Preparation of Nanoporous Carbon from Rice Husk with Improved Textural Characteristics for Hydrogen Sorption
Abstract
:1. Introduction
2. Experimental Part
2.1. Synthesis of Rice Husk-Based Nanoporous Carbon and Its Decoration with Metal Nanoparticles
2.2. Material Characterization
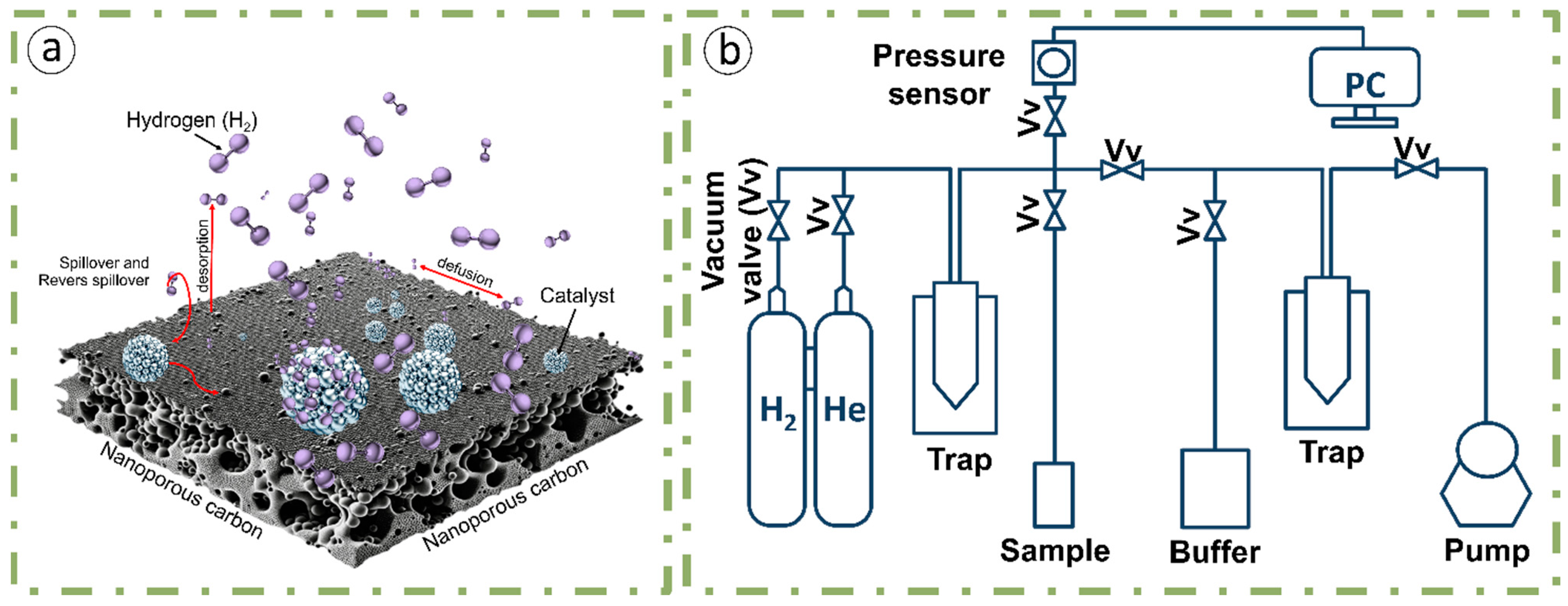
2.3. Methods of Measuring Adsorption Characteristics
3. Results
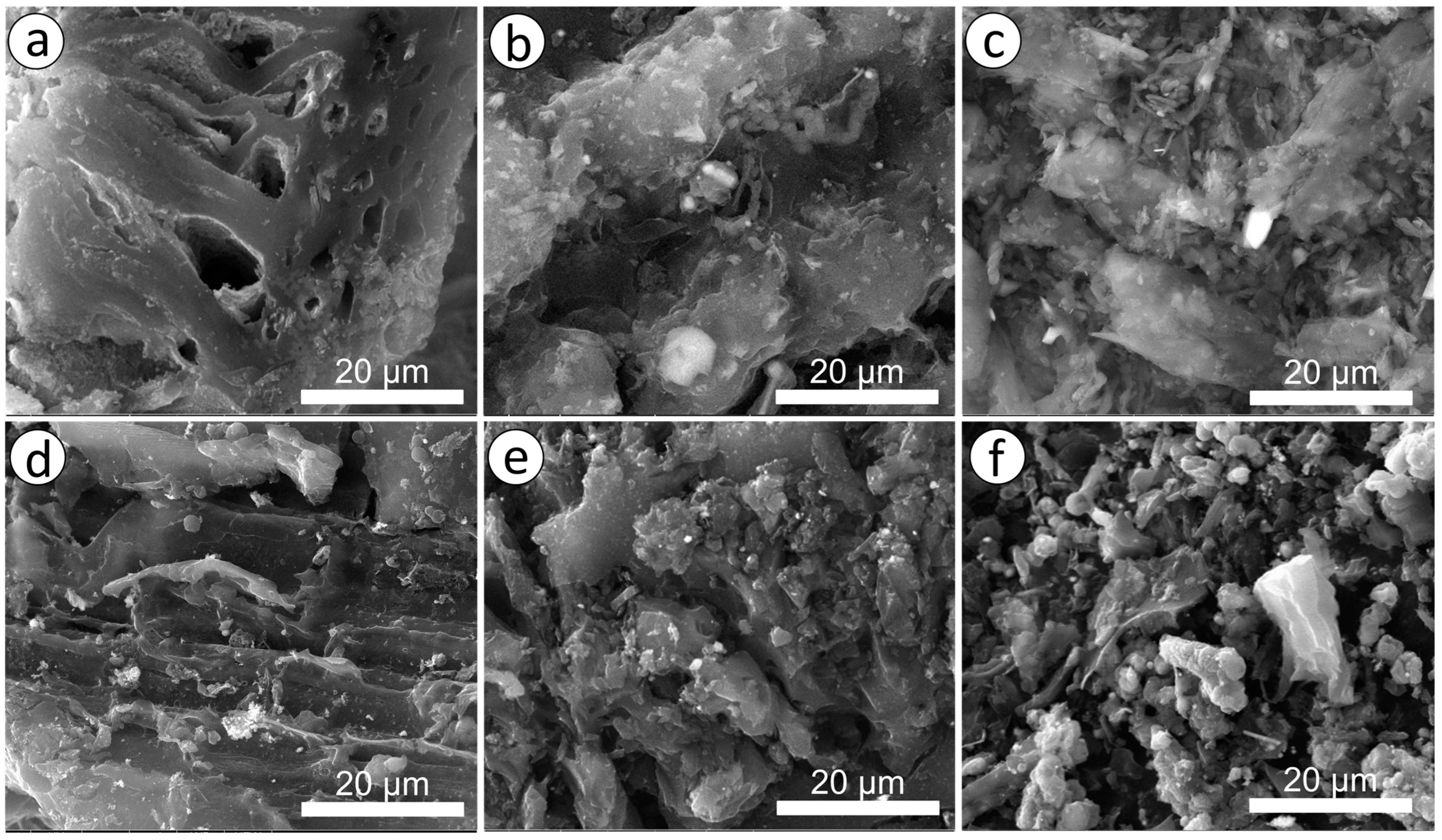
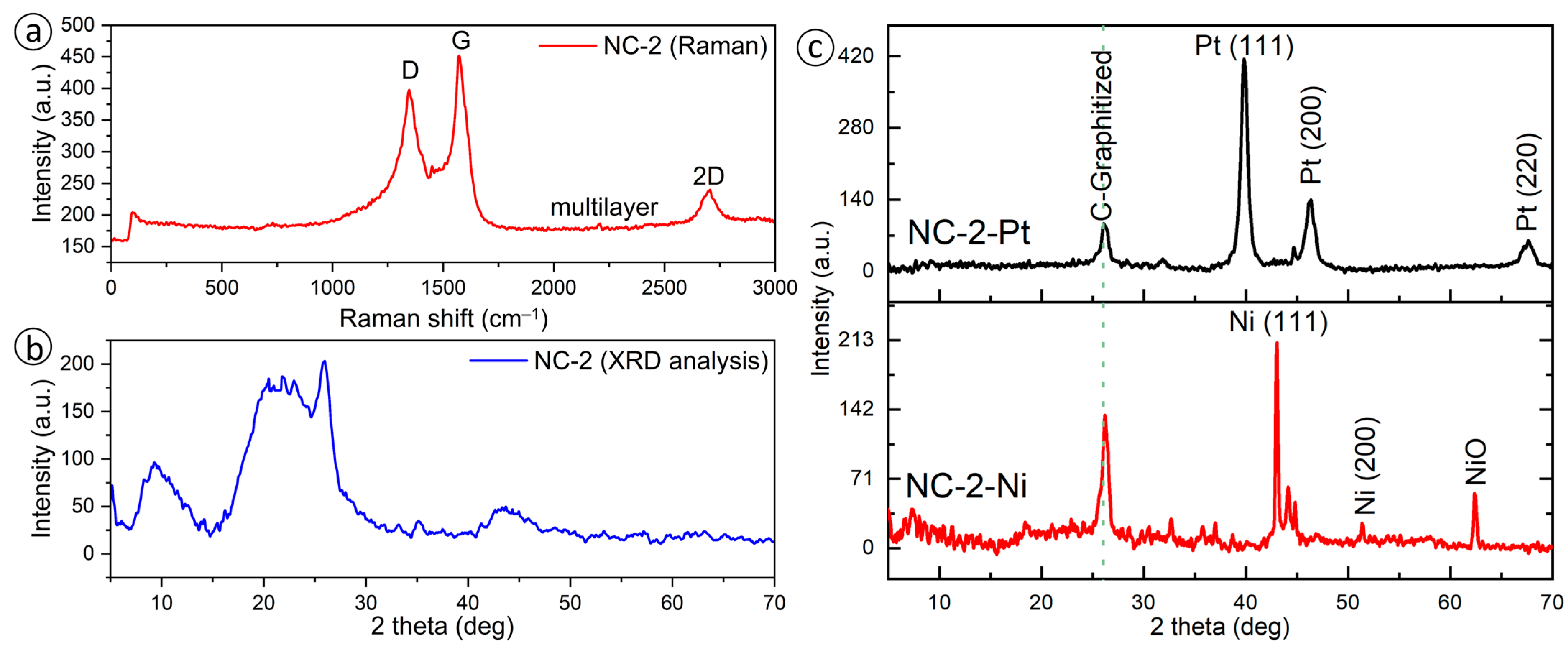
3.1. Texture Characteristics of Nanoporous Carbon
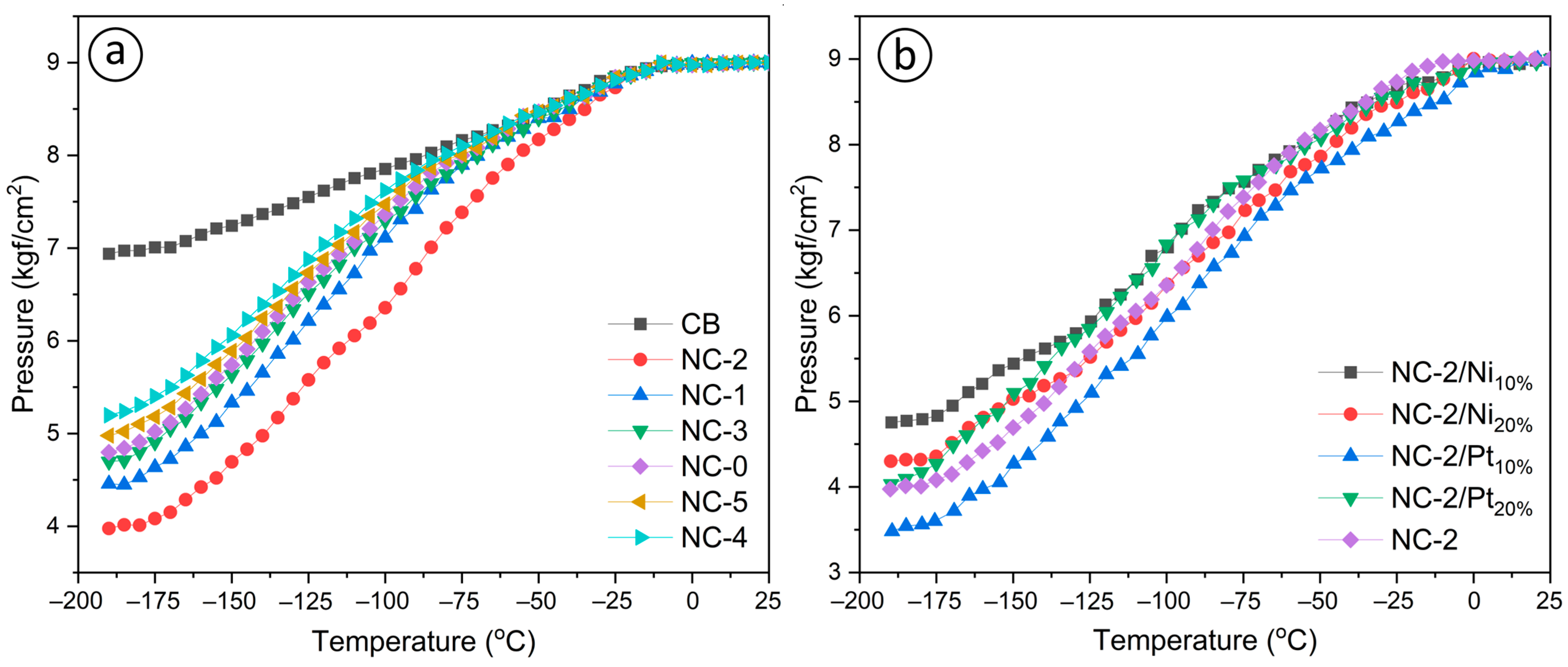
3.2. Hydrogen-Adsorption Characteristics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- The Paris Agreement. Available online: https://unfccc.int/files/essential_background/convention/application/pdf/english_paris_agreement.pdf (accessed on 16 March 2016).
- Berenguer-Murcia, Á.; Marco-Lozar, J.P.; Cazorla-Amorós, D. Hydrogen storage in porous materials: Status, milestones, and challenges. Chem. Rec. 2018, 18, 900–912. [Google Scholar] [CrossRef]
- Dutta, S.; Bhaumik, A.; Wu, K.C.-W. Hierarchically porous carbon derived from polymers and biomass: Effect of interconnected pores on energy applications. Energy Environ. Sci. 2014, 7, 3574–3592. [Google Scholar] [CrossRef]
- Ramirez-Vidal, P.; Sdanghi, G.; Celzard, A.; Fierro, V. High hydrogen release by cryo-adsorption and compression on porous materials. Int. J. Hydrogen Energy 2022, 47, 8892–8915. [Google Scholar] [CrossRef]
