Tuning the Tensile and Shear Properties of a Scar Healing Composite for Mechanotherapy
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials and Rationale for Our Choice of Materials
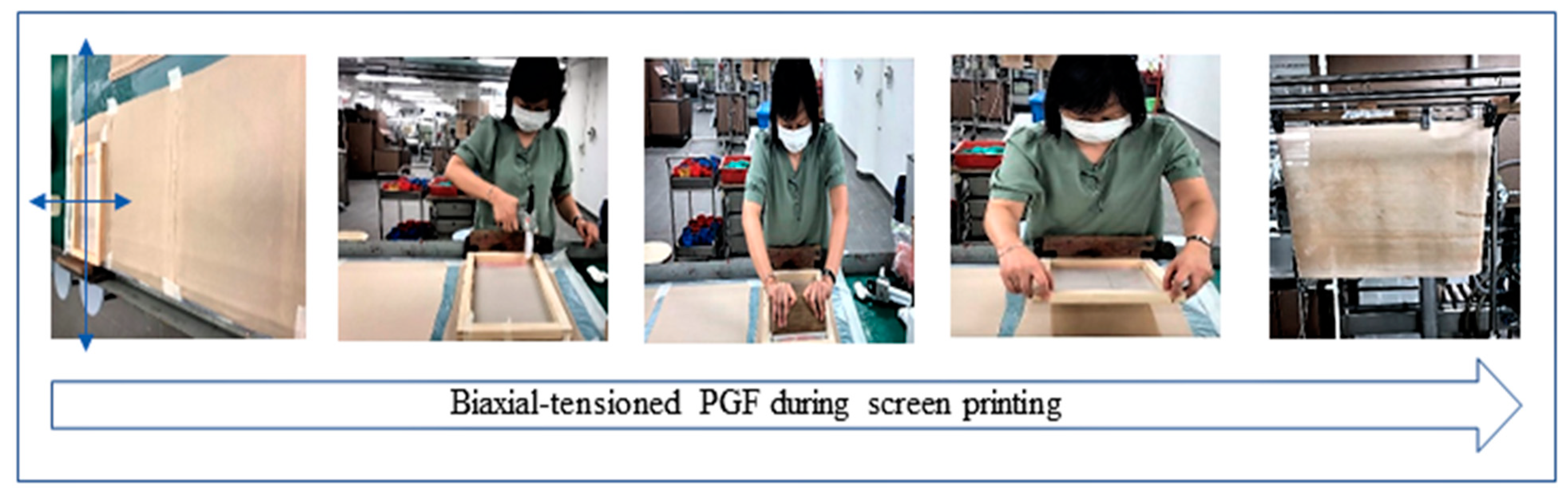
2.2. Preparation of PGF-Biopor®AB
2.3. Mechanical Property Tunability
2.4. Surface and Compression Properties
3. Results and Discussion
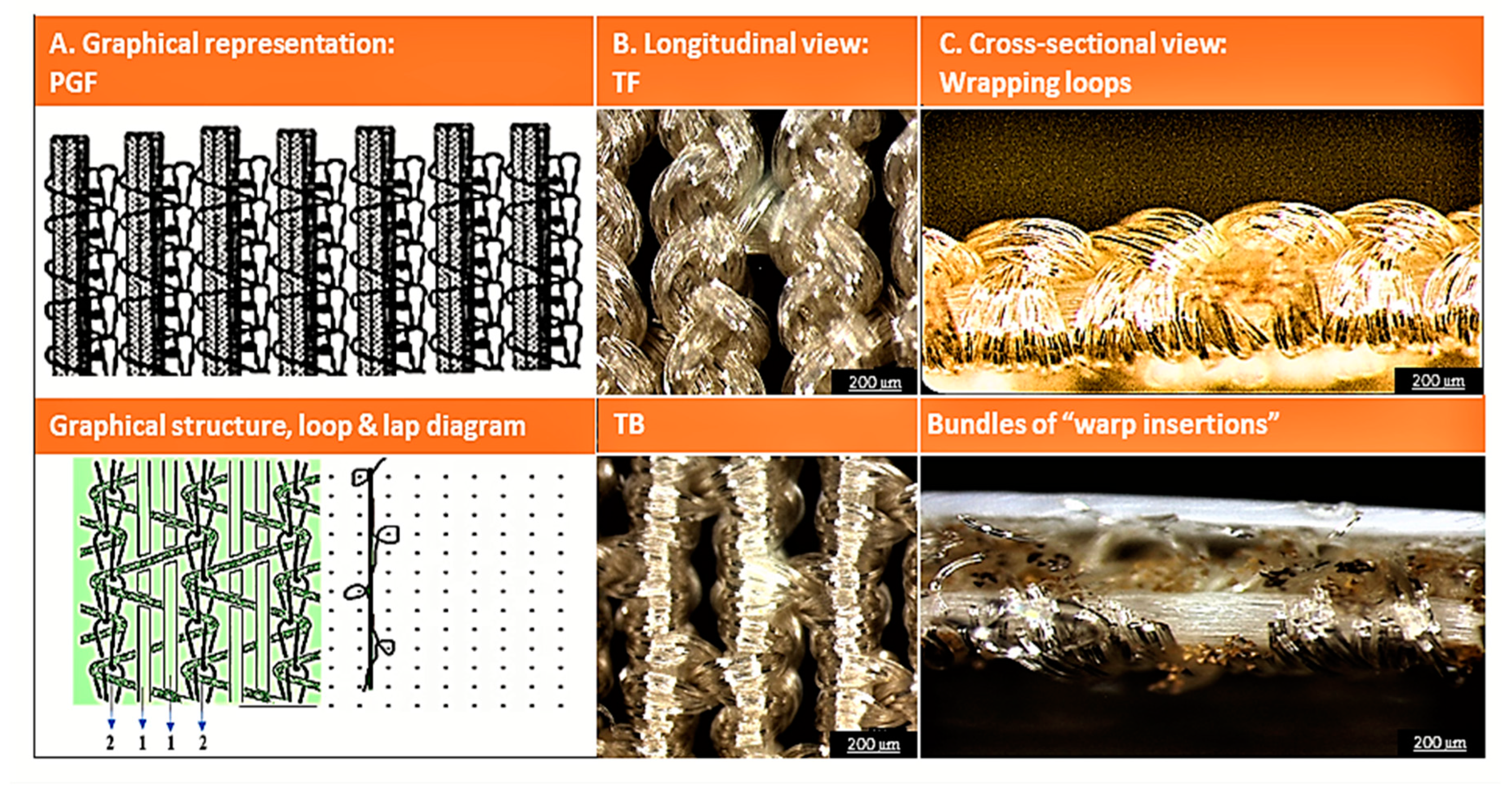
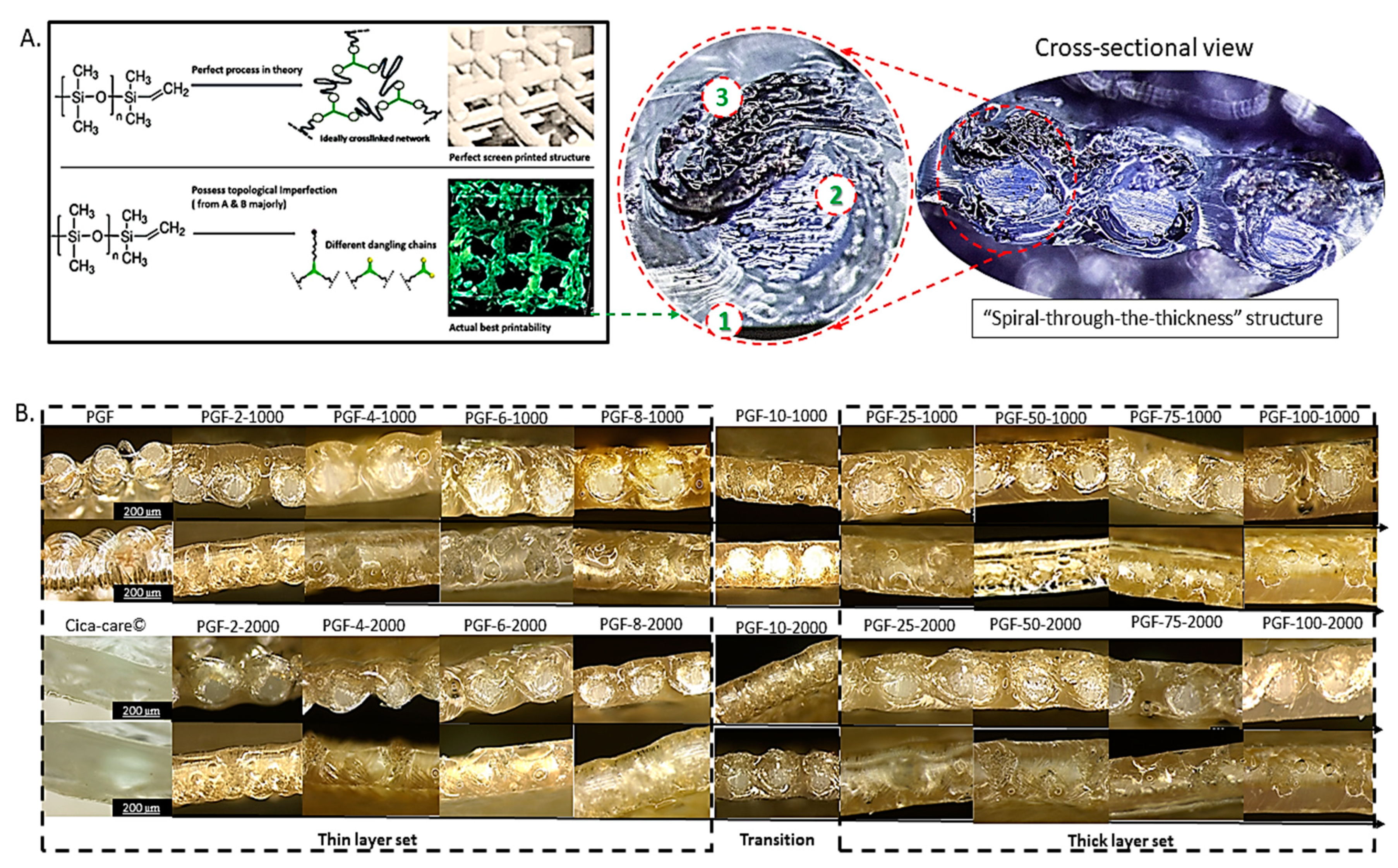
3.1. Structural Analysis
3.1.1. Topological Features
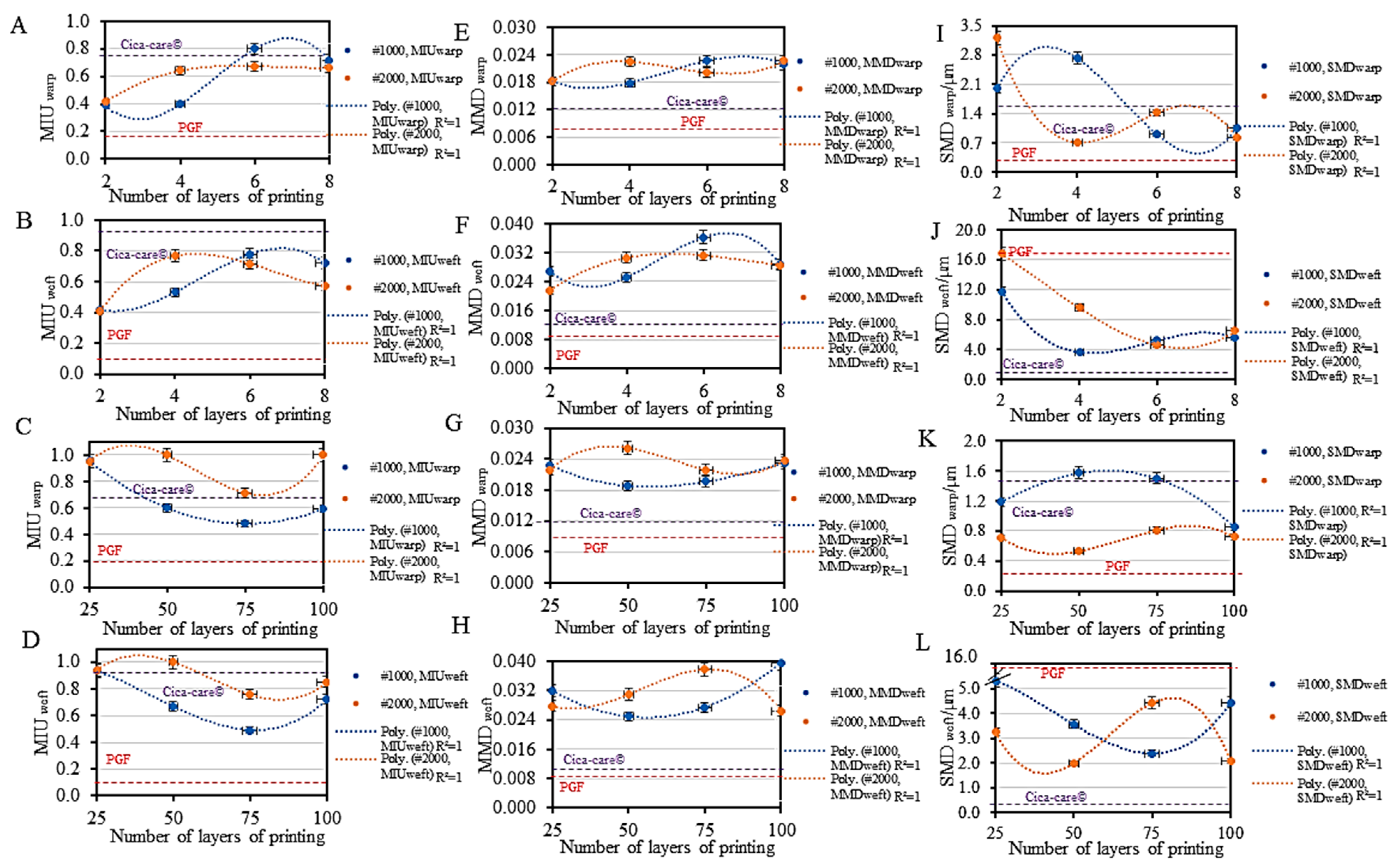
3.1.2. Surface Friction
3.2. Mechanical Property Tunability
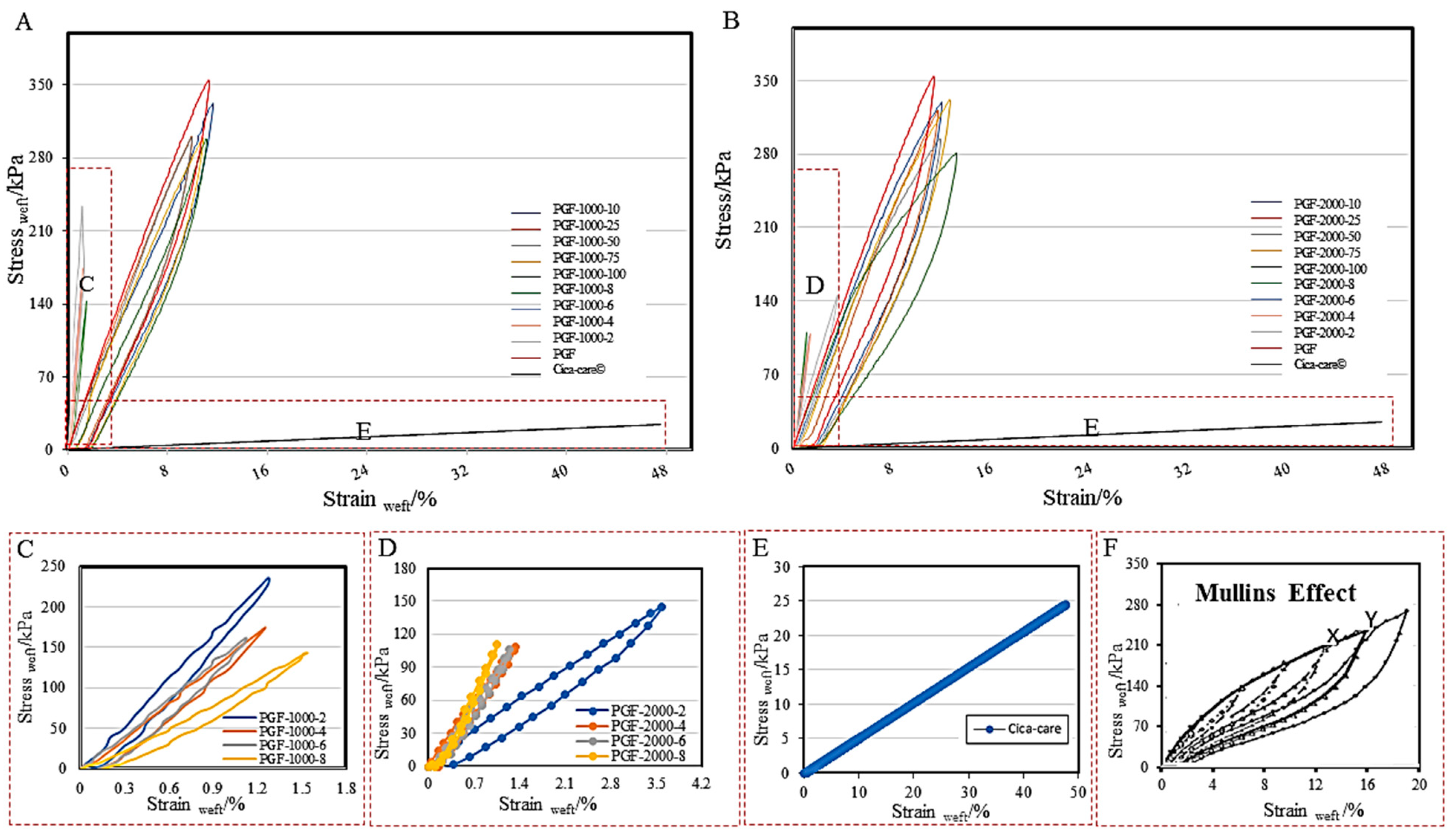
3.2.1. Tensile Tunability
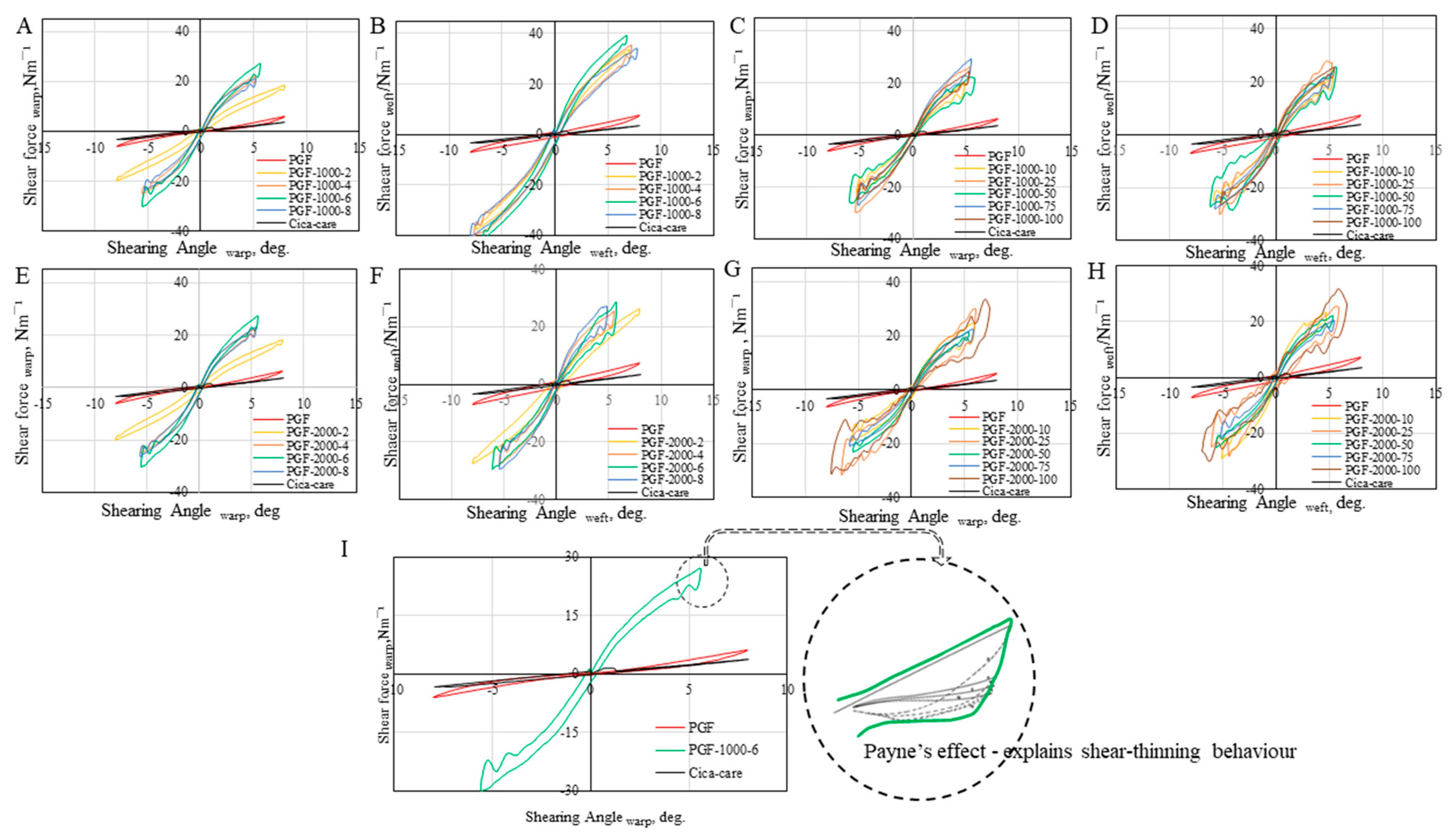
3.2.2. Shear Tunability
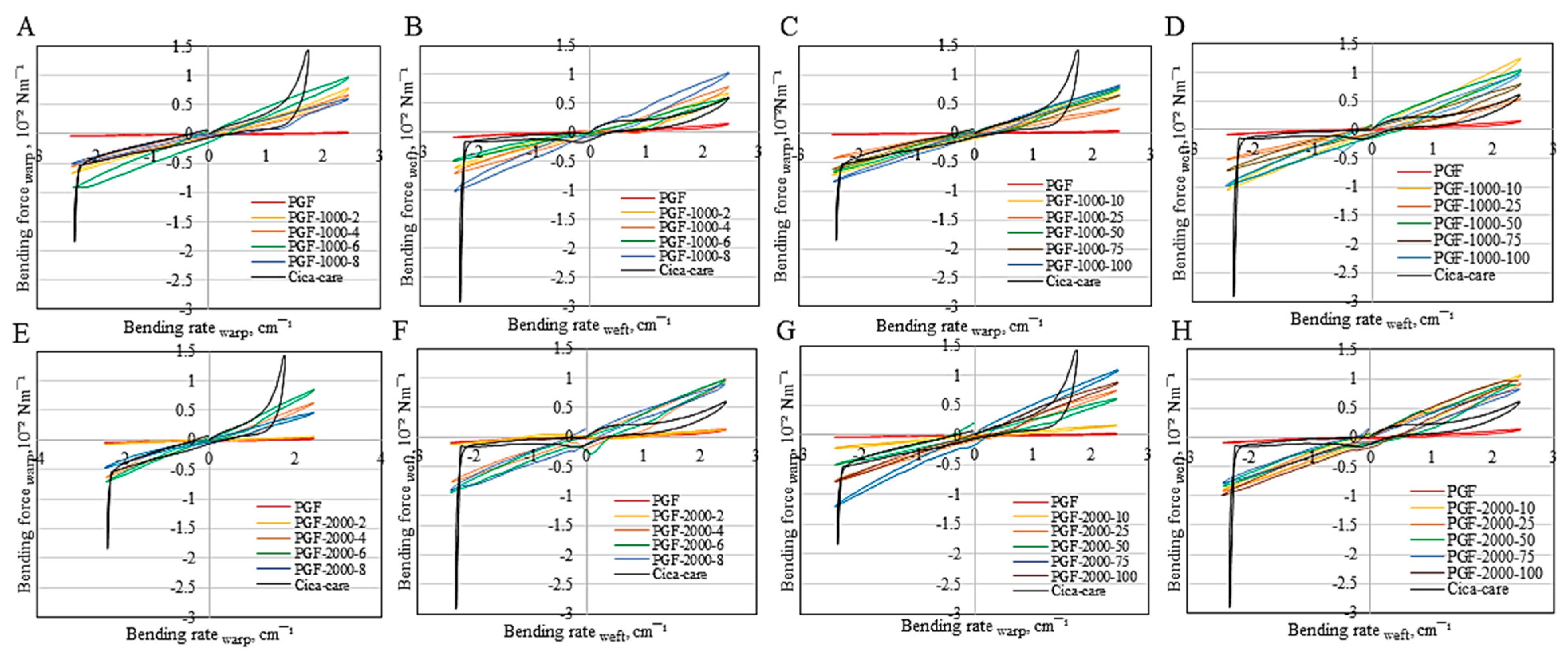
3.2.3. Non-Tuneable Bending Properties
3.3. Mechano-Therapeutic Performance
3.3.1. Load Sharing in Internal Pressure Redistribution
3.3.2. Force Reduction upon Tension Shielding and Pressure Redistribution
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghosh, T.K.; Zhou, N. Characterization of fabric bending behaviour: A review of measurement principles. Indian J. Fibre Text. Res. 2003, 28, 471–476. [Google Scholar]
- Davidson, M.D.; Burdick, J.A.; Wells, R.G. Engineered Biomaterial Platforms to Study Fibrosis. Adv. Healthc. Mater. 2020, 9, 1901682. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.G.; da Silva, L.P.; Marques, A.P. Skin mechanobiology and biomechanics: From homeostasis to wound healing. In Advances in Biomechanics and Tissue Regeneration; Academic Press: Cambridge, MA, USA, 2019; pp. 343–360. [Google Scholar]
- Swanson, E. Tension shielding with the embrace device: Does it really improve scars? Plast. Reconstr. Surg. 2014, 134, 662e–664e. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Brown, J.; Khosrotehrani, K.; Bayat, A.; Shafiee, A. Skin biomechanics: A potential therapeutic intervention target to reduce scarring. Burn. Trauma 2022, 10, tkac036. [Google Scholar] [CrossRef] [PubMed]
- Barnes, L.A.; Marshall, C.D.; Leavitt, T.; Hu, M.S.; Moore, A.L.; Gonzalez, J.G.; Longaker, M.T.; Gurtner, G.C. Mechanical Forces in Cutaneous Wound Healing: Emerging Therapies to Minimize Scar Formation. Adv. Wound Care 2018, 7, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.K.; Lin, H.H.; Hans, I.; Harn, C.; Hughes, M.W.; Tang, M.J.; Yang, C.C. Mechanical forces in skin disorders. J. Dermatol. Sci. 2018, 90, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Kuehlmann, B.; Bonham, C.A.; Zucal, I.; Prantl, L.; Gurtner, G.C. Mechanotransduction in wound healing and fibrosis. J. Clin. Med. 2020, 9, 1423. