TiO2-CoFe2O4 and TiO2-CuFe2O4 Composite Films: A New Approach to Synthesis, Characterization, and Optical and Photocatalytic Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Ferrites
2.3. Fabrication of the PEO-Coatings
2.4. Ferrite Films Fabrication on the PEO-Coatings
2.5. Characterization of the Powders and Coatings
2.6. Photocatalytic Tests
3. Results and Discussion
3.1. Films Fabrication Method
3.2. Morphology
3.3. X-ray Diffraction
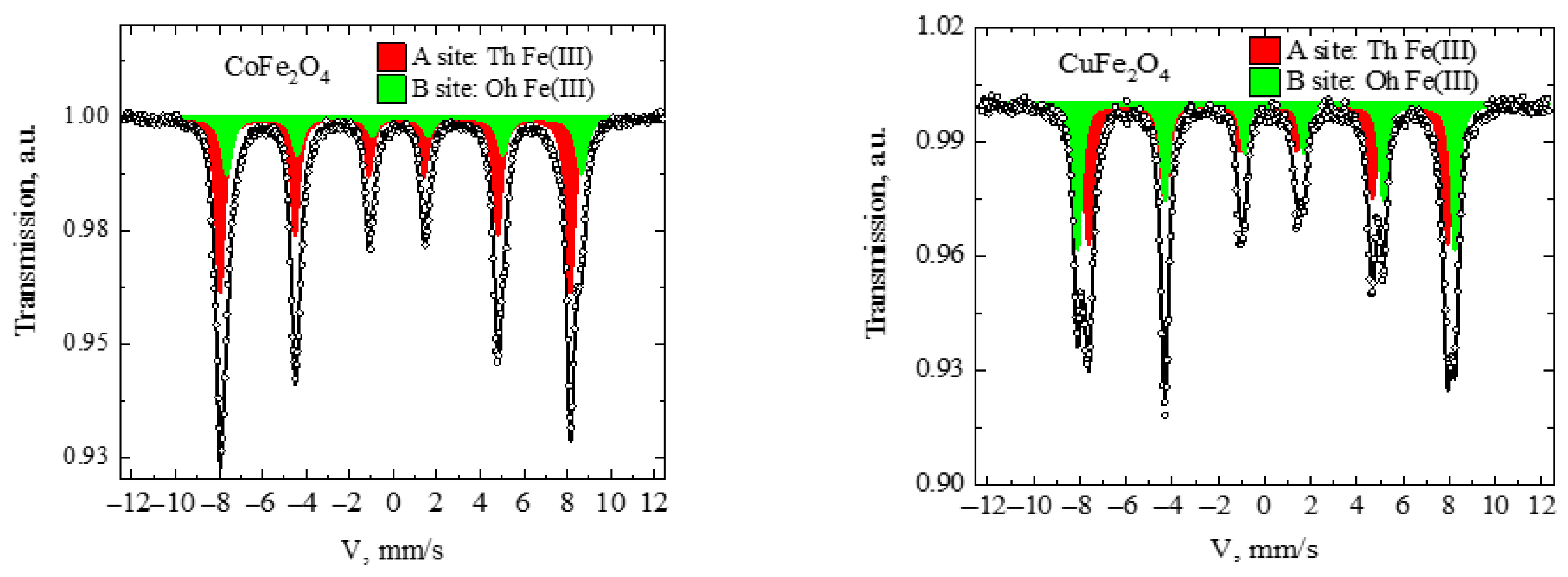
3.4. Mössbauer Spectroscopy
3.5. Indigo Carmine Degradation
3.6. Optoelectronic Properties
3.7. Charge Carrier Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hazra, S.; Ghosh, N.N. Preparation of Nanoferrites and Their Applications. J. Nanosci. Nanotechnol. 2014, 14, 1983–2000. [Google Scholar] [CrossRef] [PubMed]
- Yanagihara, H.; Sharmin, S.; Niizeki, T.; Kita, E. Magnetic Properties of Spinel Ferrite Thin Films Grown by Reactive Sputtering. Mater. Trans. 2016, 57, 777–780. [Google Scholar] [CrossRef] [Green Version]
- Khairy, M.; Bayoumy, W.A.; Selima, S.S.; Mousa, M.A. Studies on Characterization, Magnetic and Electrochemical Properties of Nano-Size Pure and Mixed Ternary Transition Metal Ferrites Prepared by the Auto-Combustion Method. J. Mater. Res. 2020, 35, 2652–2663. [Google Scholar] [CrossRef]
- Du, H.; Akakuru, O.U.; Yao, C.; Yang, F.; Wu, A. Transition Metal Ion-Doped Ferrites Nanoparticles for Bioimaging and Cancer Therapy. Transl. Oncol. 2022, 15, 101264. [Google Scholar] [CrossRef]
- Tamboli, Q.Y.; Patange, S.M.; Mohanta, Y.K.; Sharma, R.; Zakde, K.R. Green Synthesis of Cobalt Ferrite Nanoparticles: An Emerging Material for Environmental and Biomedical Applications. J. Nanomater. 2023, 2023, 9770212. [Google Scholar] [CrossRef]
- Chen, H.D.; Xu, J.K.; Wei, J.Q.; Wang, P.F.; Han, Y.B.; Xu, J.C.; Hong, B.; Jin, H.X.; Jin, D.F.; Peng, X.L.; et al. Mesoporous CoFe2O4 Nanowires: Nanocasting Synthesis, Magnetic Separation and Enhanced Catalytic Degradation for Ciprofloxacin. J. Phys. Chem. Solids 2019, 132, 138–144. [Google Scholar] [CrossRef]
- Najwa, N.; Jani, M.; Rahmah, A.; Ahmad, D.; Adnan, R. CuFe2O4 as a Heterogeneous Fenton Catalyst for the Removal of Ciprofloxacin from Aqueous Solution at Natural PH. Malays. J. Catal. 2021, 5, 31–37. [Google Scholar]
- Swathi, S.; Yuvakkumar, R.; Kumar, P.S.; Ravi, G.; Velauthapillai, D. Annealing Temperature Effect on Cobalt Ferrite Nanoparticles for Photocatalytic Degradation. Chemosphere 2021, 281, 130903. [Google Scholar] [CrossRef]
