Effect of Both the Phase Composition and Modification Methods on Structural-Adsorption Parameters of Dispersed Silicas
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Phase Compositions
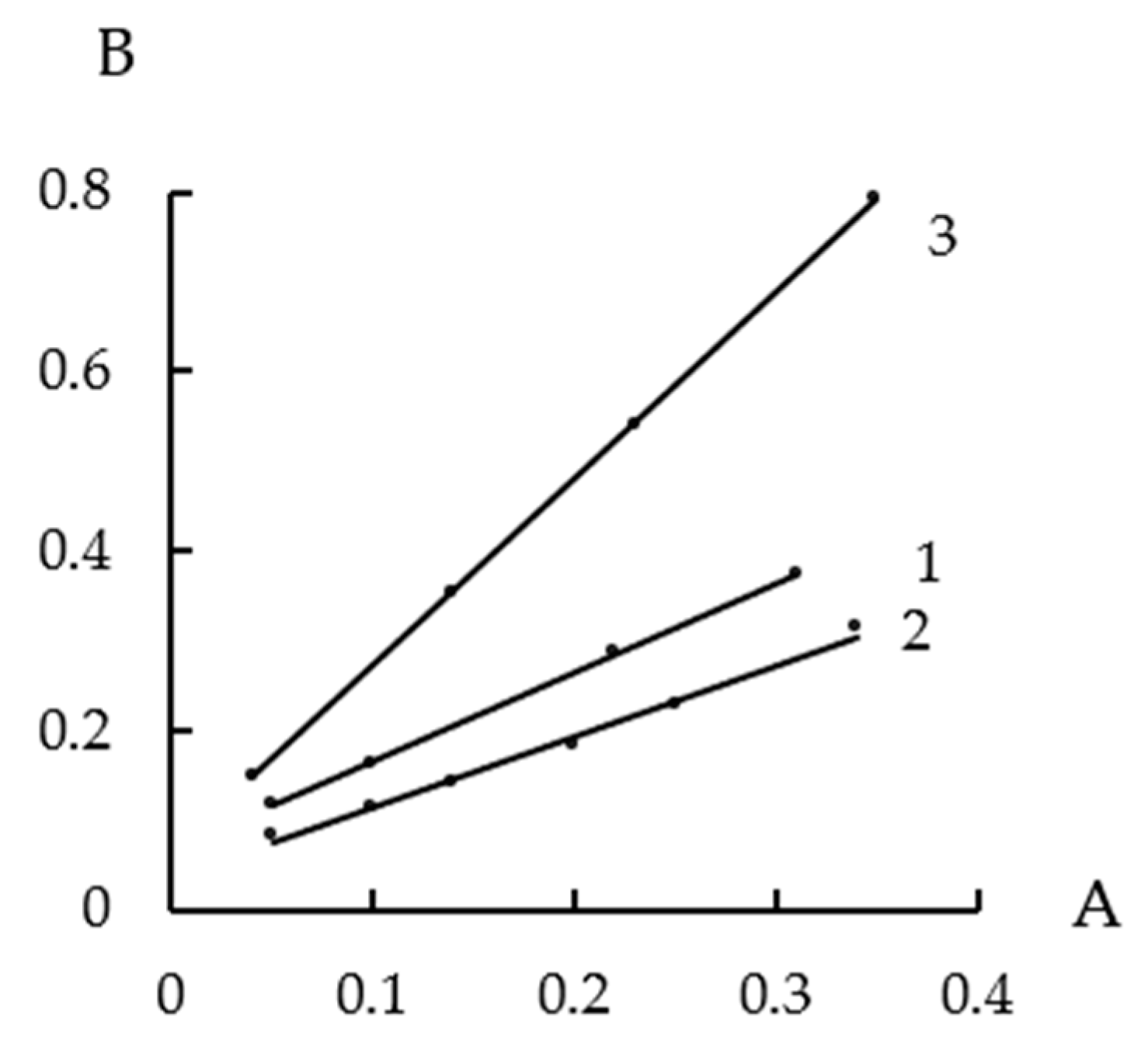
3.2. Water Vapor Sorption
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Koloborodov, V.G. Development of adsorption cryogenic investigations. Probl. Atom. Sci. Technol. 2006, 4, 38–46. [Google Scholar]
- Choudhury, B.; Saha, B.B.; Chatterjee, P.K.; Sarkar, J.P. An overview of developments in adsorption refrigeration systems towards a sustainable way of cooling. Appl. Energy 2013, 104, 554–567. [Google Scholar] [CrossRef]
- Younes, M.M.; El-Sharkawy, I.I.; Kabeel, A.; Saha, B.B. A review on adsorbent-adsorbate pairs for cooling applications. Appl. Therm. Eng. 2017, 114, 394–414. [Google Scholar] [CrossRef]
- Saha, B.B.; El-Sharkawy, I.I.; Shahzad, M.W.; Thu, K.; Ang, L.; Ng, K.C. Fundamental and application aspects of adsorption cooling and desalination. Appl. Therm. Eng. 2016, 97, 68–76. [Google Scholar] [CrossRef] [Green Version]
- Rakitskaya, T.L.; Ennan, А.A.; Volkova, V.Y. Low-Temperature Catalytic Air Purification from Carbon Monoxide; Ecology: Odessa, Ukraine, 2005; p. 191. ISBN 966-8740-13-0. [Google Scholar]
- Rakitskaya, T.L.; Ennan, А.A. Phosphine. Physicochemical Properties and Practicalities of Catching; Astroprint: Odessa, Ukraine, 2012; p. 2081. ISBN 978-966-190-554-1. [Google Scholar]
- Kiose, T.A.; Volkova, V.Y.; Ennan, А.A. Use of Ukrainian natural aluminosilicates for development of new metal-complex catalysts for air purification from toxic gaseous substances. Energotechnol. Resour.-Sav. 2009, 6, 18–23. [Google Scholar]
- Rakitskaya, T.L.; Truba, A.S.; Kiose, T.A.; Raskola, L.A. Formation mechanisms of 3d metal complexes on porous supports and their catalytic activity in redox-reactions. ONU Herald Chem. 2015, 20, 27–48. [Google Scholar] [CrossRef]
- Tarasevich, Y.I. Natural Sorbents in the Process. of Water Purification; Naukova Dumka: Kiev, Ukraine, 1981; p. 208. [Google Scholar]
- Sheng, G.; Hu, J.; Wang, X. Sorption properties of Th(IV) on the raw diatomite-Effects of contact time, pH, ionic strength and temperature. Appl. Radiat. Isot. 2008, 66, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Sljivic, M.; Smiciklas, I.; Pejanovic, S.; Plecas, I. Comparative study of Cu2+ adsorption on a zeolite, a clay and a diatomite from Serbia. Appl. Clay Sci. 2009, 43, 33–40. [Google Scholar] [CrossRef]
