Bio-Based White Eggshell as a Value-Added Filler in Poly(Lactic Acid) Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Eggshell Processing
2.3. Preparation of Composites
2.4. Composite Characterization
2.4.1. Mechanical Testing
2.4.2. Morphological Analysis
2.4.3. Statistical Analysis
2.4.4. Water Absorption
2.4.5. pH Change of PLA Composites
3. Results and Discussion
3.1. Mechanical Properties
3.1.1. Tensile Property
3.1.2. Flexural Property
3.1.3. Impact Property
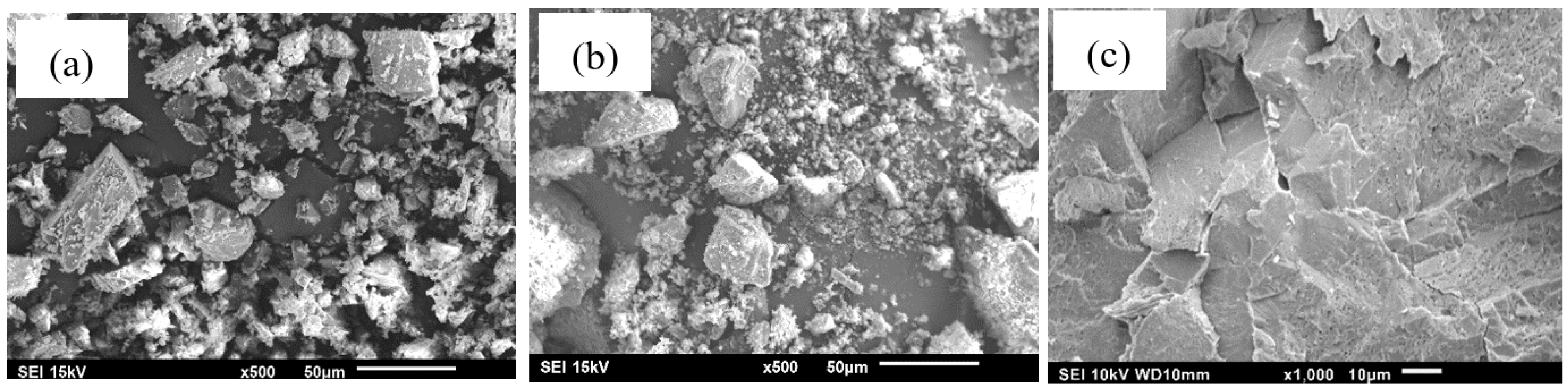
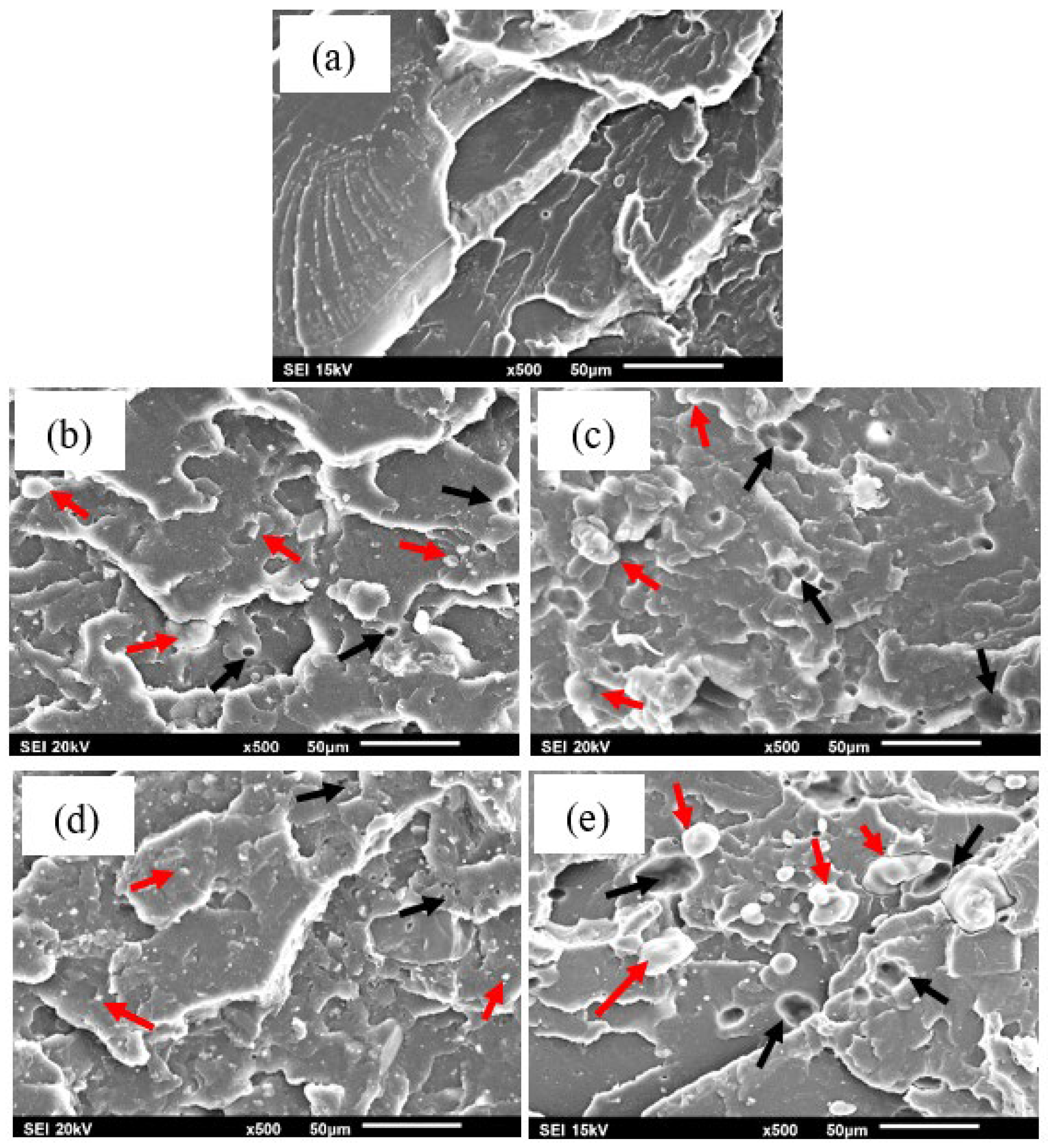
3.1.4. Morphological Analysis
3.1.5. Statistical Analysis
3.1.6. Water Absorption
3.1.7. Weight Loss
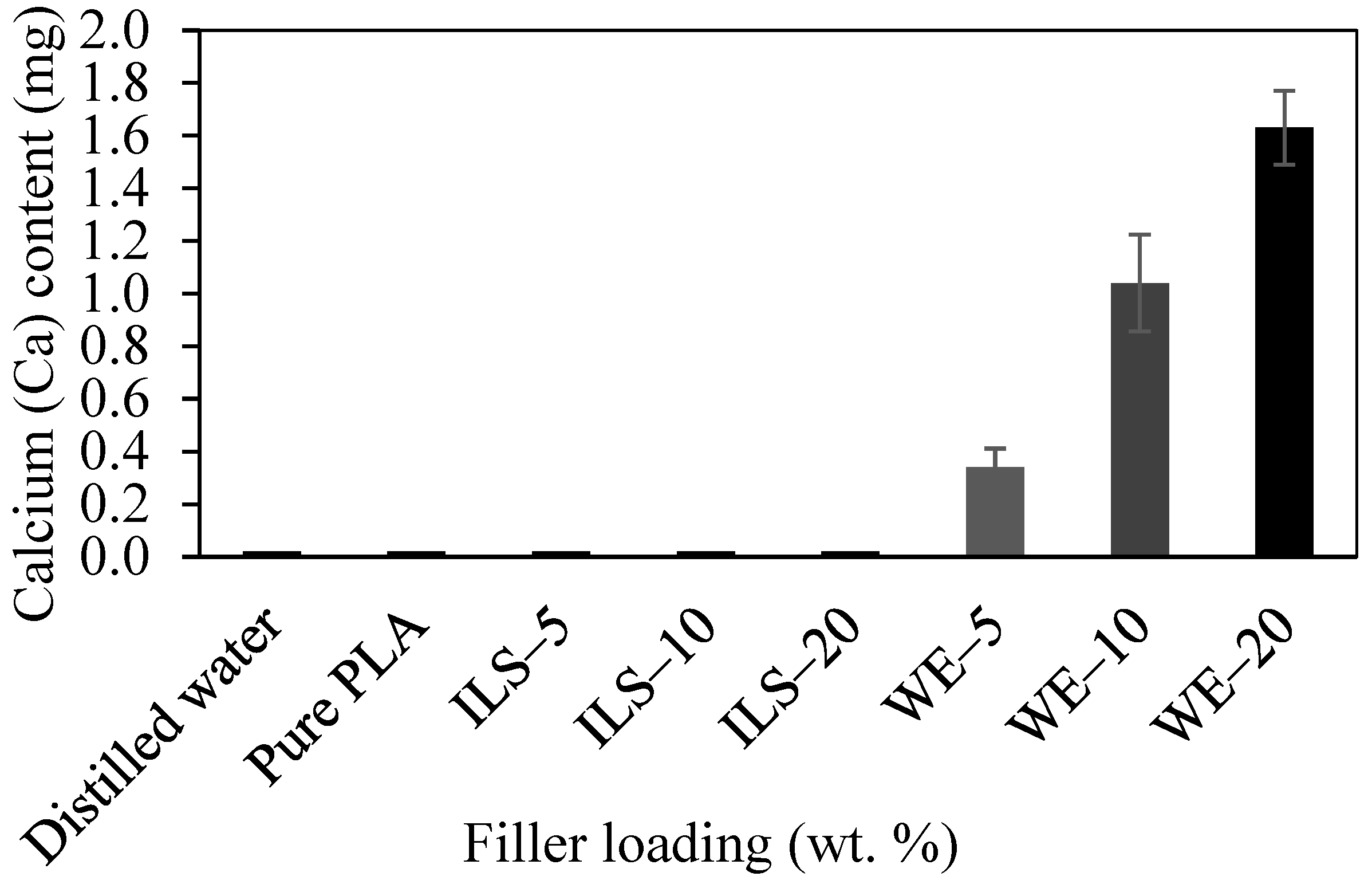
3.1.8. Leaching
3.1.9. pH Change of PLA Composites
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sangeetha, V.H.; Harekrishna, D.; Varghese, T.O.; Nayak, S.K. State of the art and future prospectives of poly (lactic acid) based blends and composites. Polym. Compos. 2018, 39, 81–101. [Google Scholar] [CrossRef]
- da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef]
- Araújo, A.; Oliveira, M.; Oliveira, R.; Botelho, G.; Machado, A.V. Biodegradation assessment of PLA and its nanocomposites. Environ. Sci. Pollut. Res. 2014, 21, 9477–9486. [Google Scholar] [CrossRef] [PubMed]
- Elsawy, M.A.; Kim, K.H.; Park, J.W.; Deep, A. Hydrolytic degradation of polylactic acid (PLA) and its composites. Renew. Sust. Energ. Rev. 2017, 79, 1346–1352. [Google Scholar] [CrossRef]
- Móczó, J.; Pukánszky, B. Polymer micro and nanocomposites: Structure, interactions, properties. J. Ind. Eng. Chem. 2008, 14, 535–563. [Google Scholar] [CrossRef]
- Tanniru, M.; Misra, R.D.K. On enhanced impact strength of calcium carbonate-reinforced high-density polyethylene composites. Mater. Sci. Eng. A 2005, 405, 178–193. [Google Scholar] [CrossRef]
- Bomal, Y.; Godard, P. Melt viscosity of calcium-carbonate-filled low density polyethylene: Influence of matrix-filler and particle-particle interactions. Polym. Eng. Sci. 1996, 36, 237–243. [Google Scholar] [CrossRef]