- Zaharieva, K.; Rives, V.; Tsvetkov, M.; Cherkezova-Zheleva, Z.; Kunev, B.; Trujillano, R.; Mitov, I.; Milanova, M. Preparation, Characterization and Application of Nanosized Copper Ferrite Photocatalysts for Dye Degradation under UV Irradiation. Mater. Chem. Phys. 2015, 160, 271–278. [Google Scholar] [CrossRef]
- Motora, K.G.; Wu, C.M.; Naseem, S. Magnetic Recyclable Self-Floating Solar Light-Driven WO2.72/Fe3O4 Nanocomposites Immobilized by Janus Membrane for Photocatalysis of Inorganic and Organic Pollutants. J. Ind. Eng. Chem. 2021, 102, 25–34. [Google Scholar] [CrossRef]
- Li, K.; Zhong, Y.; Luo, S.; Deng, W. Fabrication of Powder and Modular H3PW12O40/Ag3PO4 Composites: Novel Visible-Light Photocatalysts for Ultra-Fast Degradation of Organic Pollutants in Water. Appl. Catal. B Environ. 2020, 278, 119313. [Google Scholar] [CrossRef]
- Tao, W.; Wang, M.; Ali, R.; Nie, S.; Zeng, Q.; Yang, R.; Lau, W.M.; He, L.; Tang, H.; Jian, X. Multi-Layered Porous Hierarchical TiO2/g-C3N4 Hybrid Coating for Enhanced Visible Light Photocatalysis. Appl. Surf. Sci. 2019, 495, 143435. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.; Xue, W.; Cheng, G.; Zheng, R.; Cheng, Y. Structures and Properties of Ceramic Films on TiAl Intermetallic Compound Fabricated by Microarc Oxidation. Surf. Coat. Technol. 2007, 201, 5556–5559. [Google Scholar] [CrossRef]
- Terleeva, O.P.; Belevantsev, V.I.; Slonova, A.I.; Boguta, D.L.; Rudnev, V.S. Comparison Analysis of Formation and Some Characteristics of Microplasma Coatings on Aluminum and Titanium Alloys. Prot. Met. 2006, 42, 272–278. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, B.; Lei, T.; Guo, L. Dependence of Growth Features of Microarc Oxidation Coatings of Titanium Alloy on Control Modes of Alternate Pulse. Mater. Lett. 2004, 58, 1907–1911. [Google Scholar] [CrossRef]
- Zhang, W.; Du, K.; Yan, C.; Wang, F. Preparation and Characterization of a Novel Si-Incorporated Ceramic Film on Pure Titanium by Plasma Electrolytic Oxidation. Appl. Surf. Sci. 2008, 254, 5216–5223. [Google Scholar] [CrossRef]
- Rudnev, V.S.; Yu Ustinov, A.; Lukiyanchuk, I.V.; Kharitonskii, P.V.; Frolov, A.M.; Morozova, V.P.; Tkachenko, I.A.; Sergienko, A.V.I. Magnetic Properties of Plasma Electrolytic Iron-Containing Oxide Coatings on Aluminum. Dokl. Phys. Chem. 2009, 428, 189–192. [Google Scholar] [CrossRef]
