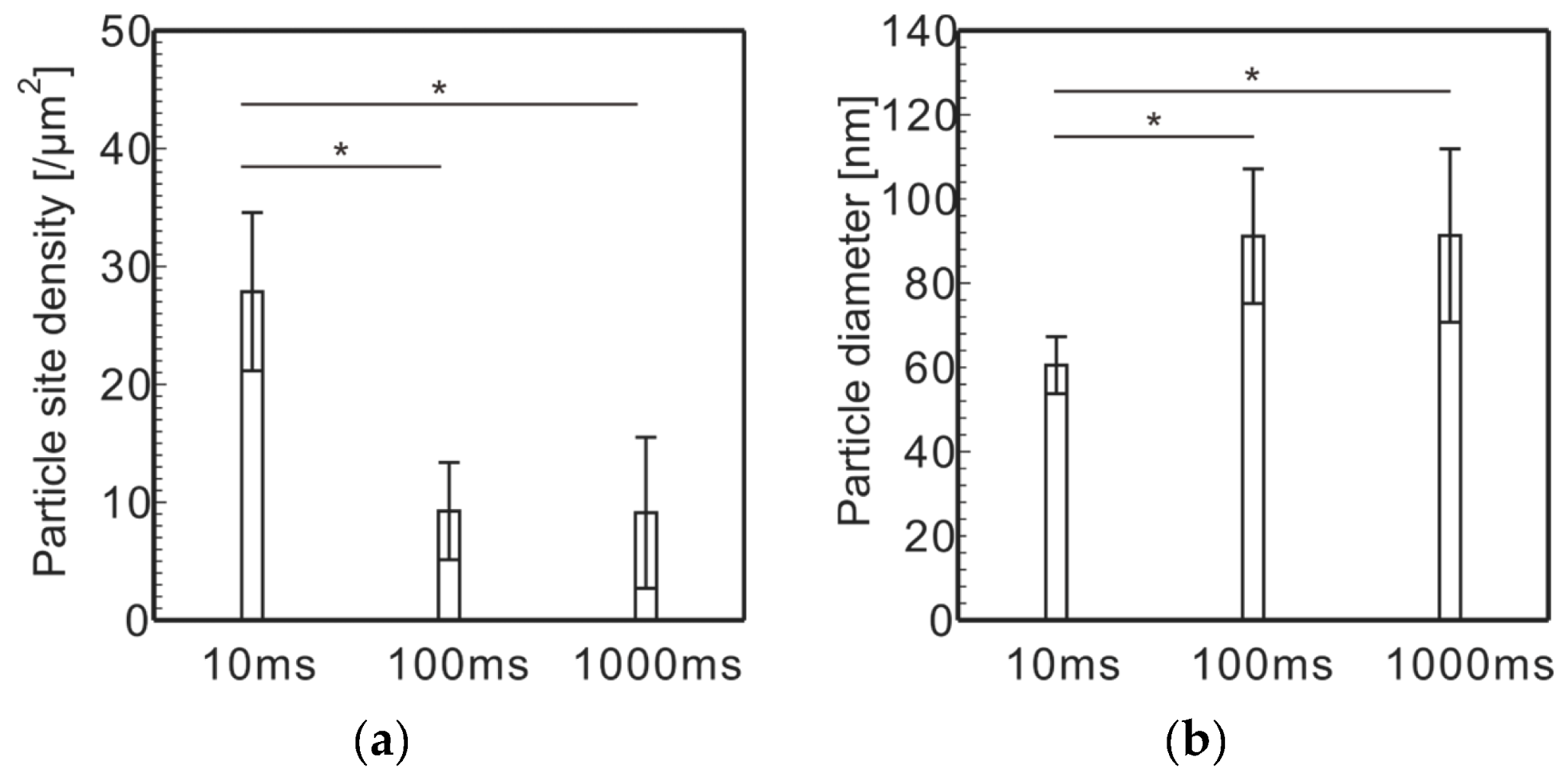
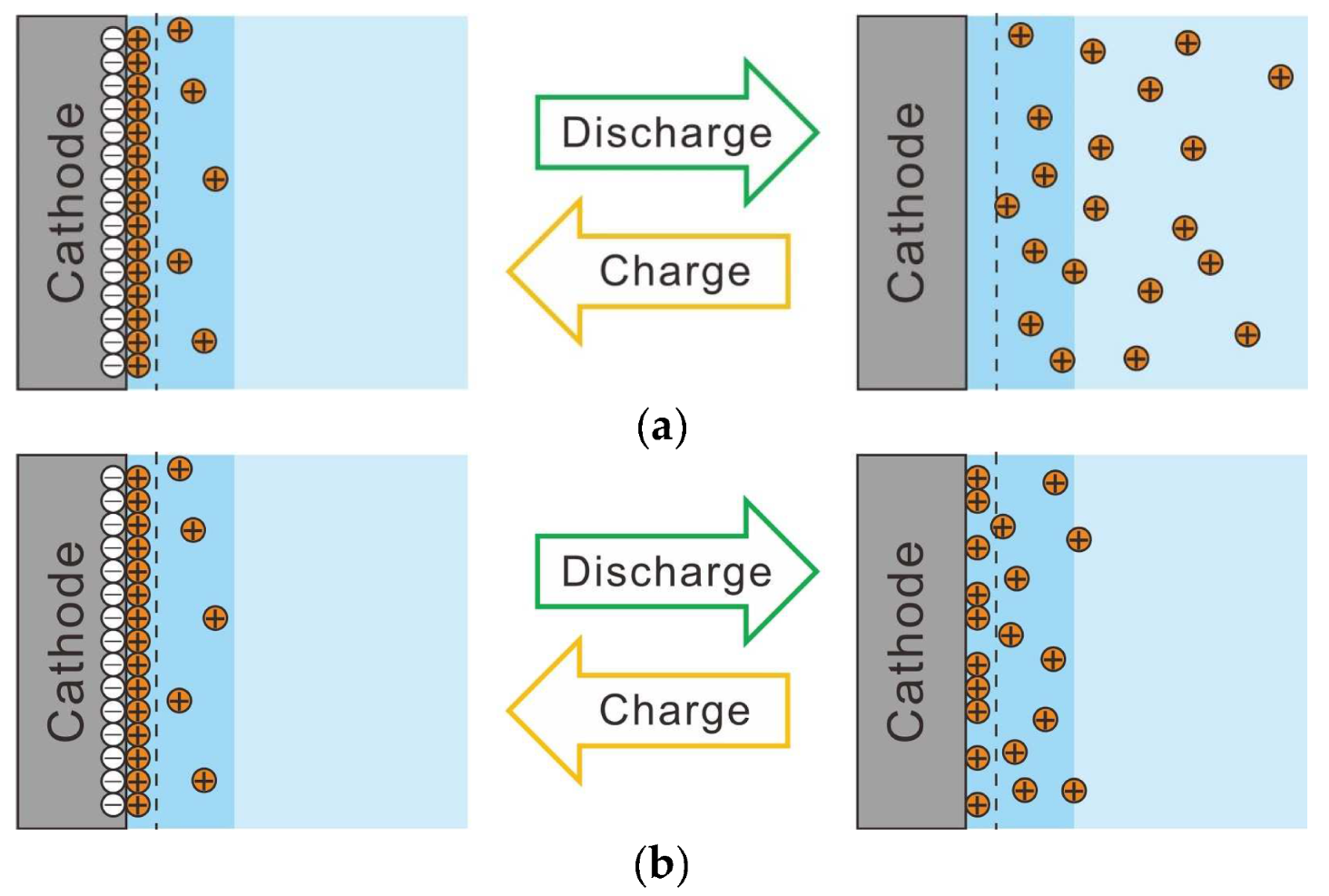
- Electrolytically plating Ni onto carbon fibers via the PC after the removal of the sizing agent attached to the surface of carbon fiber enabled Ni particles with sparser site densities and larger diameters to be plated rather than those plated via a direct current.