- Kwon, S.; Kim, K.J.; Kim, H.; Kundu, P.P.; Kim, T.J.; Lee, Y.K.; Lee, B.H.; Choe, S. Tensile property and interfacial dewetting in the calcite filled HDPE, LDPE, and LLDPE composites. Polymer 2002, 43, 6901–6909. [Google Scholar] [CrossRef]
- Misra, R.D.K.; Nerikar, P.; Bertrand, K.; Murphy, D. Some aspects of surface deformation and fracture of 5–20% calcium carbonate-reinforced polyethylene composites. Mater. Sci. Eng. A 2004, 384, 284–298. [Google Scholar] [CrossRef]
- Ji-Zhao, L. Tensile, flow, and thermal properties of CaCO3-filled LDPE/LLDPE composites. J. Appl. Polym. Sci. 2007, 104, 1692–1696. [Google Scholar]
- Nowaczyk, G.; Głowinkowski, S.; Jurga, S. Rheological and NMR studies of polyethylene/calcium carbonate composites. Solid State Nucl. Magn. Reason. 2004, 25, 194–199. [Google Scholar] [CrossRef]
- Osman, M.A.; Atallah, A. Surfactant chain length and tensile properties of calcium carbonate-polyethylene composites. Macromol. Chem. Phys. 2007, 208, 87–93. [Google Scholar] [CrossRef]
- Kao, N.; Chandra, A.; Bhattacharya, S. Melt strength of calcium carbonate filled polypropylene melts. Polym. Int. 2002, 51, 1385–1389. [Google Scholar] [CrossRef]
- Weon, J.I.; Gam, K.T.; Boo, W.J.; Sue, H.J.; Chan, C.M. Impact-toughening mechanisms of calcium carbonate-reinforced polypropylene nanocomposite. J. Appl. Polym. Sci. 2006, 99, 3070–3076. [Google Scholar] [CrossRef]
- Serra-Parareda, F.; Alba, J.; Tarrés, Q.; Espinach, F.X.; Mutjé, P.; Delgado-Aguilar, M. Characterization of CaCO3 filled poly (lactic) acid and bio polyethylene materials for building applications. Polymers 2021, 13, 3323. [Google Scholar] [CrossRef]
- Cree, D.; Rutter, A. Sustainable bio-inspired limestone eggshell powder for potential industrialized applications. ACS Sustain. Chem. Eng. 2015, 3, 941–949. [Google Scholar] [CrossRef]
- ISO 3262-5:1998; Extenders for paints—Specifications and Methods of Test—Part 5: Natural Crystalline Calcium. ISO: Geneva, Switzerland, 1998.
- Statistics Canada. The Daily–Poultry and Egg Statistics, May 2022 and Annual 2021; Statistics Canada: Ottawa, Canada, 2022.
