Development of an Optical System for Non-Contact Type Measurement of Heart Rate and Heart Rate Variability
Abstract
:1. Introduction
- A novel design of a self-mixing optical homodyne and heterodyne detection setup for measuring the vibrations of chest wall or wrist position is proposed;
- A comparison of results of homodyne and heterodyne techniques with available contact type measurement techniques, such as an electrocardiogram (ECG), is studied;
- The feature extraction of the vibrocardiogram (VCG) signal for the monitoring of HR and HRV is obtained.
2. Optical Coherent Detection Technique
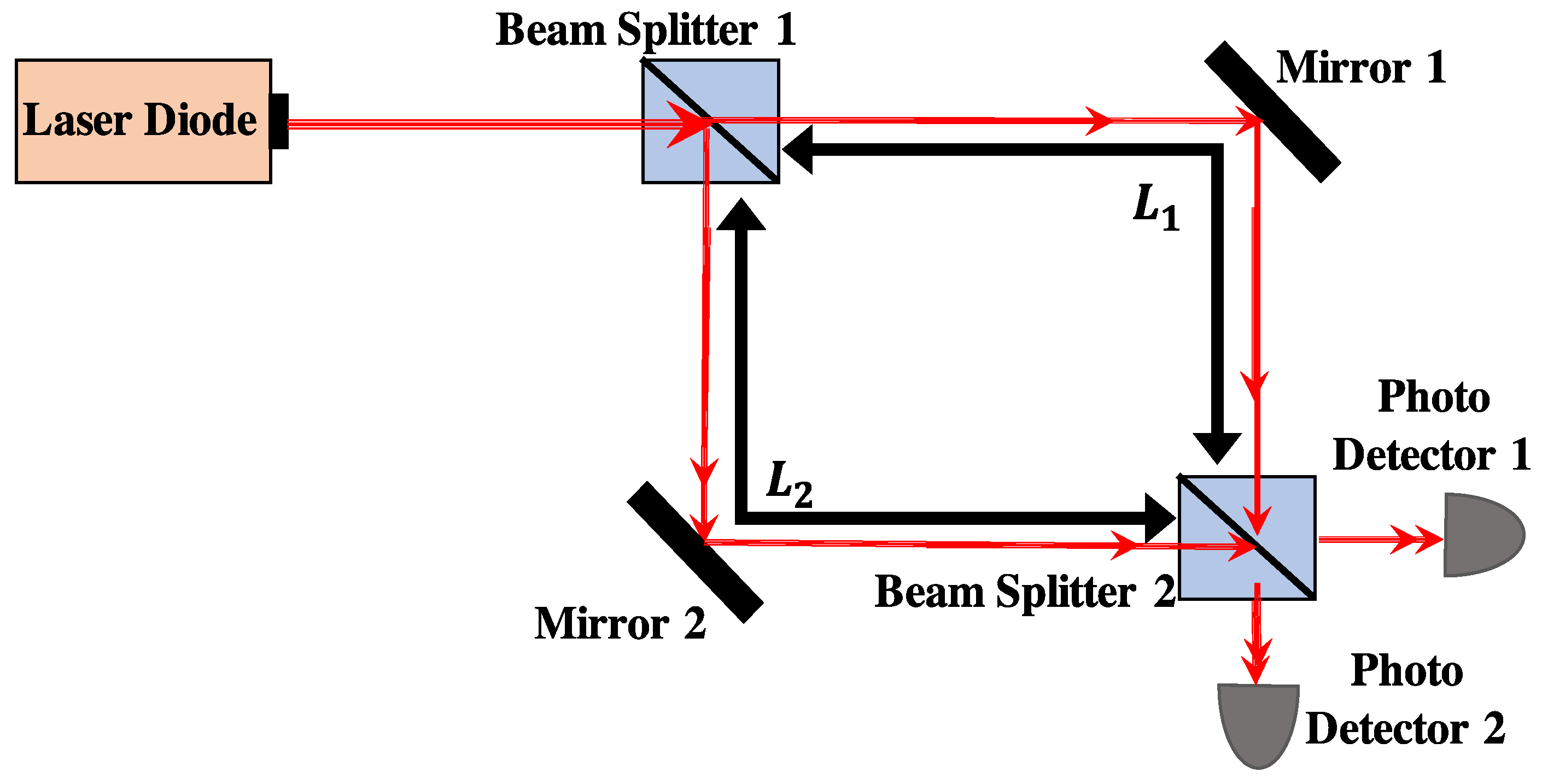
2.1. Optical Homodyne Detection
2.2. Heart Rate Detection with Self-Mixing Optical Homodyne Technique