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Holfeld, J.; Schaden, W.; Orgill, D.; Ogawa, R. Mechanotherapy: Revisiting physical therapy and recruiting mechanobiology for a new era in medicine. Trends Mol. Med. 2013, 19, 555–564. [Google Scholar] [CrossRef]
- Seo, B.R.; Mooney, D.J. Recent and future strategies of mechanotherapy for tissue regenerative rehabilitation. ACS Biomater. Sci. Eng. 2022, 8, 4639–4642. [Google Scholar] [CrossRef]
- Ma, S.; Tang, Q.; Han, X.; Feng, Q.; Song, J.; Setchi, R.; Liu, Y.; Liu, Y.; Goulas, A.; Engstrøm, D.S.; et al. Manufacturability, mechanical properties, mass-transport properties and biocompatibility of triply periodic minimal surface (TPMS) porous scaffolds fabricated by selective laser melting. Mater. Des. 2020, 195, 109034. [Google Scholar] [CrossRef]
- Chan, C.K.; Jiang, X.Y.; Liew, K.L.; Chan, L.K.; Wong, W.K.; Lau, M.P. Evaluation of mechanical properties of uniform fabrics in garment manufacturing. J. Mater. Process. Technol. 2006, 174, 183–189. [Google Scholar] [CrossRef]
- Fletcher, J. Pressure ulcer education 4: Selection and use of support surfaces. Nurs. Times 2020, 116, 41–43. [Google Scholar]
- Gómez-González, M.; Latorre, E.; Arroyo, M.; Trepat, X. Measuring mechanical stress in living tissues. Nat. Rev. Phys. 2020, 2, 300–317. [Google Scholar] [CrossRef]
- Forbes, K.J.; Hjortsoe, I.M.; Nenova, T. The Shocks Matter: Improving Our Estimates of Exchange Rate Pass-Through. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=2689737 (accessed on 10 November 2023).
- Fernandes, M.G.; da Silva, L.P.; Cerqueira, M.T.; Ibañez, R.; Murphy, C.M.; Reis, R.L.; Marques, A.P. Mechanomodulatory biomaterials prospects in scar prevention and treatment. Acta Biomater. 2022, 150, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Klare, M. Elastomer Materials. Germany Patent DE 10 2011 115 061 A1, 11 April 2013. [Google Scholar]
- Chang, S.I.; Yoon, J.B. Shape-controlled, high fill-factor microlens arrays fabricated by a 3D diffuser lithography and plastic replication method. Opt. Express 2004, 12, 6366–6371. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Yang, H.K.; Ozcam, A.E.; Efimenko, K.; Weiger, M.C.; Genzer, J.; Haugh, J.M. Poly (vinylmethylsilox ane) elastomer networks as functional materials for cell adhesion and migration studies. Biomacromolecules 2011, 12, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, R. Keloid and hypertrophic scars are the result of chronic inflammation in the reticular dermis. Int. J. Mol. Sci. 2017, 18, 606. [Google Scholar] [CrossRef]