- Roszak, R.; Firlej, L.; Roszak, S.; Pfeife, P.; Kuchta, B. Hydrogen storage by adsorption in porous materials: Is it possible? Colloids Surf. A Physicochem. Eng. Asp. 2016, 496, 69–76. [Google Scholar] [CrossRef]
- Mohan, M.; Sharma, V.K.; Kumar, E.A.; Gayathri, V. Hydrogen storage in carbon materials—A review. Energy Storage 2019, 1, e35. [Google Scholar] [CrossRef]
- Heinemann, N.; Alcalde, J.; Miocic, J.M.; Hangx, S.J.T.; Kallmeyer, J.; Ostertag-Henning, C.; Hassanpouryouzband, A.; Thaysen, E.M.; Strobel, G.J.; Schmidt-Hattenberger, C.; et al. Enabling large-scale hydrogen storage in porous media–the scientific challenges. Energy Environ. Sci. 2021, 14, 853–864. [Google Scholar] [CrossRef]
- Broom, D.P.; Webb, C.J.; Fanourgakis, G.S.; Froudakis, G.E.; Trikalitis, P.N.; Hirscher, M. Concepts for improving hydrogen storage in nanoporous materials. Int. J. Hydrogen Energy 2019, 44, 7768–7779. [Google Scholar] [CrossRef]
- Zhang, L.; Allendorf, M.D.; Balderas-Xicohténcatl, R.; Broom, D.P.; Fanourgakis, G.S.; Froudakis, G.E.; Gennett, T.; Hurst, K.E.; Ling, S.; Milanese, C.; et al. Fundamentals of hydrogen storage in nanoporous materials. Prog. Energy 2022, 4, 042013. [Google Scholar] [CrossRef]
- Germain, J.; Fréchet, J.M.J.; Svec, F. Nanoporous polymers for hydrogen storage. Small 2009, 5, 1098–1111. [Google Scholar] [CrossRef]
- Yu, X.; Tang, Z.; Sun, D.; Ouyang, L.; Zhu, M. Recent advances and remaining challenges of nanostructured materials for hydrogen storage applications. Prog. Mater. Sci. 2017, 88, 1–48. [Google Scholar] [CrossRef]
