Heterogeneous Hierarchical Self-Assembly Forming Crystalline Nanocellulose–CaCO3 Hybrid Nanoparticle Biocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cellulose Nanocrystals
2.2. Dispersant-Free Ground Calcium Carbonate (UGCC)
2.3. Preparation of CNC and UGCC Composite Suspensions
2.4. Conductivity Measurements
2.5. Rheometry
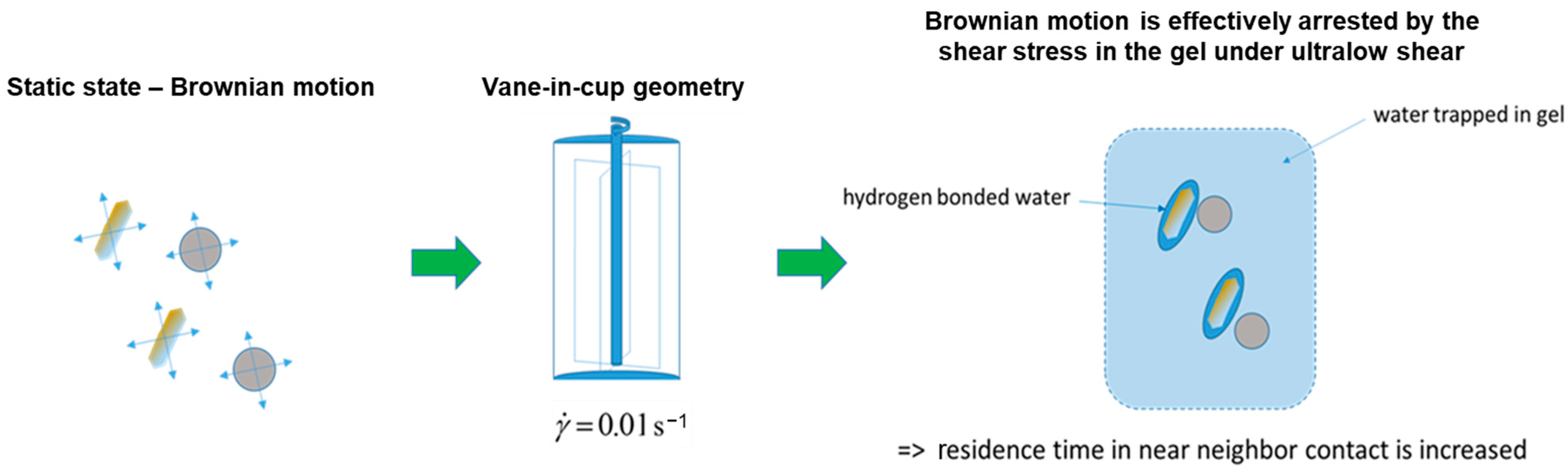
2.5.1. Agglomerate Build-Up
2.5.2. Suspension Structure Recovery
2.5.3. Consecutive Yield Stress
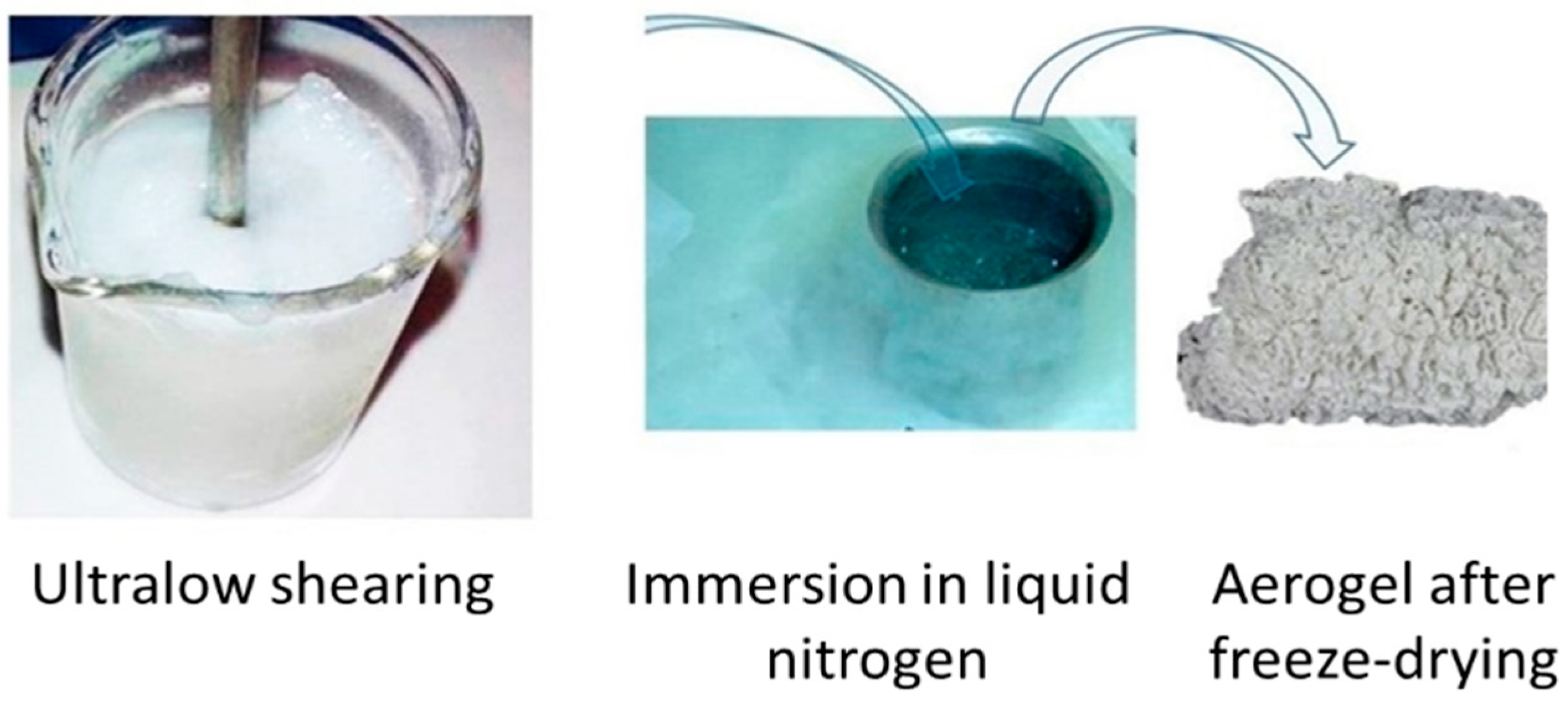
2.6. Freeze-Dried Aerogels for Optical and Electron Microscopy Imaging
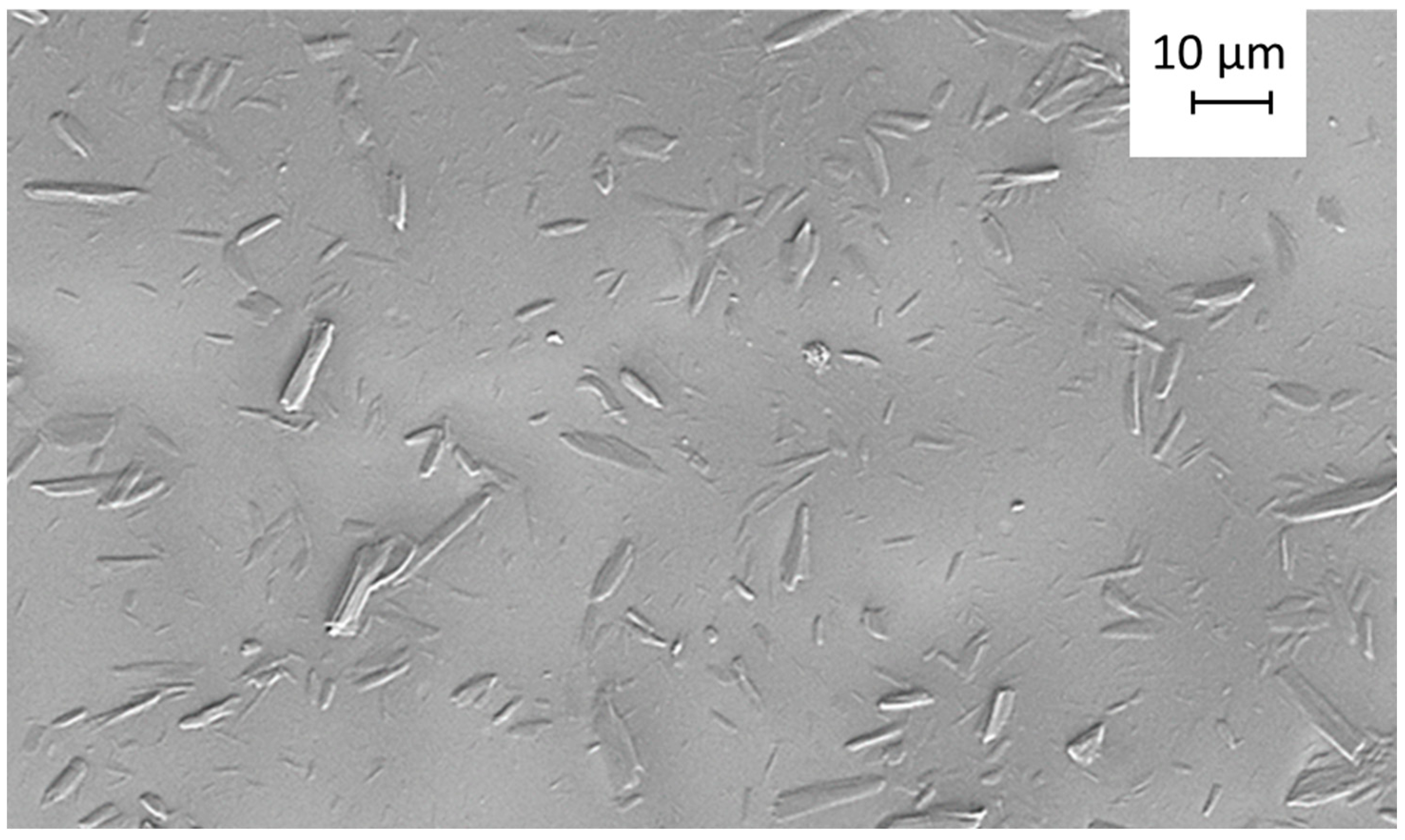
2.6.1. Morphological Characterization of Particles
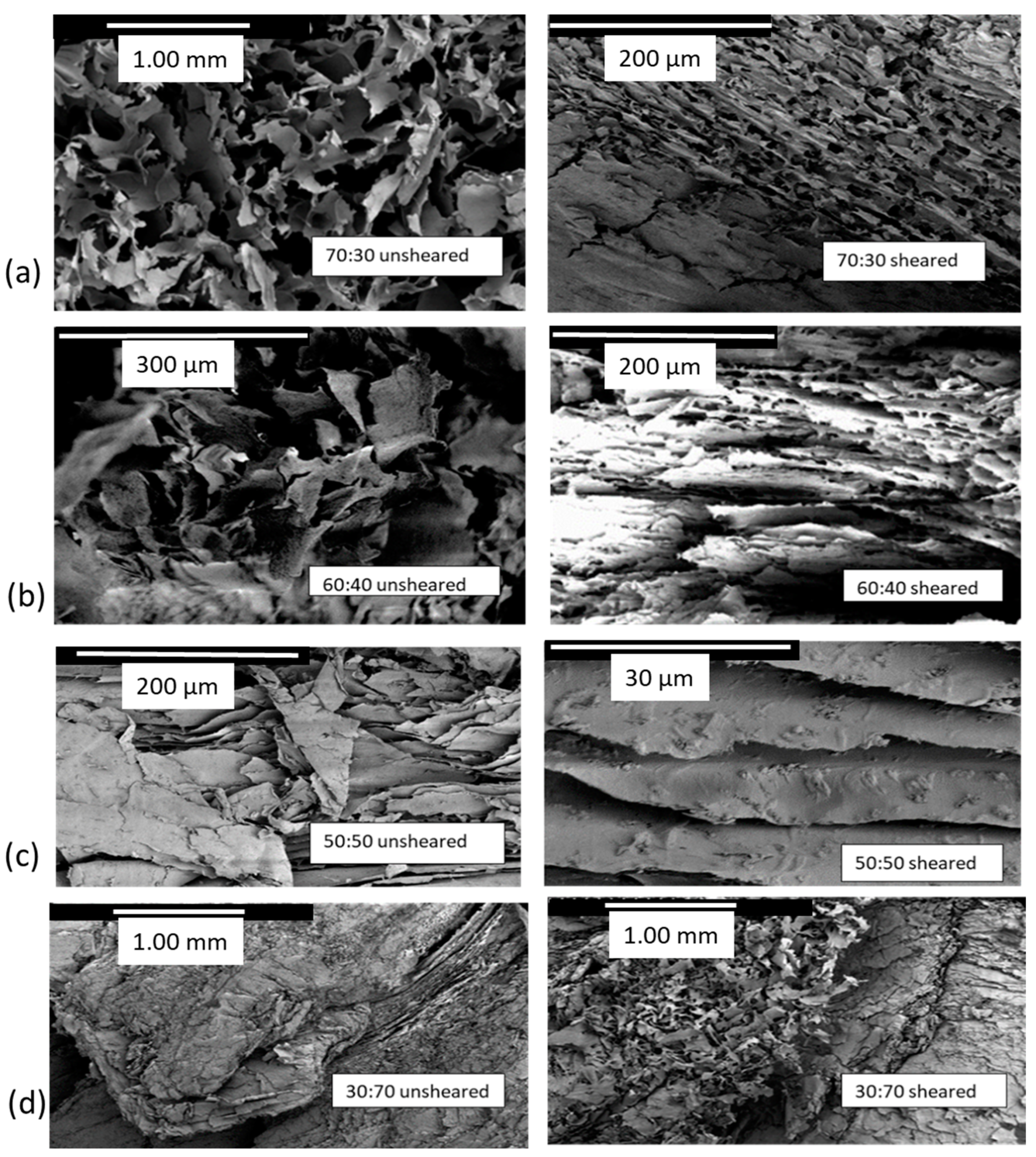
2.6.2. Scanning Electron Microscopy
3. Results
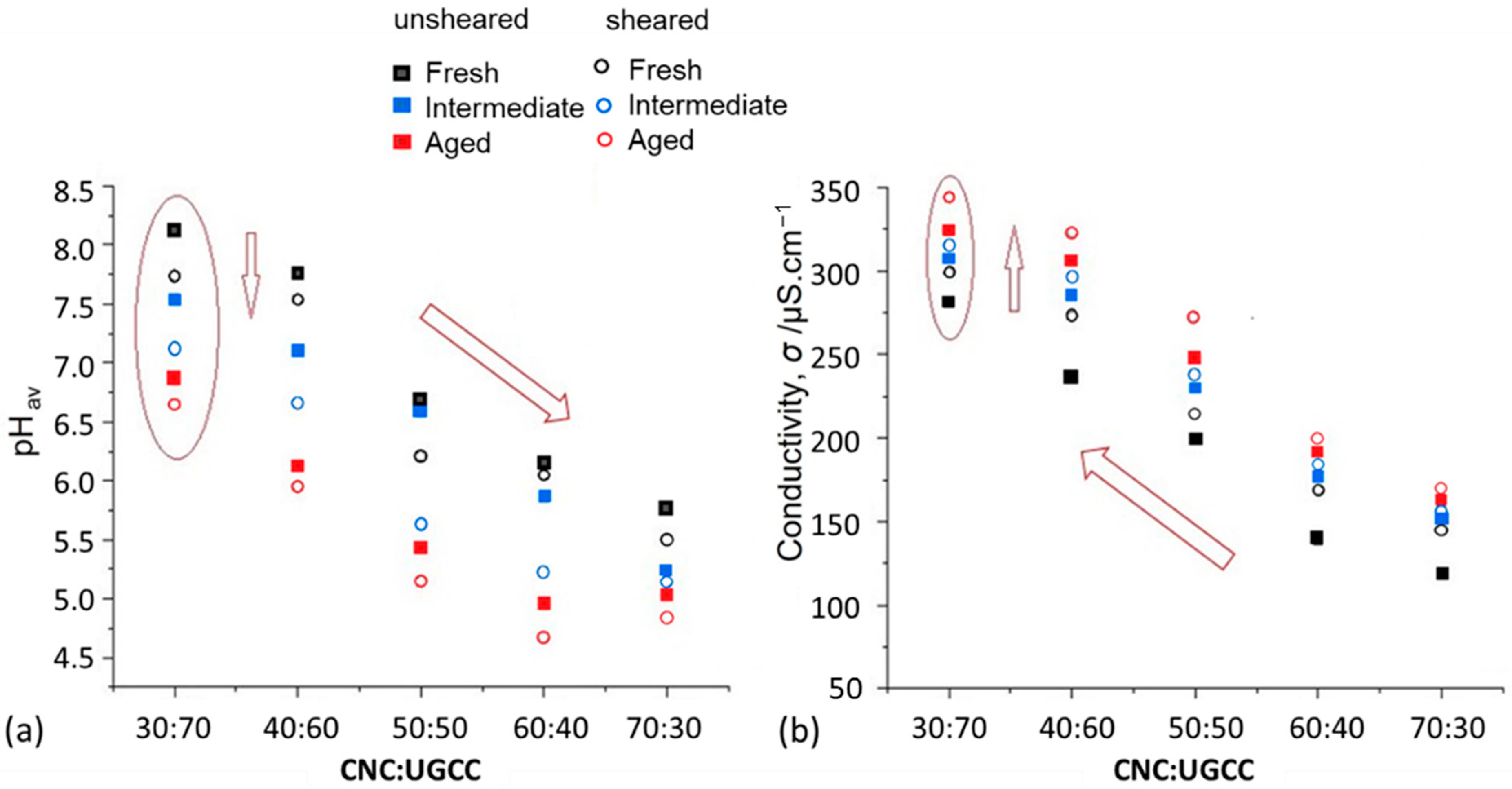
3.1. pH and Conductivity (Zeta Potential, ζ)
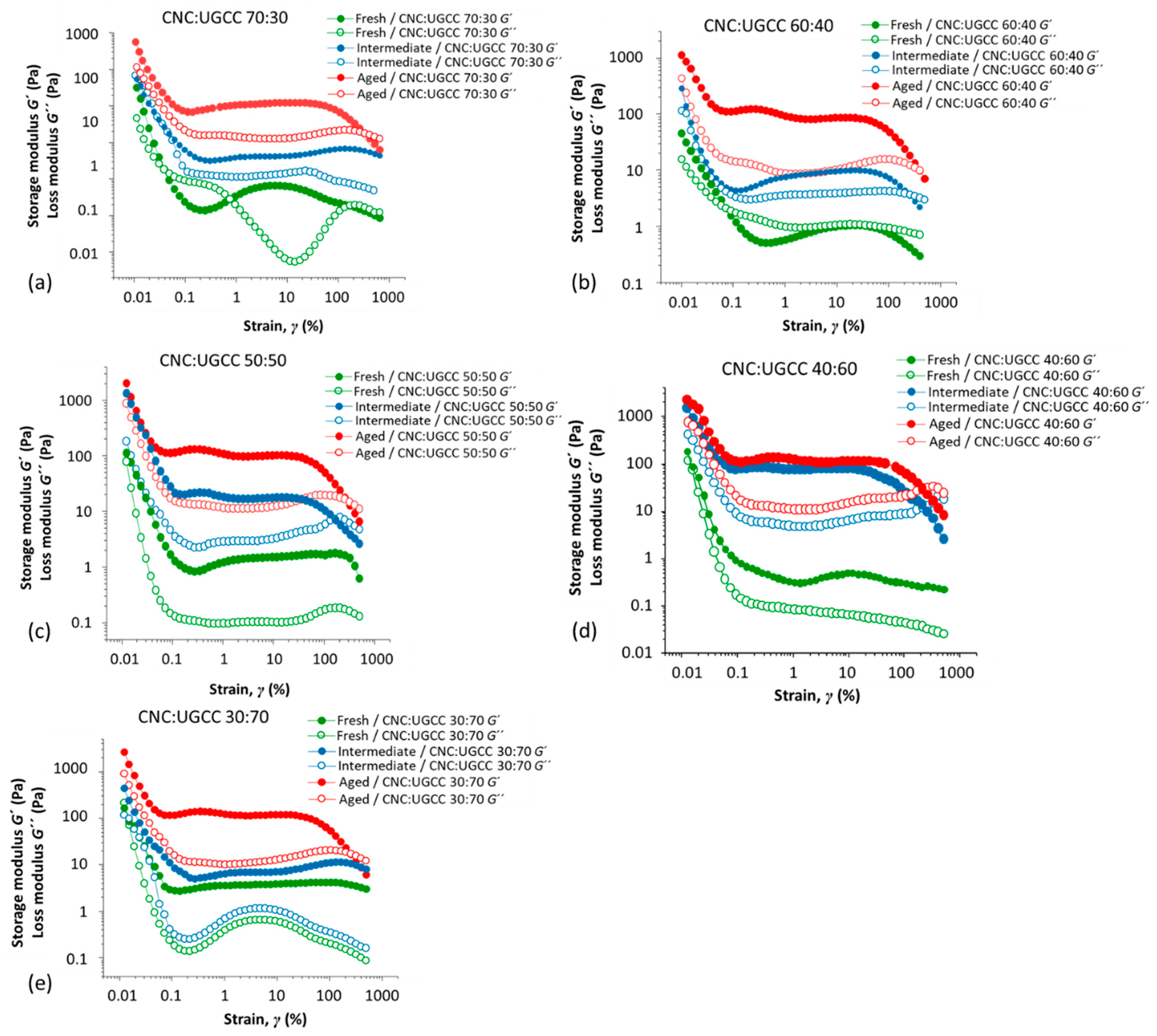
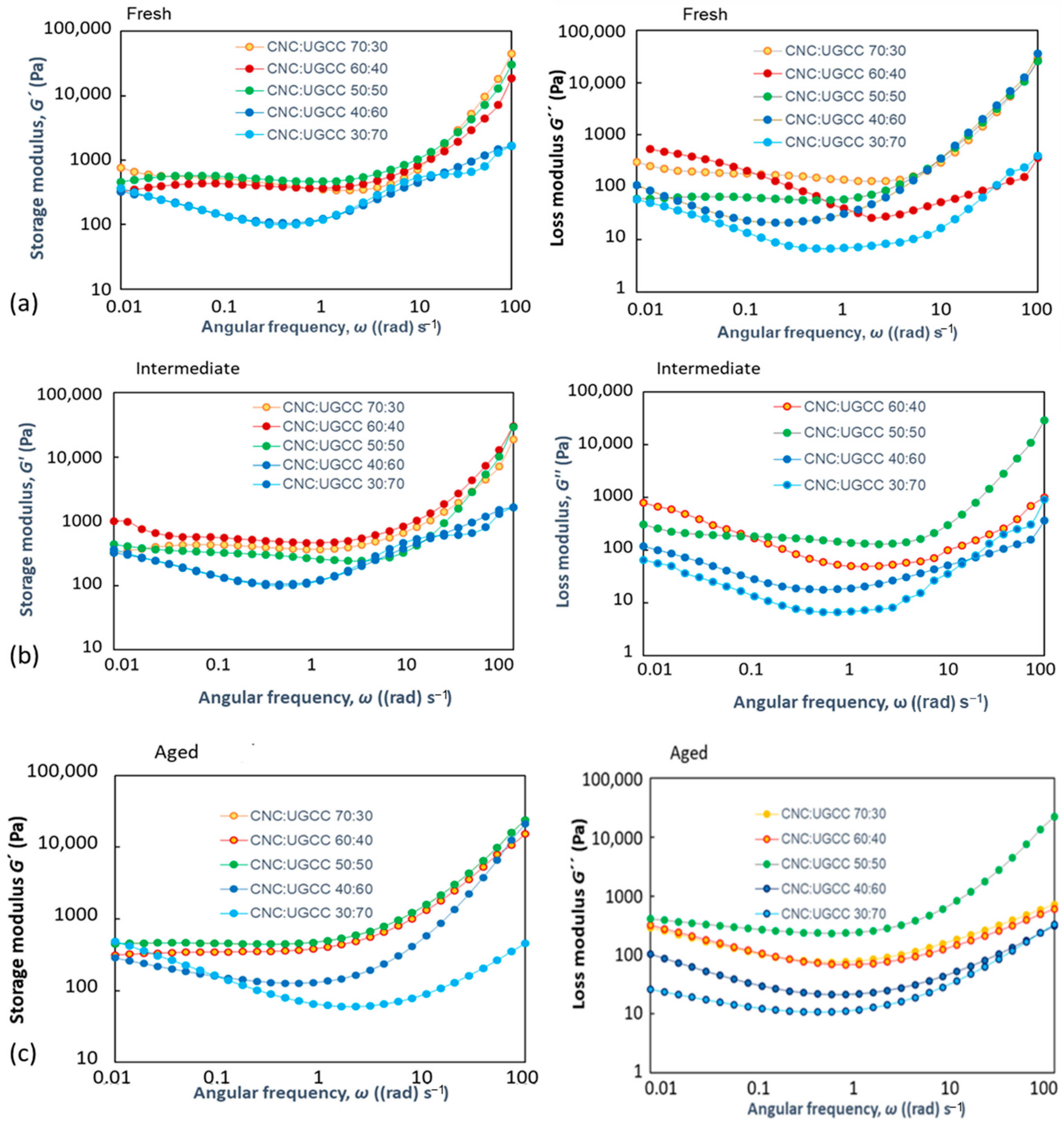
3.2. Rheological Behavior
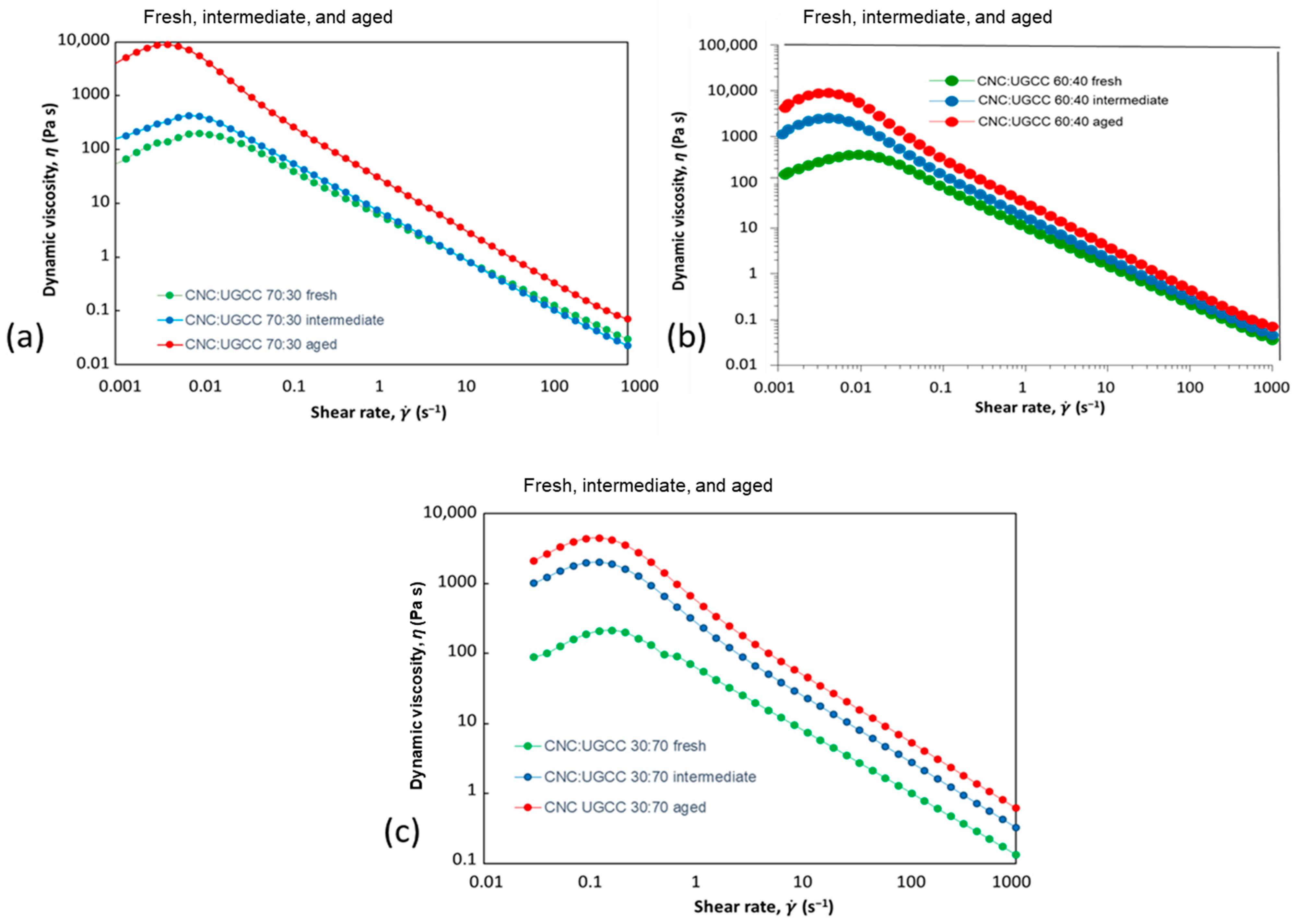
3.2.1. CNC Alone
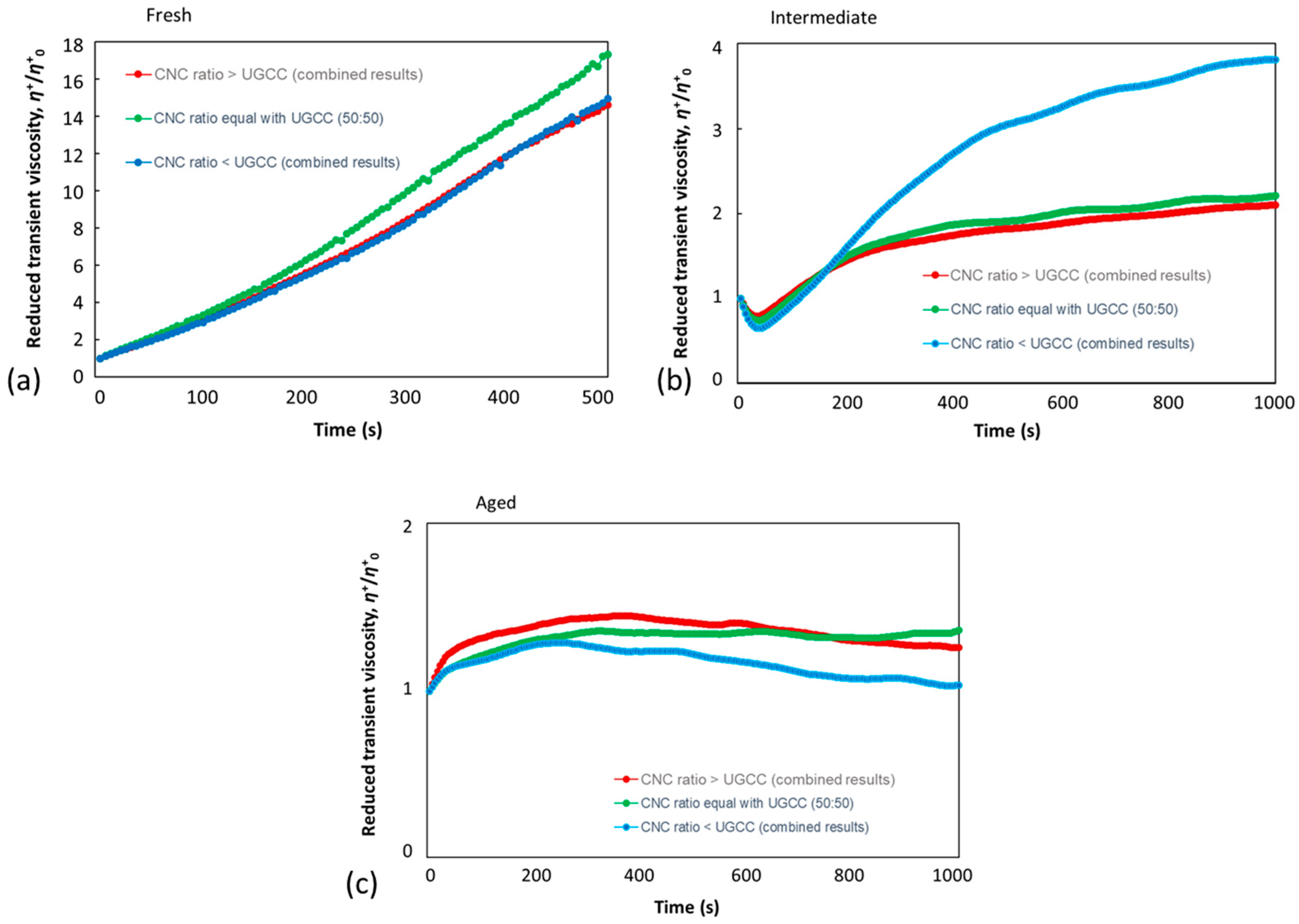
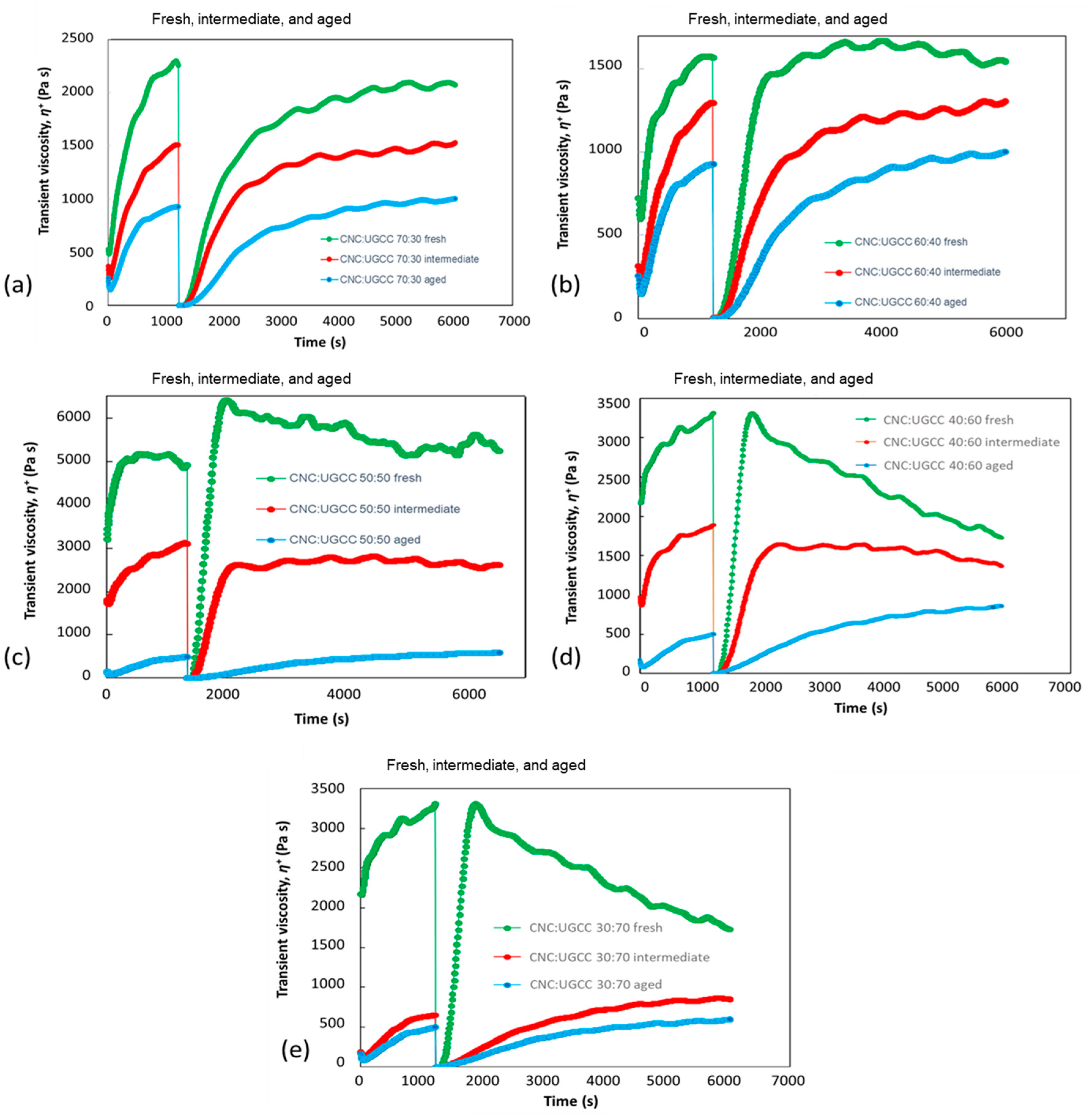
3.2.2. CNC–UGCC Combinations
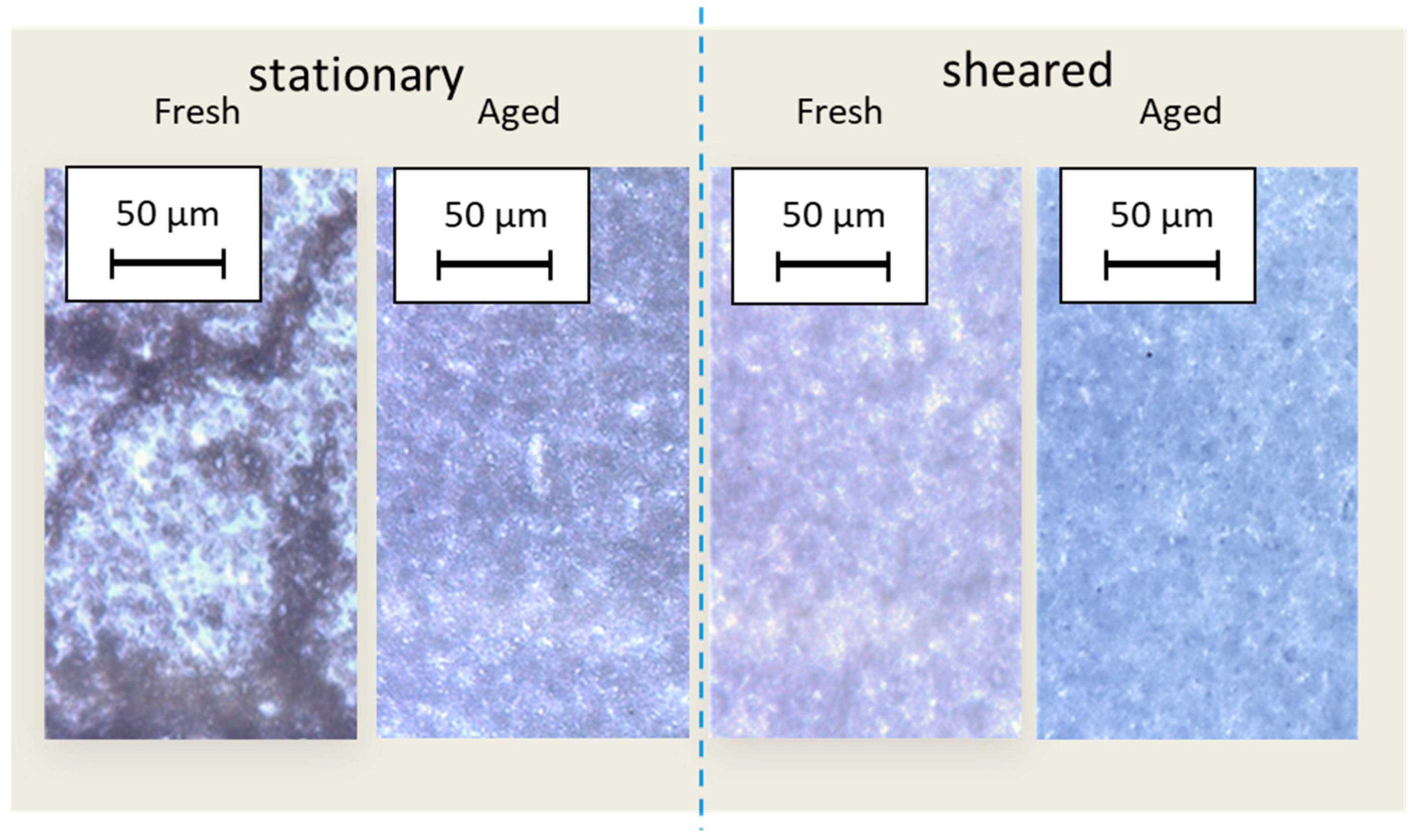
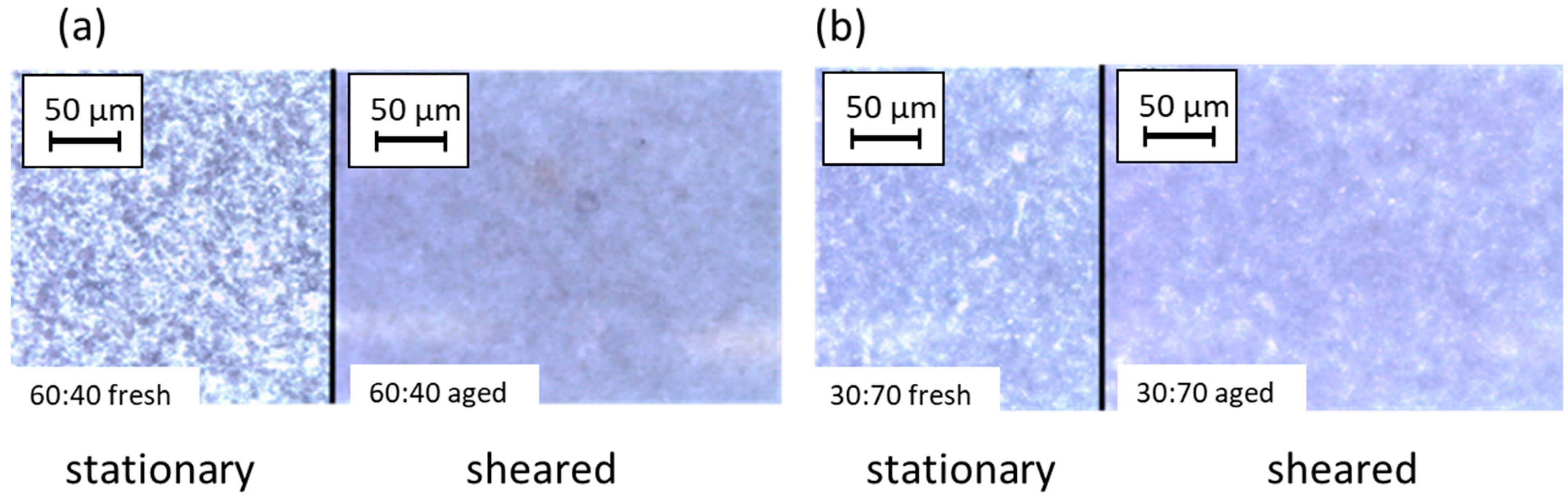
3.3. Microscopy Following the Structure Interactions
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Osong, S.H.; Norgren, S.; Engstrand, P. Processing of wood-based microfibrillated cellulose and nanofibrillated cellulose, and applications relating to papermaking: A review. Cellulose 2015, 23, 93–123. [Google Scholar] [CrossRef]
- Rantanen, J.; Dimic-Misic, K.; Kuusisto, J.; Maloney, T. The effect of micro and nanofibrillated cellulose water uptake on high filler content composite paper properties and furnish dewatering. Cellulose 2016, 22, 4003–4015. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Q. Recent development in applications of cellulose nanocrystals for advanced polymer-based nanocomposites by novel fabrication strategies. In Nanocrystals-Synthesis, Characterization and Applications; Neralla, S., Ed.; InTechOpen: London, UK, 2012; pp. 103–120. [Google Scholar] [CrossRef]
