Overcoming Therapeutic Inertia in Type 2 Diabetes: Exploring Machine Learning-Based Scenario Simulation for Improving Short-Term Glycemic Control
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Eligibility Criteria
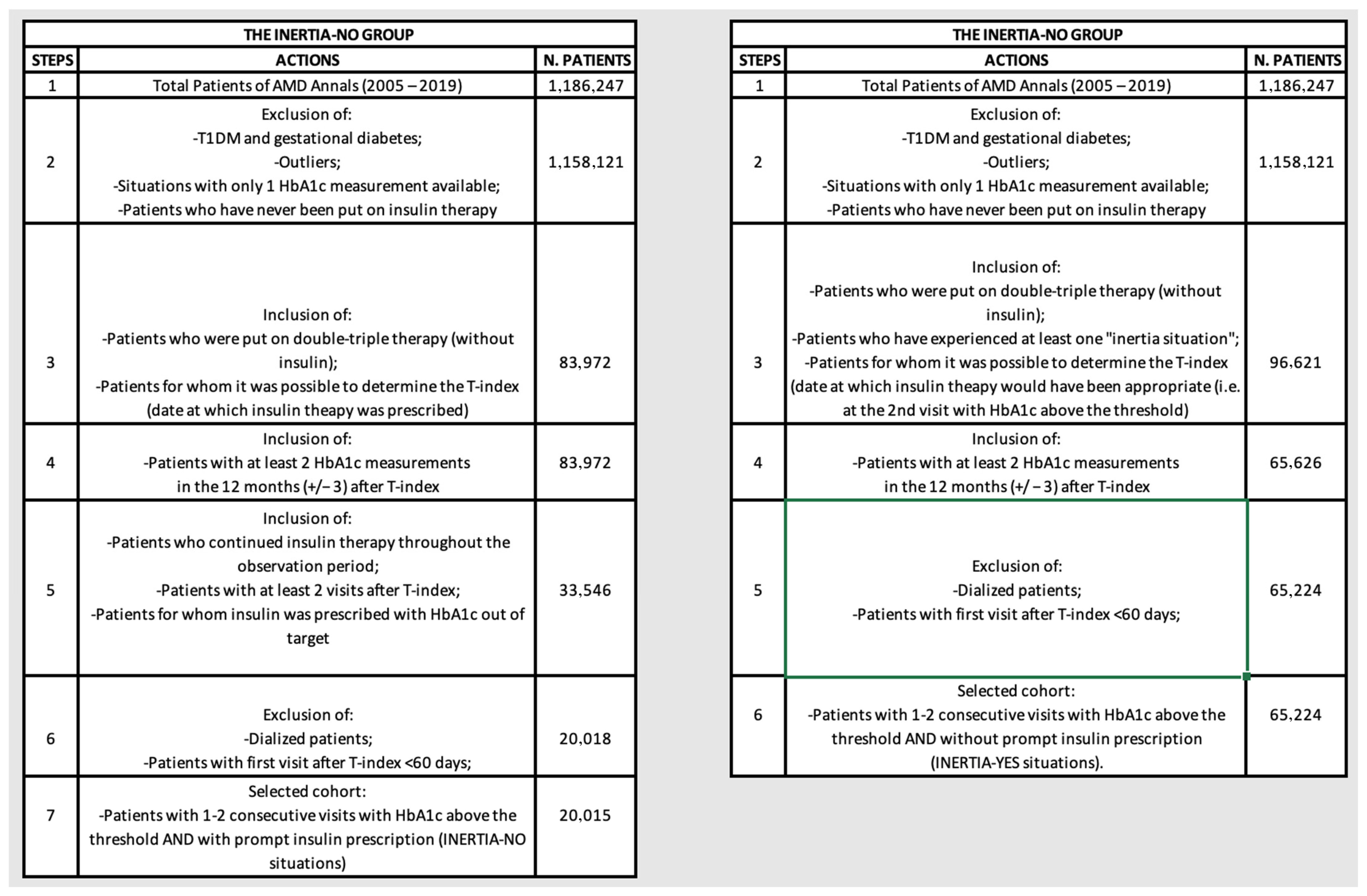
2.1.1. Inclusion Criteria
2.1.2. Exclusion Criteria
2.2. Database Description
2.3. Machine Learning Analysis
2.3.1. General Criteria
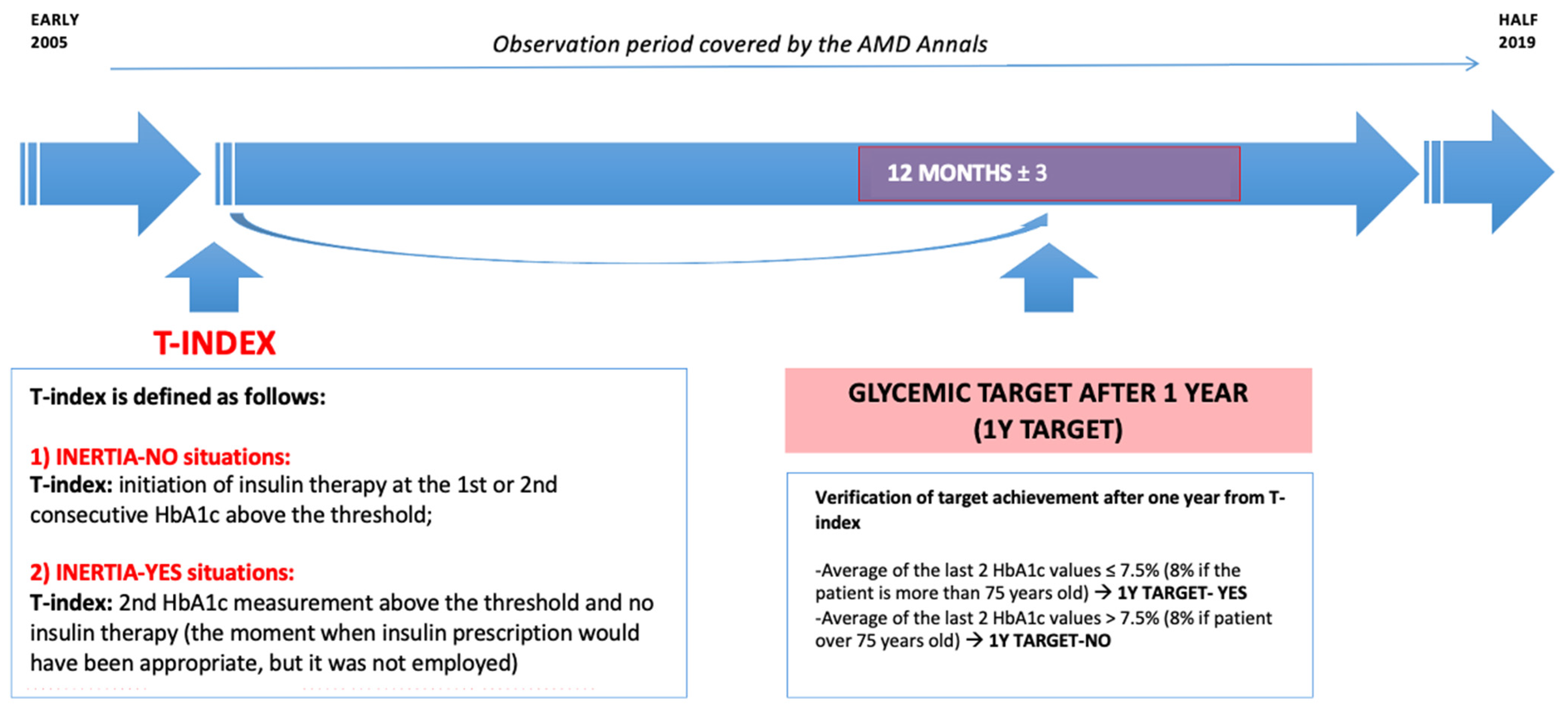
2.3.2. Definition of the Outcomes
2.3.3. Logic Learning Machine Characteristics
- (1)
- Training phase: starting from the input variables, LLM technology builds a model composed of a set of intelligible rules employing 70% of the available data.
- (2)
- Validation phase: assessment of the model’s performance using the remaining 30% of data, computing metrics including sensitivity, specificity, precision, accuracy, and the ROC-AUC are calculated.
- (3)
- Feature ranking creation: the LLM automatically identifies and transparently produces a ranking of the most pertinent variables explaining the initial premise.
- (4)
- Display of threshold values: the LLM explicitly indicates threshold values for the variables selected in the feature ranking.
- (5)
- Prediction: In addition to delivering outcome-related responses, the model elucidates the rationale behind predictions, considering specific variables characterizing the individual. For instance: For a given patient, the likelihood of achieving a certain target is influenced by a set of specific factors.
2.3.4. How LLM Has Been Used for the Scenario Simulation What-If Analysis
3. Results
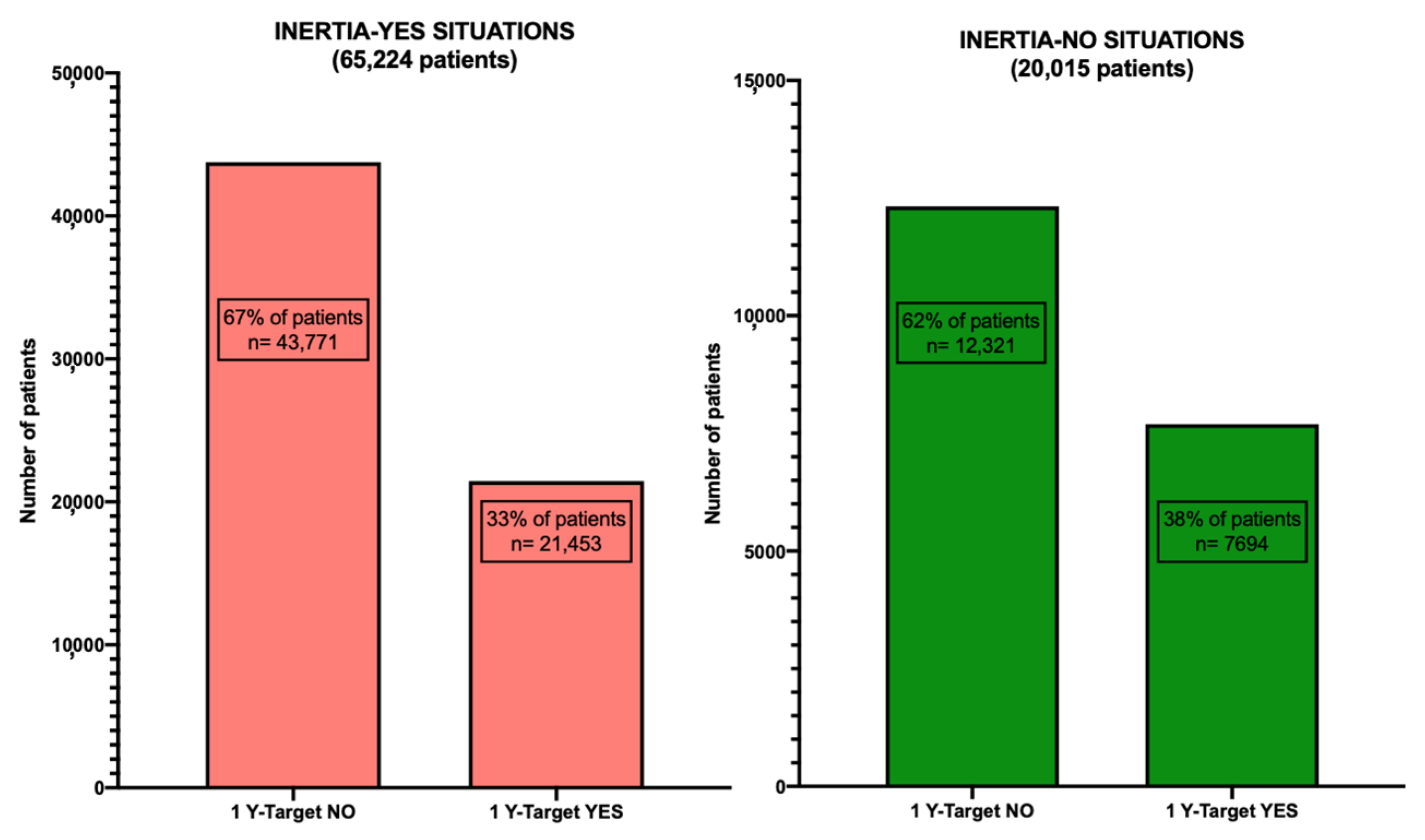
3.1. General Characteristics of the Patients
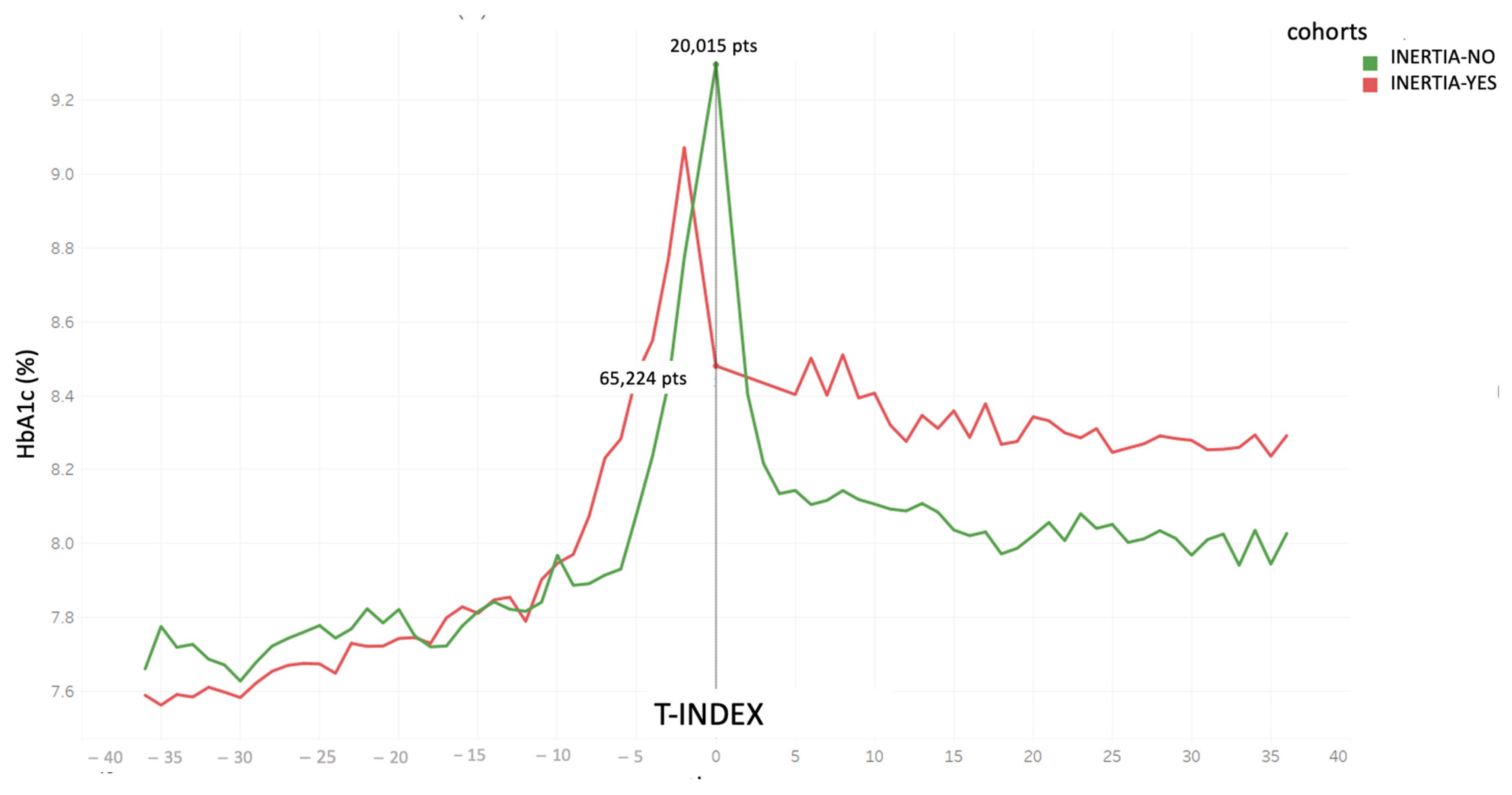
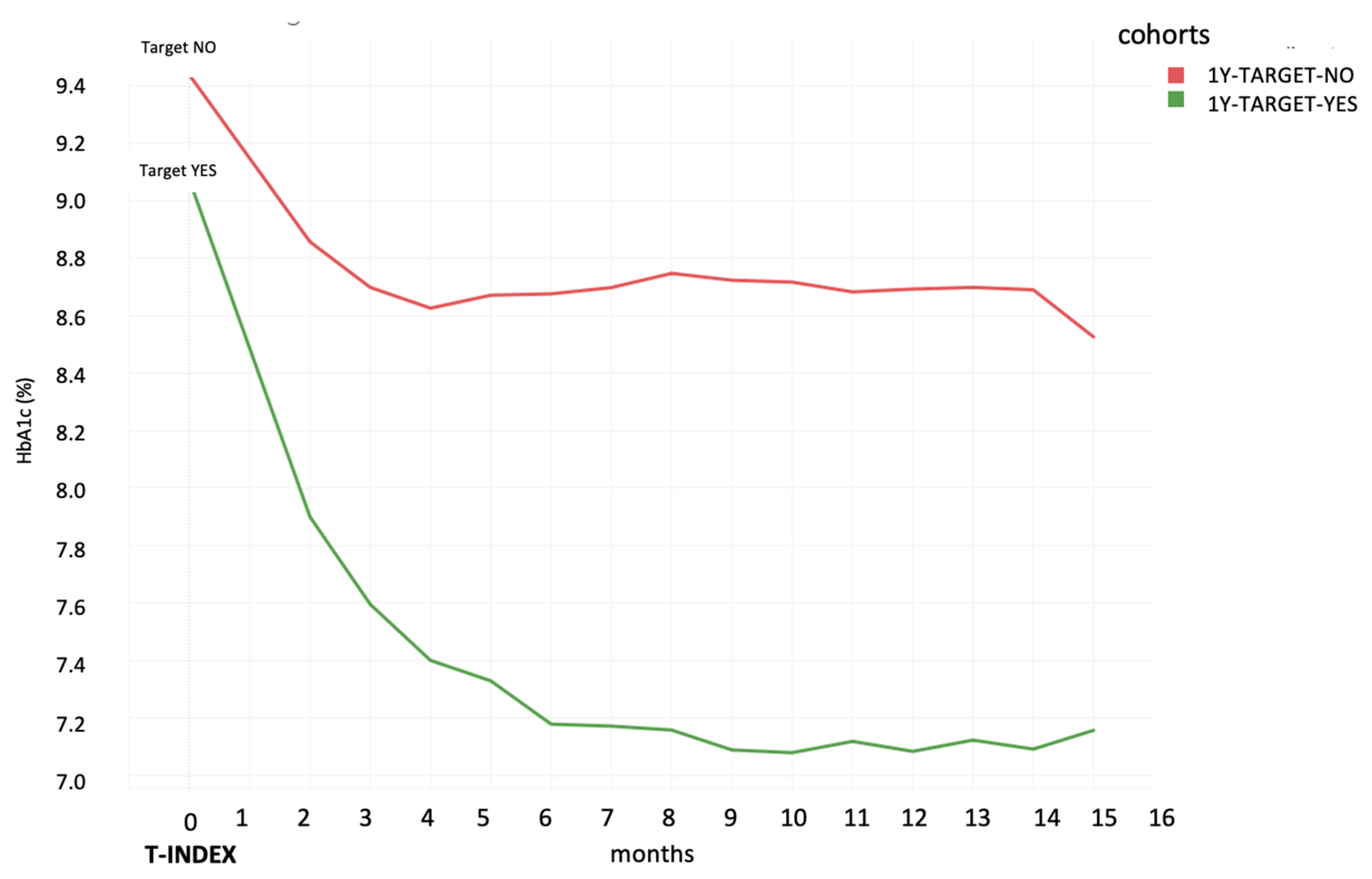
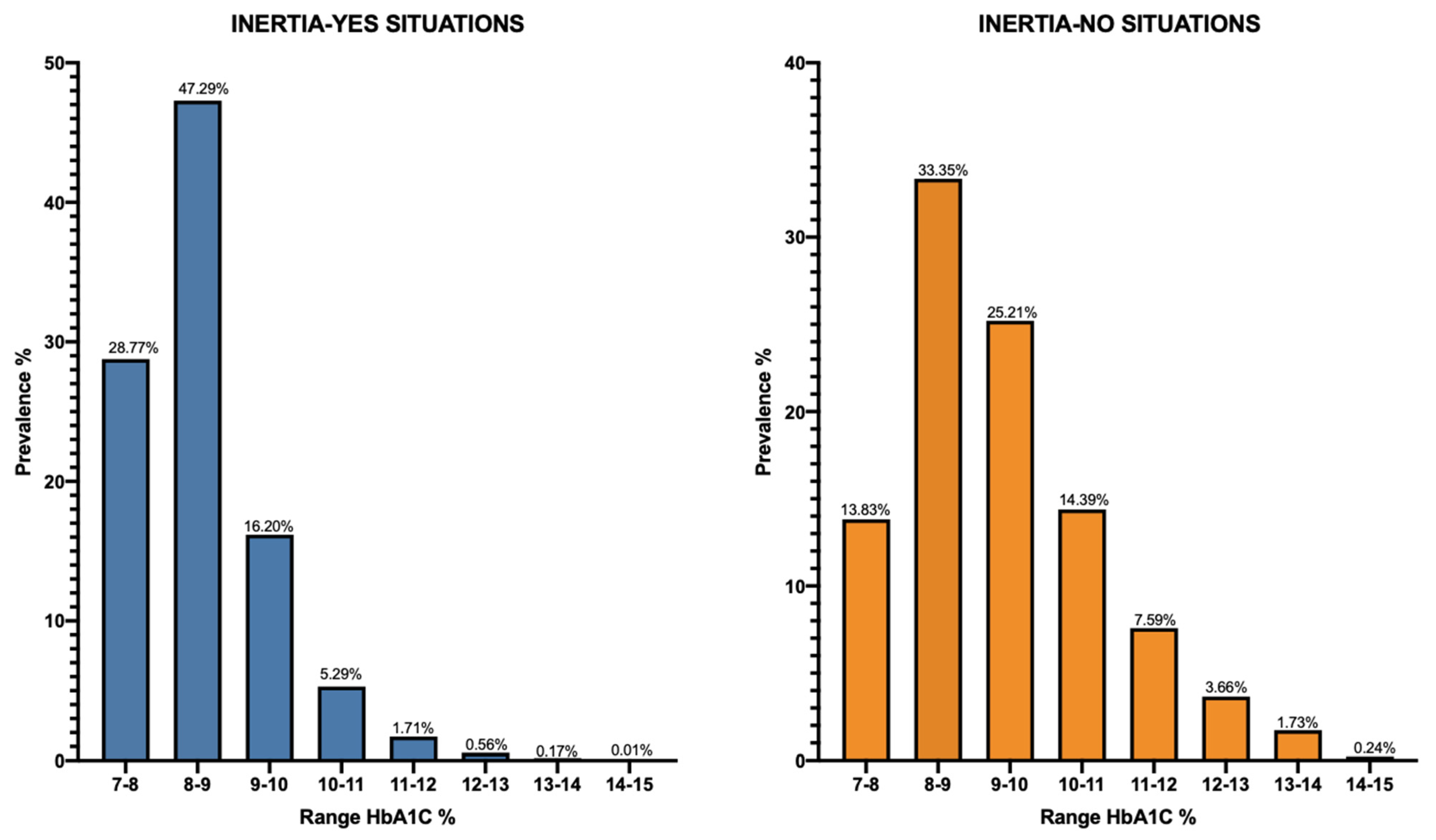
3.2. Comparison of HbA1c Average Levels among the Different Cohorts and Subgroups of Patients
3.3. LLM Analysis
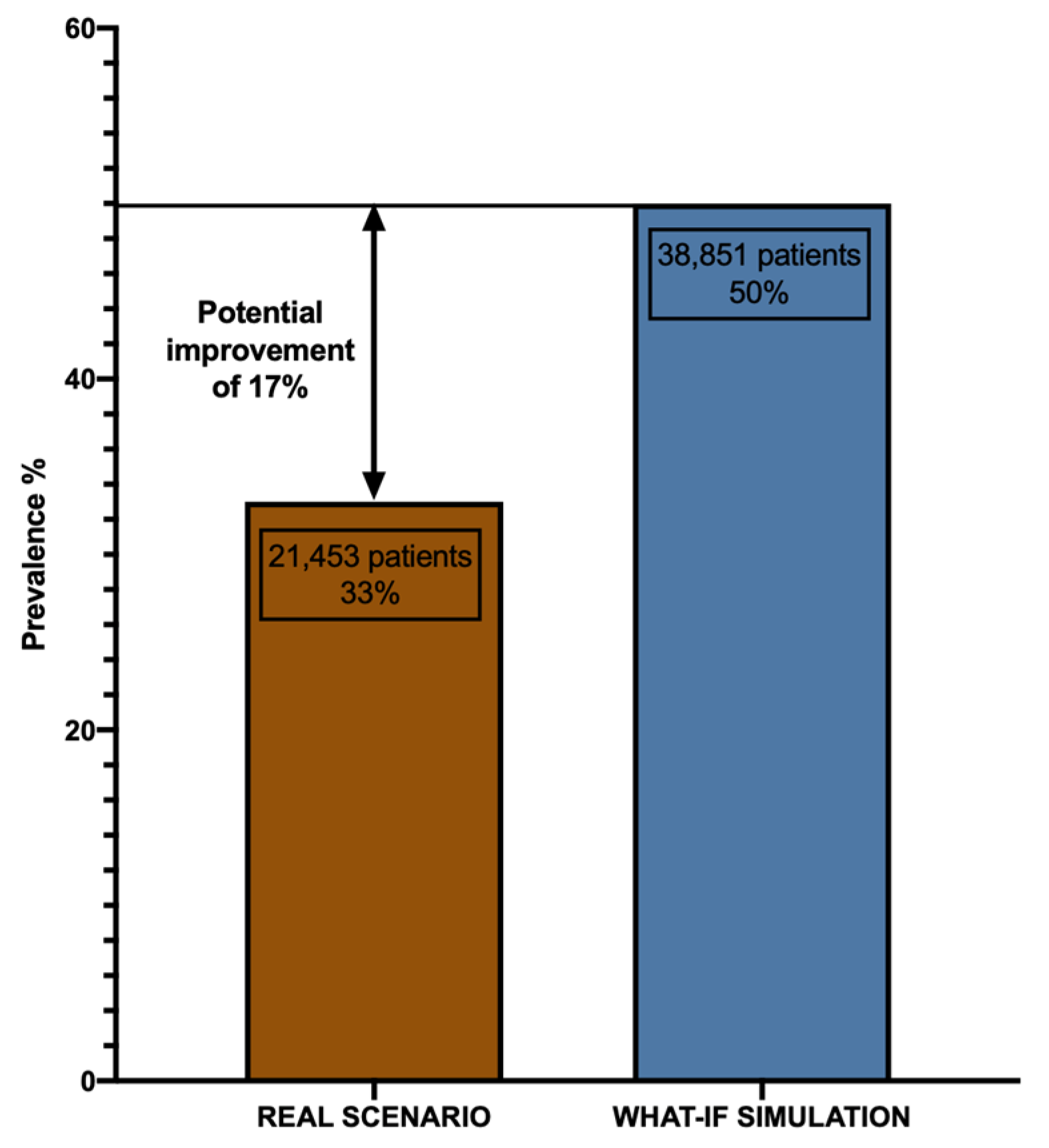