- To electrolytically plate Ni with PC, it is necessary to shorten the plating time per pulse cycle to about 10 ms and lengthen the off time per pulse cycle to about 100 ms in order to plate Ni particles with sparse site densities and large diameters.
- Using Ni particles with sparse site densities, CNTs with sparse site densities can be grafted.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Das, R.; Shahnavaz, Z.; Ali, M.E.; Islam, M.M.; Abd Hamid, S.B. Can We Optimize Arc Discharge and Laser Ablation for Well-Controlled Carbon Nanotube Synthesis? Nanoscale Res. Lett. 2016, 11, 510. [Google Scholar] [CrossRef] [Green Version]
- Termine, S.; Trompeta, A.-F.A.; Dragatogiannis, D.A.; Charitidis, C.A. Novel CNTs grafting on carbon fibres through CVD: Investigation of epoxy matrix/fibre interface via nanoindentation. MATEC Web Conf. 2019, 304, 8. [Google Scholar] [CrossRef]
- Pozegic, T.R.; Jayawardena, K.D.G.I.; Chen, J.-S.; Anguita, J.V.; Ballocchi, P.; Stolojan, V.; Silva, S.R.P.; Hamerton, I. Development of sizing-free multi-functional carbon fiber nanocomposites. Compos. Part A Appl. Sci. Manuf. 2016, 90, 306–319. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Xu, H.; Liu, C.; Mi, L.; Shen, C. The effect of double grafted interface layer on the properties of carbon fiber reinforced polyamide 66 composites. Compos. Sci. Technol. 2018, 168, 20–27. [Google Scholar] [CrossRef]
- Naito, K. Development of high-performance and multi-functional carbon fibers. JSPP 2012, 24, 127–134. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Su, S. Optimization of Process Conditions for Continuous Growth of CNTs on the Surface of Carbon Fibers. J. Compos. Sci. 2021, 5, 111. [Google Scholar] [CrossRef]
- Badakhsh, A.; An, K.-H.; Kim, B.-J. Enhanced Surface Energetics of CNT-Grafted Carbon Fibers for Superior Electrical and Mechanical Properties in CFRPs. Polymers 2020, 12, 1432. [Google Scholar] [CrossRef]
- Lee, G.; Ko, K.D.; Yu, Y.C.; Lee, J.; Yu, W.-R.; Youk, J.H. A facile method for preparing CNT-grafted carbon fibers and improved tensile strength of their composites. Compos. Part A Appl. Sci. Manuf. 2015, 69, 132–1389. [Google Scholar] [CrossRef]
- Naito, K.; Nagai, C. Effect of carbon nanotube surface modification on tensile properties of carbon fiber epoxy impregnated bundle composites. Polym. Polym. Compos. 2022, 10, 1176–1179. [Google Scholar] [CrossRef]
- Komissarov, I.; Shaman, Y.; Fedotova, J.; Shulitski, B.; Zavadsky, S.; Kasiuk, J.; Karoza, A.; Pyatlitski, A.; Zhigulin, D.; Aleshkevych, P.; et al. Structural and magnetic investigation of single wall carbon nanotube films with iron based nanoparticles inclusions synthesized by CVD technique from ferrocene/ethanol solution. Phys. Status Solidi C 2013, 10, 1176–1179. [Google Scholar] [CrossRef]
- Li, W.Z.; Wang, D.Z.; Yang, S.X.; Wen, J.G.; Ren, Z.F. Controlled growth of carbon nanotubes on graphite foil by chemical vapor deposition. Chem. Phys. Lett. 2001, 335, 141–149. [Google Scholar] [CrossRef]
- Zhu, S.; Su, C.-H.; Lehoczky, S.L.; Muntele, I.; Ila, D. Carbon nanotube growth on carbon fibers. Diam. Relat. Mater. 2003, 12, 1825–1828. [Google Scholar] [CrossRef]