- Firlej, L.; Pfeifer, P.; Kuchta, B. Understanding universal adsorption limits for hydrogen storage in nanoporous systems. Adv. Mater. 2013, 25, 5971–5974. [Google Scholar] [CrossRef] [PubMed]
- Kuchta, B.; Firlej, L.; Cepel, R.; Pfeifer, P.; Wexler, C. Structural and energetic factors in designing a nanoporous sorbent for hydrogen storage. Colloids Surf. A Physicochem. Eng. Asp. 2010, 357, 61–66. [Google Scholar] [CrossRef]
- Heo, Y.-J.; Park, S.-J. Synthesis of activated carbon derived from rice husks for improving hydrogen storage capacity. J. Ind. Eng. Chem. 2015, 31, 330–334. [Google Scholar] [CrossRef]
- Sethia, G.; Sayari, A. Activated carbon with optimum pore size distribution for hydrogen storage. Carbon 2016, 99, 289–294. [Google Scholar] [CrossRef]
- Huang, J.; Liang, Y.; Dong, H.; Hu, H.; Yu, P.; Peng, L.; Zheng, M.; Xiao, Y.; Liu, Y. Revealing contribution of pore size to high hydrogen storage capacity. Int. J. Hydrogen Energy 2018, 43, 18077–18082. [Google Scholar] [CrossRef]
- Geng, Z.; Zhang, C.; Wang, D.; Zhou, X.; Cai, M. Pore size effects of nanoporous carbons with ultra-high surface area on high-pressure hydrogen storage. J. Energy Chem. 2015, 24, 1–8. [Google Scholar] [CrossRef]
- Lee, H.-M.; Heo, Y.-J.; An, K.-H.; Jung, S.-C.; Chung, D.C.; Park, S.-J.; Kim, B.-J. A study on optimal pore range for high pressure hydrogen storage behaviors by porous hard carbon materials prepared from a polymeric precursor. Int. J. Hydrogen Energy 2018, 43, 5894–5902. [Google Scholar] [CrossRef]