3.4. What-If Scenario Simulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, Regional, and National Prevalence of Overweight and Obesity in Children and Adults during 1980–2013: A Systematic Analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes–Global Burden of Disease and Forecasted Trends. JEGH 2019, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.; Di Bartolo, P.; Candido, R.; Lucisano, G.; Manicardi, V.; Giandalia, A.; Nicolucci, A.; Rocca, A.; Rossi, M.C.; Di Cianni, G. The AMD ANNALS: A Continuous Initiative for the Improvement of Type 2 Diabetes Care. Diabetes Res. Clin. Pract. 2023, 199, 110672. [Google Scholar] [CrossRef] [PubMed]
- Khunti, K.; Ceriello, A.; Cos, X.; De Block, C. Achievement of Guideline Targets for Blood Pressure, Lipid, and Glycaemic Control in Type 2 Diabetes: A Meta-Analysis. Diabetes Res. Clin. Pract. 2018, 137, 137–148. [Google Scholar] [CrossRef]
- Mauricio, D.; Meneghini, L.; Seufert, J.; Liao, L.; Wang, H.; Tong, L.; Cali, A.; Stella, P.; Carita, P.; Khunti, K. Glycaemic Control and Hypoglycaemia Burden in Patients with Type 2 Diabetes Initiating Basal Insulin in E Urope and the USA. Diabetes Obes. Metab. 2017, 19, 1155–1164. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; Puckett, C.; Triplitt, C.; Maggs, D.; Adams, J.; Cersosimo, E.; DeFronzo, R.A. Initial Combination Therapy with Metformin, Pioglitazone and Exenatide Is More Effective than Sequential Add-on Therapy in Subjects with New-onset Diabetes. Results from the E Fficacy and D Urability of I Nitial C Ombination T Herapy for T Ype 2 D Iabetes ( EDICT ): A Randomized Trial. Diabetes Obes. Metab. 2015, 17, 268–275. [Google Scholar] [CrossRef]
- Hayward, R.A.; Reaven, P.D.; Wiitala, W.L.; Bahn, G.D.; Reda, D.J.; Ge, L.; McCarren, M.; Duckworth, W.C.; Emanuele, N.V. Follow-up of Glycemic Control and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2015, 372, 2197–2206. [Google Scholar] [CrossRef]
