Asphaltenes at Oil/Gas Interfaces: Foamability Even with No Significant Surface Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Asphaltenes Extraction
2.2.2. Crude Oil Model Solutions Preparation
2.2.3. Tensiometry for Surface Tension and Evaporation Measurements
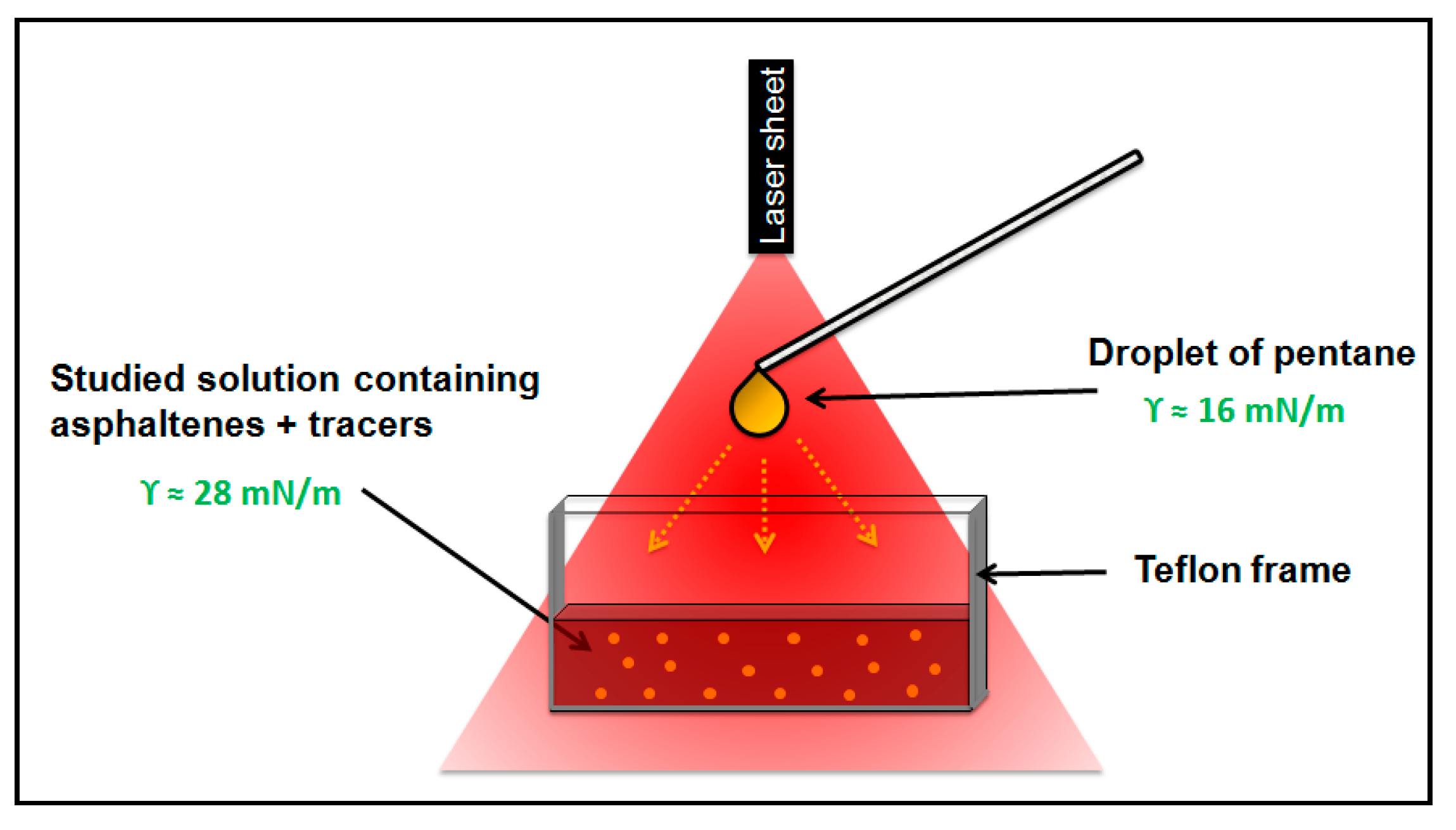
2.2.4. Marangoni Flow Method
- -
- A decane/toluene (45/55 wt%) mixture containing 0.0025, 0.05, 0.1, 1.5 or 5 wt% of asphaltenes TR
- -
- toluene containing 2 or 5 wt% of asphaltenes TR
- -
- A decane/toluene (35/65 wt%) mixture containing 0.02, 0.3, 0.1 or 1.5 wt% of asphaltenes MO
2.2.5. Foamability Tests
- -
- The lower part consisted of a bubbly liquid of height .