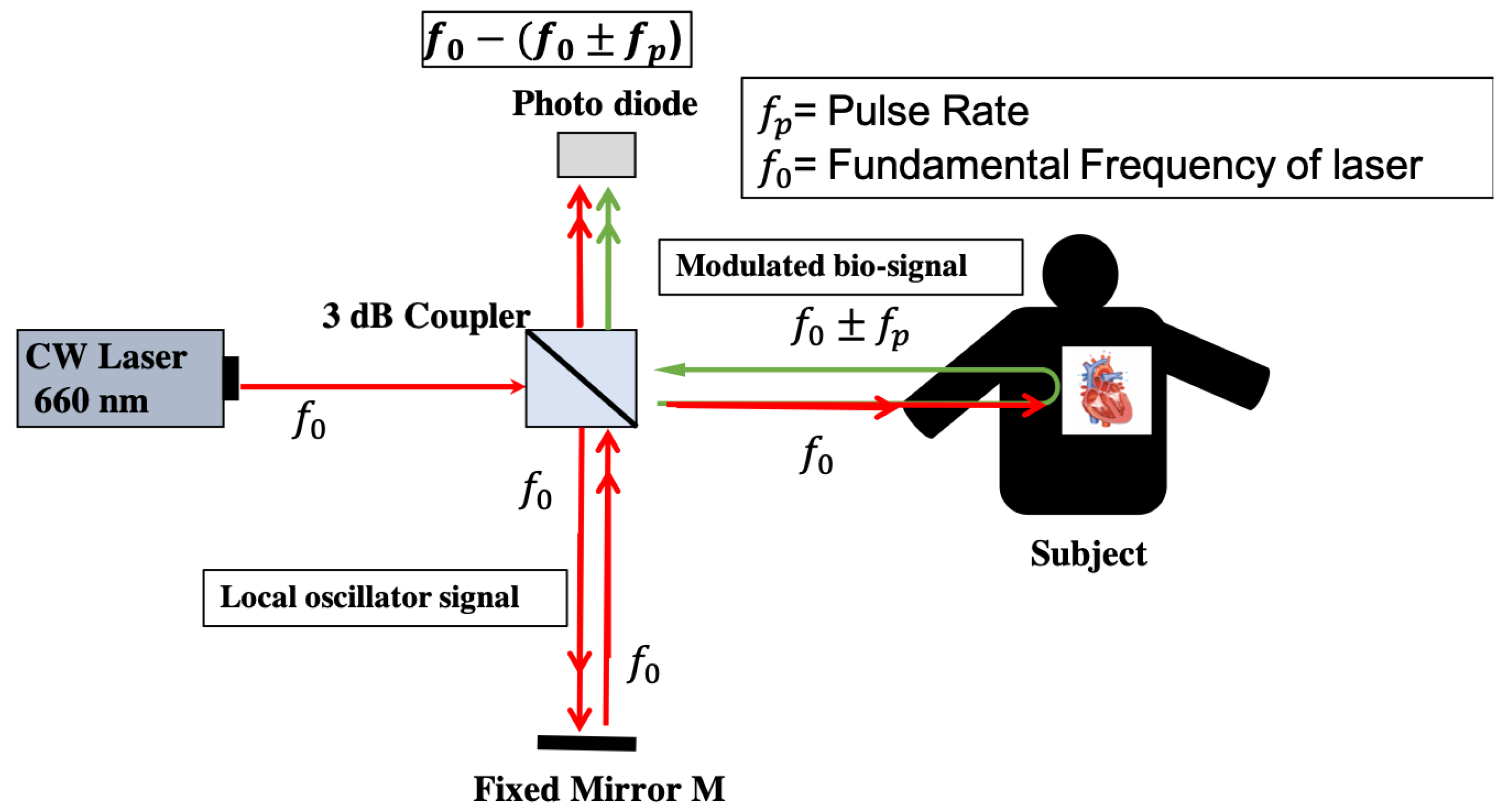
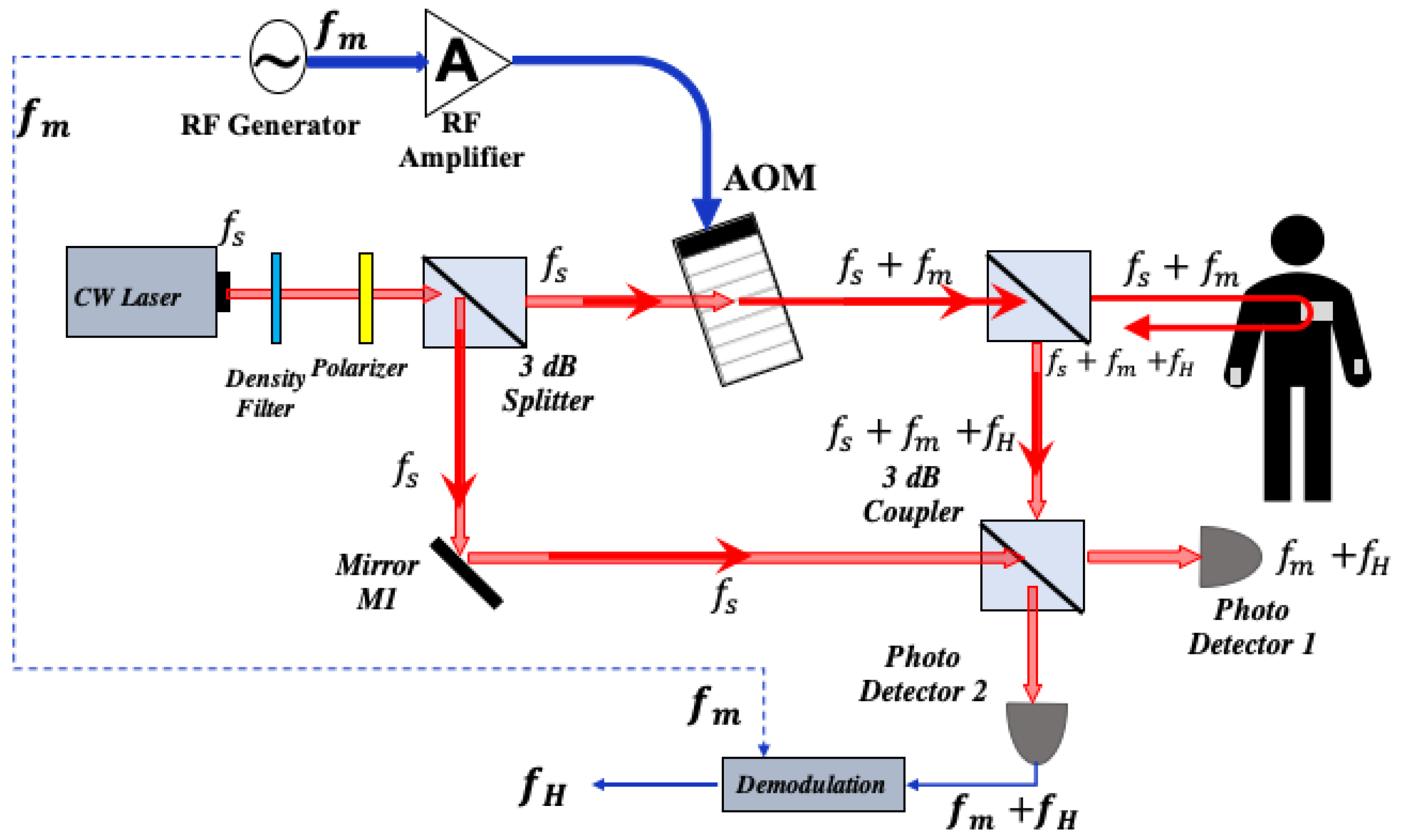
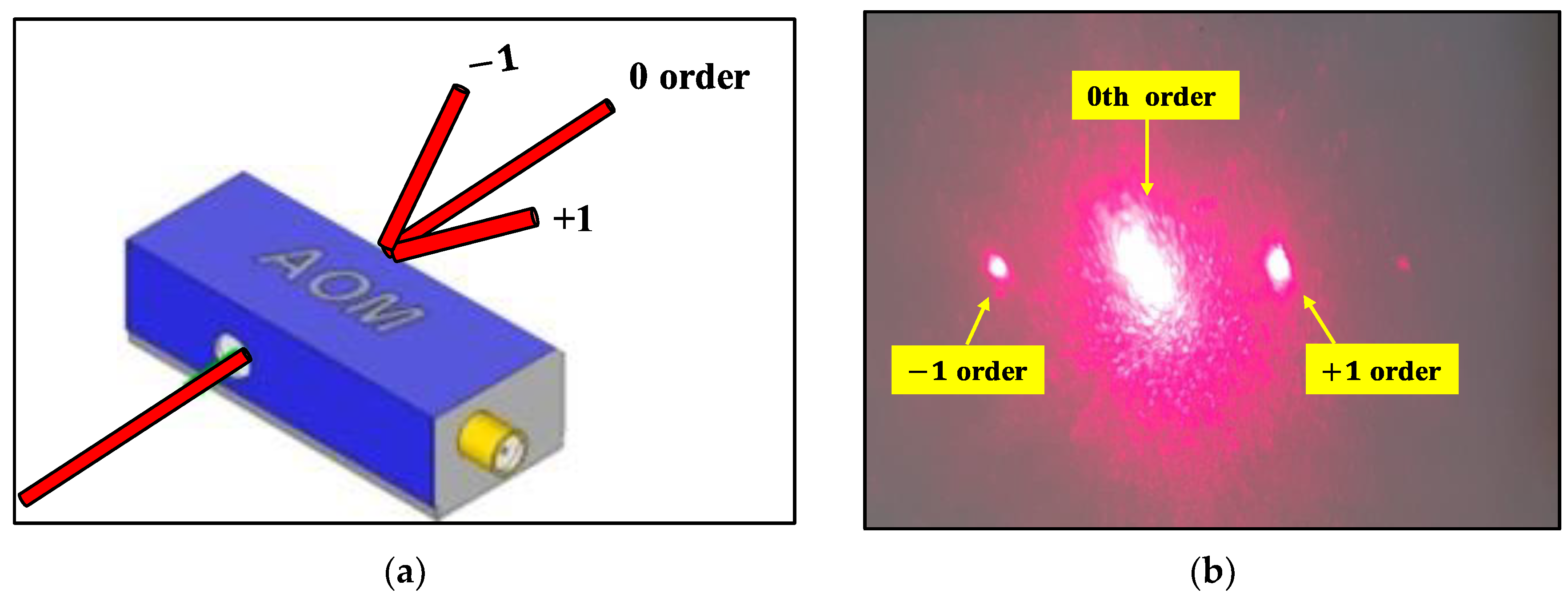
2.3. Optical Heterodyne Detection Technique
2.4. Heart Rate Detection with Self Mixing Optical Heterodyne Technique