- McCoy, R.G.; O’Connor, P.J. Overcoming Therapeutic Inertia in Type 2 Diabetes Care—Timing, Context, and Appropriateness of Treatment Intensification. JAMA Netw. Open 2021, 4, e2130926. [Google Scholar] [CrossRef]
- Rattelman, C.R.; Ciemins, E.L.; Stempniewicz, N.; Mocarski, M.; Ganguly, R.; Cuddeback, J.K. A Retrospective Analysis of Therapeutic Inertia in Type 2 Diabetes Management Across a Diverse Population of Health Care Organizations in the USA. Diabetes Ther. 2021, 12, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Khunti, S.; Khunti, K.; Seidu, S. Therapeutic Inertia in Type 2 Diabetes: Prevalence, Causes, Consequences and Methods to Overcome Inertia. Ther. Adv. Endocrinol. 2019, 10, 204201881984469. [Google Scholar] [CrossRef] [PubMed]
- Gabbay, R.A.; Kendall, D.; Beebe, C.; Cuddeback, J.; Hobbs, T.; Khan, N.D.; Leal, S.; Miller, E.; Novak, L.M.; Rajpathak, S.N.; et al. Addressing Therapeutic Inertia in 2020 and Beyond: A 3-Year Initiative of the American Diabetes Association. Clin. Diabetes 2020, 38, 371–381. [Google Scholar] [CrossRef]
- Powell, R.E.; Zaccardi, F.; Beebe, C.; Chen, X.M.; Crawford, A.; Cuddeback, J.; Gabbay, R.A.; Kissela, L.; Litchman, M.L.; Mehta, R.; et al. Strategies for Overcoming Therapeutic Inertia in Type 2 Diabetes: A Systematic Review and Meta-analysis. Diabetes Obes. Metab. 2021, 23, 2137–2154. [Google Scholar] [CrossRef] [PubMed]
- Correa, M.F.; Li, Y.; Kum, H.-C.; Lawley, M.A. Assessing the Effect of Clinical Inertia on Diabetes Outcomes: A Modeling Approach. J. Gen. Intern. Med. 2019, 34, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Del Ser, J.; Barredo-Arrieta, A.; Díaz-Rodríguez, N.; Herrera, F.; Saranti, A.; Holzinger, A. On Generating Trustworthy Counterfactual Explanations. Inf. Sci. 2024, 655, 119898. [Google Scholar] [CrossRef]