- Moud, A.A.; Milad, K.; Amir, S.-N.; Seyed, H.H. Suspensions and hydrogels of cellulose nanocrystals (CNCs): Characterization using microscopy and rheology. Cellulose 2022, 29, 3621–3653. [Google Scholar] [CrossRef]
- Le, E.A.; Wang, W. Improved Processing and Methods for Manufacturing Cellulose Nanocrystal Films. In Proceedings of the 2014 International Symposium on Optomechatronic Technologies, Seattle, WA, USA, 5–7 November 2014; pp. 337–341. [Google Scholar] [CrossRef]
- Shankaran, D.R. Chapter 14—Cellulose Nanocrystals for Health Care Applications. In Micro and Nano Technologies, Applications of Nanomaterials; Sneha, M.B., Oluwatobi, S.O., Nandakumar, K., Sabu, T., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 415–459. [Google Scholar]
- Hartmann, R.; Kinnunen, P.; Illikainen, M. Cellulose-mineral interactions based on the DLVO theory and their correlation with flotability. Min. Eng. 2018, 22, 44–52. [Google Scholar] [CrossRef]
- Hartmann, R.; Rinne, T.; Serna-Guerrero, R. On the Colloidal Behavior of Cellulose Nanocrystals as a Hydrophobization Reagent for Mineral Particles. Langmuir 2021, 37, 2322–2333. [Google Scholar] [CrossRef] [PubMed]
- Bendersky, M.; Santore, M.M.; Davis, J.M. Statistically-based DLVO approach to the dynamic interaction of colloidal microparticles with topographically and chemically heterogeneous collectors. J. Colloid Interface Sci. 2015, 449, 443–451. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Rojas, O.J. Colloidal stability and aggregation of lignocellulosic materials in aqueous suspension: A review. BioResources 2008, 3, 1419–1491. [Google Scholar]
- Siqueira, G.; Bras, J.; Dufresne, A. Cellulosic bionanocomposites: A review of preparation, properties and applications. Polymers 2010, 2, 728–765. [Google Scholar] [CrossRef]
- Siró, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Ambrogi, V. A New Challenge for the Old Excipient Calcium Carbonate: To Improve the Dissolution Rate of Poorly Soluble Drugs. Pharmaceutics 2023, 15, 300. [Google Scholar] [CrossRef]
- Watcharamul, S.; Lerddamrongchai, S.; Siripongpreda, T.; Rodtassana, C.; Nuisin, R.; Kiatkamjornwong, S. Effects of Carboxymethyl Cellulose/Nano-Calcium Carbonate Hydrogel Amendment of Loamy Sand Soil for Maize Growth. ACS Agric. Sci. Technol. 2022, 2, 1071–1080. [Google Scholar] [CrossRef]
