Investigating Forkhead Box O Transcription Factor 1 Gene’s Relation to Immunoglobulin E in House Dust Mite-Allergic Asthma Patients
Abstract
:Highlights
- Subcutaneous immunotherapy is a long-term effective immunotherapy;
- Immunity biomarkers such as IgE, FoxO1, and Sirtuin 1 are important biomarkers that have potential roles in the pathogenesis of asthma and remission of clinical symptoms.
- There is correlation between IgE, FoxO1, and Sirtuin 1;
- Further broader-scale studies are needed to determine a novel protocol for the control and remission of clinical symptoms of HDM-allergic asthma.
Abstract
1. Introduction
2. Patients and Method
2.1. Study Design and Ethical Considerations
2.1.1. Sample Size
2.1.2. Inclusion and Exclusion Criteria
2.1.3. Assessment of Severity of Asthma
2.1.4. Pulmonary Function Test
2.1.5. Skin Prick Test
2.2. Samples
2.3. Measurement of the mRNA Expression Levels of FoxO1 and Sirtuin 1
2.3.1. Separation of Peripheral Blood Mononuclear Cells (PBMCs)
2.3.2. RNA Extraction and Isolation
2.3.3. Synthesis of the First-Strand Complementary DNA
2.3.4. Quantitative Analysis of FoxO1, SIRT1, and 18S rRNA Genes
2.4. Enzyme-Linked Immunosorbent Assay
2.5. Statistical Analysis
3. Results
Participants Criteria
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ando, T.; Kitaura, J. Tuning IgE: IgE-Associating Molecules and Their Effects on IgE-Dependent Mast Cell Reactions. Cells 2021, 10, 1697. [Google Scholar] [CrossRef]
- Arlian, L.G.; Bernstein, D.; Bernstein, I.L.; Friedman, S.; Grant, A.; Lieberman, P.; Lopez, M.; Metzger, J.; Platts-Mills, T.; Schatz, M.; et al. Prevalence of dust mites in the homes of people with asthma living in eight different geographic areas of the United States. J. Allergy Clin. Immunol. 1992, 90, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Australasian Society of Clinical Immunology and Allergy (ASCIA). Skin Prick Testing Guide for Diagnosis of Allergic Disease. 2020. Available online: https://www.allergy.org.au/images/ASCIA_HP_SPT_Guide_2020.pdf (accessed on 16 June 2023).
- Bachert, C.; Zhang, N. Chronic rhinosinusitis and asthma: Novel understanding of the role of IgE ‘above atopy’. J. Intern. Med. 2012, 272, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Baioumy, S.A.; Elgendy, A.; Ibrahim, S.M.; Taha, S.I.; Fouad, S.H. Association between serum zonulin level and severity of house dust mite allergic asthma. Allergy Asthma Clin. Immunol. 2021, 17, 86. [Google Scholar] [CrossRef] [PubMed]
- Beeh, K.M.; Ksoll, M.; Buhl, R. Elevation of total serum immunoglobulin E is associated with asthma in nonallergic individuals. Eur. Respir. J. 2000, 16, 609. [Google Scholar] [CrossRef]
- Bernstein, I.L.; Li, J.T.; Bernstein, D.I.; Hamilton, R.; Spector, S.L.; Tan, R.; Sicherer, S.; Golden, D.B.K.; Khan, D.A.; Nicklas, R.A.; et al. Allergy Diagnostic Testing: An Updated Practice Parameter. Ann. Allergy Asthma Immunol. 2008, 100, S1–S148. [Google Scholar] [CrossRef] [PubMed]
- Bijanzadeh, M.; Mahesh, P.A.; Ramachandra, N.B. An understanding of the genetic basis of asthma. Indian J. Med. Res. 2011, 134, 149–161. [Google Scholar] [PubMed]
- Blank, U.; Huang, H.; Kawakami, T. The high affinity IgE receptor: A signaling update. Curr. Opin. Immunol. 2021, 72, 51–58. [Google Scholar] [CrossRef]
- de Blay, F.; Gherasim, A.; Casale, T.B.; Doyen, V.; Bernstein, D. Which patients with asthma are most likely to benefit from allergen immunotherapy? J. Allergy Clin. Immunol. 2022, 149, 833–843. [Google Scholar] [CrossRef]