- -
- The upper part consisted of a foam of height .
2.3. Summary of the Investigated Systems and Used Techniques
3. Results
3.1. Surface Tension for Oil Mixtures Containing Asphaltenes: Layer Formation and Evaporation
3.2. Evaluation of the Surface Activity with the Marangoni Flow Method
3.3. Foamability
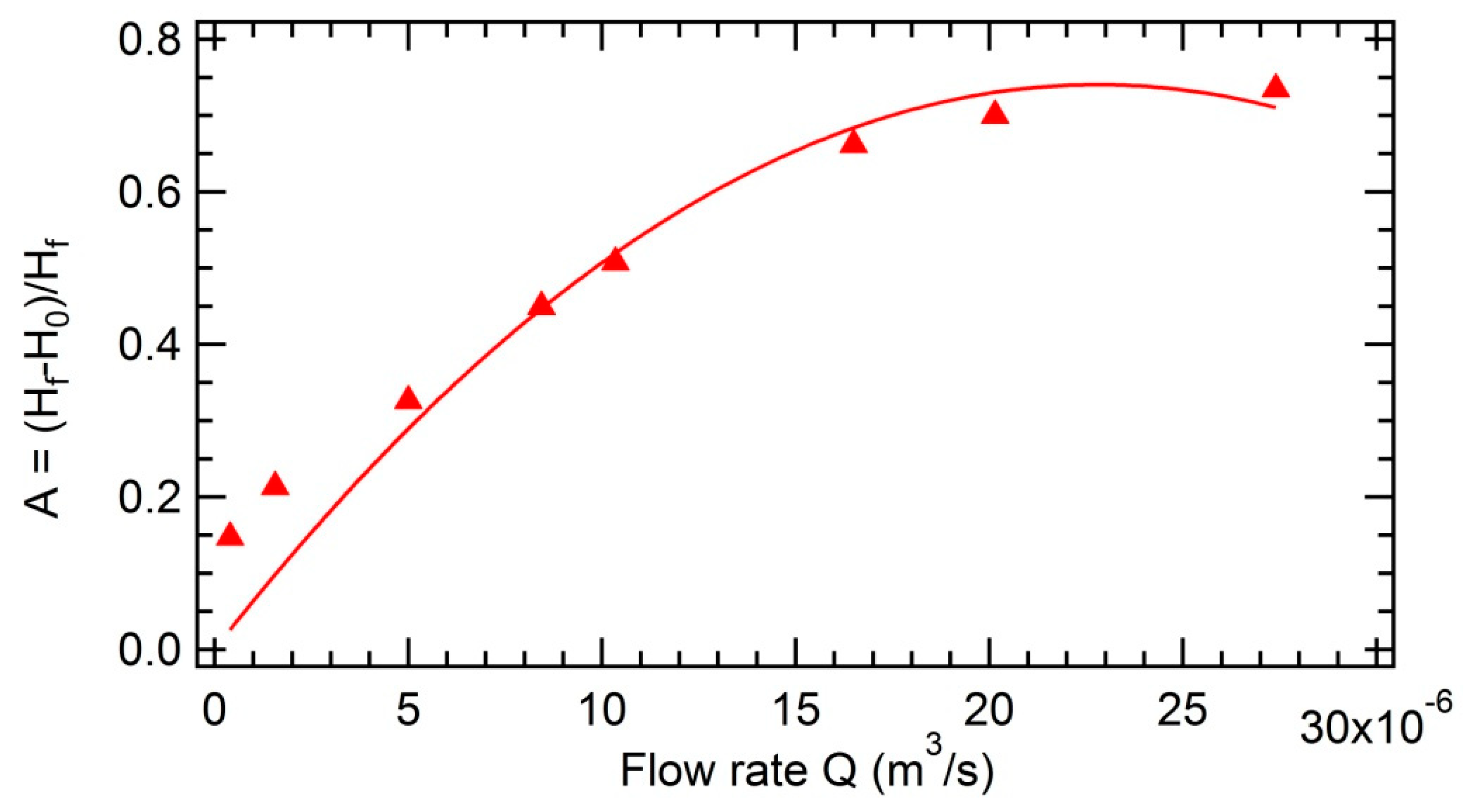