- Doyle-Delgado, K.; Chamberlain, J.J.; Shubrook, J.H.; Skolnik, N.; Trujillo, J. Pharmacologic Approaches to Glycemic Treatment of Type 2 Diabetes: Synopsis of the 2020 American Diabetes Association’s Standards of Medical Care in Diabetes Clinical Guideline. Ann. Intern. Med. 2020, 173, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Augstein, P.; Heinke, P.; Vogt, L.; Vogt, R.; Rackow, C.; Kohnert, K.-D.; Salzsieder, E. Q-Score: Development of a New Metric for Continuous Glucose Monitoring That Enables Stratification of Antihyperglycaemic Therapies. BMC Endocr. Disord. 2015, 15, 22. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Rossi, M.C.; De Cosmo, S.; Lucisano, G.; Pontremoli, R.; Fioretto, P.; Giorda, C.; Pacilli, A.; Viazzi, F.; Russo, G.; et al. Overall Quality of Care Predicts the Variability of Key Risk Factors for Complications in Type 2 Diabetes: An Observational, Longitudinal Retrospective Study. Diabetes Care 2019, 42, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Abu Hassan, H.; Tohid, H.; Mohd Amin, R.; Long Bidin, M.B.; Muthupalaniappen, L.; Omar, K. Factors Influencing Insulin Acceptance among Type 2 Diabetes Mellitus Patients in a Primary Care Clinic: A Qualitative Exploration. BMC Fam Pr. 2013, 14, 164. [Google Scholar] [CrossRef] [PubMed]
- Guerci, B.; Drouin, P.; Grangé, V.; Bougnères, P.; Fontaine, P.; Kerlan, V.; Passa, P.; Thivolet, C.; Vialettes, B.; Charbonnel, B. Self-Monitoring of Blood Glucose Significantly Improves Metabolic Control in Patients with Type 2 Diabetes Mellitus: The Auto-Surveillance Intervention Active (ASIA) Study. Diabetes Metab. 2003, 29, 587–594. [Google Scholar] [CrossRef]
- Sastre, J.; Pinés, P.J.; Del Val, F.; Moreno-Fernandez, J.; Gonzalez López, J.; Quiroga, I.; Herranz, S.; López Gallardo, G.; Calderón, D.; López López, J. Metabolic Control and Treatment Regimens in Patients with Type 1 Diabetes in Castilla-La Mancha, 10 Years Later: The 2020 DIACAM1 Study. Endocrinol. Diabetes Y Nutr. 2022, 69, 483–492. [Google Scholar] [CrossRef]