- Khraishen, A.M.; Al-Degs, Y.; Mcminn, A.M. Remediation of wastewater containing heavy metals using raw and modified diatomite. Chem. Eng. J. 2004, 99, 177–184. [Google Scholar] [CrossRef]
- Zaitan, H.; Chafik, T. FTIR determination of adsorption characteristics for volatile organic compounds removal on diatomite mineral compared to commercial silica. C. R. Chim. 2005, 8, 1701–1708. [Google Scholar] [CrossRef]
- Erdem, E.; Colgecen, G.; Donat, R. The removal of textile dyes by diatomite earth. J. Colloid Interface Sci. 2005, 282, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Karatepe, N.; Erdoĝan, N.; Ersoy-Meriçboyu, A.; Küçükbayrak, S. Preparation of diatomite/Ca(OH)2 sorbents and modeling their sulphation reaction. Chem. Eng. Sci. 2004, 59, 3883–3889. [Google Scholar] [CrossRef]
- Rakitskaya, T.L.; Kiose, T.A.; Kameneva, A.V. Adsorption properties of natural sorbents towards sulfur dioxide and water vapor. Chem. Phys. Technol. Surf. 2014, 5, 56–63. [Google Scholar]
- Rakitskaya, T.L.; Kiose, T.A.; Golubchik, K.O.; Kara, A.L. Adsorption-desorption properties of natural and modified tripoli towards Cu(II) and Pd(II)–components of catalysts for carbon monoxide oxidation. ONU Herald Chem. 2017, 22, 80–93. [Google Scholar] [CrossRef]
- Rakitskaya, T.L.; Kiose, T.A.; Oleksenko, L.P. Effect of water content on activity of the natural tripoli supported palladium-copper catalyst for carbon monoxide oxidation with air. ISS Chem. Chem. Technol. 2014, 3, 56–59. [Google Scholar]
- Distanov, U.G.; Mikhailov, A.S.; Konyukhova, T.P. Natural Sorbents of the USSR; Interior: Moscow, Russian, 1976; p. 206. [Google Scholar]
- Rakitskaya, T.L.; Kiose, T.A.; Golubchik, K.O.; Ennan, A.A.; Volkova, V.Y. Acid-modified clinoptilolite as a support for palladium-copper complexes catalyzing carbon monoxide oxidation with air oxygen. Chem. Cent. J. 2017, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rakitskaya, T.L.; Kiose, T.A.; Golubchik, K.O.; Oleksenko, L.P.; Dlubovskii, R.M.; Geraseva, V.G. Effect of acid-thermal modification duration on adsorption-structural properties of clinoptilolite. ONU Herald Chem. 2016, 21, 24–35. [Google Scholar] [CrossRef]