- Cordeiro, C.M.M.; Hincke, M.T. Recent patents on eggshell: Shell and membrane applications. Recent Pat. Food, Nutr. Agric. 2011, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Toro, P.; Quijada, R.; Arias, J.L.; Yazdani-Pedram, M. Mechanical and morphological studies of poly (propylene)-filled eggshell composites. Macromol. Mater. Eng. 2007, 292, 1027–1034. [Google Scholar] [CrossRef]
- Igwe, I.O.; Onuegbu, G.C. Studies on properties of egg shell and fish bone powder filled polypropylene. Am. J. Polym. Sci. 2012, 2, 56–61. [Google Scholar] [CrossRef] [Green Version]
- Iyer, K.A.; Torkelson, J.M. Green composites of polypropylene and eggshell: Effective biofiller size reduction and dispersion by single-step processing with solid-state shear pulverization. Compos. Sci. Technol. 2014, 102, 152–160. [Google Scholar] [CrossRef]
- Villarreal-Lucio, D.S.; Rivera-Armenta, J.L.; Martínez-Hernández, A.L.; Estrada-Moreno, L.A. Effect of eggshell particle size in thermal and thermomechanical properties of PP/eggshell composites. Int. J. Eng. Sci. Res. Technol. 2017, 7, 82–88. [Google Scholar]
- Yao, Z.T.; Chen, T.; Li, H.Y.; Xia, M.S.; Ye, Y.; Zheng, H. Mechanical and thermal properties of polypropylene (PP) composites filled with modified shell waste. J. Hazard. Mater. 2013, 262, 212–217. [Google Scholar]
- Supri, A.G.; Ismail, H.; Shuhadah, S. Effect of polyethylene-grafted maleic anhydride (PE-g-MAH) on properties of low density polyethylene/eggshell powder (LDPE/ESP) composites. Polym. Plast. Technol. Eng. 2010, 49, 347–353. [Google Scholar] [CrossRef]
- Nwanonenyi, S.C.; Chike-Onyegbula, C.O. Water absorption, flammability and mechanical properties of linear low density polyethylene/egg shell composite. Acad. Res. Int. 2013, 4, 352–358. [Google Scholar]
- Hussein, A.A.; Salim, R.D.; Sultan, A.A. Water absorption and mechanical properties of high–density polyethylene/egg shell composite. J. Basra. Res. 2011, 37, 36–42. [Google Scholar]
- Skórczewska, K.; Lewandowski, K.; Szewczykowski, P.; Wilczewski, S.; Szulc, J.; Stopa, P.; Nowakowska, P. Waste eggshells as a natural filler for the poly (vinyl chloride) composites. Polymers 2022, 14, 4372. [Google Scholar] [CrossRef]