- Bott, U.; Jörgens, V.; Grüsser, M.; Bender, R.; Mühlhauser, I.; Berger, M. Predictors of Glycaemic Control in Type 1 Diabetic Patients after Participation in an Intensified Treatment and Teaching Programme. Diabet. Med. 1994, 11, 362–371. [Google Scholar] [CrossRef]
- Meneghini, L.F. Early Insulin Treatment in Type 2 Diabetes. Diabetes Care 2009, 32, S266–S269. [Google Scholar] [CrossRef]
- McCormick, T.A.; Adams, J.L.; Lee, E.A.; Emptage, N.P.; Palmer-Toy, D.E.; Martin, J.P.; Broder, B.I.; Kanter, M.H.; Davis, A.C.; McGlynn, E.A. Age-Dependent Hemoglobin A1c Therapeutic Targets Reduce Diabetic Medication Changes in the Elderly. eGEMs 2019, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Shao, H.; Luo, J.; Lipska, K.; Suda, K.J.; Yudkin, J.S. Estimates of Insulin Needs and Dispensation given Wastage, Alternative Glycemic Targets, and Non-Insulin Therapies in US Populations with Type 2 Diabetes Mellitus: A Microsimulation Study. J. Diabetes Its Complicat. 2021, 35, 107839. [Google Scholar] [CrossRef]
- Annali_AMD-2018. Available online: https://aemmedi.it/wp-content/uploads/2018/11/Annali_AMD-_2018_prot.pdf (accessed on 2 February 2024).
- Verda, D.; Parodi, S.; Ferrari, E.; Muselli, M. Analyzing Gene Expression Data for Pediatric and Adult Cancer Diagnosis Using Logic Learning Machine and Standard Supervised Methods. BMC Bioinform. 2019, 20, 390. [Google Scholar] [CrossRef]
- Khunti, K.; Gomes, M.B.; Pocock, S.; Shestakova, M.V.; Pintat, S.; Fenici, P.; Hammar, N.; Medina, J. Therapeutic Inertia in the Treatment of Hyperglycaemia in Patients with Type 2 Diabetes: A Systematic Review. Diabetes Obes. Metab. 2018, 20, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Karam, S.L.; Dendy, J.; Polu, S.; Blonde, L. Overview of Therapeutic Inertia in Diabetes: Prevalence, Causes, and Consequences. Diabetes Spectr. 2020, 33, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Gavin, J.R.; Abaniel, R.M.; Virdi, N.S. Therapeutic Inertia and Delays in Insulin Intensification in Type 2 Diabetes: A Literature Review. Diabetes Spectr. 2023, 36, 379–384. [Google Scholar] [CrossRef]