- Tanaka, K.; Okuda, S.; Hinoue, Y.; Katayama, T. Effects of Water Absorption on Fiber Matrix Interfacial Shear Strength of Carbon Nanotube Grafted Carbon Fiber Reinforced Polyamide Resin. J. Compos. Sci. 2019, 3, 4. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, K.; Nishikawa, T.; Aoto, K.; Katayama, T. Effect of Carbon Nanotube Deposition Time to the Surface of Carbon Fibres on Flexural Strength of Resistance Welded Carbon Fibre Reinforced Thermoplastics Using Carbon Nanotube Grafted Carbon Fibre as Heating Element. J. Compos. Sci. 2019, 3, 9. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, K.; Habe, R.; Tanaka, M.; Katayama, T. Carbon Fiber Reinforced Thermoplastics Molding by Using Direct Resistance Heating to Carbon Nanofilaments Grafted Carbon Fiber. J. Compos. Sci. 2019, 3, 14. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, K.; Yamada, K.; Hinoue, Y.; Katayama, T. Influence of unsizing and carbon nanotube grafting of carbon fibre on fibre matrix interfacial shear strength of carbon fibre and polyamide 6. Key Eng. Mater. 2020, 827, 178–183. [Google Scholar] [CrossRef]
- Lee, G.; Sung, M.; Youk, J.H.; Lee, J.; Yu, W.R. Improved tensile strength of carbon nanotube-grafted carbon fiber reinforced composites. Compos. Struct. 2019, 220, 580–591. [Google Scholar] [CrossRef]
- Xiao, C.; Tan, Y.; Wang, X.; Gao, L.; Wang, L.; Qi, Z. Study on interfacial and mechanical improvement of carbon fiber/epoxy composites by depositing multi-walled carbon nanotubes on fibers. Chem. Phys. Lett. 2018, 703, 8–16. [Google Scholar] [CrossRef]
- Qian, H.; Bismarck, A.; Greenhalgh, E.S.; Kalinka, G.; Shaffer, M.S.P. Hierarchical composites reinforced with carbon nanotube grafted fibers: The potential assessed at the single fiber level. Chem. Mater. 2008, 20, 1862–1869. [Google Scholar] [CrossRef]
- Yao, Z.; Wang, C.; Qin, J.; Su, S.; Wang, Y.; Wang, Q.; Yu, M. Interfacial improvement of carbon fiber/epoxy composites using one-step method for grafting carbon nanotubes on the fibers at ultra-low temperatures. Carbon 2020, 164, 133–142. [Google Scholar] [CrossRef]
- Lv, P.; Feng, Y.; Zhang, P.; Chen, H.; Zhao, N.; Feng, W. Increasing the interfacial strength in carbon fiber/epoxy composites by controlling the orientation and length of carbon nanotubes grown on the fibers. Carbon 2011, 49, 4665–4673. [Google Scholar] [CrossRef]
- Tanaka, K.; Takenaka, T.; Katayama, T. Effect of grafted CNT on carbon fibers on impregnation properties of carbon fiber reinforced polyamide 6. J. Soc. Mater. Sci. 2020, 70, 670–677. [Google Scholar] [CrossRef]
- Romanov, V.S.; Lomov, S.V.; Verpoest, I.; Gorbatikh, L. Modelling evidence of stress concentration mitigation at the micro-scale in polymer composites by the addition of carbon nanotubes. Carbon 2015, 82, 184–194. [Google Scholar] [CrossRef]
- Zhang, L.; Greef, N.D.; Kalinka, G.; Bilzen, B.V.; Locquet, J.-P.; Verpoest, I.; Seo, J.W. Carbon nanotube-grafted carbon fiber polymer composites: Damage characterization on the micro-scale. Compos. Part B-Eng. 2017, 126, 202–210. [Google Scholar] [CrossRef]
- Jia, Y.; Chen, Z.; Yan, W. A numerical study on carbon nanotube–hybridized carbon fibre pullout. Compos. Sci. Technol. 2014, 91, 38–44. [Google Scholar] [CrossRef]