- Ashok, B.; Naresh, S.; Reddy, K.O.; Madhukar, K.; Cai, J.; Zhang, L.; Rajulu, A.V. Tensile and thermal properties of poly (lactic acid)/eggshell powder composite films. Int. J. Polym. Anal. Charact. 2014, 19, 245–255. [Google Scholar] [CrossRef]
- Hassan, T.A.; Rangari, V.K.; Jeelani, S. Value-added biopolymer nanocomposites from waste eggshell-based CaCO3 nanoparticles as fillers. ACS Sustain. Chem. Eng. 2014, 2, 706–717. [Google Scholar] [CrossRef]
- Hanumantharaju, H.G.; Prashanth, K.P.; Ramu, B.; Venkatesh, N.; Chethan, G.R. 3D printing of biopolymer composites investigation on effect of eggshell particles on polylactic acid matrix. Biointerface Res. Appl. Chem. 2022, 13, 1–16. [Google Scholar]
- Leclair, J.P.; Borges, N.; Cree, D.; Hof, L.A. Towards circular manufacturing: Repurposing eggshell waste as filler for poly lactic acid feedstock for 3D printing. In Proceedings of the CSME International Congress 2021, Charlottetown, PEI, Canada, 27–30 June 2021; pp. 1–6. [Google Scholar]
- Pliya, P.; Cree, D. Limestone derived eggshell powder as a replacement in Portland cement mortar. Constr. Build. Mater. 2015, 95, 1–9. [Google Scholar] [CrossRef]
- Landon, G.; Lewis, G.; Boden, G.F. The influence of particle size on the tensile strength of particulate-filled polymers. J. Mater. Sci. 1977, 12, 1605–1613. [Google Scholar]
- Tjong, S.C.; Li, R.K.Y.; Cheung, T. Mechanical behavior of CaCO3 particulate-filled β-crystalline phase polypropylene composites. Polym. Eng. Sci. 1997, 37, 166–172. [Google Scholar] [CrossRef]
- Sisakht Mohsen, R.; Saied, N.K.; Ali, Z.; Hosein, E.M.; Hasan, P. Theoretical and experimental determination of tensile properties of nanosized and micron-sized CaCO3/PA66 composites. Polym. Compos. 2009, 30, 274–280. [Google Scholar] [CrossRef]
- Dobrosielska, M.; Dobrucka, R.; Kozera, P.; Brząkalski, D.; Gabriel, E.; Głowacka, J.; Jałbrzykowski, M.; Kurzydłowski, K.J.; Przekop, R.E. Beeswax as a natural alternative to synthetic waxes for fabrication of PLA/diatomaceous earth composites. Sci. Rep. 2023, 13, 1161. [Google Scholar] [CrossRef]
- Riley, A.M.; Paynter, C.D.; McGenity, P.M.; Adams, J.M. Factors affecting the impact properties of mineral filled polypropylene. Plast. Rubber Process Appl. 1990, 14, 85–93. [Google Scholar]