- Puzey, G. The use of pressure garments on hypertrophic scars. J. Tissue Viability 2002, 2002, 11–15. [Google Scholar] [CrossRef]
- Lui, K.C.; Noor, N.; Kan, C.W.; Wang, X. A self-pumping composite dressing improved hypertrophic scar healing with dual therapy and active-fluid transport. J. Compos. Sci. 2023, 7, 192. [Google Scholar] [CrossRef]
- Ly, N.G.; Denby, E.F. A CSIRO inter-laboratory trial of the KES-F for measuring fabric properties. J. Text. Inst. 1988, 79, 198–219. [Google Scholar] [CrossRef]
- McNeely, M.R.; Sputea, M.K.; Tusneem, N.A.; Oliphant, A.R. Sample processing with hydrophobic microfluidics. J. Assoc. Lab. Autom. 1999, 4, 30–33. [Google Scholar] [CrossRef]
- Hong, S.; Minary-Jolandan, M.; Naraghi, M. Controlling the wettability and adhesion of carbon fibres with polymer interfaces via grafted nanofibers. Compos. Sci. Technol. 2015, 117, 130–138. [Google Scholar] [CrossRef]
- Andersson, C.H.; Dartman, T.; Gredinger, P.; Asplund, J.; Strandqvist, H. Flexible composites, strength, deformation, and fracture processes. 1. reinforcement structures and tensile strength. Mech. Compos. Mater. 1998, 34, 525–536. [Google Scholar] [CrossRef]
- Huang, H.; Kamm, R.D.; Lee, R.T. Cell mechanics and mechanotransduction: Pathways, probes, and physiology. Am. J. Physiol.-Cell Physiol. 2004, 287, C1–C11. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, S.; Gale, R. Skin adhesives and skin adhesion: 1. Transdermal drug delivery systems. Biomaterials 1998, 19, 1119–1136. [Google Scholar] [CrossRef]
- Verdier-Sévrain, S.; Bonté, F. Skin hydration: A review on its molecular mechanisms. J. Cosmet. Dermatol. 2007, 6, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Brown, G.O.; Burris, D.L.; Korley, L.T.; Epps, T.H., III. Coating architects: Manipulating multiscale structures to optimize interfacial properties for coating applications. Am. Chem. Soc. Appl. Polym. Mater. 2019, 1, 2249–2266. [Google Scholar] [CrossRef]
- Lahey, T.J.; Heppler, G.R. Mechanical modelling of fabrics in bending. J. Appl. Mech. 2004, 71, 32–40. [Google Scholar] [CrossRef]
- Owen, M.J.; Dvornic, P.R. (Eds.) Silicone Surface Science, Advances in Silicon Science 4; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Kawabata, S.; Niwa, M. Objective measurement of fabric mechanical property and quality: Its application to textile and clothing manufacturing. Int. J. Cloth. Sci. Technol. 1991, 3, 7–18. [Google Scholar] [CrossRef]
