Biodegradation of Aqueous Superabsorbents: Kinetic Assessment Using Biological Oxygen Demand Analysis
Abstract
:1. Introduction
- ⚬
- development of the kinetic theory of BOD analysis for assessing the biodegradability of gel-forming organic materials;
- ⚬
- obtaining kinetic BOD curves for the studied hydrogels in long-term (60–120 days) incubation experiments based on the VELP BOD analytical equipment;
- ⚬
- mathematical processing of BOD curves for calculating the half-life indicators of the studied aqueous superabsorbents;
- ⚬
- comparative analysis of the obtained results and controlling factors (composition, biocidal additives, incubation conditions) of biodegradability for gel-forming soil conditioners.
2. Materials and Methods
2.1. Tested Composite Gel-Forming Materials
2.2. BOD Analysis Method and VELP Equipment
3. Results
3.1. Kinetic Theory of BOD Analysis for Assessing the Biodegradability of Gel-Forming Organic Materials
3.2. Experimental Results
3.2.1. Respirometric BOD Curves
3.2.2. BOD Analysis and Evaluation of the Biodegradability of Composites
3.2.3. Half-Life of Acrylic Composite Superabsorbents
4. Discussion
4.1. Analysis of the BOD Kinetic Approach for Assessing the Biodegradation of Hydrogels
4.2. Comparison of the Obtained Experimental Results with Known Data
4.3. Composites with Biocides
4.4. Methodological Aspects of Assessing Biodegradability
5. Conclusions
- ⚬
- A new methodology for quantifying the biodegradability of organic gel-forming materials based on precision VELP equipment for BOD analysis is proposed.
- ⚬
- This methodology uses the original kinetic model of real-time BOD curves obtained in long-term (60–120 days) incubation experiments with automatic manometric monitoring of BOD dynamics.
- ⚬
- The kinetic constants of the long-term dynamics of BOD and biodegradation of the studied organic materials are identical values underlying the calculation of the half-life of these materials.
- ⚬
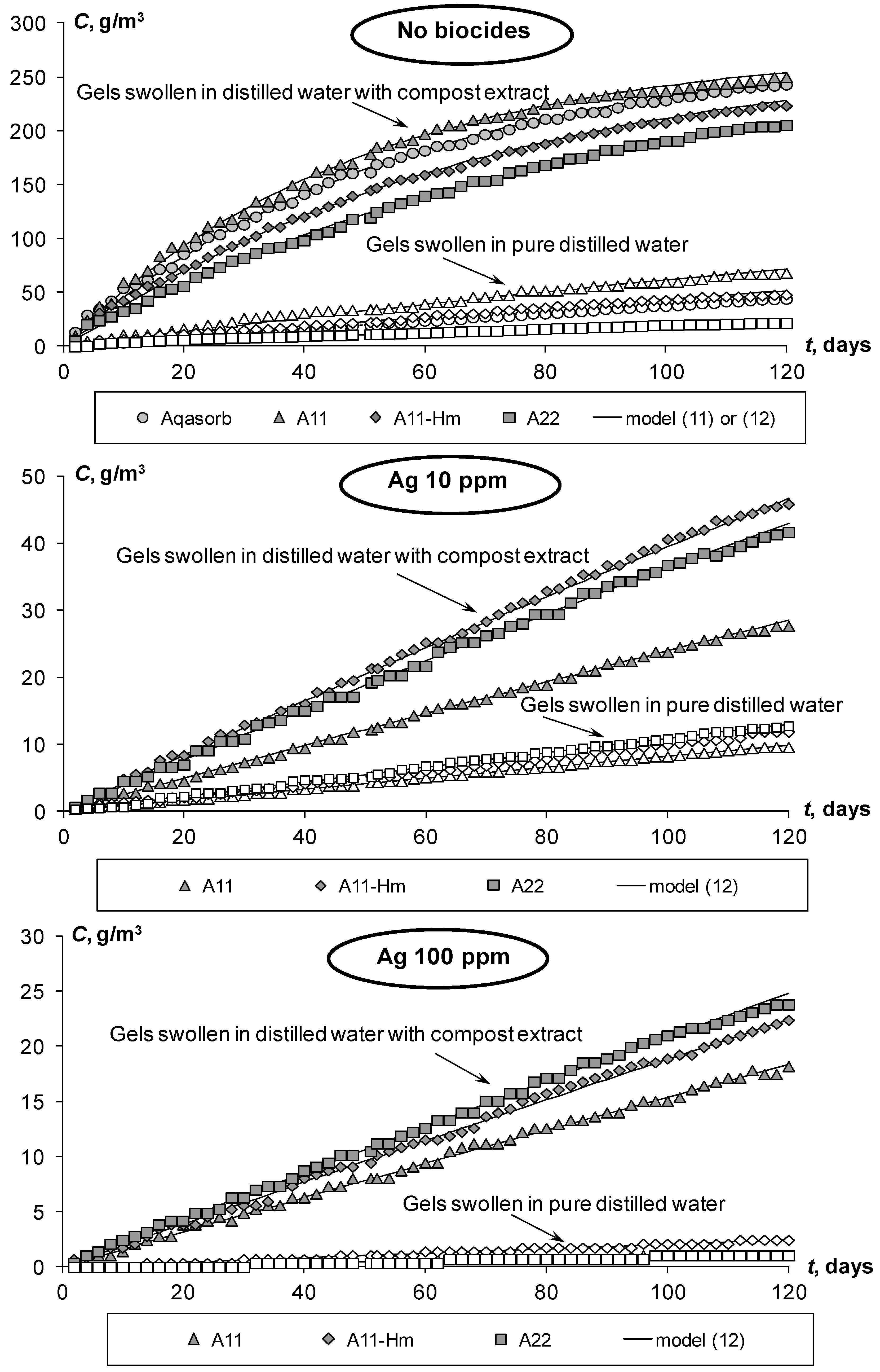