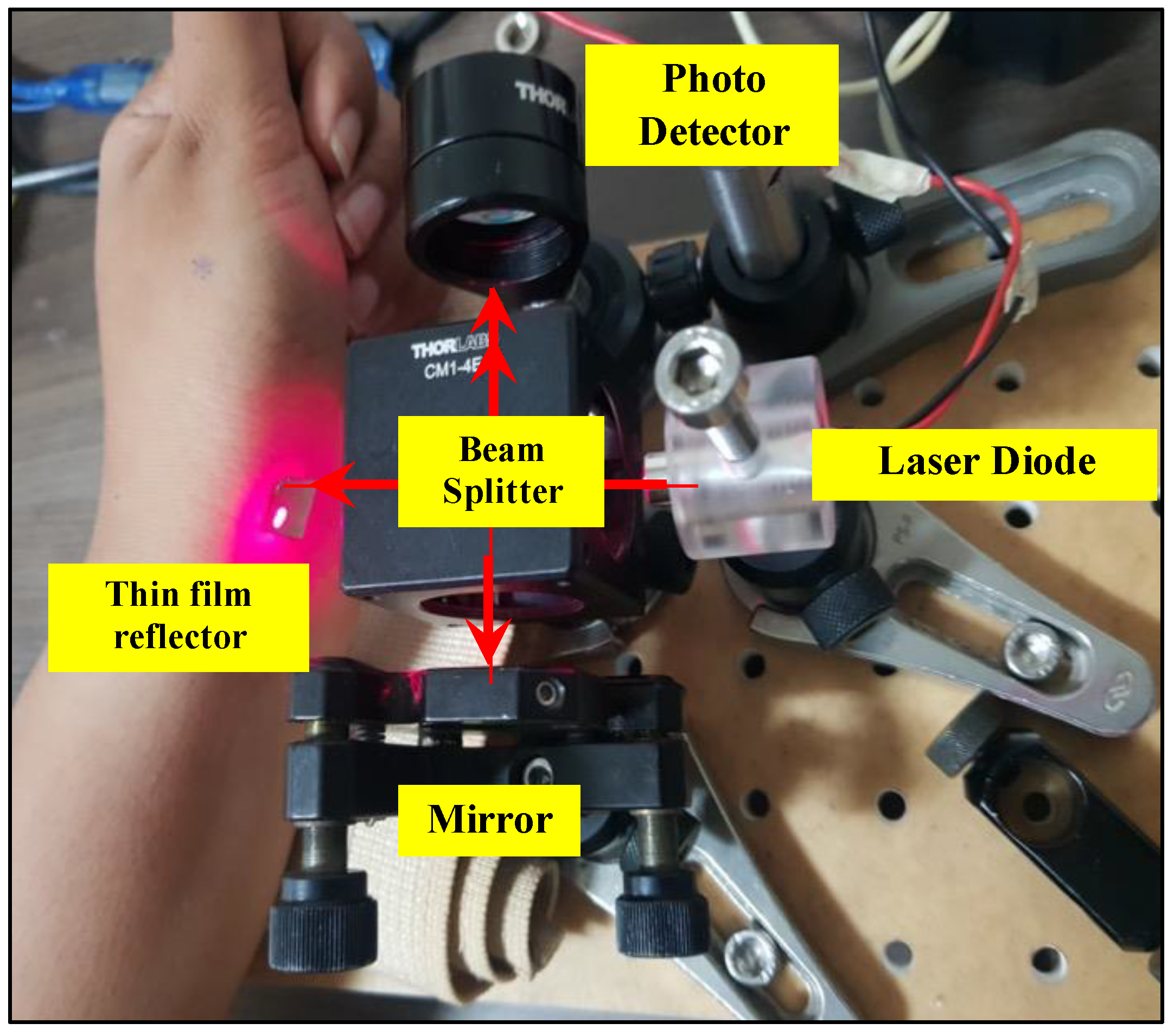
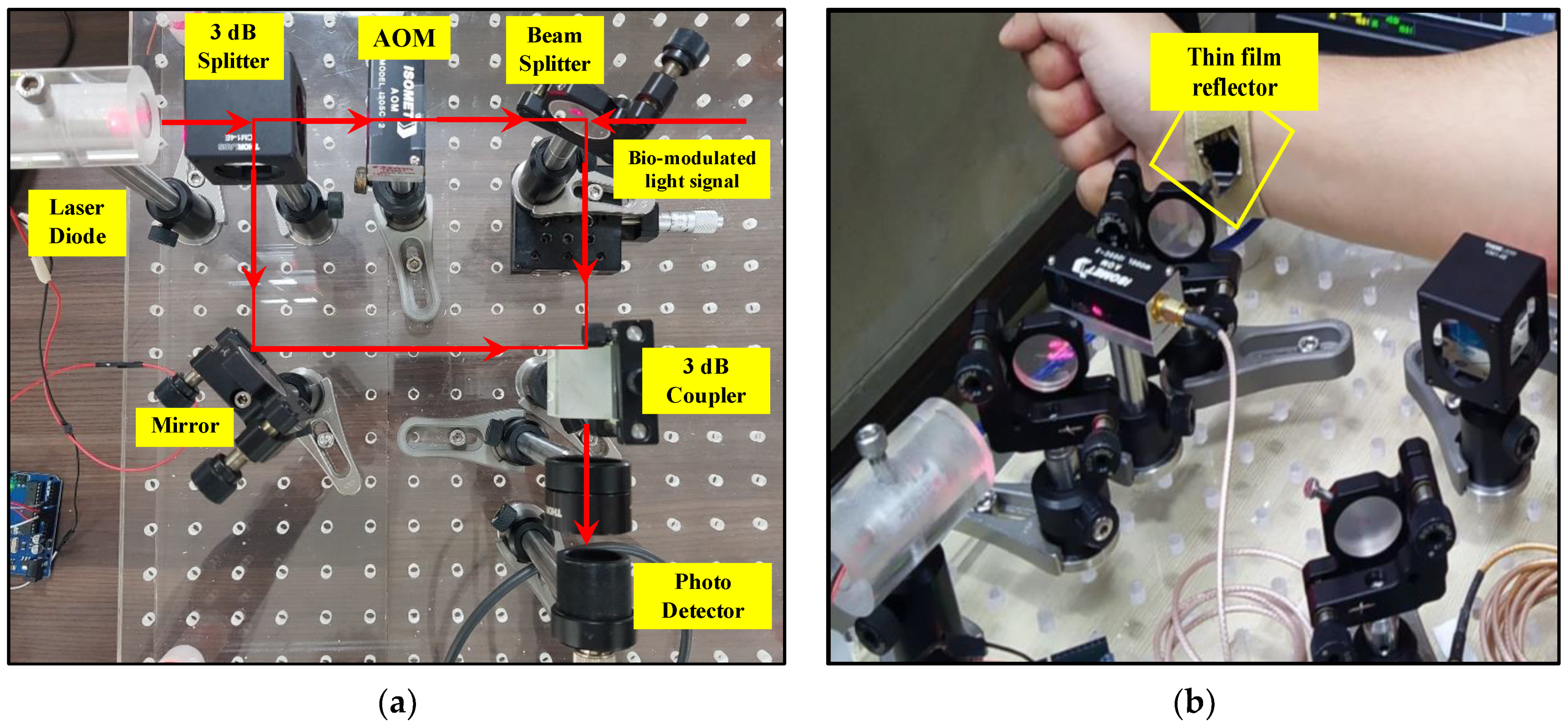
3. Experimental Setup of Optical Homodyne and Heterodyne Detection
3.1. Experiment for Heart Rate Detection with Self-Mixing Optical Homodyne Technique