- Blink, S.E.; Fu, Y.-X. IgE regulates T helper cell differentiation through FcγRIII mediated dendritic cell cytokine modulation. Cell. Immunol. 2010, 264, 54–60. [Google Scholar] [CrossRef]
- Brunet, A.; Sweeney, L.B.; Sturgill, J.F.; Chua, K.F.; Greer, P.L.; Lin, Y.; Tran, H.; Ross, S.E.; Mostoslavsky, R.; Cohen, H.Y.; et al. Stress-Dependent Regulation of FOXO Transcription Factors by the SIRT1 Deacetylase. Science 2004, 303, 2011–2015. [Google Scholar] [CrossRef] [PubMed]
- Burrows, B.; Martinez, F.D.; Halonen, M.; Barbee, R.A.; Cline, M.G. Association of Asthma with Serum IgE Levels and Skin-Test Reactivity to Allergens. N. Engl. J. Med. 1989, 320, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.A.; Wallace, C.C.; Wolstenholme, J. Analysis of the mitochondrial 12S rRNA gene supports a two-clade hypothesis of the evolutionary history of scleractinian corals. Mol. Phylogenet. Evol. 2002, 23, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, K.; Jiao, S.; Cai, N.; Zhao, X.; Zou, H.; Xie, Y.; Wang, Z.; Zhong, M.; Wei, L. High levels of SIRT1 expression enhance tumorigenesis and associate with a poor prognosis of colorectal carcinoma patients. Sci. Rep. 2014, 4, 7481. [Google Scholar] [CrossRef] [PubMed]
- Childs, C.E.; Munblit, D.; Ulfman, L.; Gómez-Gallego, C.; Lehtoranta, L.; Recker, T.; Salminen, S.; Tiemessen, M.; Collado, M.C. Potential Biomarkers, Risk Factors, and Their Associations with IgE-Mediated Food Allergy in Early Life: A Narrative Review. Adv. Nutr. 2022, 13, 633–651. [Google Scholar] [CrossRef]
- Gandhi, P.K.; Kenzik, K.M.; Thompson, L.A.; DeWalt, D.A.; Revicki, D.A.; Shenkman, E.A.; Huang, I.-C. Exploring factors influencing asthma control and asthma-specific health-related quality of life among children. Respir. Res. 2013, 14, 26. [Google Scholar] [CrossRef]
- Chung, S.; Kim, J.Y.; Song, M.A.; Park, G.Y.; Lee, Y.G.; Karpurapu, M.; Englert, J.A.; Ballinger, M.N.; Pabla, N.; Chung, H.Y.; et al. FoxO1 is a critical regulator of M2-like macrophage activation in allergic asthma. Allergy Eur. J. Allergy Clin. Immunol. 2019, 74, 535–548. [Google Scholar] [CrossRef]
- Colley, T.; Mercado, N.; Kunori, Y.; Brightling, C.; Bhavsar, P.K.; Barnes, P.J.; Ito, K. Defective sirtuin-1 increases IL-4 expression through acetylation of GATA-3 in patients with severe asthma. J. Allergy Clin. Immunol. 2016, 137, 1595–1597.e7. [Google Scholar] [CrossRef]
- Custovic, A. To what extent is allergen exposure a risk factor for the development of allergic disease? Clin. Exp. Allergy 2015, 45, 54–62. [Google Scholar] [CrossRef]
- Dilmac, S.; Kuscu, N.; Caner, A.; Yildirim, S.; Yoldas, B.; Farooqi, A.A.; Tanriover, G. SIRT1/FOXO Signaling Pathway in Breast Cancer Progression and Metastasis. Int. J. Mol. Sci. 2022, 23, 10227. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Shi, H.; Li, J.; Dong, Y.; Liang, J.; Ye, J.; Kong, S.; Zhang, S.; Zhong, T.; Yuan, Z.; et al. Mst1/Mst2 Regulate Development and Function of Regulatory T Cells through Modulation of Foxo1/Foxo3 Stability in Autoimmune Disease. J. Immunol. 2014, 192, 1525–1535. [Google Scholar] [CrossRef] [PubMed]
- Farhan, M.; Wang, H.; Gaur, U.; Little, P.J.; Xu, J.; Zheng, W. FOXO Signaling Pathways as Therapeutic Targets in Cancer. Int. J. Biol. Sci. 2017, 13, 815–827. [Google Scholar] [CrossRef]
- Froidure, A.; Mouthuy, J.; Durham, S.R.; Chanez, P.; Sibille, Y.; Pilette, C. Asthma phenotypes and IgE responses. Eur. Respir. J. 2016, 47, 304–319. [Google Scholar] [CrossRef]
- National Institutes of Health. Global Initiative for Asthma—GINA. Global Strategy for Asthma Management and Prevention Revision. 2020. Available online: https://ginasthma.org/ (accessed on 10 October 2021).