- Schenker, M.; Schoelkopf, J.; Mangin, P.; Gane, P.A.C. Pigmented micro-nanofibrillated cellulose (MNFC) as packaging composite material: A first assessment. In Proceedings of the Tappi PaperCon 2015 Conference, Atlanta, GA, USA, 19–22 April 2015. [Google Scholar]
- Krajewska, B. Urease-aided calcium carbonate mineralization for engineering applications: A review. J. Adv. Res. 2018, 13, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.S.; Wang, F.; Becker, W.C.; Edwards, M.A. A 21st-Century Perspective on Calcium Carbonate Formation in Potable Water Systems. Environ. Eng. Sci. 2018, 35, 143–158. [Google Scholar] [CrossRef]
- Habibi, Y. Key advances in the chemical modification of nanocelluloses. Chem. Soc. Rev. 2014, 43, 1519–1542. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Revol, J.F.; Bradford, H.; Giasson, J.; Marchessault, R.H.; Gray, D.G. Helicoidal self-ordering of cellulose microfibrils in aqueous suspension. Int. J. Biol. Macromol. 1992, 14, 170–172. [Google Scholar] [CrossRef]
- Majoinen, J.; Kontturi, E.; Ikkala, O.; Gray, D.G. SEM imaging of chiral nematic films cast from cellulose nanocrystal suspensions. Cellulose 2012, 19, 1599–1605. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, Y.; Hu, J.; Yang, J.; Feng, F.; Yu, Y.; Liu, P.; Men, Y.; Zhang, J. Origin of vacuum-assisted chiral self-assembly of cellulose nanocrystals. Carbohydr. Polym. 2020, 245, 116459. [Google Scholar] [CrossRef]
- Dimic-Misic, K.; Hummel, M.; Paltakari, J.; Sixta, H.; Maloney, T.C.; Gane, P.A.C. From colloidal spheres to nanofibrils: Extensional flow properties of mineral pigment and mixtures with micro and nanofibrils under progressive double layer suppression. J. Colloid Interface Sci. 2015, 446, 31–43. [Google Scholar] [CrossRef]
- Molnes, S.N.; Paso, K.G.; Strand, S.; Syverud, K. The effects of pH, time and temperature on the stability and viscosity of cellulose nanocrystal (CNC) dispersions: Implications for use in enhanced oil recovery. Cellulose 2017, 24, 4479–4491. [Google Scholar] [CrossRef]
