Synthesis of Submicrocontainers with “Green” Biocide and Study of Their Antimicrobial Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
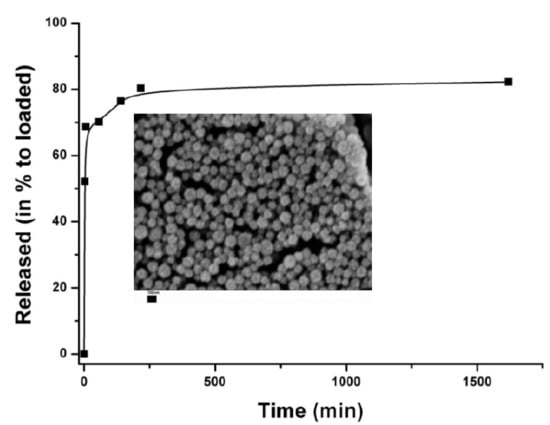
3.1. Formation of Submicrocontainers
3.2. Properties of Submicrocontainers
4. Сonclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Soliman, E.A.; El-Moghazy, A.Y.; El-Din, M.S.M.; Massoud, M.A. Microencapsulation of Essential Oils within Alginate: Formulation and in vitro Evaluation of Antifungal Activity. J. Encapsul. Adsorpt. Sci. 2013, 3, 48–55. [Google Scholar] [CrossRef]
- de Jesus, M.B.; Radaic, A.; Zuhorn, I.S. Microemulsion extrusion technique: A new method to produce lipid nanoparticles. J. Nanopart. Res. 2013, 15, 1960. [Google Scholar] [CrossRef]
- Kim, B.; Peppas, N.A. In vitro release behavior and stability of insulin in complexation hydrogels as oral drug delivery carriers. Int. J. Pharm. 2003, 266, 29–37. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Elgohary, M.M.; Kamel, N.M. Chapter Six—Implications of Protein- and Peptide-Based Nanoparticles as Potential Vehicles for Anticancer Drugs. Adv. Protein Struct. Biol. 2015, 98, 169–221. [Google Scholar]
- Song, L.; Zhi, Z.; Pickup, J.C. Nanolayer encapsulation of insulin- chitosan complexes improves efficiency of oral insulin delivery. Int. J. Nanomed. 2014, 9, 2127–2136. [Google Scholar]
- Chevalier, Y.; Bolzinger, M. Emulsions stabilized with solid nanoparticles: Pickering emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Zhou, H.; Shi, T.; Zhou, X. Preparation of polystyrene/SiO2 microsphere via Pickering emulsion polymerization: Synergistic effect of SiO2 concentrations and initiator sorts. Appl. Surf. Sci. 2013, 266, 33–38. [Google Scholar] [CrossRef]
- Yin, D.; Ma, L.; Liu, J.; Zhang, Q. Pickering emulsion: A novel template for microencapsulated phase change materials with polymer–silica hybrid shell. Energy 2014, 64, 575–581. [Google Scholar] [CrossRef]
- Frelichowska, J.; Bolzinger, M.; Chevalier, Y. Pickering emulsions with bare silica. Colloids Surf. A Physicochem. Eng. Asp. 2009, 343, 70–74. [Google Scholar] [CrossRef]
- Sharipova, A.; Aidarova, S.; Mucic, N.; Miller, R. Dilational rheology of polymer/surfactant mixtures at water/hexane interface. Colloids Surf. A Physicochem. Eng. Asp. 2011, 391, 130–134. [Google Scholar] [CrossRef]
- Sharipova, A.; Aidarova, S.; Cernoch, P.; Miller, R. Effect of surfactant hydrophobicity on the interfacial properties of polyallylamine hydrochloride/sodium alkylsulphate at water/hexane interface. Colloids Surf. A Physicochem. Eng. Asp. 2013, 438, 141–147. [Google Scholar] [CrossRef]
- Kaewsaneha, C.; Tangboriboonrat, P.; Polpanich, D.; Eissa, M.; Elaissari, A. Preparation of Janus colloidal particles via Pickering emulsion: An overview. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 35–42. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Sun, P.; Yang, C. Pickering emulsions stabilized by native starch granules. Colloids Surf. A Physicochem. Eng. Asp. 2013, 431, 142–149. [Google Scholar] [CrossRef]
- Zou, S.; Yang, Y.; Liu, H.; Wang, C. Synergistic stabilization and tunable structures of Pickering high internal phase emulsions by nanoparticles and surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 1–9. [Google Scholar] [CrossRef]