- Graves, D.T.; Milovanova, T.N. Mucosal Immunity and the FOXO1 Transcription Factors. Front. Immunol. 2019, 10, 2530. [Google Scholar] [CrossRef]
- Guan, R.; Cai, Z.; Wang, J.; Ding, M.; Li, Z.; Xu, J.; Li, Y.; Li, J.; Yao, H.; Liu, W.; et al. Hydrogen sulfide attenuates mitochondrial dysfunction-induced cellular senescence and apoptosis in alveolar epithelial cells by upregulating sirtuin 1. Aging 2019, 11, 11844–11864. [Google Scholar] [CrossRef]
- Harada, Y.; Harada, Y.; Elly, C.; Ying, G.; Paik, J.-H.; DePinho, R.A.; Liu, Y.-C. Transcription factors Foxo3a and Foxo1 couple the E3 ligase Cbl-b to the induction of Foxp3 expression in induced regulatory T cells. J. Exp. Med. 2010, 207, 1381–1391. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.; Santner-Nanan, B.; Hu, M.; Skarratt, K.; Lee, C.H.; Stormon, M.; Wong, M.; Fuller, S.J.; Nanan, R. IL-10 Potentiates Differentiation of Human Induced Regulatory T Cells via STAT3 and Foxo1. J. Immunol. 2015, 195, 3665–3674. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Durham, S.R.; Ying, S.; Kimmitt, P.; Barkans, J.; Assoufi, B.; Pfister, R.; Menz, G.; Robinson, D.S.; Kay, A.B.; et al. IL-4 and IL-5 mRNA and protein in bronchial biopsies from patients with atopic and nonatopic asthma: Evidence against “intrinsic” asthma being a distinct immunopathologic entity. Am. J. Respir. Crit. Care Med. 1996, 154, 1497–1504. [Google Scholar] [CrossRef]
- Ichikawa, T.; Hayashi, R.; Suzuki, K.; Imanishi, S.; Kambara, K.; Okazawa, S.; Inomata, M.; Yamada, T.; Yamazaki, Y.; Koshimizu, Y.; et al. Sirtuin 1 activator SRT1720 suppresses inflammation in an ovalbumin-induced mouse model of asthma. Respirology 2013, 18, 332–339. [Google Scholar] [CrossRef]
- Jiang, Y.; Deng, S.; Hu, X.; Luo, L.; Zhang, Y.; Zhang, D.; Li, X.; Feng, J. Identification of potential biomarkers and immune infiltration characteristics in severe asthma. Int. J. Immunopathol. Pharmacol. 2022, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, T.; Galli, S.J. Regulation of mast-cell and basophil function and survival by IgE. Nat. Rev. Immunol. 2002, 2, 773–786. [Google Scholar] [CrossRef]