- The experiments revealed a strong contrast between half-life values of the studied aquatic superabsorbents depending on their incubation conditions. Pure hydrogels swollen in distilled water have rather high half-life values, ranging from 0.8 ± 0.2 to 2.4 ± 1.6 years. The addition of a small amount of fresh aqueous extract from organic compost to the liquid phase of the gels sharply (8–15 times) reduces the half-life of these materials up to 33–60 days.
- ⚬
- These results show that acrylic gel-forming superabsorbents of the same chemical composition can, depending on environmental conditions, be either “non-biodegradable” or “biodegradable” according to the European EN 13432 and the American ASTM 6400 biodegradability standards.
- ⚬
- Embedding silver biocides into the polymer matrix of the studied composite superabsorbents effectively (up to 20–35 times) reduces the rate of their biodegradation, which can be used in soil engineering technologies to prolong the service life of these water-saving soil conditioners.
6. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Rosenkranz, F.; Chamy, R. (Eds.) Biodegradation—Life of Science; Pontificial Catholic University of Valparaiso Publ.: Valparaíso, Chile; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef] [Green Version]
- Smagin, A.V.; Sadovnikova, N.B.; Vasenev, V.I.; Smagina, M.V. Biodegradation of Some Organic Materials in Soils and Soil Constructions: Experiments, Modeling and Prevention. Materials 2018, 11, 1889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, P.N.; Parmar, K.G.; Nakum, A.N.; Patel, M.N.; Patel, P.R.; Patel, V.R.; Sen, D.J. Biodegradable Polymers: An Ecofriendly Approach. In: Newer Millenium. Asian J. Biomed. Pharm. Sci. 2011, 1, 23–39. [Google Scholar]
- Certini, G.; Scalenghe, R. (Eds.) Soils: Basic Concepts and Future Challenges; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Dale, T. Total, chemical and biological oxygen consumption of the sediments in Lindåspollence, Western Norway. Mar. Biol. 1978, 49, 333–341. [Google Scholar] [CrossRef]
- Van der Linden, A.M.A.; Boesten, J.J.T.I.; Brock, T.C.M.; Van Eekelen, G.M.A.; Ter Horst, M.M.S.; De Jong, F.M.W.; Montforts, M.H.M.M.; Pol, J.W. Evaluation of the 2006 Proposal for Risk Assessment of Persistence of Plant Protection Products in Soil. RIVM Report 601712002/2008. 2008. Available online: https://www.rivm.nl (accessed on 1 February 2023).