3.3.1. Impact of the Concentration of Asphaltenes on the Foamability
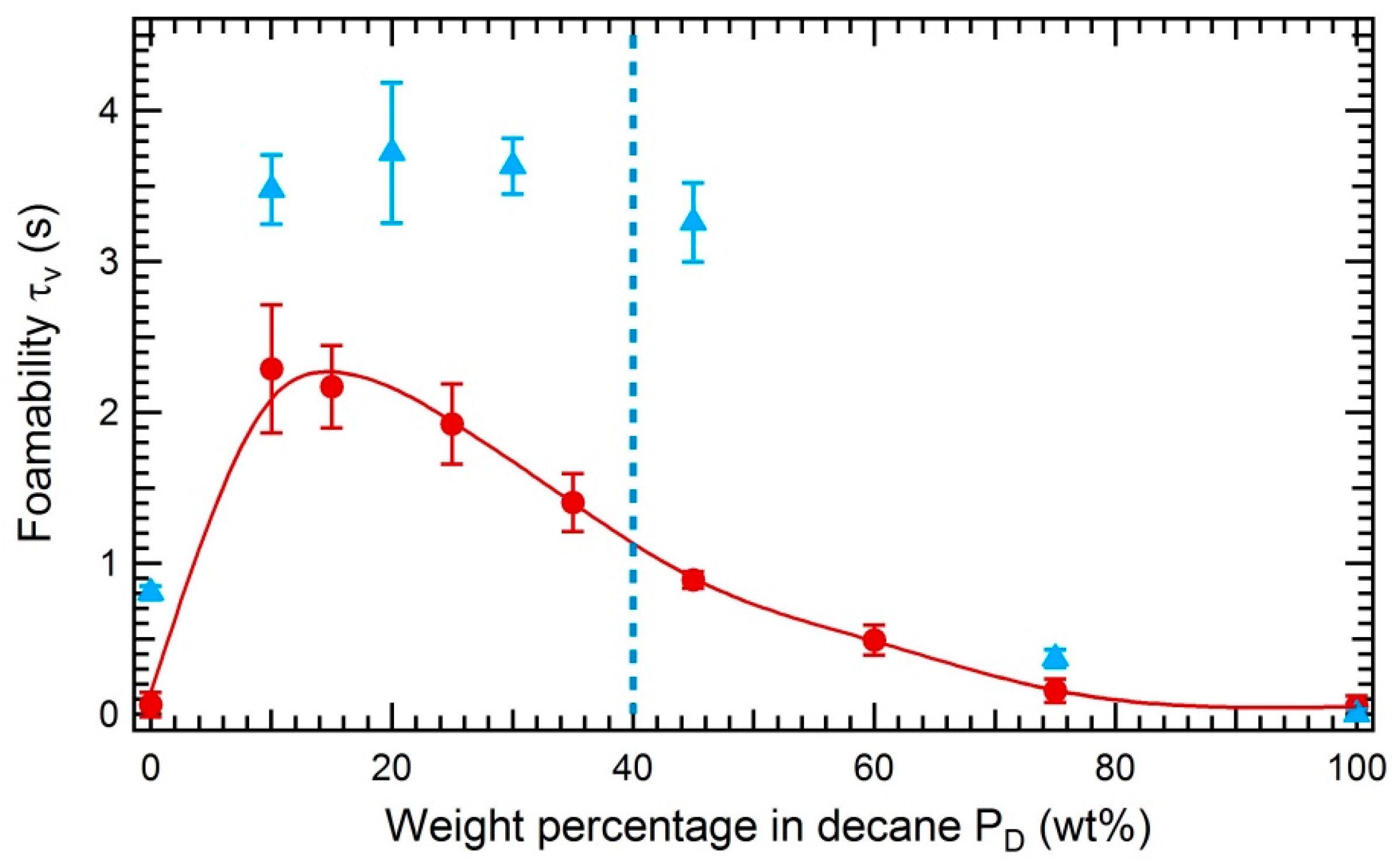
3.3.2. Impact of the Solubility of Asphaltenes on the Foamability
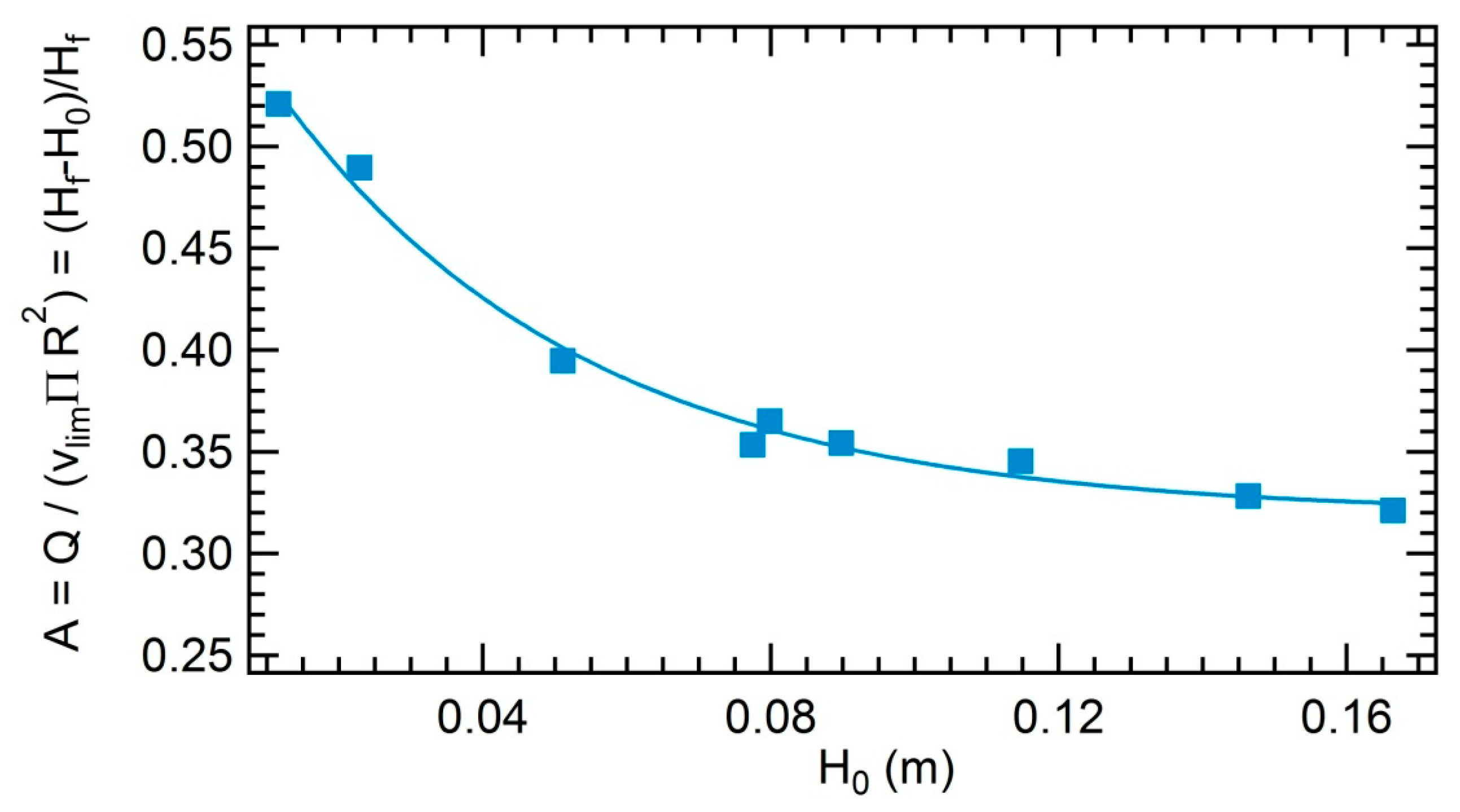
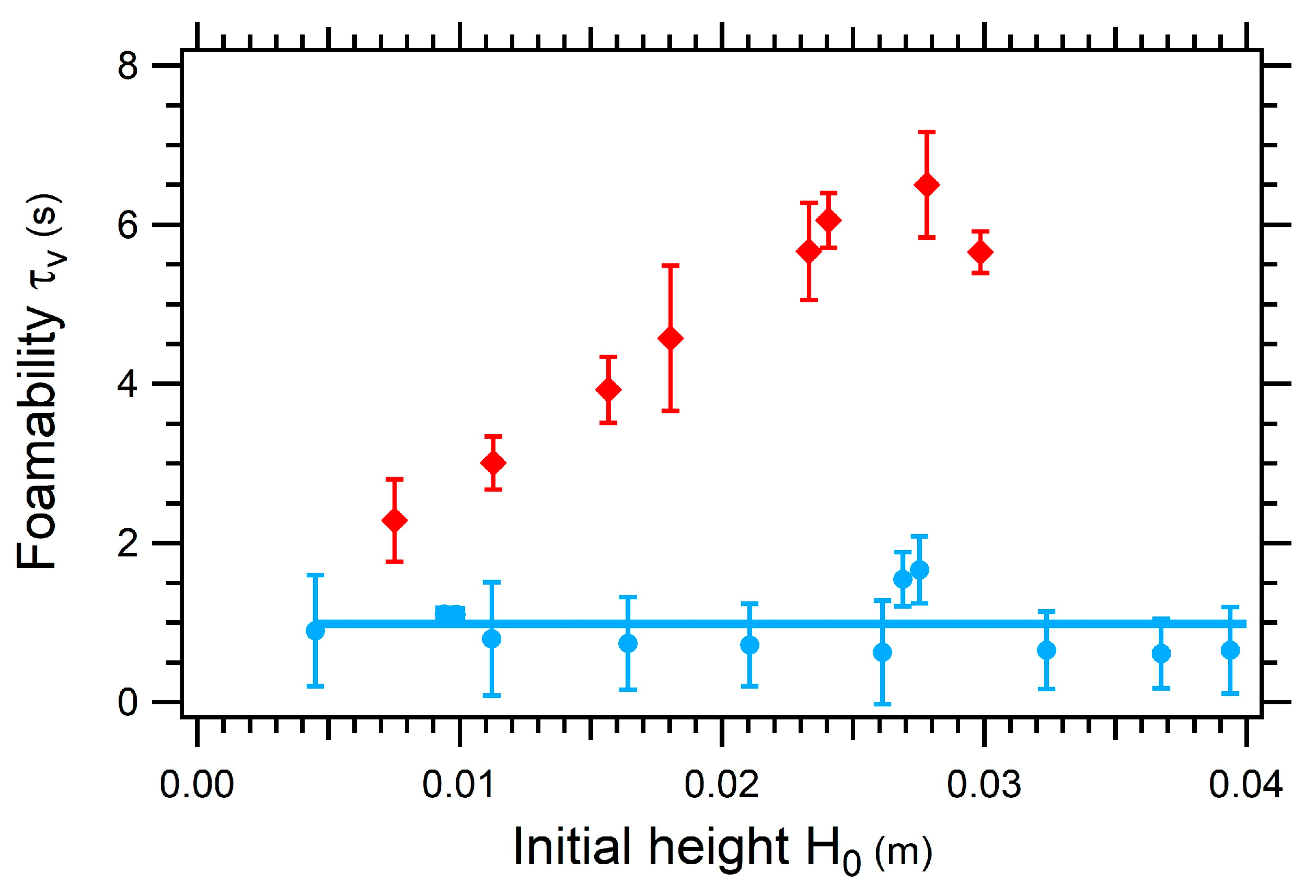
3.3.3. Impact of the Initial Height on the Foamability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
- -
- The upper part composed of foam which volume is equal to
- -
- The lower part composed of bubbly liquid which liquid volume is equal to Thus, we obtain:
Appendix B
Appendix C
References
- McCain, W.D., Jr. Properties of Petroleum Fluids, 3rd ed.; PennWell Corporation: Tulsa, OK, USA, 2017; ISBN 978-1-59370-373-8. [Google Scholar]
- Wauquier, J.-P. All Petroleum Refining. Vol. 1 Crude Oil. Petroleum Products. Process Flowsheets. Available online: http://www.editionstechnip.com/fr/catalogue-detail/525/petroleum-refining-vol-1-crude-oil-petroleum-products-process-flowsheets.html (accessed on 18 November 2018).