- Khan, M.A. Regulatory T cells mediated immunomodulation during asthma: A therapeutic standpoint. J. Transl. Med. 2020, 18, 456. [Google Scholar] [CrossRef]
- Kim, S.R.; Lee, K.S.; Park, S.J.; Min, K.H.; Choe, Y.H.; Moon, H.; Yoo, W.H.; Chae, H.-J.; Han, M.K.; Lee, Y.C. Involvement of sirtuin 1 in airway inflammation and hyperresponsiveness of allergic airway disease. J. Allergy Clin. Immunol. 2010, 125, 449–460.e14. [Google Scholar] [CrossRef] [PubMed]
- Lallemand, F.; Vacher, S.; de Koning, L.; Petitalot, A.; Briaux, A.; Driouch, K.; Callens, C.; Schnitzler, A.; Lecerf, C.; Oulie-Bard, F.; et al. The high protein expression of FOXO3, but not that of FOXO1, is associated with markers of good prognosis. Sci. Rep. 2020, 10, 6920. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Lee, J.H.; Choi, Y.M.; Choi, H.; Cho, H.D.; Cha, G.H.; Lee, Y.H.; Jo, E.K.; Park, B.H.; et al. SIRT1 Promotes Host Protective Immunity against Toxoplasma gondii by Controlling the FoxO-Autophagy Axis via the AMPK and PI3K/AKT Signalling Pathways. Int. J. Mol. Sci. 2022, 23, 13578. [Google Scholar] [CrossRef]
- Luo, G.; Jian, Z.; Zhu, Y.; Zhu, Y.; Chen, B.; Ma, R.; Tang, F.; Xiao, Y. Sirt1 promotes autophagy and inhibits apoptosis to protect cardiomyocytes from hypoxic stress. Int. J. Mol. Med. 2019, 43, 2033–2043. [Google Scholar] [CrossRef] [PubMed]
- McKnight, C.G.; Jude, J.A.; Zhu, Z.; Panettieri, R.A.; Finkelman, F.D. House dust mite-induced allergic airway disease is independent of IgE and FceRIa. Am. J. Respir. Cell Mol. Biol. 2017, 57, 674–682. [Google Scholar] [CrossRef]
- Medema, R.H.; Jäättelä, M. Cytosolic FoxO1: Alive and killing. Nat. Cell Biol. 2010, 12, 642–643. [Google Scholar] [CrossRef] [PubMed]
- Ng, F.; Tang, B.L. Sirtuins’ modulation of autophagy. J. Cell. Physiol. 2013, 228, 2262–2270. [Google Scholar] [CrossRef] [PubMed]