- Hoseini, A.; Yarmand, B. Immobilization of Fe2O3/TiO2 Photocatalyst on the Metallic Substrate via Plasma Electrolytic Oxidation Process: Degradation Efficiency. J. Nanopart. Res. 2020, 22, 312. [Google Scholar] [CrossRef]
- Kim, Y.S.; Shin, K.R.; Kim, G.W.; Ko, Y.G.; Shin, D.H. Photocatalytic Activity of TiO2 Film Containing Fe2O3 via Plasma Electrolytic Oxidation. Surf. Eng. 2016, 32, 443–447. [Google Scholar] [CrossRef]
- Tang, X.; Jin, L.; Wei, R.; Zhu, X.; Yang, J.; Dai, J.; Song, W.; Zhu, X.; Sun, Y. High-Coercivity CoFe2O4 Thin Films on Si Substrates by Sol-Gel. J. Magn. Magn. Mater. 2017, 422, 255–261. [Google Scholar] [CrossRef]
- Wang, X.W.; Zhang, Y.Q.; Meng, H.; Wang, Z.J.; Zhang, Z.D. Perpendicular Magnetic Anisotropy in 70 nm CoFe2O4 Thin Films Fabricated on SiO2/Si(100) by the Sol–Gel Method. J. Alloys Compd. 2011, 509, 7803–7807. [Google Scholar] [CrossRef]
- Shi, M.; Zuo, R.; Xu, Y.; Jiang, Y.; Yu, G.; Su, H.; Zhong, J. Preparation and Characterization of CoFe2O4 Powders and Films via the Sol–Gel Method. J. Alloys Compd. 2012, 512, 165–170. [Google Scholar] [CrossRef]
- Ferreira, L.S.; Silva, T.R.; Santos, J.R.D.; Silva, V.D.; Raimundo, R.A.; Morales, M.A.; Macedo, D.A. Structure, Magnetic Behavior and OER Activity of CoFe2O4 Powders Obtained Using Agar-Agar from Red Seaweed (Rhodophyta). Mater. Chem. Phys. 2019, 237, 121847. [Google Scholar] [CrossRef]
- Grilikhes, S.Y. Metal Degreasing, Etching and Polishing; Mashinostroenie: Leningrad, Russia, 1977. [Google Scholar]
- Vasil’eva, M.S.; Rudnev, V.S.; Sklyarenko, O.E.; Tyrina, L.M.; Kondrikov, N.B. Titanium-Supported Nickel-Copper Oxide Catalysts for Oxidation of Carbon(II) Oxide. Russ. J. Gen. Chem. 2010, 80, 1557–1562. [Google Scholar] [CrossRef]
- Zi, Z.; Sun, Y.; Zhu, X.; Yang, Z.; Dai, J.; Song, W. Synthesis and Magnetic Properties of CoFe2O4 Ferrite Nanoparticles. J. Magn. Magn. Mater. 2009, 321, 1251–1255. [Google Scholar] [CrossRef]
- Wang, W.H.; Ren, X. Flux Growth of High-Quality CoFe2O4 Single Crystals and Their Characterization. J. Cryst. Growth 2006, 289, 605–608. [Google Scholar] [CrossRef]
- Jiang, J.Z.; Goya, G.F.; Rechenberg, H.R. Magnetic Properties of Nanostructured CuFe2O4. J. Phys. Condens. Matter 1999, 11, 4063–4078. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Liu, L.; Jia, D.Z.; Pan, W. Simple Synthesis of CuFe2O4 Nanoparticles as Gas-Sensing Materials. Sens. Actuators B Chem. 2007, 125, 144–148. [Google Scholar] [CrossRef]