- Manning, F.S.; Thompson, R.E. Oilfield Processing of Petroleum: Crude oil; PennWell Books: Tulsa, OK, USA, 1991; ISBN 978-0-87814-354-2. [Google Scholar]
- Langevin, D.; Argillier, J.-F. Interfacial behavior of asphaltenes. Adv. Colloid Interf. Sci. 2016, 233, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Mullins, O.C.; Sabbah, H.; Eyssautier, J.; Pomerantz, A.E.; Barré, L.; Andrews, A.B.; Ruiz-Morales, Y.; Mostowfi, F.; McFarlane, R.; Goual, L.; et al. Advances in Asphaltene Science and the Yen–Mullins Model. Energy Fuels 2012, 26, 3986–4003. [Google Scholar] [CrossRef]
- Chacón-Patiño, M.L.; Rowland, S.M.; Rodgers, R.P. Advances in Asphaltene Petroleomics. Part 1: Asphaltenes Are Composed of Abundant Island and Archipelago Structural Motifs. Energy Fuels 2017, 31, 13509–13518. [Google Scholar] [CrossRef]
- Yarranton, H.W.; Alboudwarej, H.; Jakher, R. Investigation of Asphaltene Association with Vapor Pressure Osmometry and Interfacial Tension Measurements. Ind. Eng. Chem. Res. 2000, 39, 2916–2924. [Google Scholar] [CrossRef]
- McLean, J.D.; Kilpatrick, P.K. Effects of Asphaltene Aggregation in Model Heptane–Toluene Mixtures on Stability of Water-in-Oil Emulsions. J. Colloid Interf. Sci. 1997, 196, 23–34. [Google Scholar] [CrossRef]
- McLean, J.D.; Kilpatrick, P.K. Effects of Asphaltene Solvency on Stability of Water-in-Crude-Oil Emulsions. J. Colloid Interf. Sci. 1997, 189, 242–253. [Google Scholar] [CrossRef]
- Kilpatrick, P.K. Water-in-Crude Oil Emulsion Stabilization: Review and Unanswered Questions. Energy Fuels 2012, 26, 4017–4026. [Google Scholar] [CrossRef]
- Gafonova, O.V.; Yarranton, H.W. The Stabilization of Water-in-Hydrocarbon Emulsions by Asphaltenes and Resins. J. Colloid Interf. Sci. 2001, 241, 469–478. [Google Scholar] [CrossRef]
- Jeribi, M.; Almir-Assad, B.; Langevin, D.; Hénaut, I.; Argillier, J.F. Adsorption Kinetics of Asphaltenes at Liquid Interfaces. J. Colloid Interf. Sci. 2002, 256, 268–272. [Google Scholar] [CrossRef]
- Yeung, A.; Dabros, T.; Czarnecki, J.; Masliyah, J. On the interfacial properties of micrometre–sized water droplets in crude oil. Proc. R. Soc. London A Math. Phy. Eng. Sci. 1999, 455, 3709–3723. [Google Scholar] [CrossRef]
- The Effects of Inorganic Solid Particles on Water and Crude Oil Emulsion Stability. Available online: https://pubs.acs.org/doi/10.1021/ie010927n (accessed on 18 November 2018).