- Oettgen, H.C.; Geha, R.S. IgE in asthma and atopy: Cellular and molecular connections. J. Clin. Investig. 1999, 104, 829–835. [Google Scholar] [CrossRef]
- Ouyang, W.; Beckett, O.; Flavell, R.A.; Li, M.O. An Essential Role of the Forkhead-Box Transcription Factor Foxo1 in Control of T Cell Homeostasis and Tolerance. Immunity 2009, 30, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Beckett, O.; Ma, Q.; Paik, J.; DePinho, R.A.; Li, M.O. Foxo proteins cooperatively control the differentiation of Foxp3+ regulatory T cells. Nat. Immunol. 2010, 11, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Liao, W.; Luo, C.T.; Yin, N.; Huse, M.; Kim, M.V.; Peng, M.; Chan, P.; Ma, Q.; Mo, Y.; et al. Novel Foxo1-dependent transcriptional programs control Treg cell function. Nature 2012, 491, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Zhong, L.; Huang, C.; Long, J.; Ye, X.; Wu, J.; Dai, W.; Lv, W.; Xie, C.; Zhang, J. Cell-bound IgE and plasma IgE as a combined clinical diagnostic indicator for allergic patients. Sci. Rep. 2020, 10, 4700. [Google Scholar] [CrossRef] [PubMed]
- Starkl, P.; Marichal, T.; Gaudenzio, N.; Reber, L.L.; Sibilano, R.; Tsai, M.; Galli, S.J. IgE antibodies, FcεRIα, and IgE-mediated local anaphylaxis can limit snake venom toxicity. J. Allergy Clin. Immunol. 2016, 137, 246–257.e11. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Chen, Q.; Meng, Z.; Sun, L.; Zhu, L.; Liu, J.; Hu, J.; Ni, Z.; Wang, X. Suppression of Sirtuin-1 Increases IL-6 Expression by Activation of the Akt Pathway During Allergic Asthma. Cell. Physiol. Biochem. 2017, 43, 1950–1960. [Google Scholar] [CrossRef]
- Tsabouri, S.; Mavroudi, A.; Feketea, G.; Guibas, G.V. Subcutaneous and Sublingual Immunotherapy in Allergic Asthma in Children. Front. Pediatr. 2017, 5, 28, Erratum in Front. Pediatr. 2017, 11, 187. [Google Scholar]