- Adjuik, T.A.; Nokes, S.E.; Montross, M.D. Biodegradability of bio-based and synthetic hydrogels as sustainable soil amendments: A review. J. Appl. Polym. Sci. 2023, 140, e53655. [Google Scholar] [CrossRef]
- Smagin, A.V.; Budnikov, V.I.; Sadovnikova, N.B.; Kirichenko, A.V.; Belyaeva, E.A.; Krivtsova, V.N. Gel-Forming Soil Conditioners of Combined Action: Laboratory Tests for Functionality and Stability. Polymers 2022, 14, 4665. [Google Scholar] [CrossRef] [PubMed]
- Puoci, F.; Iemma, F.; Spizzirri, U.G.; Cirillo, G.; Curcio, M.; Picci, N. Polymer in Agriculture: A Review. Am. J. Agric. Biol. Sci. 2008, 3, 299–314. [Google Scholar] [CrossRef] [Green Version]
- Campos, E.V.R.; de Oliveira, J.L.; Fraceto, L.F.; Singh, B. Polysaccharides as Safer Release Systems for Agrochemicals. Agron. Sustain. Dev. 2015, 35, 47–66. [Google Scholar] [CrossRef]
- Behera, S.; Mahanwar, P.A. Superabsorbent Polymers in Agriculture and Other Applications: A review. Polym. Plast. Technol. Mat. 2020, 59, 341–356. [Google Scholar] [CrossRef]
- Ostrand, M.S.; De Sutter, T.M.; Daigh, A.L.M.; Limb, R.F.; Steele, D.D. Superabsorbent polymer characteristics, properties, and applications. Agrosyst. Geosci. Environ. 2020, 3, e20074. [Google Scholar] [CrossRef]
- Lentz, R.D.; Andrawes, F.F.; Barvenik, F.W.; Koehn, A.C. Acrylamide Monomer Leaching from Polyacrylamide-treated Irrigation Furrows. J. Environ. Qual. 2008, 37, 2293–2298. [Google Scholar] [CrossRef] [Green Version]
- Environment Monograph No. 98. In Detailed Revw Paper on Biodegradability Testing; OCDE/GD(95)43; OECD Publ: Paris, France, 1995.
- ISO/DIS 14851: 2016 (E); Determination of the Ultimate Aerobic Biodegradability of Plastic Materials in an Aqueous Medium—Method by Measuring the Oxygen Demand in a Closed Respiromete; TC/61/SC5. ICS: 83.080.01. ISO Publ: Geneva, Switzerland, 2016.
- Tosin, M.; Weber, M.; Siotto, M.; Lott, C.; Degli Innocenti, F. Laboratory test methods to determine the degradation of plastics in marine environmental conditions. Front. Microbiol. 2012, 3, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smagin, A.V.; Smagina, M.V.; Sadovnikova, N.B. Biological Oxygen Demand in Soils and Litters. Eurasian Soil Sci. 2018, 51, 296–308. [Google Scholar] [CrossRef]
- Baldera-Moreno, Y.; Pino, V.; Farres, A.; Banerjee, A.; Gordillo, F.; Andler, R. Biotechnological Aspects and Mathematical Modeling of the Biodegradation of Plastics under Controlled Conditions. Polymers 2022, 14, 375. [Google Scholar] [CrossRef]
- Khaled, B.; Abdellah, A.; Noureddine, D.; Salim, H.; Sabeha, A. Modelling of biochemical oxygen demand from limited water quality variable by ANFIS using two partition methods. Water Qual. Res. J. 2018, 53, 24–40. [Google Scholar] [CrossRef]
- WTW—Laboratory & Process Instrumentation. Available online: https://www.wtw.com (accessed on 1 February 2023).