- Chacón-Patiño, M.L.; Blanco-Tirado, C.; Orrego-Ruiz, J.A.; Gómez-Escudero, A.; Combariza, M.Y. High Resolution Mass Spectrometric View of Asphaltene–SiO2 Interactions. Energy Fuels 2015, 29, 1323–1331. [Google Scholar] [CrossRef]
- Adams, J.J. Asphaltene Adsorption, a Literature Review. Energy Fuels 2014, 28, 2831–2856. [Google Scholar] [CrossRef]
- Puntervold, T.; Mamonov, A.; Aghaeifar, Z.; Frafjord, G.O.; Moldestad, G.M.; Strand, S.; Austad, T. Role of Kaolinite Clay Minerals in Enhanced Oil Recovery by Low Salinity Water Injection. Energy Fuels 2018, 32, 7374–7382. [Google Scholar] [CrossRef]
- Alzobaidi, S.; Lee, J.; Jiries, S.; Da, C.; Harris, J.; Keene, K.; Rodriguez, G.; Beckman, E.; Perry, R.; Johnston, K.P.; et al. Carbon dioxide-in-oil emulsions stabilized with silicone-alkyl surfactants for waterless hydraulic fracturing. J. Colloid Interf. Sci. 2018, 526, 253–267. [Google Scholar] [CrossRef]
- Bauget, F.; Langevin, D.; Lenormand, R. Dynamic Surface Properties of Asphaltenes and Resins at the Oil–Air Interface. J. Colloid Interf. Sci. 2001, 239, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E. Adsorbed protein layers at fluid interfaces: Interactions, structure and surface rheology. Colloid. Surf. B 1999, 15, 161–176. [Google Scholar] [CrossRef]
- Passade-Boupat, N.; Gingras, J.-P.; Desplobins, C.; Zhou, H. Could the Asphaltene Solubility Class Index Be Used as the “Wax Appearance Temperature” of Asphaltenes? Illustration through the Study of the Polydispersity of PetroPhase 2017 Asphaltenes. Energy Fuels 2018, 32, 2760–2768. [Google Scholar] [CrossRef]
- Arangalage, M.; Li, X.; Lequeux, F.; Talini, L. Dual Marangoni effects and detection of traces of surfactants. Soft Matter 2018, 14, 3378–3386. [Google Scholar] [CrossRef] [PubMed]
- Sbalzarini, I.F.; Koumoutsakos, P. Feature point tracking and trajectory analysis for video imaging in cell biology. J. Struct. Biol. 2005, 151, 182–195. [Google Scholar] [CrossRef]
- Zaki, N.N.; Poindexter, M.K.; Kilpatrick, P.K. Factors Contributing to Petroleum Foaming. 2. Synthetic Crude Oil Systems. Energy Fuels 2002, 16, 711–717. [Google Scholar] [CrossRef]
- Ross, S.; Nishioka, G. Foaminess of binary and ternary solutions. J. Phys. Chem. 1975, 79, 1561–1565. [Google Scholar] [CrossRef]
- Blazy, P.; Jdid, E.-A. Flottation-Mécanismes et Réactifs. Available online: https://www.techniques-ingenieur.fr/base-documentaire/procedes-chimie-bio-agro-th2/operations-unitaires-tri-et-traitement-des-liquides-et-des-solides-42446210/flottation-j3350/ (accessed on 18 November 2018).










| Experiment | Physical Value Measured | Decane/Toluene Ratio | Concentration of Asphaltenes |
|---|---|---|---|
| Tensiometer (pendant drop method) | Surface tension and evaporation measurements | Toluene Decane/Toluene(45/55 wt%) | 5 wt% TR 5 wt% TR |
| Marangoni experiment | Surface activity at liquid/gas interface | Decane/Toluene (45/55 wt%) | 0.0025, 0.05, 0.1, 1 and 5 wt% TR |
| Foamability test | Impact of asphaltenes concentration on foamability | Decane/Toluene (45/55 wt%) Decane/Toluene (35/65 wt%) | Between 0.001 and 5 wt% TR Between 0.01 and 5 wt% MO |
| Foamability test | Impact of asphaltenes solubility on foamability | Decane/Toluene varies from 0 to 100 wt% in decane | 5 wt% TR 3 wt% MO |
| Foamability test | Impact of the initial liquid height introduced on foamability | Decane/Toluene (45/55 wt%) | 5 wt% TR |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arangalage, M.; Gingras, J.-P.; Passade-Boupat, N.; Lequeux, F.; Talini, L. Asphaltenes at Oil/Gas Interfaces: Foamability Even with No Significant Surface Activity. Colloids Interfaces 2019, 3, 2. https://doi.org/10.3390/colloids3010002
Arangalage M, Gingras J-P, Passade-Boupat N, Lequeux F, Talini L. Asphaltenes at Oil/Gas Interfaces: Foamability Even with No Significant Surface Activity. Colloids and Interfaces. 2019; 3(1):2. https://doi.org/10.3390/colloids3010002
Chicago/Turabian StyleArangalage, Mélanie, Jean-Philippe Gingras, Nicolas Passade-Boupat, François Lequeux, and Laurence Talini. 2019. "Asphaltenes at Oil/Gas Interfaces: Foamability Even with No Significant Surface Activity" Colloids and Interfaces 3, no. 1: 2. https://doi.org/10.3390/colloids3010002