- Dimic-Misic, K.; Maloney, T.C.; Gane, P.A.C. Defining a strain-induced time constant for oriented low shear-induced structuring in high consistency MFC/NFC-filler composite suspensions. J. Appl. Polym. Sci. 2015, 132, 42827. [Google Scholar] [CrossRef]
- Khoshkava, V.; Kamal, M.R. Effect of drying conditions on cellulose nanocrystal (CNC) agglomerate porosity and dispersibility in polymer nanocomposites. Powder Technol. 2014, 261, 288–298. [Google Scholar] [CrossRef]
- Chaari, F.; Racineux, G.; Poitou, A.; Chaouche, M. Rheological behavior of sewage sludge and strain-induced dewatering. Rheol. Acta 2003, 42, 273–279. [Google Scholar] [CrossRef]
- Spicer, P.T.; Pratsinis, S.E.; Raper, J.; Amal, R.; Bushell, G.; Meesters, G. Effect of shear schedule on particle size, density, and structure during flocculation in stirred tanks. Powder Technol. 1998, 97, 26–34. [Google Scholar] [CrossRef]
- Liu, G.; Dimic-Misic, K.; Maloney, T.; Gane, P. Acid dissociation of surface bound water on cellulose nanofibrils in aqueous micro nanofibrillated cellulose (MNFC) gel revealed by adsorption of calcium carbonate nanoparticles under the application of ultralow shear. Cellulose 2017, 24, 3155–3178. [Google Scholar] [CrossRef]
- Martoïa, F.; Perge, C.; Dumont, P.J.; Orgéas, L.; Fardin, M.A.; Manneville, S.; Belgacem, M.N. Heterogeneous flow kinematics of cellulose nanofibril suspensions under shear. Soft Matter 2015, 11, 4742–4755. [Google Scholar] [CrossRef]
- Hogg, R. Flocculation and dewatering. Int. J. Miner. Process. 2000, 58, 223–236. [Google Scholar] [CrossRef]
- Tiller, F.M.; Yeh, C.S.; Tsai, C.D.; Chen, W. Generalised approach to thickening, filtration, and centrifugation. Filtr. Separat. 1987, 24, 121–126. [Google Scholar]
- Dentel, S.K.; Abu-Orf, M.M.; Walker, C.A. Optimization of slurry flocculation and dewatering based on electrokinetic and rheological phenomena. Chem. Eng. J. 2000, 801, 65–72. [Google Scholar] [CrossRef]
- Savage, N.; Diallo, M.S. Nanomaterials and Water Purification: Opportunities and Challenges. J. Nanoparticle Res. 2005, 7, 331–342. [Google Scholar] [CrossRef]
- O’Melia, C.R. Coagulation and sedimentation in lakes, reservoirs and water treatment plants. Water Sci. Technol. 1998, 37, 129–135. [Google Scholar] [CrossRef]