- Lukiyanchuk, I.V.; Vasilyeva, M.S.; Sergeev, A.A.; Nepomnyashchii, A.V.; Serov, M.M.; Krit, B.L. Features of Coalescence of Gold on the Surface of Different Supports during Catalytic Oxidation of CO. Prot. Met. Phys. Chem. Surf. 2021, 57, 1172–1179. [Google Scholar] [CrossRef]
- Joni, I.M.; Nulhakim, L.; Vanitha, M.; Panatarani, C. Characteristics of Crystalline Silica (SiO2) Particles Prepared by Simple Solution Method Using Sodium Silicate (Na2SiO3) Precursor. J. Phys. Conf. Ser. 2018, 1080, 012006. [Google Scholar] [CrossRef]
- Vasilyeva, M.S.; Lukiyanchuk, I.V.; Sergeev, A.A.; Sergeeva, K.A.; Ustinov, A.Y.; Tkachev, V.V.; Arefieva, O.D. Plasma Electrolytic Synthesis and Characterization of Oxide Coatings with MWO4 (M = Co, Ni, Cu) as Photo-Fenton Heterogeneous Catalysts. Surf. Coat. Technol. 2021, 424, 127640. [Google Scholar] [CrossRef]
- Agouriane, E.; Rabi, B.; Essoumhi, A.; Razouk, A.; Sahlaoui, M.; Costa, B.F.O.; Sajieddine, M. Structural and Magnetic Properties of CuFe2O4 Ferrite Nanoparticles Synthesized by Co-Precipitation. J. Mater. Environ. Sci. 2016, 7, 4116–4120. [Google Scholar]
- Grigorova, M.; Blythe, H.J.; Blaskov, V.; Rusanov, V.; Petkov, V.; Masheva, V.; Nihtianova, D.; Martinez, L.M.; Muñoz, J.S.; Mikhov, M. Magnetic Properties and Mössbauer Spectra of Nanosized CoFe2O4 Powders. J. Magn. Magn. Mater. 1998, 183, 163–172. [Google Scholar] [CrossRef]
- Sanchez-Lievanos, K.R.; Stair, J.L.; Knowles, K.E. Cation Distribution in Spinel Ferrite Nanocrystals: Characterization, Impact on Their Physical Properties, and Opportunities for Synthetic Control. Inorg. Chem. 2021, 60, 4291–4305. [Google Scholar] [CrossRef] [PubMed]
- Kosmulski, M. Compilation of PZC and IEP of Sparingly Soluble Metal Oxides and Hydroxides from Literature. Adv. Colloid Interface Sci. 2009, 152, 14–25. [Google Scholar] [CrossRef]
- Vergis, B.R.; Hari Krishna, R.; Kottam, N.; Nagabhushana, B.M.; Sharath, R.; Darukaprasad, B. Removal of Malachite Green from Aqueous Solution by Magnetic CuFe2O4 Nano-Adsorbent Synthesized by One Pot Solution Combustion Method. J. Nanostructure Chem. 2017, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Kosmulski, M. PH-Dependent Surface Charging and Points of Zero Charge II. Update. J. Colloid Interface Sci. 2004, 275, 214–224. [Google Scholar] [CrossRef]
- Ammar, S.; Abdelhedi, R.; Flox, C.; Arias, C.; Brillas, E. Electrochemical Degradation of the Dye Indigo Carmine at Boron-Doped Diamond Anode for Wastewaters Remediation. Environ. Chem. Lett. 2006, 4, 229–233. [Google Scholar] [CrossRef]