- VELP Scientifica. Available online: https://www.velp.com/en-us/respirometric-sensor-system-for-soil-analysis.aspx (accessed on 1 February 2023).
- Smagin, A.V.; Sadovnikova, N.B.; Smagina, M.V. Synthetic Gel Structures in Soils for Sustainable Potato Farming. Sci. Rep. 2019, 9, 18588. [Google Scholar] [CrossRef] [Green Version]
- SNF Water Science. Available online: https://www.snf.com (accessed on 1 February 2023).
- LLC EKOS-1. Available online: https://www.ekos-1.ru (accessed on 1 February 2023).
- Smagin, A.V.; Sadovnikova, N.B.; Belyaeva, E.A. Hygroscopicity of gel-forming composite materials: Thermodynamic assessment and technological significance. J. Compos. Sci. 2022, 6, 269. [Google Scholar] [CrossRef]
- Larson, R.J. Estimation of Biodegradation Potential of Xenobiotic Organic Chemicals. Appl. Environ. Microbiol. 1979, 38, 1153–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiroki, A.; Hong, P.T.T.; Nagasawa, N.; Tamada, M. Biodegradability of Blend Hydrogels Based on Carboxymethyl Cellullose and Carboxymethyl Stach. Trans. Mater. Res. Soc. 2011, 36, 397–400. [Google Scholar] [CrossRef] [Green Version]
- Zohuriaan-Mehr, M.J.; Kabiri, K. Superabsorbent polymer materials: A review. Iran. Polym. J. 2008, 17, 451–477. [Google Scholar]
- Lande, S.S.; Bosch, S.J.; Howard, P.H. Degradation and Leaching of Acrylamide in soil. J. Environ. Qual. 1979, 8, 133–137. [Google Scholar] [CrossRef]
- Abdelmagid, H.M.; Tabatabai, M.A. Decomposition of Acrylamide in Soils. J. Environ. Qual. 1982, 11, 701–704. [Google Scholar] [CrossRef]
- Shanker, R.; Ramakrishna, C.; Seth, P.K. Microbial Degradation of Acrylamide Monomer. Arch. Microb. 1990, 154, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Kay-Shoemake, J.L.; Watwood, M.E.; Sojka, R.E.; Lentz, R.D. Polyacrylamide as a Substrate for Microbial Amidase in Culture and in Soil. Soil Biol. Biochem. 1998, 30, 1647–1654. [Google Scholar] [CrossRef]
