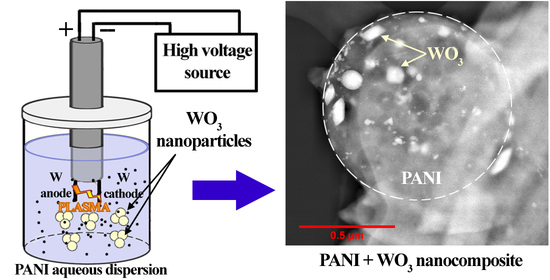
Plasma Synthesis and Characterization of PANI + WO3 Nanocomposites and their Supercapacitor Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Methods of Synthesis
2.2. Characterization
2.3. Electrochemical Characterization
3. Results and Discussion
3.1. Evidence of Electrode’s Sputtering
3.2. Characterization of Obtained Materials
3.3. Electrochemical Behavior
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skotheim, T.A. Handbook of Conducting Polymers, 2nd ed.; Marcel, D., Ed.; CRC Press: New York, NY, USA, 1998. [Google Scholar]
- Gurunathan, K.; Amalnerkar, D.P.; Trivedi, D.C. Synthesis and Characterization of Conducting Polymer Composite (PAn/TiO2) for Cathode Material in Rechargeable Battery. Mater. Lett. 2003, 57, 1642–1648. [Google Scholar] [CrossRef]
- MacDiarmid, A.; Yang, L.; Huang, W.; Humphrey, B. Polyaniline: Electrochemistry and Application to Rechargeable Batteries. Synth. Met. 1987, 18, 393–398. [Google Scholar] [CrossRef]
- Mizumoto, M.; Namba, M.; Nishimura, S.; Miyadera, H.; Koseki, M.; Kobayashi, Y. Polyaniline as an Electrode of Rechargeable Battery. Synth. Met. 1989, 28, 639–646. [Google Scholar] [CrossRef]
- Patanè, S.; Triolo, C.; Cardiano, P.; Lo Schiavo, S. Capacitive Properties of the Hydrophobic [2-(Methacryloyloxy)Ethyl]-Trimethyl Ammonium Nonafluoro-1-Butanesulfonate Poly (Ionic Liquid) Thin Film. Ionics 2017, 23, 1481–1487. [Google Scholar] [CrossRef]
- Sonawane, J.M.; Patil, S.A.; Ghosh, P.C.; Adeloju, S.B. Low-Cost Stainless-Steel Wool Anodes Modified with Polyaniline and Polypyrrole for High-Performance Microbial Fuel Cells. J. Power Sources 2018, 379, 103–114. [Google Scholar] [CrossRef]
- Wang, H.; Lin, J.; Shen, Z.X. Polyaniline (PANi) Based Electrode Materials for Energy Storage and Conversion. J. Sci. Adv. Mater. Devices 2016, 1, 225–255. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, C.; Wang, F.; Gu, Y.; Zhang, P.; Waclawik, E.R.; Du, A.; Ostrikov, K.; Wang, H. A zinc bromine “supercapattery” system combining triple functions of capacitive, pseudocapacitive and battery-type charge storage. Mater. Horiz. 2020, 7, 495–503. [Google Scholar] [CrossRef]
- Li, X.; Chen, D.; Xu, D.; Zhao, C.; Wang, Z.; Lu, H.; Na, H. SPEEKK/Polyaniline (PANI) Composite Membranes for Direct Methanol Fuel Cell Usages. J. MembR. Sci. 2006, 275, 134–140. [Google Scholar] [CrossRef]
- Yang, C.; Chen, C. Synthesis, Characterisation and Properties of Polyanilines Containing Transition Metal Ions. Synth. Met. 2005, 153, 133–136. [Google Scholar] [CrossRef]
- Anbarasan, R.; Ponprapakaran, K.; Harihara Subramani, R.; Baskaran, R.; Tung, K.-L. Synthesis, Characterization and Catalytic Activity of Copolymer/Metal Oxide Nanocomposites. Polym. Bull. 2019, 76, 4117–4138. [Google Scholar] [CrossRef]
- Bekhoukh, A.; Moulefera, I.; Sabantina, L.; Benyoucef, A. Development, Investigation, and Comparative Study of the Effects of Various Metal Oxides on Optical Electrochemical Properties Using a Doped PANI Matrix. Polymers 2021, 13, 3344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wan, M. Nanostructures of Polyaniline Composites Containing Nano-Magnet. Synth. Met. 2003, 132, 205–212. [Google Scholar] [CrossRef]