- Othman, I.; Mohamed, R.M.; Ibrahem, F.M. Study of Photocatalytic Oxidation of Indigo Carmine Dye on Mn-Supported TiO2. J. Photochem. Photobiol. A Chem. 2007, 189, 80–85. [Google Scholar] [CrossRef]
- Barka, N.; Assabbane, A.; Nounah, A.; Ichou, Y.A. Photocatalytic Degradation of Indigo Carmine in Aqueous Solution by TiO2-Coated Non-Woven Fibres. J. Hazard. Mater. 2008, 152, 1054–1059. [Google Scholar] [CrossRef]
- Vasilyeva, M.S.; Lukiyanchuk, I.V.; Sergeev, A.A.; Ustinov, A.Y.; Sergeeva, K.A.; Kuryavyi, V.G. Ti/TiO2-CoWO4-Co3(PO4)2 Composites: Plasma Electrolytic Synthesis, Optoelectronic Properties, and Solar Light-Driven Photocatalytic Activity. J. Alloys Compd. 2021, 863, 158066. [Google Scholar] [CrossRef]
- Padmanabhan, S.K.; Pal, S.; Ul Haq, E.; Licciulli, A. Nanocrystalline TiO2–Diatomite Composite Catalysts: Effect of Crystallization on the Photocatalytic Degradation of Rhodamine B. Appl. Catal. A Gen. 2014, 485, 157–162. [Google Scholar] [CrossRef]
- Daude, N.; Gout, C.; Jouanin, C. Electronic Band Structure of Titanium Dioxide. Phys. Rev. B 1977, 15, 3229–3235. [Google Scholar] [CrossRef]
- Sonu; Dutta, V.; Sharma, S.; Raizada, P.; Hosseini-Bandegharaei, A.; Gupta, V.K.; Singh, P. Review on Augmentation in Photocatalytic Activity of CoFe2O4 via Heterojunction Formation for Photocatalysis of Organic Pollutants in Water. J. Saudi Chem. Soc. 2019, 23, 1119–1136. [Google Scholar] [CrossRef]
- Syed, A.; Ahmed, B.; Elgorban, A.M.; Bahkali, A.H.; Lee, J.; Rajput, V.D.; Minkina, T. Designing Spinel CoFe2O4 Loaded Sheet-like Bi2O3 Nano-Heterostructure for Synergetic White-Light Photocatalysis with Recombination Delay and Antibacterial Applications. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127449. [Google Scholar] [CrossRef]
- Jia, Y.; Ma, H.; Liu, C. Au Nanoparticles Enhanced Z-Scheme Au-CoFe2O4/MoS2 Visible Light Photocatalyst with Magnetic Retrievability. Appl. Surf. Sci. 2019, 463, 854–862. [Google Scholar] [CrossRef]
- Zhao, W.; Jin, Y.; Gao, C.H.; Gu, W.; Jin, Z.M.; Lei, Y.L.; Liao, L.S. A Simple Method for Fabricating p–n Junction Photocatalyst CuFe2O4/Bi4Ti3O12 and Its Photocatalytic Activity. Mater. Chem. Phys. 2014, 143, 952–962. [Google Scholar] [CrossRef]
- Sonu; Dutta, V.; Sudhaik, A.; Khan, A.A.P.; Ahamad, T.; Raizada, P.; Thakur, S.; Asiri, A.M.; Singh, P. GCN/CuFe2O4/SiO2 Photocatalyst for Photo-Fenton Assisted Degradation of Organic Dyes. Mater. Res. Bull. 2023, 164, 112238. [Google Scholar] [CrossRef]