- Musacchio, N.; Zilich, R.; Ponzani, P.; Guaita, G.; Giorda, C.; Heidbreder, R.; Santin, P.; Di Cianni, G. Transparent Machine Learning Suggests a Key Driver in the Decision to Start Insulin Therapy in Individuals with Type 2 Diabetes. J. Diabetes 2023, 15, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Khunti, K.; Wolden, M.L.; Thorsted, B.L.; Andersen, M.; Davies, M.J. Clinical Inertia in People With Type 2 Diabetes. Diabetes Care 2013, 36, 3411–3417. [Google Scholar] [CrossRef]
- Martens, T.W.; Parkin, C.G. How Use of Continuous Glucose Monitoring Can Address Therapeutic Inertia in Primary Care. Postgrad. Med. 2022, 134, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Lachin, J.M.; Nathan, D.M. Understanding Metabolic Memory: The Prolonged Influence of Glycemia During the Diabetes Control and Complications Trial (DCCT) on Future Risks of Complications During the Study of the Epidemiology of Diabetes Interventions and Complications (EDIC). Diabetes Care 2021, 44, 2216–2224. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee 8. Obesity and Weight Management for the Prevention and Treatment of Type 2 Diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45, S113–S124. [Google Scholar] [CrossRef] [PubMed]
| T-INDEX | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variable | 1Y TARGET | Mean | Median | SD | % Missing | Interquartile Range | p-Value (Alpha = 0.05) | |
| Patients (n.) | INERTIA-NO GROUP (20,015) | NO (12,321–62%) | --- | --- | ||||
| YES (7694–38%) | ||||||||
| Age | NO | 66.087 | 67 | 10.4 | 0 | 59–73 | <0.0001 | |
| (y) | YES | 69.59 | 71 | 10.35 | 0 | 63–77 | ||
| HbA1c | NO | 9.43 | 9.2 | 1.2 | 0 | 8.5–10.1 | <0.0001 | |
| (%) | YES | 9.07 | 8.8 | 1.2 | 0 | 8.2–9.6 | ||