- Owuamanam, S.; Soleimani, M.; Cree, D.E. Fabrication and characterization of bio-epoxy eggshell composites. Appl. Mech. 2021, 2, 694–713. [Google Scholar] [CrossRef]
- Wagner-Amos, K.; Seymour, R.S. Effect of regional changes to shell conductance on oxygen consumption and growth of chicken embryos. Respir. Physiol. 2002, 129, 385–395. [Google Scholar]
- Yew, G.H.; Yusof, A.M.; Ishak, Z.M.; Ishiaku, U.S. Water absorption and enzymatic degradation of poly (lactic acid)/rice starch composites. Polym. Degrad. Stab. 2005, 90, 488–500. [Google Scholar]
- Aworinde, A.K.; Adeosun, S.O.; Oyawale, F.A.; Akinlabi, E.T.; Akinlabi, S.A. Comparative effects of organic and inorganic bio-fillers on the hydrophobicity of polylactic acid. Results Eng. 2020, 5, 100098. [Google Scholar] [CrossRef]
- Ndazi, B.S.; Karlssion, S. Characterization of hydrolytic degradation of polylactic acid/rice hulls composites in water at different temperatures. Express Polym. Lett. 2011, 5, 119–131. [Google Scholar] [CrossRef]
- Al-Shirawi, M.; Karimi, M.; Al-Maamari, R.S. Impact of carbonate surface mineralogy on wettability alteration using stearic acid. J. Pet. Sci. Eng. 2021, 203, 108674. [Google Scholar]
- Akbar, N.A.; Aziz, H.A.; Adlan, M.N. The characteristics of limestone and anthracite coal as filter media in treating pollutants from groundwater. Int. J. Environ. Sci. Dev. 2021, 12, 58–62. [Google Scholar]
- Vajdova, V.; Baud, P.; Wong, T.F. Compaction, dilatancy, and failure in porous carbonate rocks. J. Geophys. Res. Solid Earth 2004, 109, 1–16. [Google Scholar]
- Arzate-Vázquez, I.; Méndez-Méndez, J.V.; Flores-Johnson, E.A.; Nicolás-Bermúdez, J.; Chanona-Pérez, J.J.; Santiago-Cortés, E. Study of the porosity of calcified chicken eggshell using atomic force microscopy and image processing. Micron 2019, 118, 50–57. [Google Scholar] [CrossRef]
- Norazlina, H.; Hadi, A.A.; Qurni, A.U.; Amri, M.; Mashelmie, S.; Kamal, Y. Degradability studies of PLA nanocomposites under controlled water sorption and soil burial conditions. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Proceedings of the International Conference on Innovative Technology, Engineering and Sciences 2018 (iCITES 2018), Universiti Malaysia Pahang, Pekan Campus, Malaysia, 1–2 March 2018; IOP Publishing Ltd.: Bristol, UK, 2018; Volume 342, p. 012025. [Google Scholar]