- He, Y. A Novel Emulsion Route to Sub-Micrometer Polyaniline/Nano-ZnO Composite Fibers. Appl. Surf. Sci. 2005, 249, 1–6. [Google Scholar] [CrossRef]
- Wang, S.; Tan, Z.; Li, Y.; Sun, L.; Zhang, T. Synthesis, Characterization and Thermal Analysis of Polyaniline/ZrO2 Composites. Thermochim. Acta 2006, 441, 191–194. [Google Scholar] [CrossRef]
- Cheng, Q.; Fang, Z.; Yi, X.-S.; An, X.; Tang, B.; Xu, Y. “Ex Situ” Concept for Toughening the RTMable BMI Matrix Composites, Part I: Improving the Interlaminar Fracture Toughness. J. Appl. Polym. Sci. 2008, 109, 1625–1634. [Google Scholar] [CrossRef]
- Benykhlef, S.; Bekhoukh, A.; Berenguer, R.; Benyoucef, A.; Morallon, E. PANI-Derived Polymer/Al2O3 Nanocomposites: Synthesis, Characterization, and Electrochemical Studies. Colloid Polym. Sci. 2016, 294, 1877–1885. [Google Scholar] [CrossRef]
- Rahim, A.; Teknologi, U.; Ahmad, M.; Noor, M.; Bouzid, B.; Sapienza, L.; Carvalho, I.; Shivaji Mahavidyalaya, S.; Dickert, I.; de la Rosa, G.; et al. Synthesis of WO3-Polyaniline Composites and Their Gas Properties. Sens. Transducers J. 2010, 113, 82–94. [Google Scholar]
- Dakshayini, B.S.; Reddy, K.R.; Mishra, A.; Shetti, N.P.; Malode, S.J.; Basu, S.; Naveen, S.; Raghu, A.V. Role of Conducting Polymer and Metal Oxide-Based Hybrids for Applications in Ampereometric Sensors and Biosensors. Microchem. J. 2019, 147, 7–24. [Google Scholar] [CrossRef]
- Chidembo, A.T.; Aboutalebi, S.H.; Konstantinov, K.; Wexler, D.; Liu, H.K.; Dou, S.X. Liquid Crystalline Dispersions of Graphene-Oxide-Based Hybrids: A Practical Approach towards the Next Generation of 3D Isotropic Architectures for Energy Storage Applications. Part. Part. Syst. Charact. 2014, 31, 465–473. [Google Scholar] [CrossRef]
- Wang, H.; Ma, G.; Tong, Y.; Yang, Z. Biomass Carbon/Polyaniline Composite and WO3 Nanowire-Based Asymmetric Supercapacitor with Superior Performance. Ionics 2018, 24, 3123–3131. [Google Scholar] [CrossRef]
- Yuan, C.; Lin, H.; Lu, H.; Xing, E.; Zhang, Y.; Xie, B. Anodic Deposition and Capacitive Property of Nano-WO3·H2O/MnO2 Composite as Supercapacitor Electrode Material. Mater. Lett. 2015, 148, 167–170. [Google Scholar] [CrossRef]
- Yao, S.; Zheng, X.; Zhang, X.; Xiao, H.; Qu, F.; Wu, X. Facile Synthesis of Flexible WO3 Nanofibers as Supercapacitor Electrodes. Mater. Lett. 2017, 186, 94–97. [Google Scholar] [CrossRef]
- Wang, S.-H.; Shen, C.-Y.; Su, J.-M.; Chang, S.-W. A Room Temperature Nitric Oxide Gas Sensor Based on a Copper-Ion-Doped Polyaniline/Tungsten Oxide Nanocomposite. Sensors 2015, 15, 7084–7095. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, R.; Durucan, C.; Unalan, H.E. Ternary Nanocomposite SWNT/WO3/PANI Thin Film Electrodes for Supercapacitors. J. Alloy Compd. 2016, 658, 183–189. [Google Scholar] [CrossRef]