| HbA1c gap between visits (%) | NO | 1.09 | 1 | 1.4 | 6% | 0.2–1.9 | <0.0001 | |
| YES | 1.19 | 1 | 1.38 | 6% | 0.4–1.8 | |||
| Cholesterol (mg/dL) | NO | 179.07 | 174.20 | 45.96 | 59% | 148.8–204.1 | <0.0001 | |
| YES | 174.56 | 170.20 | 44.75 | 74% | 146.0–175.56 | |||
| LDL-C (mg/dL) | NO | 98.5 | 95 | 37.8 | 32% | 73–119 | <0.0001 | |
| YES | 96.1 | 93 | 37.4 | 30% | 72–116 | |||
| HDL-C | NO | 46.2 | 44 | 13.4 | 23% | 37–53 | <0.0001 | |
| (mg/dL) | YES | 47.2 | 45 | 14.0 | 24% | 38–55 | ||
| TGD | NO | 181.8 | 149 | 145 | 21% | 106–216 | <0.0001 | |
| (mg/dL) | YES | 161.6 | 135 | 119.5 | 22% | 96–190 | ||
| BMI | NO | 30.5 | 29.7 | 5.9 | 4% | 26.4–33.7 | <0.0001 | |
| (Kg/m2) | YES | 29.1 | 28.4 | 5.5 | 4% | 25.3–32.3 | ||
| eGFR | NO | 74.6 | 77.9 | 23.6 | 18% | 56.2–93.4 | <0.0001 | |
| (mL/min/m2) | YES | 71.0 | 73.3 | 23.4 | 16% | 52.7–71.03 | ||
| Glucose | NO | 206.7 | 199 | 62.2 | 12% | 165–241 | <0.0001 | |
| (mg/dL) | YES | 200.4 | 192 | 62.1 | 13% | 160–232 | ||
| DBP (diastolic) | NO | 78.9 | 80 | 9.8 | 15% | 70–83 | <0.0001 | |
| (mmHg) | YES | 77.8 | 80 | 9.8 | 15% | 70–80 | ||
| SBP (systolic) | NO | 137 | 140 | 19.0 | 15% | 125–150 | 0.146 | |
| (mmHg) | YES | 137 | 140 | 19.2 | 15% | 125–150 | ||
| GOT | NO | 24.5 | 20 | 22.6 | 44% | 15–26 | 0.886 | |
| (mU/mL) | YES | 24.6 | 19 | 26.7 | 43% | 15–26 | ||
| GPT | NO | 30.8 | 24 | 28.2 | 40% | 17–35 | 0.005 | |
| (mU/mL) | YES | 29.2 | 22 | 31.2 | 39% | 16–32 | ||
| Q-Score | NO | 25.1 | 24.9 | 2.5 | 20% | 23.4–26.8 | <0.0001 | |
| YES | 25.4 | 25.2 | 2.4 | 24% | 23.8–27.0 | |||
| Number of out-of-range HbA1c values in the patient’s history | NO | 5.1 | 4 | 4.2 | 0% | 2–7 | <0.0001 | |
| YES | 4.2 | 3 | 3.5 | 0% | 2–6 | |||
| Relevant Factors | “1Y target” Outcome-NO | “1Y target” Outcome-YES | Relevance (0–1) |
|---|---|---|---|
| HbA1c at current visit | >8.9% (>74 mmol/mol) | <8.9% (<74 mmol/mol) | 0.752 |
| Age at current visit | <73 | >73 | 0.601 |