- Stein, J.; Prutzman, L.C. Stress relaxation studies of model silicone RTV networks. J. Appl. Polym. Sci. 1988, 36, 511–521. [Google Scholar] [CrossRef]
- Xu, R.; Luo, G.; Xia, H.; He, W.; Zhao, J.; Liu, B.; Tan, J.; Zhou, J.; Liu, D.; Wang, Y.; et al. Novel bilayer wound dressing composed of silicone rubber with particular micropores enhanced wound re-epithelialization and contraction. Biomaterials 2015, 40, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Akovali, G. (Ed.) Handbook of Composite Fabrication; Smithers Rapra Publishing: Gaithersburg, MD, USA, 2001. [Google Scholar]
- Jang, K.I.; Chung, H.U.; Xu, S.; Lee, C.H.; Luan, H.; Jeong, J.; Cheng, H.; Kim, G.; Han, S.Y.; Lee, J.W.; et al. Soft network composite materials with deterministic and bio-inspired designs. Nat. Commun. 2015, 6, 6566. [Google Scholar] [CrossRef]
- Aljghami, M.E.; Saboor, S.; Amini-Nik, S. Emerging innovative wound dressings. Ann. Biomed. Eng. 2019, 47, 659–675. [Google Scholar] [CrossRef] [PubMed]
- Kong, N.; Peng, Q.; Li, H. Rationally designed dynamic protein hydrogels with reversibly tunable mechanical properties. Adv. Funct. Mater. 2014, 24, 7310–7317. [Google Scholar] [CrossRef]
- Cordeiro, J.M.; Nagay, B.E.; Dini, C.; Souza, J.G.; Rangel, E.C.; da Cruz, N.C.; Yang, F.; van den Beucken, J.J.J.P.; Barão, V.A. Copper source determines chemistry and topography of implant coatings to optimally couple cellular responses and antibacterial activity. Biomater. Adv. 2022, 134, 112550. [Google Scholar] [CrossRef] [PubMed]
- Fuard, D.; Tzvetkova-Chevolleau, T.; Decossas, S.; Tracqui, P.; Schiavone, P. Optimization of poly-di-methyl-siloxane (PDMS) substrates for studying cellular adhesion and motility. Microelectron. Eng. 2008, 85, 1289–1293. [Google Scholar] [CrossRef]
- De Tommasi, D.; Puglisi, G.; Saccomandi, G. A micromechanics-based model for the Mullins effect. J. Rheol. 2006, 50, 495–512. [Google Scholar] [CrossRef]
- Ogden, R.W.; Roxburgh, D.G. A pseudo–elastic model for the Mullins effect in filled rubber. Proc. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 1999, 455, 2861–2877. [Google Scholar] [CrossRef]
- Piel, M.; Théry, M. Micropatterning in Cell Biology, Part C (Chapter 3); Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Rehman, A.; Jabbar, M.; Umair, M.; Nawab, Y.; Jabbar, M.; Shaker, K. A study on the interdependence of fabric pore size and its mechanical and comfort properties. J. Nat. Fibers 2019, 16, 795–805. [Google Scholar] [CrossRef]