3.2. Experiment of Heart Rate Detection with Self Mixing Optical Heterodyne Technique
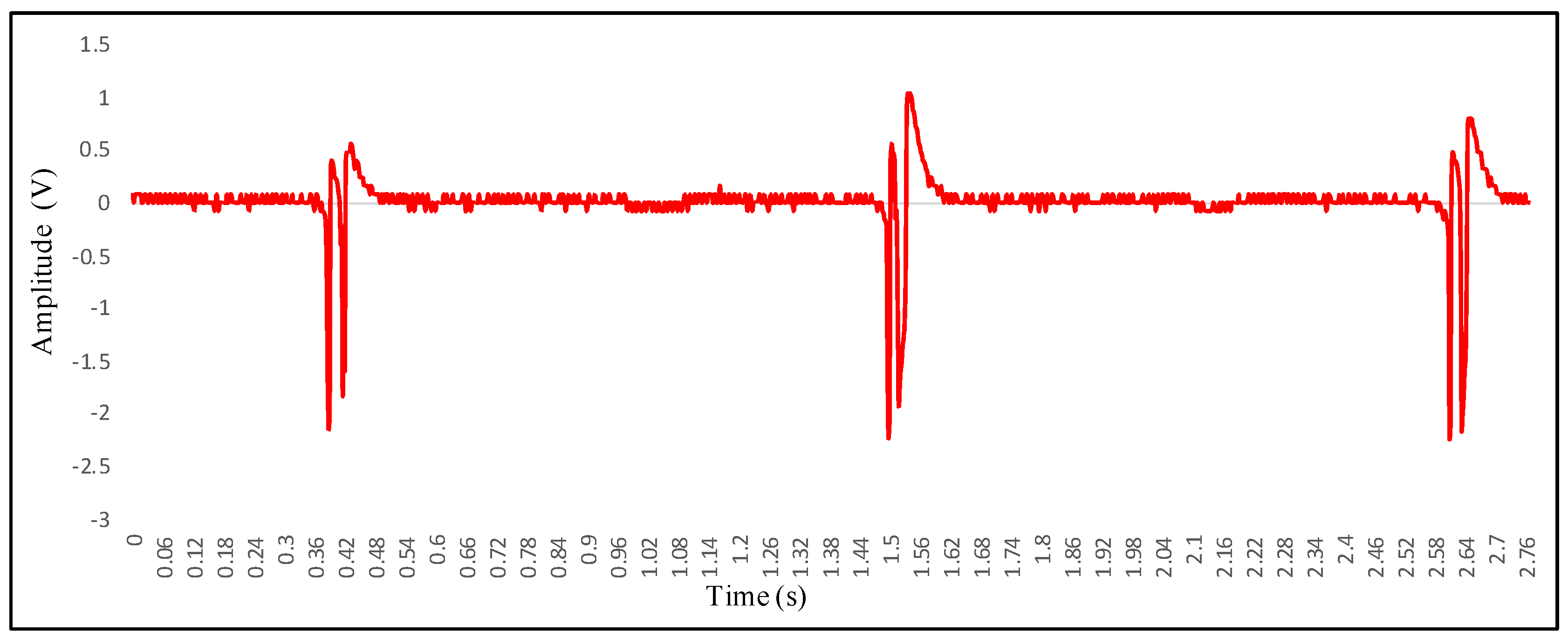
4. Measurement Results of HR and HRV with Optical Homodyne and Heterodyne Technique