- Tsilogianni, Z.; Baker, J.R.; Papaporfyriou, A.; Papaioannou, A.I.; Papathanasiou, E.; Koulouris, N.G.; Daly, L.; Ito, K.; Hillas, G.; Papiris, S.; et al. Sirtuin 1: Endocan and Sestrin 2 in Different Biological Samples in Patients with Asthma. Does Severity Make the Difference? J. Clin. Med. 2020, 9, 473. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, O. Reduction of exposure in the management of occupational asthma. Curr. Opin. Allergy Clin. Immunol. 2011, 11, 75–79. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; Ma, G.; Li, W.; Wu, J.; Lai, T.; Huang, D.; Zhao, X.; Lv, Q.; Chen, M.; et al. Increases in peripheral SIRT1: A new biological characteristic of asthma. Respirology 2015, 20, 1066–1072. [Google Scholar] [CrossRef]
- Wu, Y.; Li, W.; Hu, Y.; Liu, Y.; Sun, X. Suppression of sirtuin 1 alleviates airway inflammation through mTOR-mediated autophagy. Mol. Med. Rep. 2020, 22, 2219–2226. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liu, X.; Li, Y.; Meng, S.; Wu, F.; Yan, B.; Xue, Y.; Ma, T.; Yang, J.; Liu, J. Maternal exposure to farming environment protects offspring against allergic diseases by modulating the neonatal TLR-Tregs-Th axis. Clin. Transl. Allergy 2018, 8, 34. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Y.; Rong, W.; Fan, L.; Cai, Y.; Qu, Q.; Gao, Y.; Zhao, H. miR-221 participates in the airway epithelial cells injury in asthma via targeting SIRT1. Exp. Lung Res. 2018, 44, 272–279. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Li, W.; Zhang, J.-R.; Li, C.-Y.; Zhang, J.; Lv, X.-J. Roles of sirtuin family members in chronic obstructive pulmonary disease. Respir. Res. 2022, 23, 66. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, F.; Ding, J. As a Modulator, Multitasking Roles of SIRT1 in Respiratory Diseases. Immune Netw. 2022, 22, e21. [Google Scholar] [CrossRef] [PubMed]
- Ziyaei, T.; Berenji, F.; Jabbari-Azad, F.; Fata, A.; Jarahi, L.; Fereidouni, M. House Dust Mite Prevalence in the House of Patients with Atopic Dermatitis in Mashhad. Iran. J Arthropod-Borne Dis. 2017, 11, 309–314. [Google Scholar]