- Dimic-Misic, K.; Maloney, T.C.; Liu, G.; Gane, P. Micro nanofibrillated cellulose (MNFC) gel dewatering induced at ultralow-shear in presence of added colloidally-unstable particles. Cellulose 2017, 24, 1463–1481. [Google Scholar] [CrossRef]
- Mohtaschemi, M.; Dimic-Misic, K.; Puisto, A.; Korhonen, M.; Maloney, T.; Paltakari, J.; Alava, M.J. Rheological characterization of fibrillated cellulose suspensions via bucket vane viscometer. Cellulose 2014, 21, 1305–1312. [Google Scholar] [CrossRef]
- Haavisto, S.; Koponen, A.I.; Salmela, J. New insight into rheology and flow properties of complex fluids with Doppler optical coherence tomography. Front. Chem. 2014, 2, 27. [Google Scholar] [CrossRef] [PubMed]
- Fall, A.B.; Lindström, S.B.; Sundman, O.; Ödberg, L.; Wågberg, L. Colloidal stability of aqueous nanofibrillated cellulose dispersions. Langmuir 2011, 27, 11332–11338. [Google Scholar] [CrossRef]
- Dimic-Misic, K.; Buffiere, J.; Imani, M.; Nieminen, K.; Sixta, H.; Gane, P. Improved stabilisation of graphite nanoflake dispersions using hydrothermally-produced nanocellulose. Coll. Surf. A Physicochem. Eng. Aspects 2021, 610, 125668. [Google Scholar] [CrossRef]
- Dalpke, B.; Kerekes, R.J. The influence of fibre properties on the apparent yield stress of flocculated pulp suspensions. J. Pulp Pap. Sci. 2005, 31, 39–43. [Google Scholar]
- Horvath, A.E.; Lindström, T. The influence of colloidal interactions on fiber network strength. J. Colloid Interface Sci. 2007, 309, 511–517. [Google Scholar] [CrossRef]
- Serra, T.; Casamitjana, X. Structure of the aggregates during the process of aggregation and breakup under a shear flow. J. Colloid Interface Sci. 1998, 206, 505–511. [Google Scholar] [CrossRef]
- Khoshkava, V.; Kamal, M.R. Effect of Cellulose Nanocrystals (CNC) Particle Morphology on Dispersion and Rheological and Mechanical Properties of PP/CNC Nanocomposites. ACS Appl. Mater. Interfaces 2014, 6, 8146–8157. [Google Scholar] [CrossRef]
- Jin, H.; Nishiyama, Y.; Wada, M.; Kuga, S. Nanofibrillar cellulose aerogels. Coll. Surf. A Physicochem. Eng. Asp. 2004, 240, 63–67. [Google Scholar] [CrossRef]
- Unno, H.; Huang, X.; Akehata, T.; Hirasa, O. Gel dewatering process for biological slurry. In Polymer Gels; Springer: Berlin/Heidelberg, Germany, 1991; pp. 183–192. [Google Scholar]