- Gorrasi, G.; Pantani, R. Hydrolysis and Biodegradation of Poly(lactic acid). In Advances in Polymer Science; Springer LLC: New York, NY, USA, 2018. [Google Scholar]
- Ruivo, M.A.; Pacheco, R.R.; Sebold, M.; Giannini, M. Surface roughness and filler particles characterization of resin-based composites. Microsc. Res. Tech. 2019, 82, 1756–1767. [Google Scholar] [CrossRef]
- Suaduang, N.; Ross, S.; Ross, G.M.; Pratumshat, S.; Mahasaranon, S. Effect of spent coffee grounds filler on the physical and mechanical properties of poly (lactic acid) bio-composite films. Mater. Today: Proc. 2019, 17, 2104–2110. [Google Scholar] [CrossRef]
- Drumright, R.E.; Gruber, P.R.; Henton, D.E. Polylactic acid technology. Adv. Mater. 2000, 12, 1841–1846. [Google Scholar] [CrossRef]
- Höglund, A.; Odelius, K.; Albertsson, A.C. Crucial differences in the hydrolytic degradation between industrial polylactide and laboratory-scale poly (L-lactide). ACS Appl. Mater. Interfaces 2012, 4, 2788–2793. [Google Scholar]
- Huang, Y.; Zhang, C.; Pan, Y.; Zhou, Y.; Jiang, L.; Dan, Y. Effect of NR on the hydrolytic degradation of PLA. Polym. Degrad. Stab. 2013, 98, 943–950. [Google Scholar] [CrossRef]
- Le Duigou, A.; Davies, P.; Baley, C. Seawater ageing of flax/poly (lactic acid) biocomposites. Polym. Degrad. Stab. 2009, 94, 1151–1162. [Google Scholar]
- Berger, S.B.; Palialol, A.R.M.; Cavalli, V.; Giannini, M. Characterization of water sorption, solubility and filler particles of light-cured composite resins. Braz. Dent. J. 2009, 20, 314–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, L.; Zhang, Y. Jointly modified mechanical properties and accelerated hydrolytic degradation of PLA by interface reinforcement of PLA-WF. J. Mech. Behav. Biomed. Mater. 2018, 88, 223–230. [Google Scholar] [PubMed]
- Rodríguez-Navarro, A.B.; Marie, P.; Nys, Y.; Hincke, M.T.; Gautron, J. Amorphous calcium carbonate controls avian eggshell mineralization: A new paradigm for understanding rapid eggshell calcification. J. Struct. Biol. 2015, 3, 291–303. [Google Scholar]