- Heo, Y.-J.; Yeon, S.-H.; Park, S.-J. Defining contribution of micropore size to hydrogen physisorption behaviors: A new approach based on DFT pore volumes. Carbon 2019, 143, 288–293. [Google Scholar] [CrossRef]
- Tian, M.; Lennox, M.J.; O’Malley, A.J.; Porter, A.J.; Krüner, B.; Rudić, S.; Mays, T.J.; Düren, T.; Presser, V.; Terry, L.R.; et al. Effect of pore geometry on ultra-densified hydrogen in microporous carbons. Carbon 2020, 173, 968–979. [Google Scholar] [CrossRef]
- Hu, W.; Li, Y.; Zheng, M.; Xiao, Y.; Dong, H.; Liang, Y.; Hu, H.; Liu, Y. Degradation of biomass components to prepare porous carbon for exceptional hydrogen storage capacity. Int. J. Hydrogen Energy 2021, 46, 5418–5426. [Google Scholar] [CrossRef]
- Wang, L.F.; Yang, R.T. Hydrogen storage on carbon-based adsorbents and storage at ambient temperature by hydrogen spillover. Catal. Rev. 2010, 52, 411–461. [Google Scholar] [CrossRef]
- Chung, T.-Y.; Tsao, C.-S.; Tseng, H.-P.; Chen, C.-H.; Yu, M.-S. Effects of oxygen functional groups on the enhancement of the hydrogen spillover of Pd-doped activated carbon. J. Colloid. Interface Sci. 2015, 441, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Parambhath, V.B.; Nagar, R.; Ramaprabhu, S. Effect of nitrogen doping on hydrogen storage capacity of palladium decorated graphene. Langmuir 2012, 28, 7826–7833. [Google Scholar] [CrossRef] [PubMed]
- Vinayan, B.P.; Sethupathi, K.; Ramaprabhu, S. Facile synthesis of triangular shaped palladium nanoparticles decorated nitrogen doped graphene and their catalytic study for renewable energy applications. Int. J. Hydrogen Energy 2013, 38, 2240–2250. [Google Scholar] [CrossRef]