- Dimic-Misic, K.; Puisto, A.; Paltakari, J.; Alava, M.; Maloney, T.C. The influence of shear on the dewatering of high consistency nanofibrillated cellulose furnishes. Cellulose 2013, 20, 1853–1864. [Google Scholar] [CrossRef]
- Mikkelsen, L.H.; Mascarenhas, T.; Nielsen, P.H. Key parameters in sludge dewatering: Testing for the shear sensitivity and EPS content. Water Sci. Technol. 2002, 46, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Dimic-Misic, K.; Rantanen, J.; Maloney, T.C.; Gane, P.A.C. Gel structure phase behavior in micro nanofibrillated cellulose containing in situ precipitated calcium carbonate. J. Appl. Polym. Sci. 2016, 133, 43486. [Google Scholar] [CrossRef]
- Gebauer, D.; Oliynyk, V.; Salajkova, M.; Sort, J.; Zhou, Q.; Bergström, L.; Salazar-Alvarez, G. A transparent hybrid of nanocrystalline cellulose and amorphous calcium carbonate nanoparticles. Nanoscale 2011, 3, 3563–3566. [Google Scholar] [CrossRef]




| CNC:UGCC Ratio | Total Solids Content Fraction in Suspension (w/w%) | CNC Equivalent Solid Material (g) | CNC Fraction in Suspension (w/w%) | UGCC Equivalent Solid Material (g) | UGCC Fraction in Suspension (w/w%) | Total Equivalent Solid Material (g) |
|---|---|---|---|---|---|---|
| 70:30 | 4.03 | 0.70 | 2.82 | 0.30 | 1.21 | 24.83 |
| 60:40 | 4.55 | 0.60 | 2.73 | 0.40 | 1.82 | 22.00 |
| 50:50 | 5.22 | 0.50 | 2.61 | 0.50 | 2.61 | 19.17 |
| 40:60 | 6.12 | 0.40 | 2.45 | 0.60 | 3.67 | 16.33 |
| 30:70 | 7.41 | 0.30 | 2.22 | 0.70 | 5.19 | 13.50 |
| Component Fraction CNC:UGCC Based on Solid Weight Ratio | Total Solids Content (w/w%) | Fresh Samples (1 h at Room Temperature) | Intermediate Samples (24 h Refrigerated) | Aged Samples (72 h Refrigerated) |
|---|---|---|---|---|
| 100:0 | 3.00 |  | ||
| 70:30 | 4.03 | |||
| 60:40 | 4.55 | |||
| 50:50 | 5.12 | |||
| 40:60 | 6.12 | |||
| 30:70 | 7.41 | |||
| Material | ζ (mV) | pH |
|---|---|---|
| CNC | −27.6 | 4.6 |
| UGCC | +0.5 | 10.2 |
| Sample CNC:UGCC | Viscoelastic Moduli at 0.01 (rad) s−1 | Viscoelastic Moduli at 80 (rad) s−1 | ||
|---|---|---|---|---|
| G′ (Pa) | G″ (Pa) | G′ (Pa) | G″ (Pa) | |
| 70:30 fresh | 95.3 | 24.4 | 3127.2 | 356.5 |