- Sojka, R.E.; Entry, J.A. Influence of Polyacrylamide Application to Soil on Movement of Microorganisms in Runoff Water. Environ. Pollut. 2000, 108, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Xiong, B.; Loss, R.D.; Shields, D.; Pawlik, T.; Hochreiter, R.; Zydney, A.L.; Kumar, M. Polyacrylamide degradation and its implications in environmental systems. Clean Water 2018, 1, 17. [Google Scholar] [CrossRef]
- Turioni, C.; Guerrini, G.; Squartini, A.; Morari, F.; Maggini, M.; Gross, S. Biodegradable Hydrogels: Evaluation of Degradation as a Function of Synthesis Parameters and Environmental Conditions. Soil Syst. 2021, 5, 47. [Google Scholar] [CrossRef]
- Fontaine, S.; Mariotti, A.; Abbadie, L. The priming effect of organic matter: A question of microbial competition? Soil Biol. Biochem. 2003, 35, 837–843. [Google Scholar] [CrossRef]
- Cloutier, M.; Mantovani, D.; Rosei, F. Antibacterial Coatings: Challenges, Perspectives, and Opportunities. Trends Biotechnol. 2015, 33, 637–651. [Google Scholar] [CrossRef]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable Cellulose-based Hydrogels: Design and Applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef] [Green Version]
- De Lucca, J.; Boue, S.; Sien, T.; Cleveland, T.E.; Walsh, T.J. Silver Enhances the in Vitro Antifungal Activity of the Saponin, CAY-1. Mycoses 2011, 54, e1–e8. [Google Scholar] [CrossRef]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver Nanoparticles: The Powerful Nanoweapon Against Multidrug-Resistant Bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef]
- Kim, S.W.; Jung, J.H.; Lamsal, K.; Kim, Y.S.; Min, J.S.; Lee, Y.S. Antifungal effect of silver nanoparticles (AgNPs) against various plant pathogenic fungi. Mycobiology 2012, 40, 53–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langdon, K.A.; McLaughlin, M.J.; Kirby, J.K.; Merrington, G. Influence of Soil Properties and Soil Leaching on the Toxicity of Ionic Silver to Plants. Environ. Toxicol. Chem. 2015, 34, 2503–2512. [Google Scholar] [CrossRef] [PubMed]