- Wei, X.; Yang, X.; Xu, X.; Liu, Z.; Naraginti, S.; Wan, J. Novel Magnetically Separable Tetrahedral Ag3PO4/NrGO/CuFe2O4 Photocatalyst for Efficient Detoxification of 2,4-Dichlorophenol. Environ. Res. 2021, 201, 111519. [Google Scholar] [CrossRef]
- Chang, N.; Chen, Y.R.; Xie, F.; Liu, Y.P.; Wang, H.T. Facile Construction of Z-Scheme AgCl/Ag-Doped-ZIF-8 Heterojunction with Narrow Band Gaps for Efficient Visible-Light Photocatalysis. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126351. [Google Scholar] [CrossRef]
- Yuan, Q.; Chen, L.; Xiong, M.; He, J.; Luo, S.L.; Au, C.T.; Yin, S.F. Cu2O/BiVO4 Heterostructures: Synthesis and Application in Simultaneous Photocatalytic Oxidation of Organic Dyes and Reduction of Cr(VI) under Visible Light. Chem. Eng. J. 2014, 255, 394–402. [Google Scholar] [CrossRef]
- Steblevskaya, N.I.; Medkov, M.A.; Belobeletskaya, M.V. The Use of the Extraction–Pyrolytic Method for the Production of Oxide Functional Films and Coatings. Theor. Found. Chem. Eng. 2022, 56, 934–941. [Google Scholar] [CrossRef]
- Lubis, S.; Sheilatina; Murisna. Synthesis, Characterization and Photocatalytic Activity of α-Fe2O3/Bentonite Composite Prepared by Mechanical Milling. J. Phys. Conf. Ser. 2018, 1116, 042016. [Google Scholar] [CrossRef]
- Nawle, A.C.; Humbe, A.V.; Babrekar, M.K.; Deshmukh, S.S.; Jadhav, K.M. Deposition, Characterization, Magnetic and Optical Properties of Zn Doped CuFe2O4 Thin Films. J. Alloys Compd. 2017, 695, 1573–1582. [Google Scholar] [CrossRef]
- Zhang, Y.; Schultz, A.M.; Salvador, P.A.; Rohrer, G.S. Spatially Selective Visible Light Photocatalytic Activity of TiO2/BiFeO3 Heterostructures. J. Mater. Chem. 2011, 21, 4168. [Google Scholar] [CrossRef]
- Humayun, M.; Zada, A.; Li, Z.; Xie, M.; Zhang, X.; Qu, Y.; Raziq, F.; Jing, L. Enhanced Visible-Light Activities of Porous BiFeO3 by Coupling with Nanocrystalline TiO2 and Mechanism. Appl. Catal. B Environ. 2016, 180, 219–226. [Google Scholar] [CrossRef]
- Mishra, S.; Acharya, R.; Parida, K. Spinel-Ferrite-Decorated Graphene-Based Nanocomposites for Enhanced Photocatalytic Detoxification of Organic Dyes in Aqueous Medium: A Review. Water 2022, 15, 81. [Google Scholar] [CrossRef]
- Soto-Arreola, A.; Huerta-Flores, A.M.; Mora-Hernández, J.M.; Torres-Martínez, L.M. Comparative Study of the Photocatalytic Activity for Hydrogen Evolution of MFe2O4 (M = Cu, Ni) Prepared by Three Different Methods. J. Photochem. Photobiol. A Chem. 2018, 357, 20–29. [Google Scholar] [CrossRef]