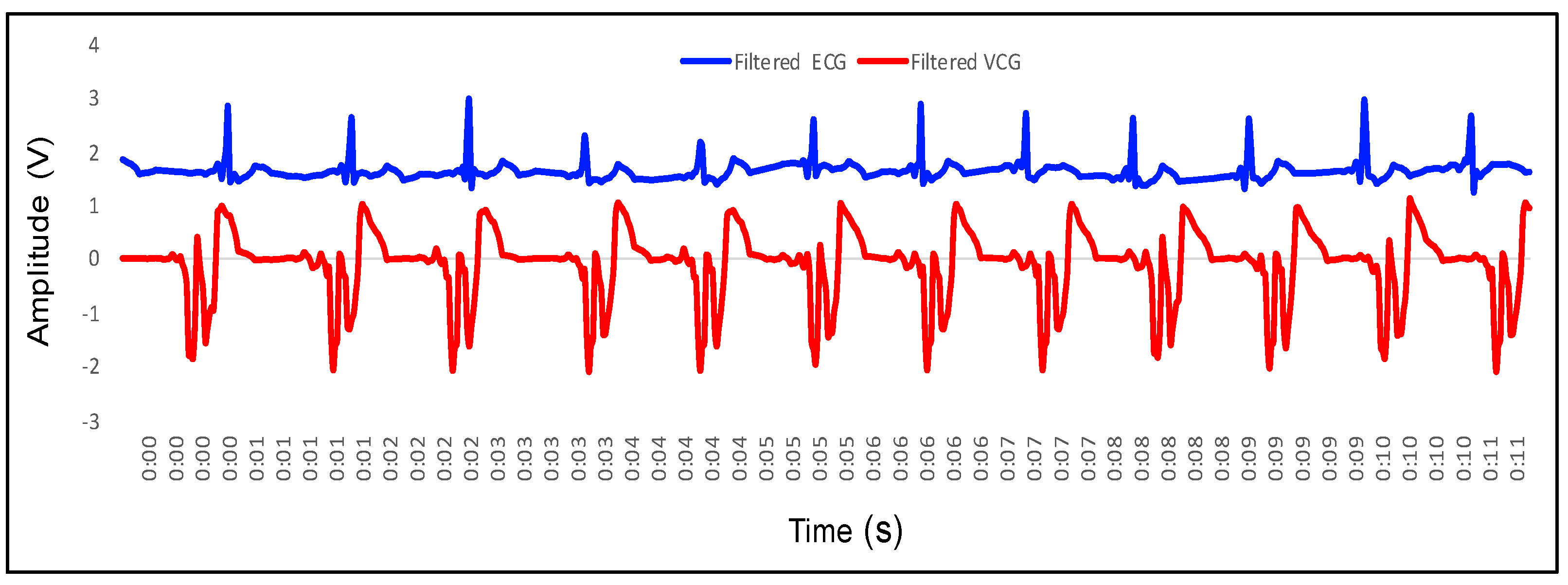
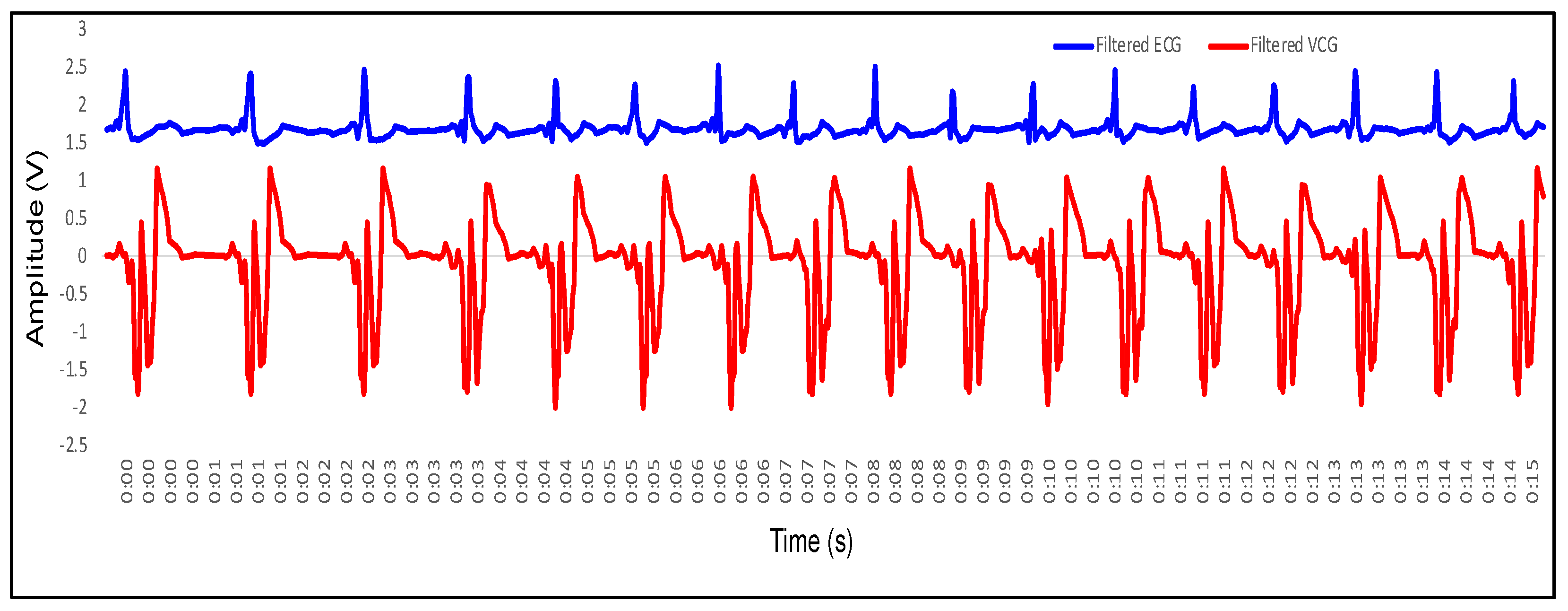
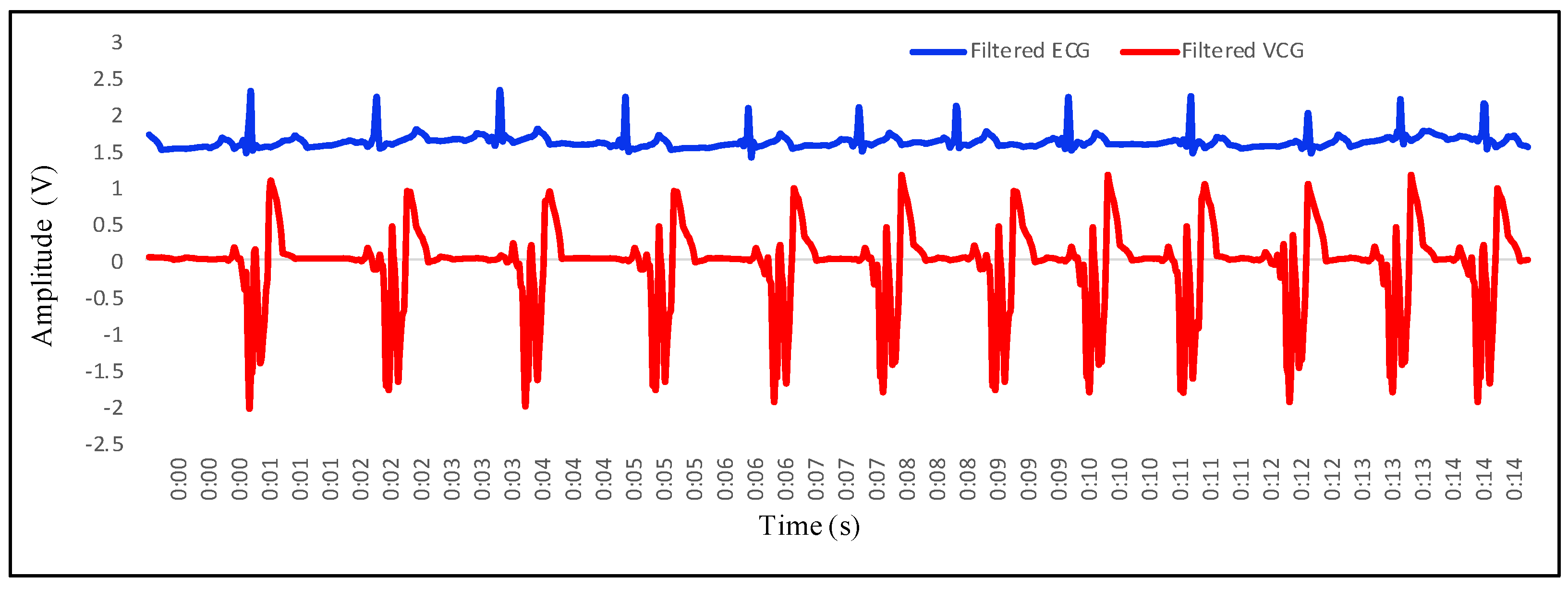
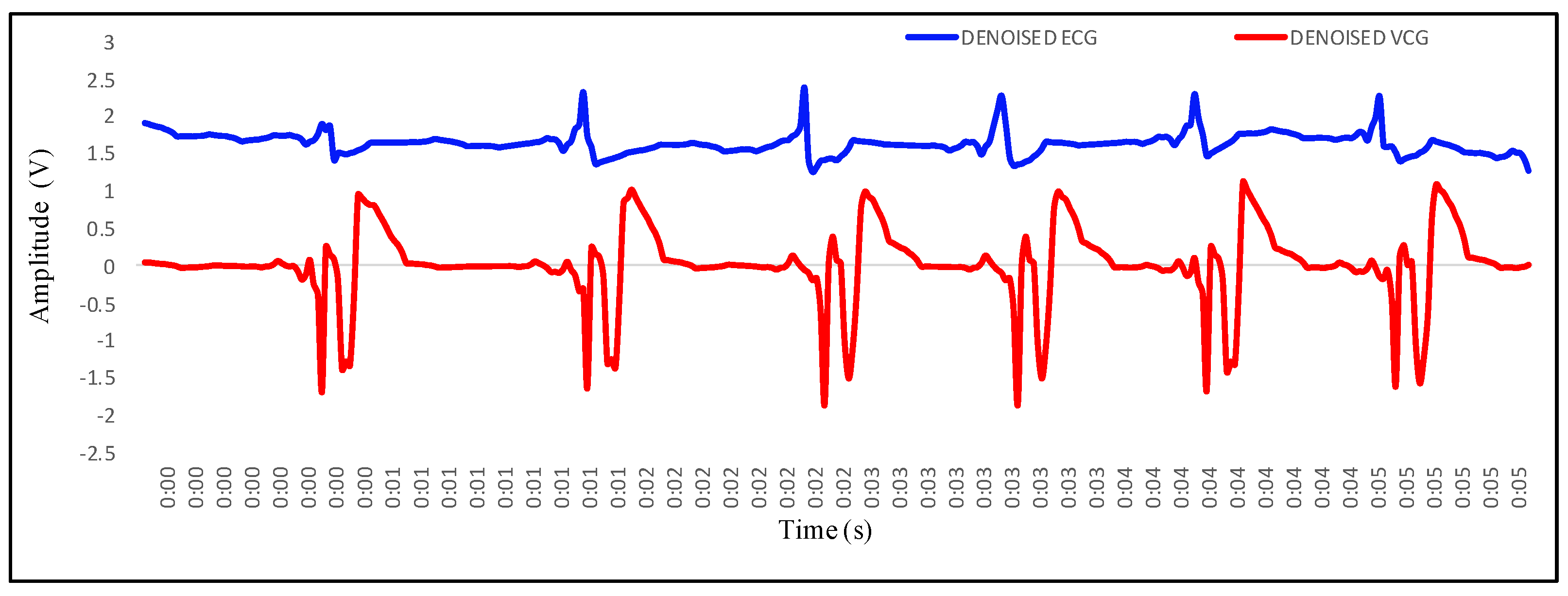
4.1. Recording of Vibrocardiogram and Electrocardiogram Signal
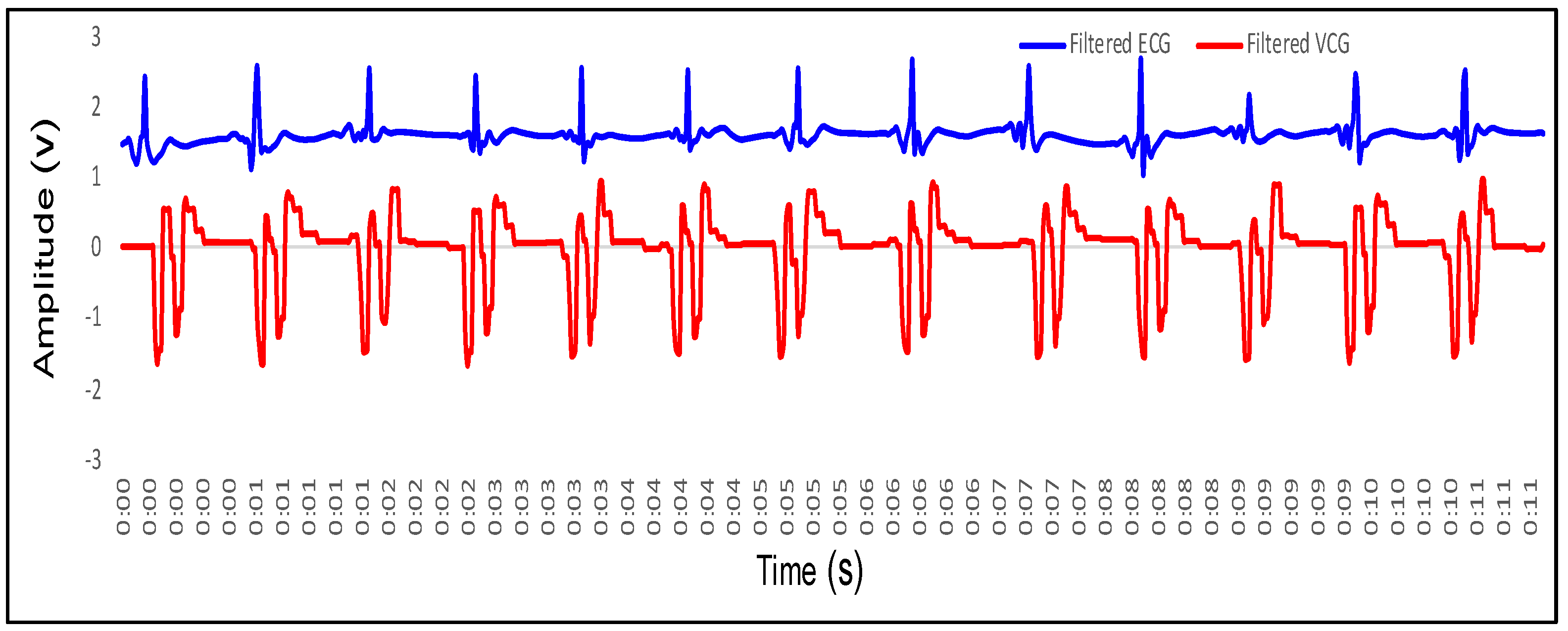