- Schlich, K.; Klawonn, T.; Terytze, K.; Hund-Rinke, K. Effects of Silver Nanoparticles and Silver Nitrate in the Earthworm Reproduction Test. Environ. Toxicol. Chem. 2013, 32, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Xiao, H.; Zhang, Y. Antimicrobial Polymeric Materials with Quaternary Ammonium and Phosphonium Salts. Int. J. Mol. Sci. 2015, 16, 3626–3655. [Google Scholar] [CrossRef] [Green Version]
- Venkatachalam, D.; Kaliappa, S. Superabsorbent polymers: A state-of-art review on their classification, synthesis, physicochemical properties, and applications. Rev. Chem. Eng. 2021, 39, 1–45. [Google Scholar] [CrossRef]
- PASCO Scientific. Available online: https://www.pasco.com (accessed on 21 February 2023).
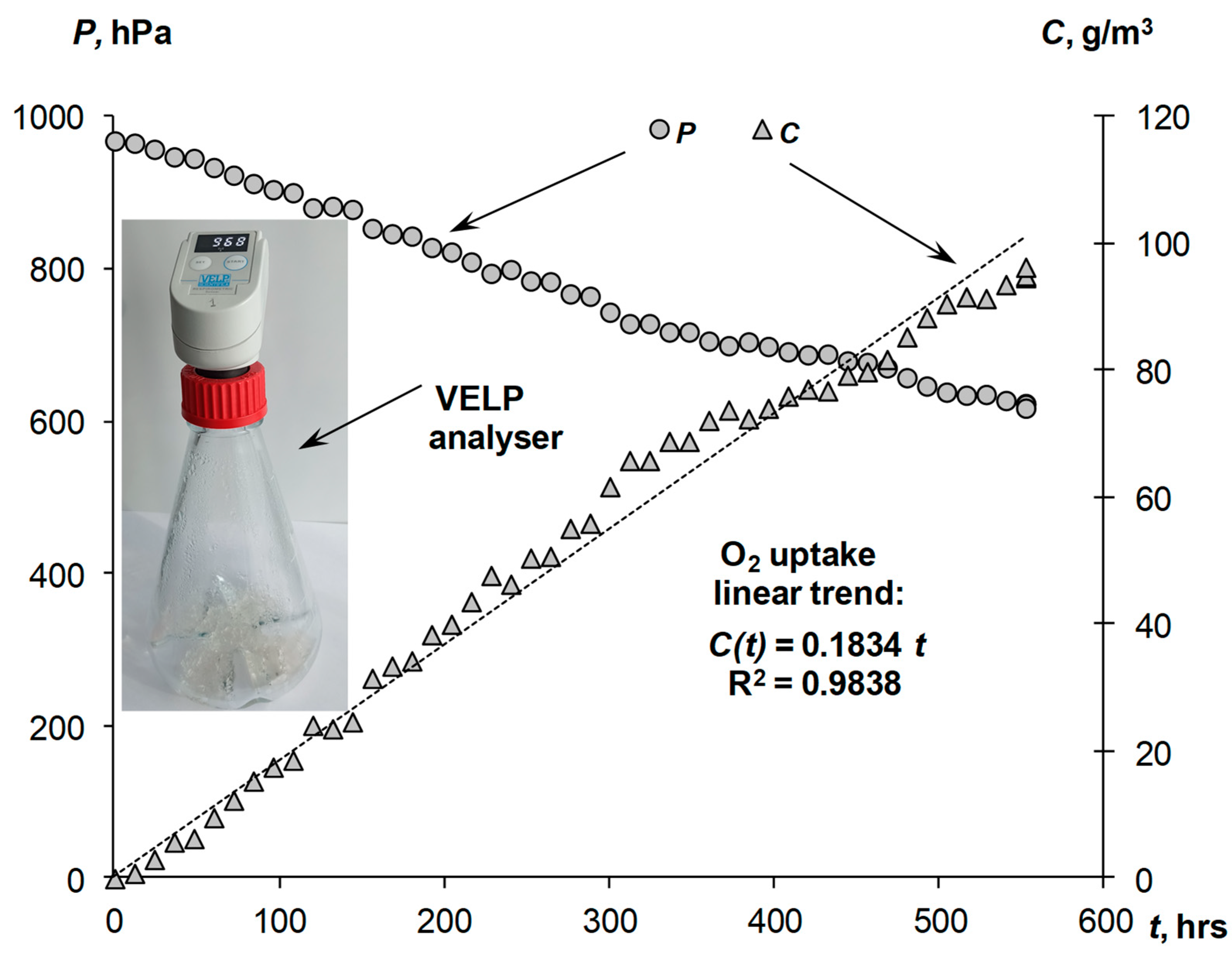

| Liquid Phase | Nonlinear Model (11) | Linear Model (12) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| R2 | s | C0 | 103·b | p-Value | R2 | s | a = C0·b | p-Value | |
| Aquasorb | |||||||||
| Com.ext. * | 0.998 | 2.2 | 278 ± 2 | 17.8 ± 0.3 | 0.0001 | 0.783 | 31.7 | 2.494 ± 0.585 | 0.0001 |
| DW ** | 0.999 | 0.4 | 278 ± 33 | 1.5 ± 0.2 | 0.0001 | 0.996 | 0.8 | 0.385 ± 0.015 | 0.0001 |
| A11 | |||||||||
| Com.ext. | 0.998 | 3.2 | 278 ± 2 | 20.3 ± 0.3 | 0.0001 | 0.716 | 37.6 | 2.631 ± 0.693 | 0.0001 |
| DW | 0.981 | 2.8 | 278 ± 70 | 2.5 ± 0.7 | 0.0008 | 0.962 | 3.9 | 0.629 ± 0.071 | 0.0001 |
| A11-Ag 10 ppm | |||||||||
| Com.ext. | 0.999 | 0.3 | 278 ± 56 | 0.9 ± 0.2 | 0.0001 | 0.998 | 0.4 | 0.239 ± 0.007 | 0.0001 |
| DW | 0.998 | 0.2 | 278 ± 206 | 0.3 ± 0.2 | 0.0008 | 0.998 | 0.1 | 0.083 ± 0.002 | 0.0001 |
| A11-Ag 100 ppm | |||||||||
| Com.ext. | 0.997 | 0.3 | 278 ± 140 | 0.6 ± 0.3 | 0.0583 | 0.996 | 0.3 | 0.156 ± 0.006 | 0.0001 |
| DW | 0.913 | 0.1 | 289 ± 686 | 0.03 ± 2.0 | 0.9874 | 0.913 | 0.1 | 0.009 ± 0.002 | 0.0001 |
| A11-Hm | |||||||||
| Com.ext. | 0.997 | 2.9 | 278 ± 3 | 14.3 ± 0.3 | 0.0001 | 0.851 | 24.7 | 2.245 ± 0.454 | 0.0001 |