- Kumar, R.; Yadav, B.C. Fabrication of Polyaniline (PANI)—Tungsten Oxide (WO3) Composite for Humidity Sensing Application. J. Inorg. Organomet. Polym. Mater. 2016, 26, 1421–1427. [Google Scholar] [CrossRef]
- Wei, H.; Yan, X.; Wu, S.; Luo, Z.; Wei, S.; Guo, Z. Electropolymerized Polyaniline Stabilized Tungsten Oxide Nanocomposite Films: Electrochromic Behavior and Electrochemical Energy Storage. J. Phys. Chem. C 2012, 116, 25052–25064. [Google Scholar] [CrossRef]
- Janáky, C.; de Tacconi, N.R.; Chanmanee, W.; Rajeshwar, K. Electrodeposited Polyaniline in a Nanoporous WO3 Matrix: An Organic/Inorganic Hybrid Exhibiting Both p- and n-Type Photoelectrochemical Activity. J. Phys. Chem. C 2012, 116, 4234–4242. [Google Scholar] [CrossRef]
- Samu, G.F.; Pencz, K.; Janáky, C.; Rajeshwar, K. On the Electrochemical Synthesis and Charge Storage Properties of WO3/Polyaniline Hybrid Nanostructures. J. Solid State Electrochem. 2015, 19, 2741–2751. [Google Scholar] [CrossRef]
- Song, X.; Yan, L.; Dai, C.; Hao, C.; Guo, H.; Xie, M.; Zhang, Y. Preparation of complementary electrochromic devices with WO3/PANI and NiO/PB double-hybrid electrodes. J. Mater. Sci. Mater. Electron. 2022, 33, 8292–8304. [Google Scholar] [CrossRef]
- Kadam, A.V.; Patil, S.B. Polyaniline Globules as a Catalyst for WO3 Nanoparticles for Supercapacitor Application. Mater. Res. Express 2018, 5, 085036. [Google Scholar] [CrossRef]
- Asim, N.; Syuhami, M.F.; Badiei, M.; Yarmo, M.A. WO3 Modification by Synthesis of Nanocomposites. APCBEE Procedia 2014, 9, 175–180. [Google Scholar] [CrossRef]
- Khlyustova, A.; Sirotkin, N.; Kraev, A.; Titov, V.; Agafonov, A. Parameters of Underwater Plasma as a Factor Determining the Structure of Oxides (Al, Cu, and Fe). Materialia 2021, 16, 101081. [Google Scholar] [CrossRef]
- Khlyustova, A.V.; Sirotkin, N.A.; Kraev, A.S.; Titov, V.A.; Agafonov, A.V. Synthesis and Characterization of Titanium Oxide Nanoparticles by Plasma in Contact with Liquid. Plasma Chem. Plasma Process. 2021, 41, 643–657. [Google Scholar] [CrossRef]
- Sirotkin, N.A.; Khlyustova, A.V.; Titov, V.A.; Krayev, A.S.; Nikitin, D.I.; Dmitrieva, O.A.; Agafonov, A.V. Synthesis and Photocatalytic Activity of WO3 Nanoparticles Prepared by Underwater Impulse Discharge. Plasma Chem. Plasma Process. 2020, 40, 571–587. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N.; Verschuren, J.; De Clerck, K.; Kiekens, P.; Leys, C. Non-Thermal Plasma Treatment of Textiles. Surf. Coat. Technol. 2008, 202, 3427–3449. [Google Scholar] [CrossRef]
- Khlyustova, A.; Sirotkin, N.; Kraev, A.; Agafonov, A.; Titov, V. Effect of Metal Oxides Added onto Polyvinyl Alcohol via Pulsed Underwater Plasma on Their Thermal, Electrical and Dielectric Properties. J. Appl. Polym. Sci. 2021, 138, 51174. [Google Scholar] [CrossRef]
- Hontañón, E.; Palomares, J.M.; Stein, M.; Guo, X.; Engeln, R.; Nirschl, H.; Kruis, F.E. The Transition from Spark to Arc Discharge and Its Implications with Respect to Nanoparticle Production. J. Nanopart. Res. 2013, 15, 1957. [Google Scholar] [CrossRef]