| 70:30 intermediate | 113.1 | 32.2 | 1789.5 | 543.3 |
| 70:30 aged | 813.8 | 96.9 | 556.3 | 19.8 |
| 60:40 fresh | 92.1 | 16.9 | 2256.5 | 194.3 |
| 60:40 intermediate | 104.9 | 19.6 | 1398.3 | 287.2 |
| 60:40 aged | 792.4 | 87.6 | 489.9 | 93.6 |
| 50:50 fresh | 82.8 | 11.3 | 1367.3 | 162.5 |
| 50:50 intermediate | 92.5 | 17.3 | 1334.6 | 235.7 |
| 50:50 aged | 728.2 | 65.0 | 421.8 | 83.6 |
| 40:60 fresh | 72.9 | 8.9 | 1268.2 | 92.6 |
| 40:60 intermediate | 84.3 | 9.9 | 1298.4 | 112.9 |
| 40:60 aged | 698.6 | 54.0 | 387.7 | 72.8 |
| 30:70 fresh | 68.5 | 7.8 | 1178.2 | 89.1 |
| 30:70 intermediate | 72.6 | 9.3 | 1267.5 | 92.4 |
| 30:70 aged | 567.4 | 35.5 | 324.8 | 69.7 |
| Sample CNC:UGCC | Complex Viscosity (Pa s) | ||
|---|---|---|---|
| η* at 0.01 (rad) s−1 | η* at 1 (rad) s−1 | η* at 100 (rad) s−1 | |
| 70:30 fresh | 398.25 | 825.47 | 8376.52 |
| 70:30 intermediate | 642.14 | 792.23 | 1428.13 |
| 70:30 aged | 1244.41 | 728.16 | 426.54 |
| 60:40 fresh | 336.34 | 728.32 | 7263.28 |
| 60:40 intermediate | 525.82 | 715.38 | 1361.12 |
| 60:40 aged | 989.72 | 531.52 | 271.49 |
| 50:50 fresh | 290.30 | 688.38 | 6374.82 |
| 50:50 intermediate | 493.06 | 614.52 | 1254.43 |
| 50:50 aged | 917.65 | 509.51 | 383.17 |
| 40:60 fresh | 81.46 | 662.53 | 2621.36 |
| 40:60 intermediate | 398.56 | 595.26 | 1108.74 |
| 40:60 aged | 884.45 | 594.12 | 465.31 |
| 30:70 fresh | 79.56 | 749.98 | 2372.93 |
| 30:70 intermediate | 382.78 | 574.74 | 957.26 |
| 30:70 aged | 921.35 | 467.24 | 384.23 |
| Sample CNF:UGCC | Transient Viscosity (Pa s) = 0.01 s−1 | |
|---|---|---|
| η+t= 0 (η0+) | η+t= 1 000 | |
| 70:30 fresh | 159.35 | 3549.98 |
| 70:30 intermediate | 1295.78 | 2281.74 |
| 70:30 aged | 4121.35 | 5250.23 |
| 60:40 fresh | 236.34 | 978.23 |
| 60:40 intermediate | 1745.62 | 3876.34 |
| 60:40 aged | 3317.42 | 5181.82 |
| 50:50 fresh | 98.30 | 1288.81 |
| 50:50 intermediate | 733.02 | 1481.56 |
| 50:50 aged | 3676.65 | 3361.84 |
| 40:60 fresh | 65.56 | 1160.57 |
| 40:60 intermediate | 294.16 | 1275.23 |
| 40:60 aged | 3502.23 | 3129.21 |
| 30:70 fresh | 46.65 | 1095.23 |
| 30:70 intermediate | 78.32 | 235.57 |
| 30:70 aged | 13,292.17 | 117.84 |