- Li, Y.; Li, Y.; Liu, S.; Tang, Y.; Mo, B.; Liao, H. New zonal structure and transition of the membrane to mammillae in the eggshell of chicken Gallus domesticus. J. Struct. Biol. 2018, 2, 162–169. [Google Scholar]
- Aylward, G.H.; Findlay, T.J.V. SI Chemical Data; John Wiley & Sons: New York, NY, USA, 1974. [Google Scholar]
- Rocca-Smith, J.R.; Chau, N.; Champion, D.; Brachais, C.H.; Marcuzzo, E.; Sensidoni, A.; Piasente, F.; Karbowiak, T.; Debeaufort, F. Effect of the state of water and relative humidity on ageing of PLA films. Food Chem. 2017, 236, 109–119. [Google Scholar] [PubMed]










| Material | ILS or WE (wt.%) | PLA (wt.%) |
|---|---|---|
| Pure PLA | 0 | 100 |
| LS-5 | 5 | 95 |
| LS-10 | 10 | 90 |
| LS-20 | 20 | 80 |
| WE-5 | 5 | 95 |
| WE-10 | 10 | 90 |
| WE-20 | 20 | 80 |
| Source of Variation | SS | df | MS | F | F-Crit |
|---|---|---|---|---|---|
| Tensile strength (MPa) | |||||
| BG | 18,212 | 12 | 151.8 | 29.17 | 1.974 |
| WG | 234.2 | 45 | 5.205 | ||
| Tensile modulus (GPa) | |||||
| BG | 6.251 | 12 | 0.5209 | 13.71 | 1.952 |
| WG | 1.900 | 50 | 0.0380 | ||
| Flexural strength (MPa) | |||||
| BG | 24,631 | 12 | 2053 | 67.87 | 1.991 |
| WG | 1270 | 42 | 30.25 | ||
| Flexural modulus (GPa) | |||||
| BG | 1.798 | 12 | 0.1499 | 15.83 | 1.991 |
| WG | 0.3975 | 42 | 0.0095 | ||
| Impact strength (kJ·m−2) | |||||
| BG | 1077 | 12 | 89.73 | 80.31 | 1.836 |
| WG | 130.7 | 117 | 1.117 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cree, D.; Soleimani, M. Bio-Based White Eggshell as a Value-Added Filler in Poly(Lactic Acid) Composites. J. Compos. Sci. 2023, 7, 278. https://doi.org/10.3390/jcs7070278
Cree D, Soleimani M. Bio-Based White Eggshell as a Value-Added Filler in Poly(Lactic Acid) Composites. Journal of Composites Science. 2023; 7(7):278. https://doi.org/10.3390/jcs7070278
Chicago/Turabian StyleCree, Duncan, and Majid Soleimani. 2023. "Bio-Based White Eggshell as a Value-Added Filler in Poly(Lactic Acid) Composites" Journal of Composites Science 7, no. 7: 278. https://doi.org/10.3390/jcs7070278