- Miron, C.; Bratescu, M.A.; Saito, N.; Takai, O. Time-Resolved Optical Emission Spectroscopy in Water Electrical Discharges. Plasma Chem. Plasma Process. 2010, 30, 619–631. [Google Scholar] [CrossRef]
- Popov, A.; Brasiunas, B.; Mikoliunaite, L.; Bagdziunas, G.; Ramanavicius, A.; Ramanaviciene, A. Comparative Study of Polyaniline (PANI), Poly(3,4-Ethylenedioxythiophene) (PEDOT) and PANI-PEDOT Films Electrochemically Deposited on Transparent Indium Thin Oxide Based Electrodes. Polymers 2019, 172, 133–141. [Google Scholar] [CrossRef]
- Deshmukh, M.A.; Patil, H.K.; Bodkhe, G.A.; Yasuzawa, M.; Koinkar, P.; Ramanaviciene, A.; Shirsat, M.D.; Ramanavicius, A. EDTA-Modified PANI/SWNTs Nanocomposite for Differential Pulse Voltammetry Based Determination of Cu(II) Ions. Sens. Actuators B Chem. 2018, 260, 331–338. [Google Scholar] [CrossRef]
- Wei, Y.; Jang, G.-W.; Hsueh, K.F.; Scherr, E.M.; MacDiarmid, A.G.; Epstein, A.J. Thermal Transitions and Mechanical Properties of Films of Chemically Prepared Polyaniline. Polymer 1992, 33, 314–322. [Google Scholar] [CrossRef]
- Bhandari, S. Polyaniline: Structure and Properties Relationship. In Polyaniline Blends, Composites, and Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2018; pp. 23–60. [Google Scholar] [CrossRef]
- Bava, A.; Gornati, R.; Cappellini, F.; Caldinelli, L.; Pollegioni, L.; Bernardini, G. D-Amino Acid Oxidase–Nanoparticle System: A Potential Novel Approach for Cancer Enzymatic Therapy. Nanomedicine 2013, 8, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- Abdiryim, T.; Xiao-Gang, Z.; Jamal, R. Comparative Studies of Solid-State Synthesized Polyaniline Doped with Inorganic Acids. Mater. Chem. Phys. 2005, 90, 367–372. [Google Scholar] [CrossRef]
- Najafi-Ashtiani, H.; Bahari, A.; Gholipour, S.; Hoseinzadeh, S. Structural, Optical and Electrical Properties of WO3-Ag Nanocomposites for the Electro-Optical Devices. Appl. Phys. A 2018, 124, 24. [Google Scholar] [CrossRef]
- Wang, Y.; Jing, X. Formation of Polyaniline Nanofibers: A Morphological Study. J. Phys. Chem. B 2008, 112, 1157–1162. [Google Scholar] [CrossRef]
- Xu, H.; Chen, X.; Zhang, J.; Wang, J.; Cao, B.; Cui, D. NO2 Gas Sensing with SnO2–ZnO/PANI Composite Thick Film Fabricated from Porous Nanosolid. Sens. Actuators B Chem. 2013, 176, 166–173. [Google Scholar] [CrossRef]
- Halasa, A.F.; Wathen, G.D.; Hsu, W.L.; Matrana, B.A.; Massie, J.M. Relationship between Interchain Spacing of Amorphous Polymers and Blend Miscibility as Determined by Wide-Angle X-ray Scattering. J. Appl. Polym. Sci. 1991, 43, 183–190. [Google Scholar] [CrossRef]
- Hassen, A.; El Sayed, A.M.; Morsi, W.M.; El-Sayed, S. Influence of Cr2O3 Nanoparticles on the Physical Properties of Polyvinyl Alcohol. J. Appl. Phys. 2012, 112, 093525. [Google Scholar] [CrossRef]
- Rahman, S.U.; Röse, P.; Shah, A.U.H.A.; Krewer, U.; Bilal, S.; Farooq, S. Exploring the Functional Properties of Sodium Phytate Doped Polyaniline Nanofibers Modified FTO Electrodes for High-Performance Binder Free Symmetric Supercapacitors. Polymers 2021, 13, 2329. [Google Scholar] [CrossRef]