- Santini, E.; Guzmán, E.; Ferrari, M.; Liggieri, L. Emulsions stabilized by the interaction of silica nanoparticles and palmitic acid at the water–hexane interface. Colloids Surf. A Physicochem. Eng. Asp. 2014, 460, 333–341. [Google Scholar] [CrossRef]
- Schmitt, V.; Ravaine, V. Surface compaction versus stretching in Pickering emulsions stabilised by microgels. Curr. Opin. Colloid Interface Sci. 2013, 18, 532–541. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, T.; Wu, S.; Zhou, H. Graphene/polystyrene nanocomposites synthesized via Pickering emulsion polymerization. J. High Perform. Polym. 2014, 26, 156–165. [Google Scholar] [CrossRef]
- Grigoriev, D.; Shchukina, E.; Tleuova, A.; Aidarova, S.; Shchukin, D. Core/shell emulsion micro- and nanocontainers for self-protecting water based coatings. Surf. Coat. Technol. 2016, 303, 299–309. [Google Scholar] [CrossRef]
- Paster, N.; Bullerman, L.B. Mould Spoilage and Mycotoxin Formation in Grains as Controlled by Physical Means. Int. J. Food Microbiol. 1988, 7, 257–265. [Google Scholar] [CrossRef]
- Paster, N.; Menasherov, M.; Ravid, U.; Juven, B. Antifungal activity of oregano and thyme essential oils applied as fumigants against fungi attacking stored grain. J. Food Prot. 1995, 58, 81–85. [Google Scholar] [CrossRef]
- Hamilton-Kemp, T.R.; Archbold, D.D.; Loughrin, J.H.; Andersen, R.A.; McCracken, C.T.; Collins, R.W.; Fallik, E. Stimulation and Inhibition of Fungal Pathogens of Plants by Natural Volatile Phytochamicals and Their Analogs. Curr. Top. Photochem. 2000, 4, 95–104. [Google Scholar]
- Bixler, G.D.; Bhushan, B. Biofouling: Lessons from nature (review). Philos. Trans. R. Soc. A 2012, 370, 2381–2417. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, S.W. Microbiologically Influenced Corrosion Handbook; Woodhead Publishing Ltd.: Cambridge, UK, 1994. [Google Scholar]
- Jacobson, A.H.; Willingham, G.L. Sea-nine antifoulant: An environmentally acceptable alternative to organotin antifoulants. Sci. Total Environ. 2000, 258, 103–110. [Google Scholar] [CrossRef]
- Schonknecht, U.; Gruycheva, J.; Mathies, H.; Bergmann, H.; Burkhardt, M. Leaching of Biocides Used in Façade Coatings under Laboratory Test Conditions. Environ. Sci. Technol. 2009, 43, 9321–9328. [Google Scholar] [CrossRef] [PubMed]
- Atarijabarzadeh, S.; Strömberg, E.; Karlsson, S. Inhibition of biofilm formation on silicone rubber samples using various antimicrobial agents. Int. Biodeterior. Biodegrad. 2011, 65, 1111–1118. [Google Scholar] [CrossRef]
- Markarian. Rethinking Biocides for Plastics, Compounding World. July 2013, pp. 16–22. Available online: http://www.nanobiomatters.com/wordpress/wp-content/uploads/2013/07/CWJuly2013.pdf (accessed on July 2013).
- Negroni, A.; Zanaroli, G.; Ruzzi, M.; Fava, F. Biological fate of Diuron and Sea-nine® 211 and their effect on primary microbial activities in slurries of a contaminated sediment from Venice Lagoon. Ann. Microbiol. 2010, 60, 321–327. [Google Scholar] [CrossRef]
- Marine Paint Annual Report 2008; University of Gothenburg, Plant and Environmental Sciences: Gothenburg, Sweden, 2008.