- Qian, W.; Hu, X.; He, W.; Zhan, R.; Liu, M.; Zhou, D.; Huang, Y.; Hu, X.; Wang, Z.; Fei, G.; et al. Polydimethylsiloxane incorporated with reduced graphene oxide (rGO) sheets for wound dressing application: Preparation and characterization. Colloids Surf. B: Biointerfaces 2018, 166, 61–71. [Google Scholar] [CrossRef]
- Chen, J.; Irianto, J.; Inamdar, S.; Pravincumar, P.; Lee, D.A.; Bader, D.L.; Knight, M.M. Cell mechanics, structure, and function are regulated by the stiffness of the three-dimensional microenvironment. Biophys. J. 2012, 103, 1188–1197. [Google Scholar] [CrossRef] [PubMed]
- Thostenson, E.T.; Chou, T.W. Aligned multi-walled carbon nanotube-reinforced composites: Processing and mechanical characterization. J. Phys. D Appl. Phys. 2002, 35, L77. [Google Scholar] [CrossRef]
- Santos, E.; Orive, G.; Hernández, R.M.; Pedraz, J.L. Cell-Biomaterial Interaction: Strategies to Mimic the Extracellular Matrix. In On Biomimetics; IntechOpen: London, UK, 2011. [Google Scholar]
- Sarkar, D.; Zhao, W.; Schaefer, S.; Ankrum, J.A.; Teo, G.S.; Pereira, M.N.; Lino Ferreira Karp, J.M. Overview of tissue engineering concepts and applications. In Biomaterials Science; Academic Press: Cambridge, MA, USA, 2013; pp. 1122–1137. [Google Scholar]
- Jorba, I.; Beltrán, G.; Falcones, B.; Suki, B.; Farré, R.; García-Aznar, J.M.; Navajas, D. Nonlinear elasticity of the lung extracellular microenvironment is regulated by macroscale tissue strain. Acta Biomater. 2019, 92, 265–276. [Google Scholar] [CrossRef]
- Mukhopadhyay, M. Mechanics of Composite Materials and Structures; Universities Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Ahmadzadeh, S.M.H.; Hukins, D.W. Feasibility of using mixtures of silicone elastomers and silicone oils to model the mechanical behaviour of biological tissues. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2014, 228, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Harwood, J.A.C.; Mullins, L.; Payne, A.R. Stress softening in natural rubber vulcanizates. Part II. Stress softening effects in pure gum and filler loaded rubbers. J. Appl. Polym. Sci. 1965, 9, 3011–3021. [Google Scholar] [CrossRef]
- Yunus, D.E.; He, R.; Shi, W.; Kaya, O.; Liu, Y. Short fibre reinforced 3D printed ceramic composite with shear induced alignment. Ceram. Int. 2017, 43, 11766–11772. [Google Scholar] [CrossRef]