- Popov, A.; Brasiunas, B.; Damaskaite, A.; Plikusiene, I.; Ramanavicius, A.; Ramanaviciene, A. Electrodeposited Gold Nanostructures for the Enhancement of Electrochromic Properties of PANI–PEDOT Film Deposited on Transparent Electrode. Polymers 2020, 12, 2778. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y.; Dunn, B. Where Do Batteries End and Supercapacitors Begin? Science 2014, 343, 1210–1211. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.U.; Röse, P.; Surati, M.; Shah, A.U.H.A.; Krewer, U.; Bilal, S. 3D Polyaniline Nanofibers Anchored on Carbon Paper for High-Performance and Light-Weight Supercapacitors. Polymers 2020, 12, 2705. [Google Scholar] [CrossRef] [PubMed]
- Noori, A.; El-Kady, M.F.; Rahmanifar, M.S.; Kaner, R.B.; Mousavi, M.F. Towards establishing standard performance metrics for batteries, supercapacitors and beyond. Chem. Soc. Rev. 2019, 48, 1272–1341. [Google Scholar] [CrossRef] [PubMed]








| Sample | υ, g/h | Concentration, wt% | |
|---|---|---|---|
| Anode | Cathode | ||
| PANI + WO3 0.25 A | 0.324 | 0.039 | 3.03 |
| PANI + WO3 0.8 A | 0.546 | 0.067 | 5.11 |
| Wavenumber [cm−1] | Assignments |
|---|---|
| 3473 | N-H stretching vibration |
| 3210 | O-H stretching |
| 2992 | symmetric -CH2- stretching vibration credited to pyranose ring |
| 2855 | N-H vibration |
| 1665 | C=N stretching vibrations of quinoid ring |
| 1583 | C=C stretching vibrations of quinoid ring |
| 1481 | C=C stretching vibrations of benzoid ring |
| 1306 | C–N stretching vibrations of aromatic ring |
| 1249 | C–N stretching vibrations in aromatic primary amine |
| 1125, 1027 | in-plane bending vibrations of aromatic C–H |
| 795 | aromatic C–H out of plane bending |
| 665, 595 | W-O Stretching |
| Sample | DPANI, nm | DWO3, nm | R, nm |
|---|---|---|---|
| PANI | 10.5 | --- | 0.81 |
| PANI + WO3 0.25 A | 9.4 | 30 | 0.79 |
| PANI + WO3 0.8 A | 9.1 | 34 | 0.80 |
| Sample | SBET, m2g−1 | SBJH, m2g−1 | Dp, nm | Vp, cm3g−1 |
|---|---|---|---|---|
| PANI | 15.26 | 8.04 | 2.99 | 0.0181 |
| PANI + WO3 0.25 A | 16.51 | 9.57 | 3.42 | 0.0265 |
| PANI + WO3 0.8 A | 16.74 | 9.73 | 3.81 | 0.0268 |
| Sample | Potential Window (V) | Sweep Rate (V/s) | Specific Capacitance (F/g) |
|---|---|---|---|
| PANI | −0.1–1.0 | 0.1 | 27.45 |
| PANI + WO3 0.25 A | −0.1–1.0 | 0.1 | 83.22 |
| PANI + WO3 0.8 A | −0.1–1.0 | 0.1 | 87.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirotkin, N.; Khlyustova, A. Plasma Synthesis and Characterization of PANI + WO3 Nanocomposites and their Supercapacitor Applications. J. Compos. Sci. 2023, 7, 174. https://doi.org/10.3390/jcs7040174
Sirotkin N, Khlyustova A. Plasma Synthesis and Characterization of PANI + WO3 Nanocomposites and their Supercapacitor Applications. Journal of Composites Science. 2023; 7(4):174. https://doi.org/10.3390/jcs7040174
Chicago/Turabian StyleSirotkin, Nikolay, and Anna Khlyustova. 2023. "Plasma Synthesis and Characterization of PANI + WO3 Nanocomposites and their Supercapacitor Applications" Journal of Composites Science 7, no. 4: 174. https://doi.org/10.3390/jcs7040174