- Li, T.; Chen, C.; Brozena, A.H.; Zhu, J.Y.; Xu, L.; Driemeier, C.; Dai, J.; Rojas, O.J.; Isogai, A.; Wågberg, L.; et al. Developing fibrillated cellulose as a sustainable technological material. Nature 2021, 590, 47–56. [Google Scholar] [CrossRef]
- Opia, A.C.; Hamid, M.K.B.A.; Syahrullail, S.; Rahim, A.B.A.; Johnson, C.A.N. Biomass as a potential source of sustainable fuel, chemical and tribological materials—Overview. Mater. Today Proc. 2020, 39, 922–9928. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Martin, L.P.; Nagle, D.; Rosen, M. Effect of particle size distribution upon specific surface area and ultrasonic velocity in sintered ceramic powders. Mater. Sci. Eng. A 1998, 246, 151–160. [Google Scholar] [CrossRef]
- Nanda, S.S.; Kim, M.J.; Yeom, K.S.; An, S.S.A.; Ju, H.; Yi, D.K. Raman spectrum of graphene with its versatile future perspectives. TrAC Trends Anal. Chem. 2016, 80, 125–131. [Google Scholar] [CrossRef]



| Sample Designation | SBET, m2/g | SBET, m2/g D-R Method | VBJH, cm3/g | RBJH Å |
|---|---|---|---|---|
| NC-0 | 2386 | 2754 | 0.251 | 19.251 |
| NC-1 | 2735 | 2649 | 0.706 | 17.136 |
| NC-2 | 2713 | 3099 | 1.625 | 17.198 |
| NC-3 | 2353 | 2133 | 1.715 | 19.259 |
| NC-4 | 2482 | 2863 | 2.215 | 19.263 |
| NC-5 | 2007 | 2436 | 0.525 | 19.201 |
| Nanoporous Carbon Sample | NC-0 | NC-1 | NC-2 | NC-3 | NC-4 | NC-5 |
|---|---|---|---|---|---|---|
| Amount of adsorbed hydrogen in mass.% at ≈−190 °C | 2.31 | 3.22 | 3.71 | 2.93 | 2.81 | 2.52 |