| Number of out-of-range HbA1c values in the patient’s history | >9 | <7 | 0.298 |
| HbA1c gap from previous visit | >+1.45% (>+14.6 mmol/mol) | <+1.45% (<+14.6 mmol/mol) | 0.136 |
| Q-Score | <24 | No threshold | 0.058 |
| BMI | >34 | <25 | 0.052 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicoletta, M.; Zilich, R.; Masi, D.; Baccetti, F.; Nreu, B.; Giorda, C.B.; Guaita, G.; Morviducci, L.; Muselli, M.; Ozzello, A.; et al. Overcoming Therapeutic Inertia in Type 2 Diabetes: Exploring Machine Learning-Based Scenario Simulation for Improving Short-Term Glycemic Control. Mach. Learn. Knowl. Extr. 2024, 6, 420-434. https://doi.org/10.3390/make6010021
Nicoletta M, Zilich R, Masi D, Baccetti F, Nreu B, Giorda CB, Guaita G, Morviducci L, Muselli M, Ozzello A, et al. Overcoming Therapeutic Inertia in Type 2 Diabetes: Exploring Machine Learning-Based Scenario Simulation for Improving Short-Term Glycemic Control. Machine Learning and Knowledge Extraction. 2024; 6(1):420-434. https://doi.org/10.3390/make6010021
Chicago/Turabian StyleNicoletta, Musacchio, Rita Zilich, Davide Masi, Fabio Baccetti, Besmir Nreu, Carlo Bruno Giorda, Giacomo Guaita, Lelio Morviducci, Marco Muselli, Alessandro Ozzello, and et al. 2024. "Overcoming Therapeutic Inertia in Type 2 Diabetes: Exploring Machine Learning-Based Scenario Simulation for Improving Short-Term Glycemic Control" Machine Learning and Knowledge Extraction 6, no. 1: 420-434. https://doi.org/10.3390/make6010021
APA StyleNicoletta, M., Zilich, R., Masi, D., Baccetti, F., Nreu, B., Giorda, C. B., Guaita, G., Morviducci, L., Muselli, M., Ozzello, A., Pisani, F., Ponzani, P., Rossi, A., Santin, P., Verda, D., Cianni, G. D., & Candido, R. (2024). Overcoming Therapeutic Inertia in Type 2 Diabetes: Exploring Machine Learning-Based Scenario Simulation for Improving Short-Term Glycemic Control. Machine Learning and Knowledge Extraction, 6(1), 420-434. https://doi.org/10.3390/make6010021