- Tu, Y.; Huang, Z.P.; Wang, D.Z.; Wen, J.G.; Ren, Z.F. Growth of aligned carbon nanotubes with controlled site density. Appl. Phys. Lett. 2002, 80, 4018–4020. [Google Scholar] [CrossRef]
- Cheung, C.L.; Kurtz, A.; Park, H.; Lieber, C.M. Diameter-controlled synthesis of carbon nanotubes. J. Phys. Chem. B 2002, 106, 2429–2433. [Google Scholar] [CrossRef]
- Gakis, G.P.; Termine, S.; Trompeta, A.-F.A.; Aviziotis, I.G.; Charitidis, C.A. Unraveling the mechanisms of carbon nanotube growth by chemical vapor deposition. J. Chem. Eng. 2022, 445, 1. [Google Scholar] [CrossRef]
- Kato, T.; Hatakeyama, R. Growth of Single-Walled Carbon Nanotubes by Plasma CVD. J. Nanotechnol. 2010, 2010, 256906. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, K.; Okumura, Y.; Katayama, T. Effect of carbon nanotubes deposition form on carbon fiber and polyamide resin interfacial strength. J. Soc. Mater. Sci. 2016, 65, 586–591. [Google Scholar] [CrossRef] [Green Version]
- Natter, H.; Hempelmann, R. Nanocrystalline copper by pulsed electrodeposition: The effects of organic additives, bath temperature, and pH. J. Phys. Chem. 1996, 100, 19525–19532. [Google Scholar] [CrossRef]
- Puippe, J.-C.; SA, S.G. Qualitative Approach to Pulse Plating. NASF Surf. Technol. White Pap. 2021, 85, 6–14. [Google Scholar]
- Lan, L.T.; Ohno, I.I.; Haruyama, S. Parusu mekki ni okeru kakuhassei oyobi seichou. (Generation and growth of nuclei in pulse current plating). Denki Kagaku Oyobi Kogyo Butsuri Kagaku 1983, 51, 167–168. (In Japanese) [Google Scholar] [CrossRef]
- Tanaka, K.; Okuda, S.; Katayama, T. Effect of Air Oxidation of Carbon Fiber on Interfacial Shear Strength of Carbon Fiber Reinforced Thermoplastics. J. Soc. Mater. Sci. 2020, 69, 358–364. [Google Scholar] [CrossRef]
- Ogata, M. Some problems in pulsed electrodeposition. J. Met. Finish. Soc. Jpn. 1988, 39, 180–184. [Google Scholar] [CrossRef]
- En’yo, M. Recent research on hydrogen overvoltage. Catalysis 1956, 13, 155–177. [Google Scholar]
- Shimao, K. Basic principles of electrophoresis. Seibutsu Butsuri Kagaku 1997, 41, 1–11. [Google Scholar] [CrossRef]







| Current (mA) | ON-Time (ms) | OFF-Time (ms) | Actual Total Plating Time (s) |
|---|---|---|---|
| 100 | 10 | 10 | 5 |
| 200 | 50 | 100 | 10 |
| 300 | 100 | 1000 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, K.; Kyoyama, S. The Effect of Pulse Current on Electrolytically Plating Nickel as a Catalyst for Grafting Carbon Nanotubes onto Carbon Fibers via the Chemical Vapor Deposition Method. J. Compos. Sci. 2023, 7, 88. https://doi.org/10.3390/jcs7020088
Tanaka K, Kyoyama S. The Effect of Pulse Current on Electrolytically Plating Nickel as a Catalyst for Grafting Carbon Nanotubes onto Carbon Fibers via the Chemical Vapor Deposition Method. Journal of Composites Science. 2023; 7(2):88. https://doi.org/10.3390/jcs7020088
Chicago/Turabian StyleTanaka, Kazuto, and Shuhei Kyoyama. 2023. "The Effect of Pulse Current on Electrolytically Plating Nickel as a Catalyst for Grafting Carbon Nanotubes onto Carbon Fibers via the Chemical Vapor Deposition Method" Journal of Composites Science 7, no. 2: 88. https://doi.org/10.3390/jcs7020088