- Renò, F.; Sabbatini, M.; Lombardi, F.; Stella, M.; Pezzuto, C.; Magliacani, G.; Cannas, M. In vitro mechanical compression induces apoptosis and regulates cytokines release in hypertrophic scars. Wound Repair Regen. 2003, 11, 331–336. [Google Scholar] [CrossRef]
- Lomov, S.V.; Belov, E.B.; Bischoff, T.; Ghosh, S.B.; Chi, T.T.; Verpoest, I. Carbon composites based on multiaxial multiply stitched preforms. Part 1. Geometry of the preform. Compos. Part A Appl. Sci. Manuf. 2002, 33, 1171–1183. [Google Scholar] [CrossRef]
- Krupp, T.; dos Santos, B.D.; Gama, L.A.; Silva, J.R.; Arrais-Silva, W.W.; de Souza, N.C.; Américo, M.F.; de Souza Souto, P.C. Natural rubber-propolis membrane improves wound healing in second-degree burning model. Int. J. Biol. Macromol. 2019, 131, 980–988. [Google Scholar] [CrossRef]
- Song, S.; Drotlef, D.M.; Majidi, C.; Sitti, M. Controllable load sharing for soft adhesive interfaces on three-dimensional surfaces. Proc. Natl. Acad. Sci. USA 2017, 114, E4344–E4353. [Google Scholar] [CrossRef]
- Gupta, A.; Mahesh, S.; Keralavarma, S.M. Strength distribution of large unidirectional composite patches with realistic load sharing. Phys. Rev. E 2017, 96, 043002. [Google Scholar] [CrossRef] [PubMed]
- Habeeb, C.I.; Mahesh, S. Strength distribution of planar local load-sharing bundles. Phys. Rev. E 2015, 92, 022125. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Bari, M.M.; McQueen, R.H.; Nguyen, H.; Wismer, W.V.; de la Mata, A.P.; Harynuk, J.J. Synthetic clothing and the problem with odour: Comparison of nylon and polyester fabrics. Cloth. Text. Res. J. 2018, 36, 251–266. [Google Scholar] [CrossRef]



| Catalyst Component (A) | Basic Component (B) |
|---|---|
| 58.9% by weight of vinyl-end polydimethylsiloxane 8.9 wt.% of a polydimethylsiloxane oil 12.0% by weight of a paraffin oil 21.7% by weight of a fumed silica (Surface-treated) with a BET in surface area > 50 m2/g 0.4% by weight of a platinum catalyst | 54.4% by weight of vinyl-end polydimethylsiloxane (with viscosities of 150–65 K mPas) 3.1% by weight of a polydimethylsiloxane oil 7.6% by weight of a paraffin oil 21.2 wt.% Of a fumed silica (surface-treated) with a BET in surface area > 50 m2/g 11.8% by weight of a polymethyl hydrogen siloxane having a SiH content of 2.3 mmol/g |
| The procedure of manufacture: a mixture of base component (A + B) and catalyst, each individually mixed homogeneously for 30 min. Each A and B mixture (A:B = 1:1) was then degassed in a vacuum for 15 min. | |
| SAMPLES | TSwarp stress/KPA | TSweft stress/KPA | TSwarp strain/% | TSweft strain/% | Ewarp/ KPA | Eweft/ KPA |