| DW | 0.987 | 1.6 | 278 ± 90 | 1.7 ± 0.6 | 0.0061 | 0.943 | 2.1 | 0.441 ± 0.038 | 0.0001 |
| A11-Hm-Ag 10 ppm | |||||||||
| Com.ext. | 0.999 | 0.5 | 278 ± 31 | 1.5 ± 0.2 | 0.0001 | 0.997 | 0.7 | 0.402 ± 0.014 | 0.0001 |
| DW | 0.997 | 0.2 | 278 ± 206 | 0.4 ± 0.3 | 0.1891 | 0.997 | 0.2 | 0.100 ± 0.003 | 0.0001 |
| A11-Hm-Ag 100 ppm | |||||||||
| Com.ext. | 0.998 | 0.3 | 278 ± 96 | 0.7 ± 0.3 | 0.0071 | 0.998 | 0.3 | 0.191 ± 0.006 | 0.0001 |
| DW | 0.989 | 0.1 | 289 ± 353 | 0.1 ± 0.9 | 0.9353 | 0.979 | 0.1 | 0.020 ± 0.002 | 0.0001 |
| A22 | |||||||||
| Com.ext. | 0.998 | 2.3 | 278 ± 3 | 11.6 ± 0.2 | 0.0001 | 0.917 | 17.4 | 2.011 ± 0.321 | 0.0001 |
| DW | 0.952 | 1.4 | 278 ± 375 | 0.8 ± 1.1 | 0.4773 | 0.943 | 1.5 | 0.205 ± 0.027 | 0.0001 |
| A22-Ag 10 ppm | |||||||||
| Com.ext. | 0.998 | 0.5 | 278 ± 44 | 1.4 ± 0.2 | 0.0001 | 0.996 | 0.8 | 0.365 ± 0.015 | 0.0001 |
| DW | 0.998 | 0.2 | 278 ± 184 | 0.4 ± 0.3 | 0.1427 | 0.997 | 0.2 | 0.109 ± 0.004 | 0.0001 |
| A22-Ag 100 ppm | |||||||||
| Com.ext. | 0.998 | 0.53 | 278 ± 79 | 0.8 ± 0.2 | 0.0013 | 0.997 | 0.4 | 0.208 ± 0.007 | 0.0001 |
| DW | 0.905 | 0.1 | 289 ± 683 | 0.03 ± 2.1 | 0.9886 | 0.905 | 0.1 | 0.009 ± 0.002 | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smagin, A.V.; Sadovnikova, N.B.; Budnikov, V.I. Biodegradation of Aqueous Superabsorbents: Kinetic Assessment Using Biological Oxygen Demand Analysis. J. Compos. Sci. 2023, 7, 164. https://doi.org/10.3390/jcs7040164
Smagin AV, Sadovnikova NB, Budnikov VI. Biodegradation of Aqueous Superabsorbents: Kinetic Assessment Using Biological Oxygen Demand Analysis. Journal of Composites Science. 2023; 7(4):164. https://doi.org/10.3390/jcs7040164
Chicago/Turabian StyleSmagin, Andrey V., Nadezhda B. Sadovnikova, and Viktor I. Budnikov. 2023. "Biodegradation of Aqueous Superabsorbents: Kinetic Assessment Using Biological Oxygen Demand Analysis" Journal of Composites Science 7, no. 4: 164. https://doi.org/10.3390/jcs7040164