| Sample | Atomic Ratios in Samples Fe:Cu/Co | Cu/Co, at % | Fe, at % | O, at % | Ti, at % | Si, at % |
|---|---|---|---|---|---|---|
| CoFe2O4 | 2:1.15 | 17.64 | 30.75 | 51.60 | ||
| CuFe2O4 | 2:1.07 | 16.16 | 30.09 | 53.74 | ||
| CoFe2O4-PEO | 2:1.01 | 2.98 | 5.93 | 64.40 | 6.00 | 20.69 |
| CuFe2O4-PEO | 2:1.04 | 2.43 | 4.66 | 66.31 | 6.67 | 19.93 |
| Sample | δ, mm/s | Δ, mm/s | H, kOe | Γ, mm/s | Relative area, % | Assignment |
|---|---|---|---|---|---|---|
| CoFe2O4 | 0.23 | −0.05 | 498.8 | 0.45 | 67.73 | A site: Th Fe(III) |
| 0.52 | 0.17 | 505.1 | 0.51 | 32.27 | B site: Oh Fe(III) | |
| CuFe2O4 | 0.26 | −0.02 | 481.7 | 0.47 | 61.58 | A site: Th Fe(III) |
| 0.36 | −0.33 | 507.6 | 0.37 | 38.42 | B site: Oh Fe(III) |
| Sample | UV | VIS | ||
|---|---|---|---|---|
| k, min−1 | R2 | k, min−1 | R2 | |
| Blank IC | 0.57 × 10−3 | 0.99 | 0.41 × 10−3 | 0.99 |
| CoFe2O4-PEO | 5.00 × 10−3 | 0.99 | 3.02 × 10−3 | 0.99 |
| CuFe2O4-PEO | 7.97 × 10−3 | 0.99 | 3.60 × 10−3 | 0.99 |
| Sample | X, eV | Eg, eV (Ref) | ECB, eV | EVB, eV |
|---|---|---|---|---|
| TiO2 | 5.81 | 3.11 [53] | −0.25 | 2.87 |
| CoFe2O4 | 5.81 | 1.72 [54] | −0.45 | 2.17 |
| CuFe2O4 | 5.85 | 2.04 [55] | −0.33 | 2.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balatskiy, D.; Budnikova, Y.; Bratskaya, S.; Vasilyeva, M. TiO2-CoFe2O4 and TiO2-CuFe2O4 Composite Films: A New Approach to Synthesis, Characterization, and Optical and Photocatalytic Properties. J. Compos. Sci. 2023, 7, 295. https://doi.org/10.3390/jcs7070295
Balatskiy D, Budnikova Y, Bratskaya S, Vasilyeva M. TiO2-CoFe2O4 and TiO2-CuFe2O4 Composite Films: A New Approach to Synthesis, Characterization, and Optical and Photocatalytic Properties. Journal of Composites Science. 2023; 7(7):295. https://doi.org/10.3390/jcs7070295
Chicago/Turabian StyleBalatskiy, Denis, Yulia Budnikova, Svetlana Bratskaya, and Marina Vasilyeva. 2023. "TiO2-CoFe2O4 and TiO2-CuFe2O4 Composite Films: A New Approach to Synthesis, Characterization, and Optical and Photocatalytic Properties" Journal of Composites Science 7, no. 7: 295. https://doi.org/10.3390/jcs7070295
APA StyleBalatskiy, D., Budnikova, Y., Bratskaya, S., & Vasilyeva, M. (2023). TiO2-CoFe2O4 and TiO2-CuFe2O4 Composite Films: A New Approach to Synthesis, Characterization, and Optical and Photocatalytic Properties. Journal of Composites Science, 7(7), 295. https://doi.org/10.3390/jcs7070295