|---|---|---|---|---|---|---|
| CICA-CARE® | 4.94 | 41.94 | 12.87 | 12.99 | 0.065 | 1.00 |
| PGF-1000-2 PGF-2000-2 | 233.36 111.71 | 235.68 145.08 | 1.27 2.54 | 1.27 3.59 | 184.8 42.64 | 187.24 39.99 |
| PGF-1000-4 PGF-2000-4 | 173.66 110.37 | 173.66 108.93 | 1.17 1.54 | 1.25 1.34 | 149.83 71.53 | 142.89 80.96 |
| PGF-1000-6 PGF-2000-6 | 161.27 105.28 | 161.27 106.67 | 1.71 1.44 | 1.12 1.25 | 92.22 73.55 | 145.90 87.64 |
| PGF-1000-8 PGF-2000-8 | 144.47 110.37 | 143.05 110.37 | 1.59 0.90 | 1.54 1.05 | 93.10 123.50 | 94.95 112.29 |
| PGF-1000-25 PGF-2000-25 | 263.89 320.96 | 300.87 320.96 | 25.04 30.24 | 9.94 11.65 | 9.83 9.03 | 30.44 30.32 |
| PGF-1000-50 PGF-2000-50 | 295.19 296.30 | 299.22 294.84 | 24.82 26.58 | 9.97 11.82 | 10.36 10.66 | 31.07 26.22 |
| PGF-1000-75 PGF-2000-75 | 349.80 333.75 | 299.22 332.10 | 31.73 36.47 | 10.87 12.63 | 9.75 8.38 | 29.03 27.89 |
| PGF-1000-100 PGF-2000-100 | 299.22 281.22 | 299.22 281.22 | 25.77 25.72 | 11.11 13.19 | 10.59 9.54 | 27.10 20.90 |
| Samples | Density | TEwarp, TEweft | EWR | Samples | Density | TEwarp, TEweft | EWR | ||
|---|---|---|---|---|---|---|---|---|---|
| Unit | g.m−3 | 103.gf.cm−2 | g.m−3 | 103.gf.cm−2 | |||||
| PGF | 0.60 | 0.89 | 204.14 | 0.0037 | PGF | 0.60 | 0.89 | 204.14 | 0.0037 |
| Cica-care® | 4.28 | 5.23 | 1735.89 | 0.0027 | Cica-care® | 4.33 | 5.23 | 1735.89 | 0.0028 |
| PGF-1000-2 | 1.76 | 1.76 | 453.18 | 0.0039 | PGF-2000-2 | 1.64 | 5.25 | 375.63 | 0.0092 |
| PGF-1000-4 | 1.47 | 1.59 | 479.71 | 0.0032 | PGF-2000-4 | 0.77 | 0.78 | 439.64 | 0.0018 |
| PGF-1000-6 | 3.17 | 0.26 | 487.89 | 0.0035 | PGF-2000-6 | 0.66 | 0.73 | 525.68 | 0.0013 |
| PGF-1000-8 | 31.81 | 13.33 | 501.88 | 0.0450 | PGF-2000-8 | 0.42 | 0.43 | 514.45 | 0.0008 |
| PGF-1000-25 | 172.09 | 60.59 | 511.82 | 0.2270 | PGF-2000-25 | 225.97 | 98.57 | 573.88 | 0.2828 |
| PGF-1000-50 | 140.87 | 79.43 | 577.50 | 0.1907 | PGF-2000-50 | 185.05 | 76.40 | 638.93 | 0.2046 |
| PGF-1000-75 | 194.64 | 63.54 | 573.52 | 0.2251 | PGF-2000-75 | 248.43 | 85.14 | 554.22 | 0.3009 |
| PGF-1000-100 | 139.19 | 62.53 | 528.26 | 0.1909 | PGF-2000-100 | 175.81 | 70.39 | 580.60 | 0.2120 |
| Sample# | Gwarp | Gweft | 2HGwarp | 2HGweft | Sample# | Gwarp | Gweft | 2HGwarp | 2HGweft |
|---|---|---|---|---|---|---|---|---|---|
| Cica-care© | 0.89 | 0.85 | 3.06 | 0.82 | Cica-care© | 0.89 | 0.85 | 3.06 | 0.82 |
| PGF | 0.60 | 0.75 | 0.41 | 1.85 | PGF | 0.60 | 0.75 | 0.41 | 1.85 |
| PGF-1000-2 | 4.37 | 6.87 | 5.64 | 6.05 | PGF-2000-2 | 4.61 | 4.45 | 3.30 | 3.96 |