- Zou, B.; Fu, Y.; Cao, C.; Pan, D.; Wang, W.; Kong, L. Gentiopicroside ameliorates ovalbumin-induced airway inflammation in a mouse model of allergic asthma via regulating SIRT1/NF-κB signaling pathway. Pulm. Pharmacol. Ther. 2021, 68, 102034. [Google Scholar] [CrossRef]
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef] [PubMed]

| Primer | Sense Primer Sequence | Antisense Primer | Product Size (bp) |
|---|---|---|---|
| FoxO1 | 5′-GTCAAGAGCGTGCCCTACTTCA-3′ | 5′-TGAACTTGCTGTGTAGGGACAGATTAT-3′ | 102 [22] |
| SIRT1 | 5′-TGCTGGCCTAATAGAGTGGCA-3′ | 5′-CTCAGCGCCATGGAAAATGT-3′ | 101 [23] |
| 18S rRNA | 5′-AGT CCC TGC CCT TTG TAC ACA-3′ | 5′-GAT CCG AGG GCC TCA CTA AAC-3′ | 69 [24] |
| PCR Step | Temperature | Time | Repeat Cycles |
|---|---|---|---|
| Initial denaturation | 95 °C | 10 min | |
| Denaturation | 95 °C | 15 s | 40 |
| Annealing | 60 °C | 30 s | |
| Extension | 72 °C | 30 s | 30 |
| Criteria | HV n = 40 | HDM/AA n = 40 | HDM/SCIT n = 40 | F | p Value |
|---|---|---|---|---|---|
| AGE Mean ± SD | 30.75 ± 9.8 | 29.60 ± 11.5 | 34.08 ± 12.3 | 0.337 | ns $ |
| Gender male/female | 17/23 (42.5/57.5%) | 18/22 (45/55%) | 20/20 (50/50%) | - | |
| Residence Urban Rural | 25 (62%) 15 (37.5%) | 22 (55%) 18 (45%) | 23 (57.5%) 17 (42.5%) | - | |
| Family history of asthma Negative Positive | 31 (77.5%) 9 (22.5%) | 8 (20%) 32 (80%) | 9 (22.5%) 31 (77.5%) | - | |
| Skin prick test | 0% | 100% | 100% | - | |
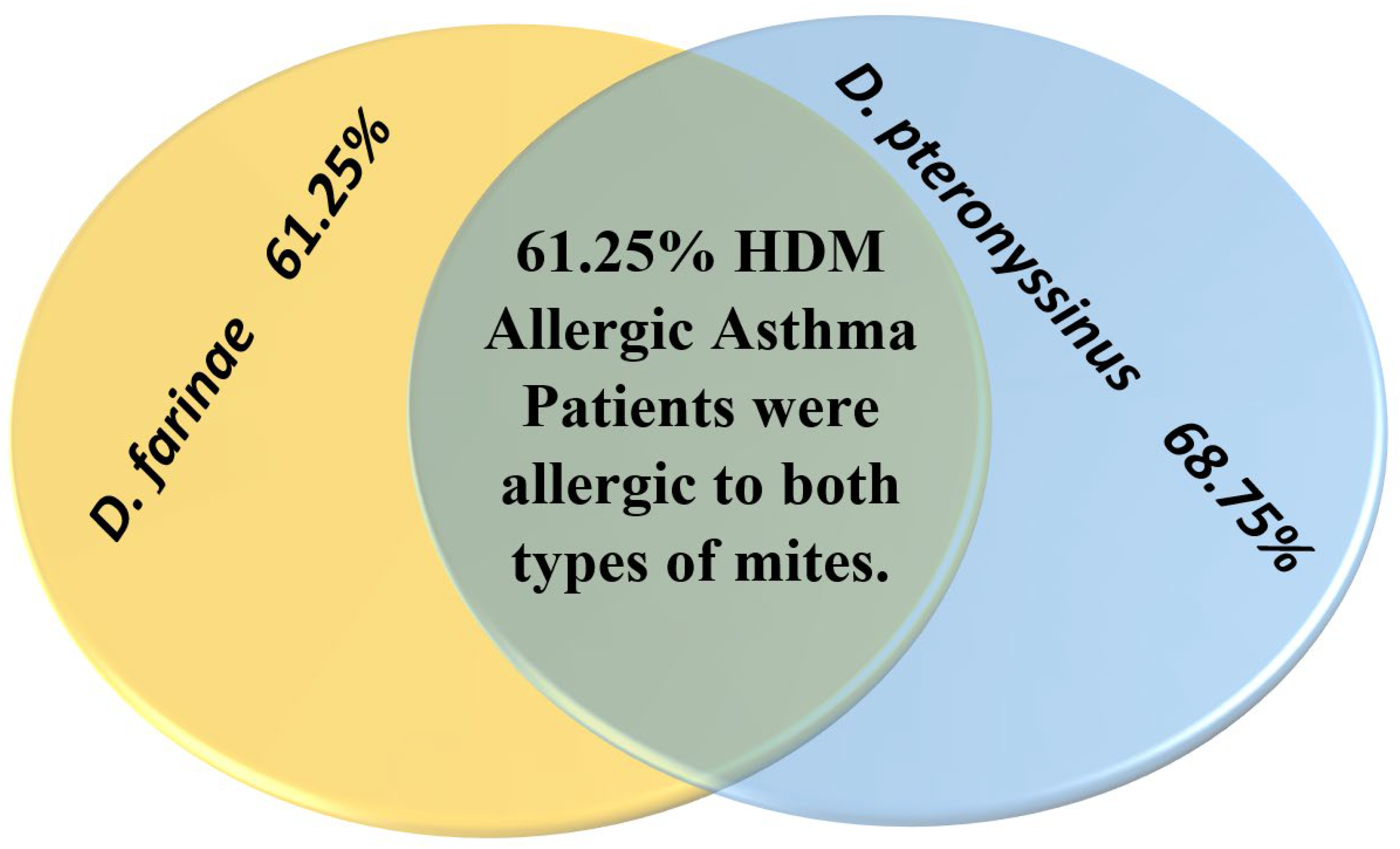
| House dust mites D. pteronyssinus D. farinae Both | 0% - - - | 100% 28 24 24 | 100% 27 25 25 | - | |
| Severity of asthma Grade I Grade II | 10 30 | 29 11 | - | ||
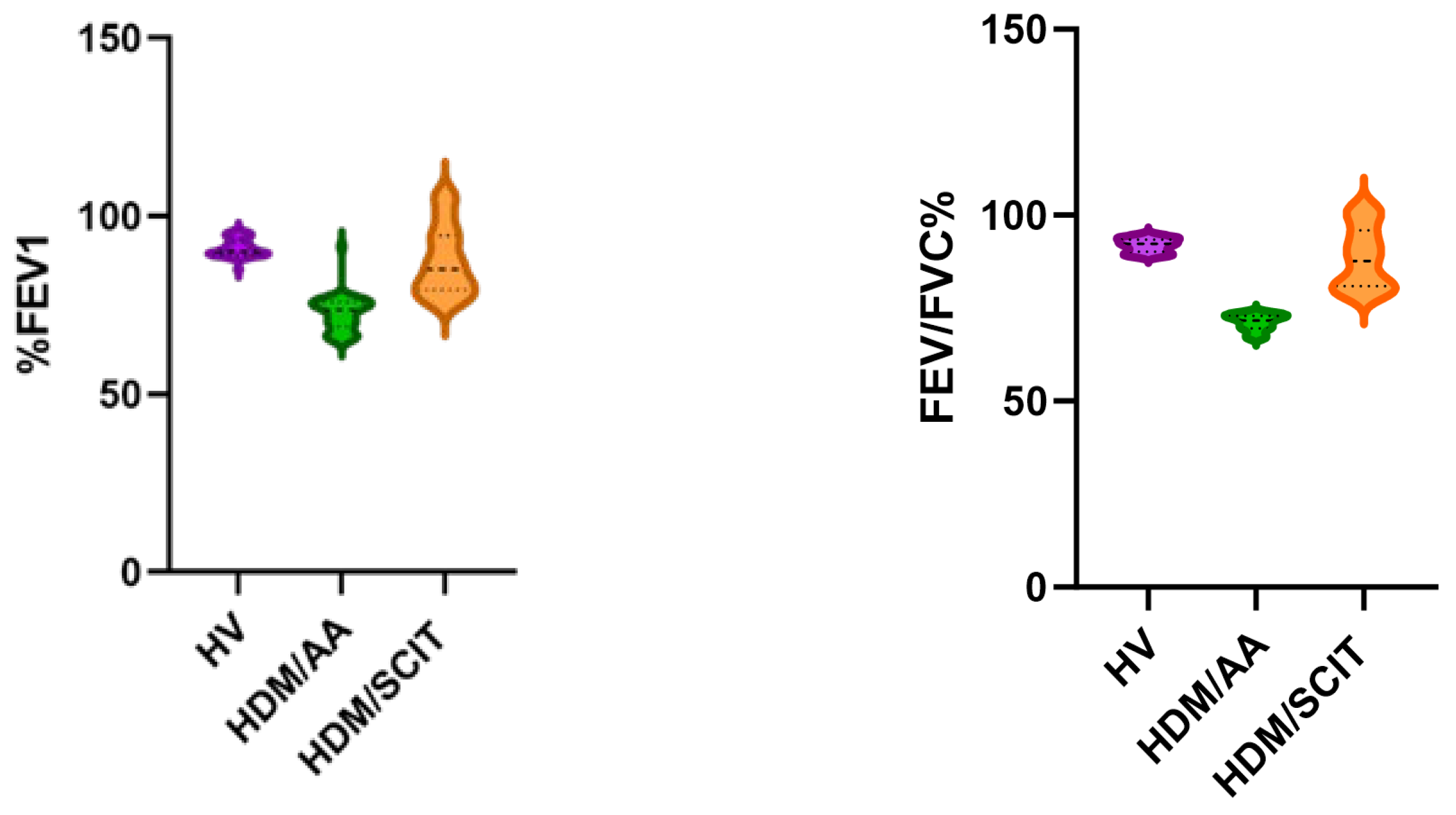
| FEV1 (% of predicted) | 91.10 ± 9.666 | 72.62 ± 4.943 | 87.49 ± 2.52 | - | <0.0001 **# |
| FEV1/FVC (%) | 91.91 ± 1.849 | 71.14 ± 2.141 | 88.59 ± 8.247 | - | <0.0001 **# |
| Total serum IgE (ng/mL) Mean ± SD | 158.3 ± 73.1 | 921.3 ± 317.3 | 798.2 ± 204.0 | - | <0.0001 **# |
| FoxO1 gene expression (ng/mL) Mean ± SD | 1.29 ± 0.76 | 1.33 ± 0.43 | 1.82 ± 0.49 | - | <0.0001 **# |