- Rakitskaya, T.L.; Dzhiga, A.M.; Kiose, T.A. Adsorption and physicochemical properties of natural and modified montmorillonite forms. ONU Herald Chem. 2017, 22, 38–54. [Google Scholar] [CrossRef]
- Rakitskaya, T.L.; Truba, A.S.; Ennan, A.A.; Dlubovskii, R.M.; Volkova, V.Y. Water vapor adsorption by nanostructured polyphase compositions based on the solid component of welding aerosol. Adsorpt. Sci. Technol. 2017, 35, 389–395. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity; Academic Press: New York, NY, USA, 1982. [Google Scholar]
- Wang, C.; Cao, L.; Huang, J. Influences of acid and heat treatments on the structure and water vapor adsorption property of natural zeolite. Surf. Interface Anal. 2017, 49, 1249–1255. [Google Scholar] [CrossRef]
- Yamamoto, S.; Kendelewicz, T.; Newberg, J.T. Water adsorption on α-Fe2O3(0001) at near ambient conditions. J. Phys. Chem. 2017, 114, 2256–2266. [Google Scholar] [CrossRef]
- Song, X.; Boily, J.F. Water vapor adsorption on goethite. Environ. Sci. Technol. 2013, 47, 7171–7177. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Xu, Z.; Wang, C.; Gao, Y. Shape evolution of metal nanoparticles in water vapor environment. Nano Lett. 2016, 16, 2628–2632. [Google Scholar] [CrossRef] [PubMed]
- Stakebake, J.L.; Steward, L.M. Water vapor adsorption on plutonium dioxide. J. Colloid Interface Sci. 1973, 42, 328–333. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; Selim, A.Q. Heat treatment of natural diatomite. Physicochem. Probl. Miner. Process. 2012, 48, 413–424. [Google Scholar] [CrossRef]


| Sample | Phases | Phase Content, wt % | D, nm | Sample | Phases | Phase Content, wt % | D, nm |
|---|---|---|---|---|---|---|---|
| N-Tr(K-I) | β-crist | 30.8 | 11 | H2O-Tr(K-II) | β-crist | 42.1 | 8 |
| α-trid | 22.2 | 11 | α-trid | 29.9 | 6 | ||
| α-quartz | 31.9 | 248 | α-quartz | 20.3 | 248 | ||
| N-Tr(K-II) | β-crist | 36.0 | 9 | 3H-Tr(K-II) | β-crist | 44.7 | 8 |
| α-trid | 29.2 | 48 | α-trid | 25.7 | 7 | ||
| α-quartz | 23.4 | 126 | α-quartz | 23.8 | 248 | ||
| N-Tr(MP) | calcite | 47.3 | 555 | 1000-Tr(K-II) | β-crist | 19.6 | 21 |
| β-crist | 35.5 | 8 | α-trid | 39.7 | 13 | ||
| α-trid | 16.2 | 26 | α-quartz | 25.9 | >1000 | ||