- Breuer, K.; Mayer, F.; Scherer, C.; Schwerd, R.; Sedlbauer, K. Wirkstoffauswaschung aus hydrophoben Fassadenbeschichtungen: Verkapselte versus unverkapselte Biozid Systeme. Bauphysik 2012, 34, 19–23. [Google Scholar] [CrossRef]
- European Union. Commission implementing regulation (EU) No. 437/2014 approving 4,5-dichloro-2-octyl-2h-isothiazol-3-one as an existing active substance for use in biocidal products for product-type 21. Off. J. Eur. Union L 2015, 128, 64–67. [Google Scholar]
- Morley, J.O.; Kapur, A.J.O.; Charlton, M.H. Kinetic studies on the reactions of 3-isothiazolones with 2-methyl-2-propanethiol. Int. J. Chem. Kinet. 2007, 39, 254–260. [Google Scholar] [CrossRef]
- Arning, J.; Dringen, J.; Schmidt, M.; Thiessen, A.; Stolte, S.; Matzke, M.; Bottin-Weber, U.; Ceasar-Geertz, B.; Jastorff, B.; Ranke, J. Structure-activity relationships for the impact of selected isothiazol-3-one biocides on glutathione metabolism and glutathione reductase of the human liver cell line Hep G2. J. Toxicol. 2008, 246, 203–212. [Google Scholar] [CrossRef]
- Norwegian Climate and Pollution Agency. Competent Authority Report 4,5-Dichloro-2-octyl-2H-isothiazol-3-one (DCOIT) PT21; Climate and Pollution Agency: Oslo, Norway, 2010. [Google Scholar]
- Martinez, K.; Ferrer, I.; Hernando, M.D.; Fernandez-Alba, A.R.; Marce, R.M.; Borrull, F.; Barcel, D. Occurrence of antifouling biocides in the Spanish Mediterranean marine environment. J. Environ. Technol. 2001, 22, 543–552. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; del Toro-Sanchez, L.; Alvarez-Parrilla, E.; Gonzalez-Aguilar, G.A. High Relative Humidity In-Package of Fresh-Cut Fruits and Vegetables: Advantage or Disadvantage Considering Microbiological Problems and Antimicrobial Delivering Systems. J. Food Sci. 2008, 73, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.-H.; Bao, Y.; Yang, X.-Z.; Zhu, Y.-H.; Wang, J. Delivery of antibiotics with polymeric particles. Adv. Drug Deliv. Rev. 2014, 78, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Cheow, W.S.; Hadinoto, K. Factors affecting drug encapsulation and stability of lipidpolymer hybrid nanoparticles. Colloids Surf. B Biointerfaces 2011, 85, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Dzhakasheva, M.A.; Rysbaeva, G.S.; Esimova, A.M.; Narymbaeva, Z.K.; Elemanova, Z.R. Selection of Aspergillus Awamori Strain for Reception of the Highly Active Producer of the Complex of Pectolytic Enzyme; News of NAS RK, Biological and Medical Series; No. 5; Academy of Science of Republic of Kazakhstan: Almaty, Kazakhstan, 2017; pp. 165–173. [Google Scholar]
- Sacanna, S.; Kegel, W.K.; Philipse, A.P. Spontaneous oil-in-water emulsification induced by charge-stabilized dispersions of various inorganic colloids. Langmuir 2007, 23, 10486–10492. [Google Scholar] [CrossRef] [PubMed]
- Tleuova, A.; Aidarova, S.; Sharipova, A.; Bekturganova, N.; Schenderlein, M.; Grigoriev, D. Using profile analysis tensiometry for monitoring auto-oscillations caused by the hydrolysis of 3-(trimethoxysilyl) propyl methacrylate when contacting water. Colloids Surf. A Physicochem. Eng. Asp. 2016, 505, 18–22. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Pantoja, M.; Velasco, F.; Broekema, D.; Abenojar, J.; del Real, J.C. The Influence of pH on the Hydrolysis Process of γ-Methacryloxypropyltrimethoxysilane, Analyzed by FT-IR, and the Silanization of Electrogalvanized Steel. J. Adhes. Sci. Technol. 2014, 24, 1131–1143. [Google Scholar] [CrossRef]








| No. | Sample | Diameter of inhibition zone of microorganism growth, cm: Aspergillus niger (diameter of the full inhibition zone 5 cm) |
| 1 | Control nutrient medium without biocide | + |
| 2 | Biocide in free form | 2.0–2,6 |
| 3 | Submicrocontainer with biocide (1.6 g TPM, 0.16 g DCOIT, 2 g Ludox) | 3–3.5 |
| 4 | Empty capsule without biocide (TPM and Ludox) | + |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aidarova, S.B.; Sharipova, A.A.; Issayeva, A.B.; Mutaliyeva, B.Z.; Tleuova, A.B.; Grigoriev, D.O.; Kudasova, D.; Dzhakasheva, M.; Miller, R. Synthesis of Submicrocontainers with “Green” Biocide and Study of Their Antimicrobial Activity. Colloids Interfaces 2018, 2, 67. https://doi.org/10.3390/colloids2040067
Aidarova SB, Sharipova AA, Issayeva AB, Mutaliyeva BZ, Tleuova AB, Grigoriev DO, Kudasova D, Dzhakasheva M, Miller R. Synthesis of Submicrocontainers with “Green” Biocide and Study of Their Antimicrobial Activity. Colloids and Interfaces. 2018; 2(4):67. https://doi.org/10.3390/colloids2040067
Chicago/Turabian StyleAidarova, Saule B., Altynay A. Sharipova, Assem B. Issayeva, Botagoz Zh. Mutaliyeva, Aiym B. Tleuova, Dmitry O. Grigoriev, Dariga Kudasova, Madina Dzhakasheva, and Reinhard Miller. 2018. "Synthesis of Submicrocontainers with “Green” Biocide and Study of Their Antimicrobial Activity" Colloids and Interfaces 2, no. 4: 67. https://doi.org/10.3390/colloids2040067