| Pressure difference, kgf/cm2 | 1.921 | 2.653 | 3.14 | 2.442 | 2.325 | 2.133 |
| Mass of adsorbed hydrogen, g | 0.035 | 0.048 | 0.055 | 0.044 | 0.042 | 0.037 |
| Type of Material | Initial Pressure in the System, kgf/cm2 | Pressure in the System after Combining the Sample Vessel with the Pipeline, kgf/cm2 | Mass of Adsorbed Hydrogen, g | Amount of Adsorbed Hydrogen, wt.% |
|---|---|---|---|---|
| NC-2 | 9.009 | 8.341 | 0.0121 | 0.81 |
| NC-2/Ni10% | 8.996 | 8.320 | 0.0124 | 0.83 |
| NC-2/Pt10% | 9.005 | 8.094 | 0.0167 | 1.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lesbayev, B.; Rakhymzhan, N.; Ustayeva, G.; Maral, Y.; Atamanov, M.; Auyelkhankyzy, M.; Zhamash, A. Preparation of Nanoporous Carbon from Rice Husk with Improved Textural Characteristics for Hydrogen Sorption. J. Compos. Sci. 2024, 8, 74. https://doi.org/10.3390/jcs8020074
Lesbayev B, Rakhymzhan N, Ustayeva G, Maral Y, Atamanov M, Auyelkhankyzy M, Zhamash A. Preparation of Nanoporous Carbon from Rice Husk with Improved Textural Characteristics for Hydrogen Sorption. Journal of Composites Science. 2024; 8(2):74. https://doi.org/10.3390/jcs8020074
Chicago/Turabian StyleLesbayev, Bakhytzhan, Nurgali Rakhymzhan, Gaukhar Ustayeva, Yerkebulan Maral, Meiram Atamanov, Moldir Auyelkhankyzy, and Ayazhan Zhamash. 2024. "Preparation of Nanoporous Carbon from Rice Husk with Improved Textural Characteristics for Hydrogen Sorption" Journal of Composites Science 8, no. 2: 74. https://doi.org/10.3390/jcs8020074