| SIRT1 gene expression (ng/mL) Mean ± SD | 1.6 ± 0.52 | 1.2 ± 0.31 | 2.03 ± 0.43 | 41.59 | <0.0001 **$ |
| Compared Groups | HV vs. HDM/AA | HV vs. HDM/SCIT | HDM/AA vs. HDM/SCIT |
|---|---|---|---|
| FEV # | <0.0001 ** | 0.1679 ns | <0.0001 ** |
| FEV1/FVC% # | <0.0001 ** | >0.9999 ns | <0.0001 ** |
| Total serum IgE (ng/mL) # | <0.0001 ** | <0.0001 ** | >0.9999 ns |
| FoxO1 gene expression (ng/mL) # | >0.9999 ns | <0.0001 ** | 0.0004 ** |
| Sirtuin 1 gene expression (ng/mL) $ | 0.0002 ** | <0.0001 ** | <0.0001 ** |
| HV | HDM/AA | HDM/SCIT | ||||
|---|---|---|---|---|---|---|
| Characteristics | r | p-Value | r | p-Value | r | p-Value |
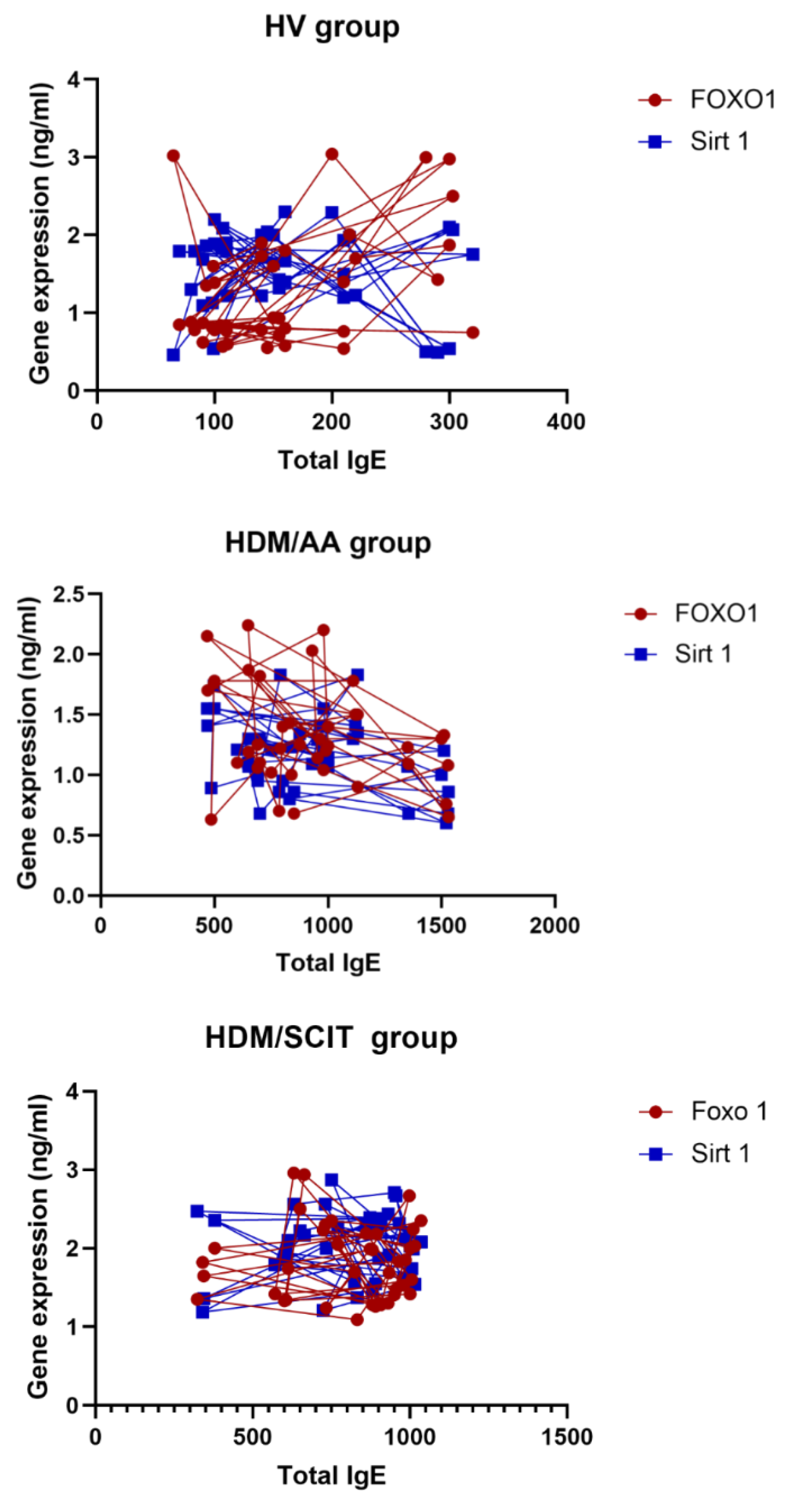
| FoxO1 (ng/mL) | 0.4338 | 0.0052 ** | −0.3108 | 0.0510 ns | −0.01030 | 0.9497 ns |
| SIRT1 (ng/mL) | −0.1120 | 0.4915 ns | −0.3321 | 0.0363 * | 0.08908 | 0.5847 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, R.A.; Fakhr, A.E.; Baioumy, S.A. Investigating Forkhead Box O Transcription Factor 1 Gene’s Relation to Immunoglobulin E in House Dust Mite-Allergic Asthma Patients. Adv. Respir. Med. 2023, 91, 532-545. https://doi.org/10.3390/arm91060039
Mohamed RA, Fakhr AE, Baioumy SA. Investigating Forkhead Box O Transcription Factor 1 Gene’s Relation to Immunoglobulin E in House Dust Mite-Allergic Asthma Patients. Advances in Respiratory Medicine. 2023; 91(6):532-545. https://doi.org/10.3390/arm91060039
Chicago/Turabian StyleMohamed, Rania A., Ahmed ElSadek Fakhr, and Shereen A. Baioumy. 2023. "Investigating Forkhead Box O Transcription Factor 1 Gene’s Relation to Immunoglobulin E in House Dust Mite-Allergic Asthma Patients" Advances in Respiratory Medicine 91, no. 6: 532-545. https://doi.org/10.3390/arm91060039
APA StyleMohamed, R. A., Fakhr, A. E., & Baioumy, S. A. (2023). Investigating Forkhead Box O Transcription Factor 1 Gene’s Relation to Immunoglobulin E in House Dust Mite-Allergic Asthma Patients. Advances in Respiratory Medicine, 91(6), 532-545. https://doi.org/10.3390/arm91060039