| PGF-1000-4 | 6.41 | 7.82 | 6.39 | 6.18 | PGF-2000-4 | 6.50 | 6.45 | 4.53 | 5.06 |
| PGF-1000-6 | 6.53 | 8.45 | 6.51 | 7.47 | PGF-2000-6 | 6.80 | 6.66 | 4.24 | 5.75 |
| PGF-1000-8 | 5.88 | 7.75 | 5.42 | 6.07 | PGF-2000-8 | 5.81 | 5.84 | 3.78 | 4.55 |
| PGF-1000-25 | 7.75 | 6.84 | 4.62 | 6.81 | PGF-2000-25 | 6.65 | 7.20 | 5.69 | 6.71 |
| PGF-1000-50 | 7.30 | 7.02 | 4.44 | 5.95 | PGF-2000-50 | 7.32 | 7.31 | 5.34 | 6.07 |
| PGF-1000-75 | 6.77 | 6.50 | 5.16 | 3.83 | PGF-2000-75 | 7.01 | 6.89 | 6.01 | 5.06 |
| PGF-1000-100 | 8.90 | 7.01 | 4.14 | 4.80 | PGF-2000-100 | 7.83 | 8.36 | 5.69 | 6.12 |
| Sample# | S cm2 | S’ cm2 | F’ kPa | T0 mm | TM mm | Sample# | S cm2 | S’ cm2 | F’ kPa | T0 mm | TM mm |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cica-care© | 2.000 | 2.476 | 1.97 | 2.491 | 2.012 | Cica-care© | 2.000 | 2.476 | 1.97 | 2.491 | 2.012 |
| PGF | 2.000 | 2.256 | 1.80 | 0.758 | 0.672 | PGF | 2.000 | 2.256 | 1.80 | 0.758 | 0.672 |
| PGF-1000-2 | 2.000 | 2.158 | 1.72 | 0.737 | 0.683 | PGF-2000-2 | 2.000 | 2.126 | 1.69 | 0.877 | 0.825 |
| PGF-1000-4 | 2.000 | 2.258 | 1.80 | 0.780 | 0.691 | PGF-2000-4 | 2.000 | 2.185 | 1.74 | 0.919 | 0.841 |
| PGF-1000-6 | 2.000 | 2.283 | 1.82 | 0.830 | 0.727 | PGF-2000-6 | 2.000 | 2.196 | 1.75 | 0.974 | 0.887 |
| PGF-1000-8 | 2.000 | 2.357 | 1.88 | 0.912 | 0.774 | PGF-2000-8 | 2.000 | 2.494 | 1.99 | 1.060 | 0.850 |
| PGF-1000-25 | 2.000 | 2.210 | 1.76 | 0.872 | 0.789 | PGF-2000-25 | 2.000 | 2.304 | 1.83 | 0.910 | 0.790 |
| PGF-1000-50 | 2.000 | 2.262 | 1.80 | 0.854 | 0.755 | PGF-2000-50 | 2.000 | 2.339 | 1.86 | 0.917 | 0.784 |
| PGF-1000-75 | 2.000 | 2.483 | 1,98 | 0.869 | 0.700 | PGF-2000-75 | 2.000 | 2.367 | 1.88 | 0.896 | 0.757 |
| PGF-1000-100 | 2.000 | 2.432 | 1.94 | 0.890 | 0.732 | PGF-2000-100 | 2.000 | 2.318 | 1.85 | 0.998 | 0.861 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lui, K.-C.; Wang, X.; Kan, C.-W. Tuning the Tensile and Shear Properties of a Scar Healing Composite for Mechanotherapy. J. Compos. Sci. 2024, 8, 22. https://doi.org/10.3390/jcs8010022
Lui K-C, Wang X, Kan C-W. Tuning the Tensile and Shear Properties of a Scar Healing Composite for Mechanotherapy. Journal of Composites Science. 2024; 8(1):22. https://doi.org/10.3390/jcs8010022
Chicago/Turabian StyleLui, Kam-Che, Xungai Wang, and Chi-Wai Kan. 2024. "Tuning the Tensile and Shear Properties of a Scar Healing Composite for Mechanotherapy" Journal of Composites Science 8, no. 1: 22. https://doi.org/10.3390/jcs8010022