| Sample CNC:UGCC | (at Effective Zero Shear) | k | n | ||
|---|---|---|---|---|---|
| (s−1) | (Pa s) | (Pa s) | (Pa sn) | ||
| 70:30 fresh | 0.065 | 718.5 | 687.6 | 78 | 0.92 |
| 70:30 intermediate | 0.078 | 840.6 | 722.4 | 144 | 0.87 |
| 70:30 aged | 0.163 | 4423.7 | 2871.5 | 287 | 0.85 |
| 60:40 fresh | 0.052 | 687.6 | 645.5 | 71 | 0.87 |
| 60:40 intermediate | 0.064 | 747.2 | 686.4 | 88 | 0.85 |
| 60:40 aged | 0.125 | 3523.3 | 2165.6 | 210 | 0.84 |
| 50:50 fresh | 0.004 | 615.3 | 560.5 | 68 | 0.82 |
| 50:50 intermediate | 0.008 | 633.1 | 578.5 | 85 | 0.80 |
| 50:50 aged | 0.010 | 2911.8 | 1786.7 | 188 | 0.78 |
| 40:60 fresh | 0.002 | 556.6 | 438.8 | 62 | 0.76 |
| 40:60 intermediate | 0.006 | 598.5 | 489.7 | 81 | 0.75 |
| 40:60 aged | 0.008 | 613.5 | 46.3 | 178 | 0.73 |
| 30:70 fresh | 0.002 | 425.0 | 378.5 | 58 | 0.72 |
| 30:70 intermediate | 0.003 | 515.2 | 478.2 | 72 | 0.71 |
| 30:70 aged | 0.009 | 689.0 | 589.3 | 145 | 0.67 |
| Sample CNC:UGCC | Third 3ITT Interval |
|---|---|
| Recovery Time for Transient Viscosity (s) | |
| 30:70 fresh | 2489 |
| 30:70 intermediate | 1578 |
| 30:70 aged | 1439 |
| 40:60 fresh | 2649 |
| 40:60 intermediate | 1388 |
| 40:60 aged | 1321 |
| 50:50 fresh | 4885 |
| 50:50 intermediate | 3457 |
| 50:50 aged | 2954 |
| 60:40 fresh | 2678 |
| 60:40 intermediate | 2768 |
| 60:40 aged | 2013 |
| 70:30 fresh | 2666 |
| 70:30 intermediate | 3100 |
| 70:30 aged | 3350 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liukko, S.; Dimic-Misic, K.; Ge, Y.; Gane, P. Heterogeneous Hierarchical Self-Assembly Forming Crystalline Nanocellulose–CaCO3 Hybrid Nanoparticle Biocomposites. J. Compos. Sci. 2023, 7, 333. https://doi.org/10.3390/jcs7080333
Liukko S, Dimic-Misic K, Ge Y, Gane P. Heterogeneous Hierarchical Self-Assembly Forming Crystalline Nanocellulose–CaCO3 Hybrid Nanoparticle Biocomposites. Journal of Composites Science. 2023; 7(8):333. https://doi.org/10.3390/jcs7080333
Chicago/Turabian StyleLiukko, Sirje, Katarina Dimic-Misic, Yanling Ge, and Patrick Gane. 2023. "Heterogeneous Hierarchical Self-Assembly Forming Crystalline Nanocellulose–CaCO3 Hybrid Nanoparticle Biocomposites" Journal of Composites Science 7, no. 8: 333. https://doi.org/10.3390/jcs7080333