| α-quartz | 0.6 | - | H2O-Tr(MP) | calcite | 49 | 93 | |
| H2O-Tr(K-I) | β-crist | 37.8 | 9 | β-crist | 23 | 21 | |
| α-trid | 55.6 | 18 | α-trid | 27 | 20 | ||
| α-quartz | 4.24 | 103 | α-quartz | 0.8 | >1000 | ||
| 3H-Tr (K-I) | β-crist | 20.1 | 9 | 3H-Tr(MP) | calcite | 44 | 93 |
| α-trid | 20.6 | 18 | β-crist | 31.3 | 21 | ||
| α-quartz | 50.0 | 292 | α-trid | 23.2 | 20 | ||
| 1000-Tr (K-I) | β-crist | 49.1 | 19 | α-quartz | 1.5 | >1000 | |
| α-trid | 20.4 | 20 | 1000-Tr(MP) | wollast | 45.2 | 51 | |
| α-quartz | 21.1 | 800 | β-larnite | 24.9 | 34 | ||
| α-crist | 12.2 | 6 | |||||
| α-trid | 15.2 | 8 |
| Sample | аm, mmol/g | С | а∞, mmol/g | Ssp, m2/g | Maximum Values of Pore Diameter Distribution Curves, nm |
|---|---|---|---|---|---|
| N-Tr(K-I) | 0.92 | 16.71 | 7.7 | 60 | 5.2 |
| N-Tr(K-II) | 1.29 | 18.26 | 5.7 | 84 | 3.0, 5.5, 9.7 |
| N-Tr(MP) | 0.47 | 29.88 | 4.4 | 30 | 3.4, 8.5 |
| H2O-Tr(K-I) | 1.14 | 6.02 | 7.7 | 74 | 2.5, 4.6, 8.0 |
| 3H-Tr(K-I) | 0.93 | 6.62 | 6.8 | 60 | 3.9, 7.0 |
| 1000-Tr(K-I) | 0.21 | 3.35 | 0.36 | 13 | 4.3, 9.4 |
| H2O-Tr(K-II) | 0.84 | 25.70 | 5.0 | 54 | 1.9, 5.3, 8.6 |
| 3H-Tr(K-II) | 0.96 | 13.37 | 4.1 | 62 | 3.7 |
| 1000-Tr(K-II) | 0.13 | 3.39 | 0.37 | 8 | 4.4, 8.4, 13.7 |
| H2O-Tr(MP) | 0.46 | 19.06 | 4.4 | 30 | 3.3, 8.4 |
| 3H-Tr(MP) | 0.80 | 17.30 | 5.10 | 52 | 3.3, 4.7, 8.4 |
| 1000-Tr(MP) | 0.09 | 8.59 | 0.40 | 6 | 9.5, 13.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakitskaya, T.; Kiose, T.; Golubchik, K.; Baumer, V.; Volkova, V. Effect of Both the Phase Composition and Modification Methods on Structural-Adsorption Parameters of Dispersed Silicas. Colloids Interfaces 2019, 3, 1. https://doi.org/10.3390/colloids3010001
Rakitskaya T, Kiose T, Golubchik K, Baumer V, Volkova V. Effect of Both the Phase Composition and Modification Methods on Structural-Adsorption Parameters of Dispersed Silicas. Colloids and Interfaces. 2019; 3(1):1. https://doi.org/10.3390/colloids3010001
Chicago/Turabian StyleRakitskaya, Tatyana, Tatyana Kiose, Kristina Golubchik, Viacheslav Baumer, and Vitaliya Volkova. 2019. "Effect of Both the Phase Composition and Modification Methods on Structural-Adsorption Parameters of Dispersed Silicas" Colloids and Interfaces 3, no. 1: 1. https://doi.org/10.3390/colloids3010001