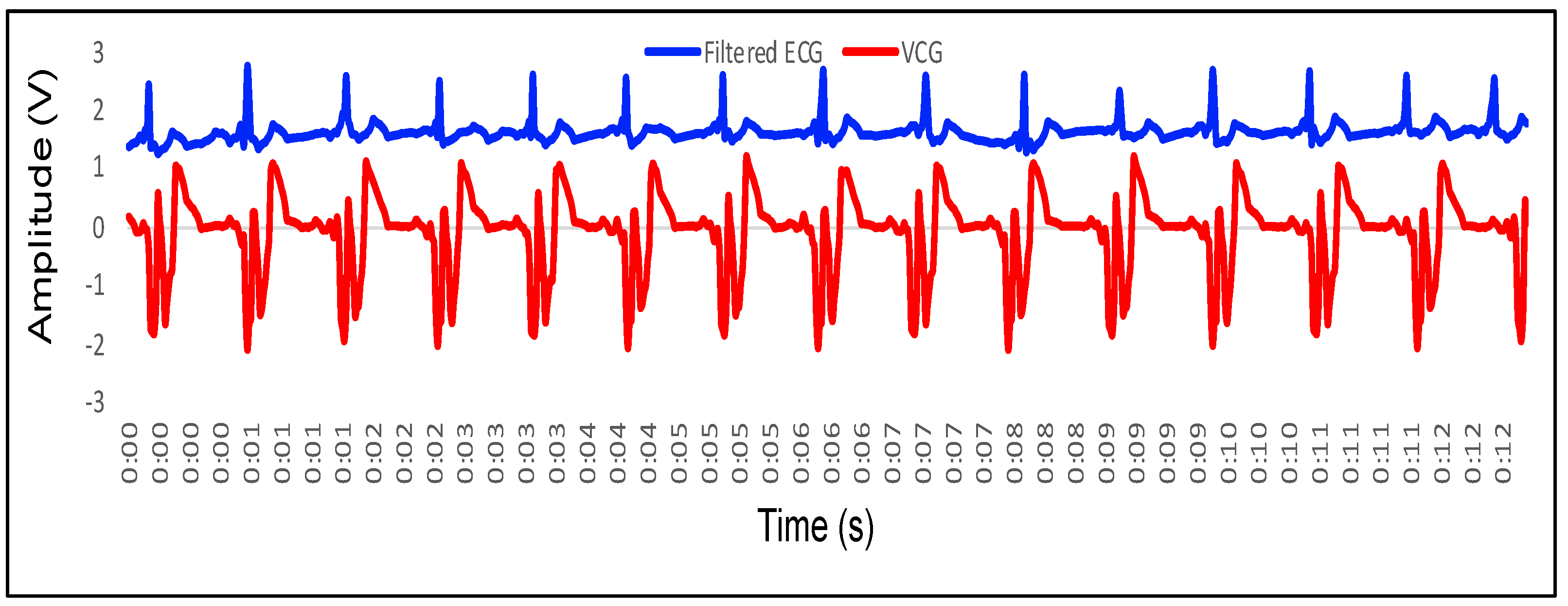
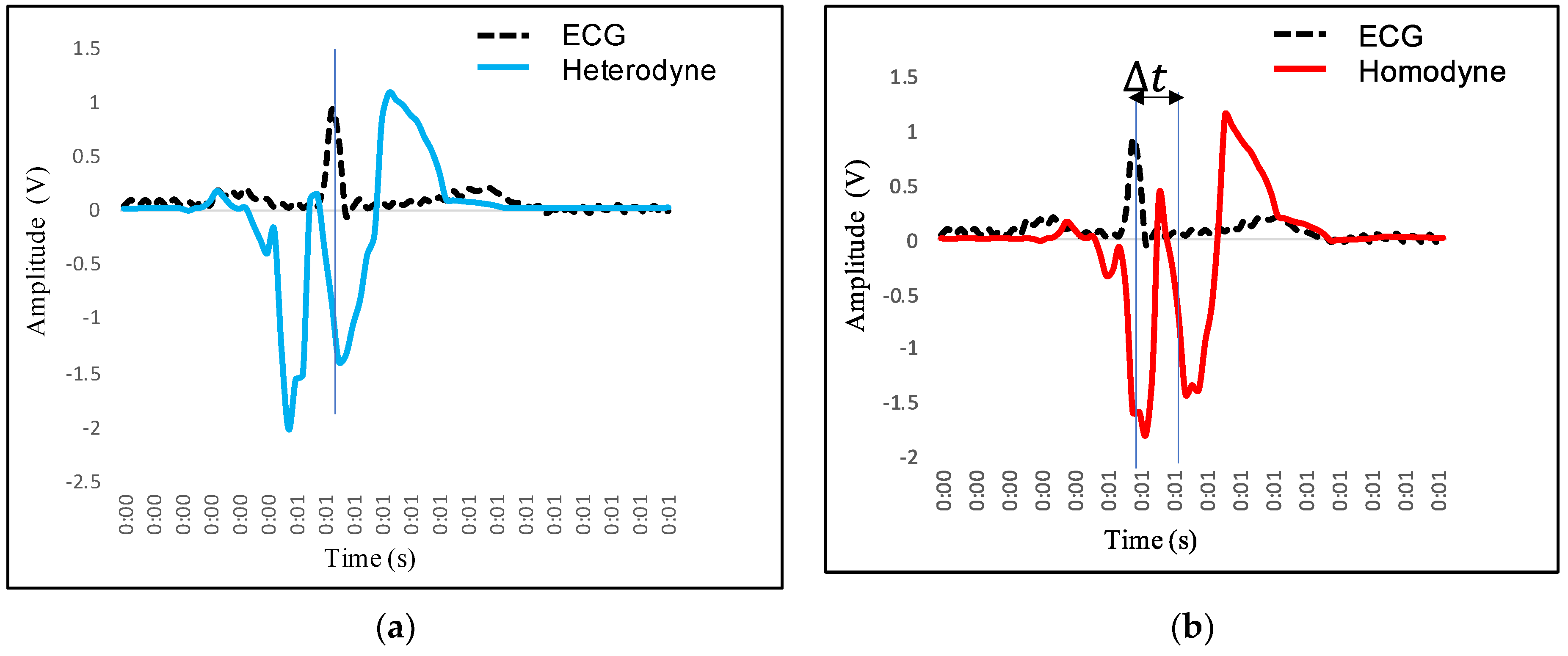
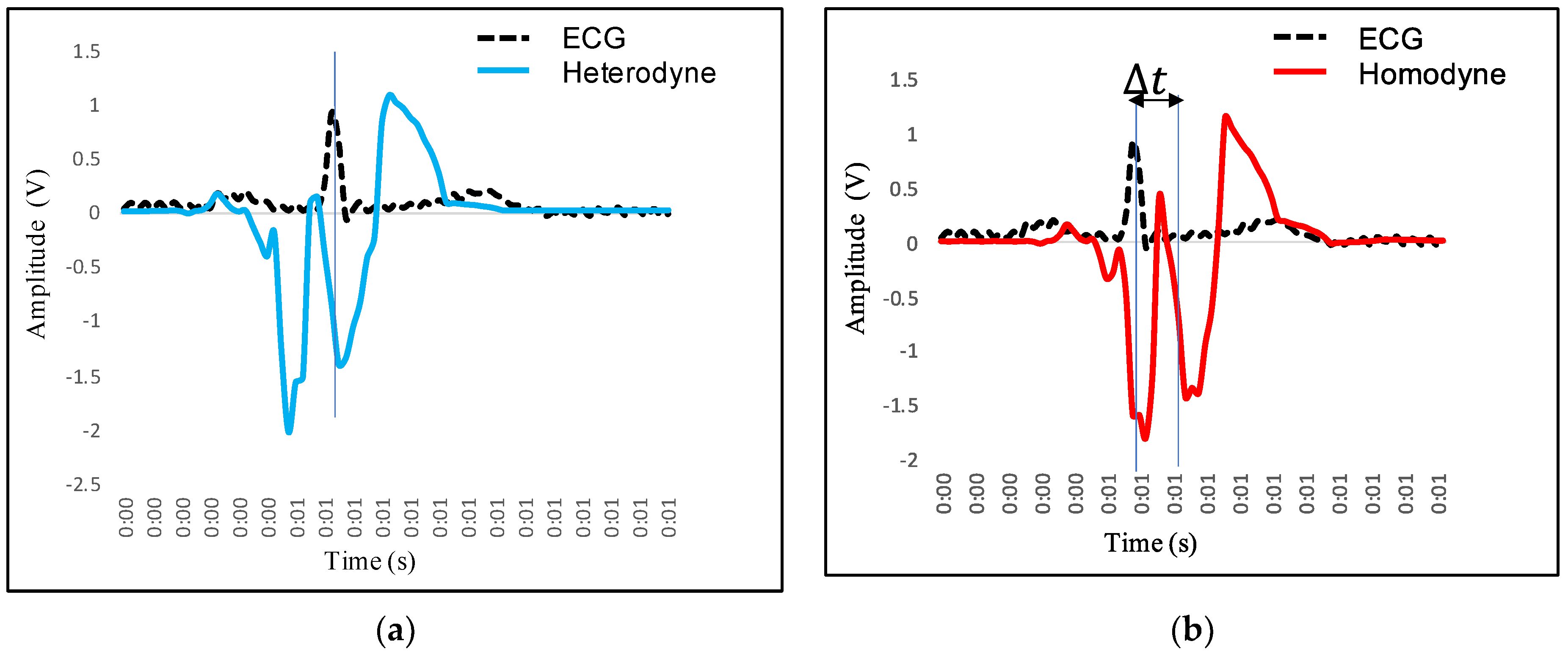
4.2. Feature Extraction in VCG and Its Mapping with ECG
- 1.
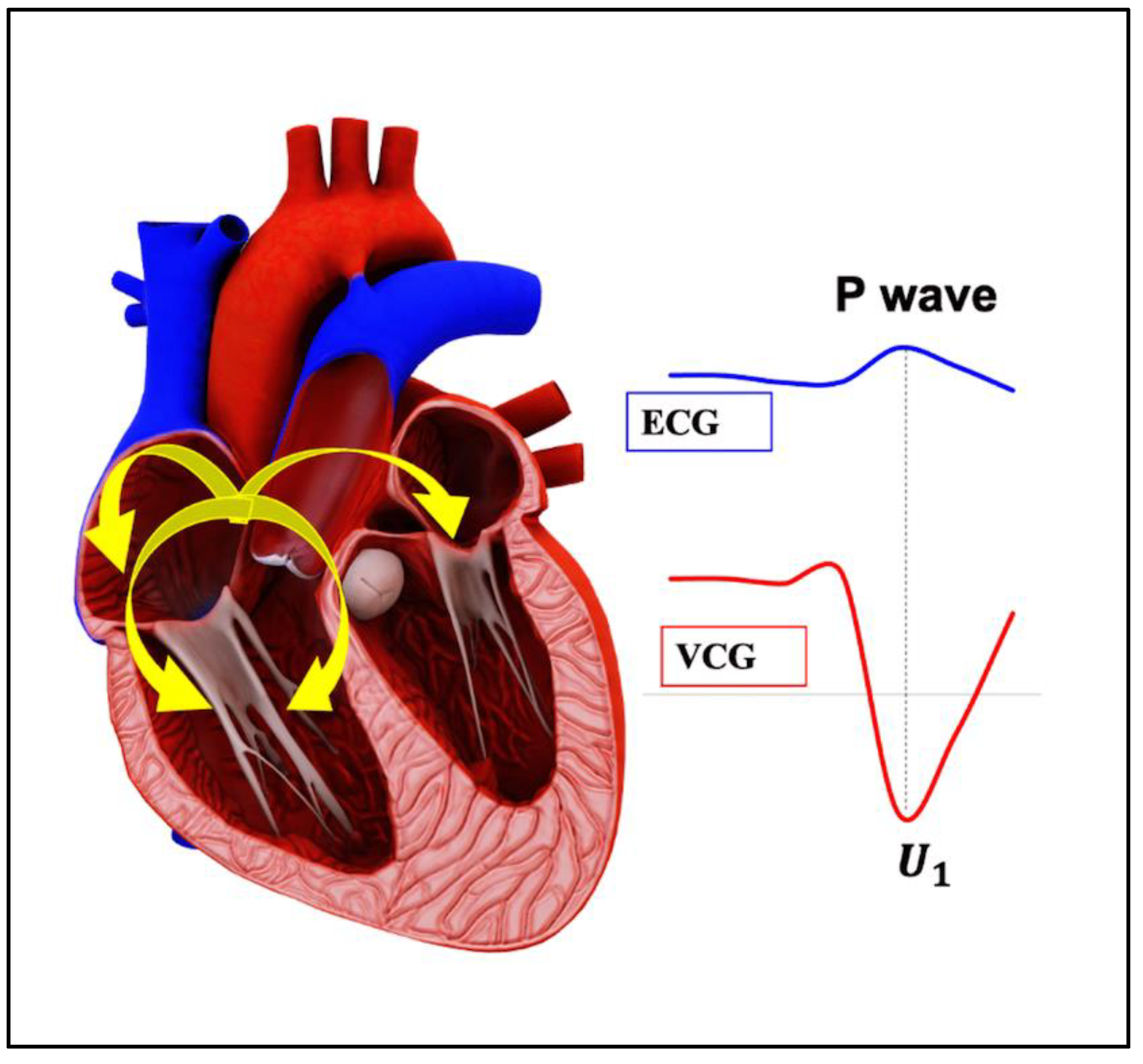
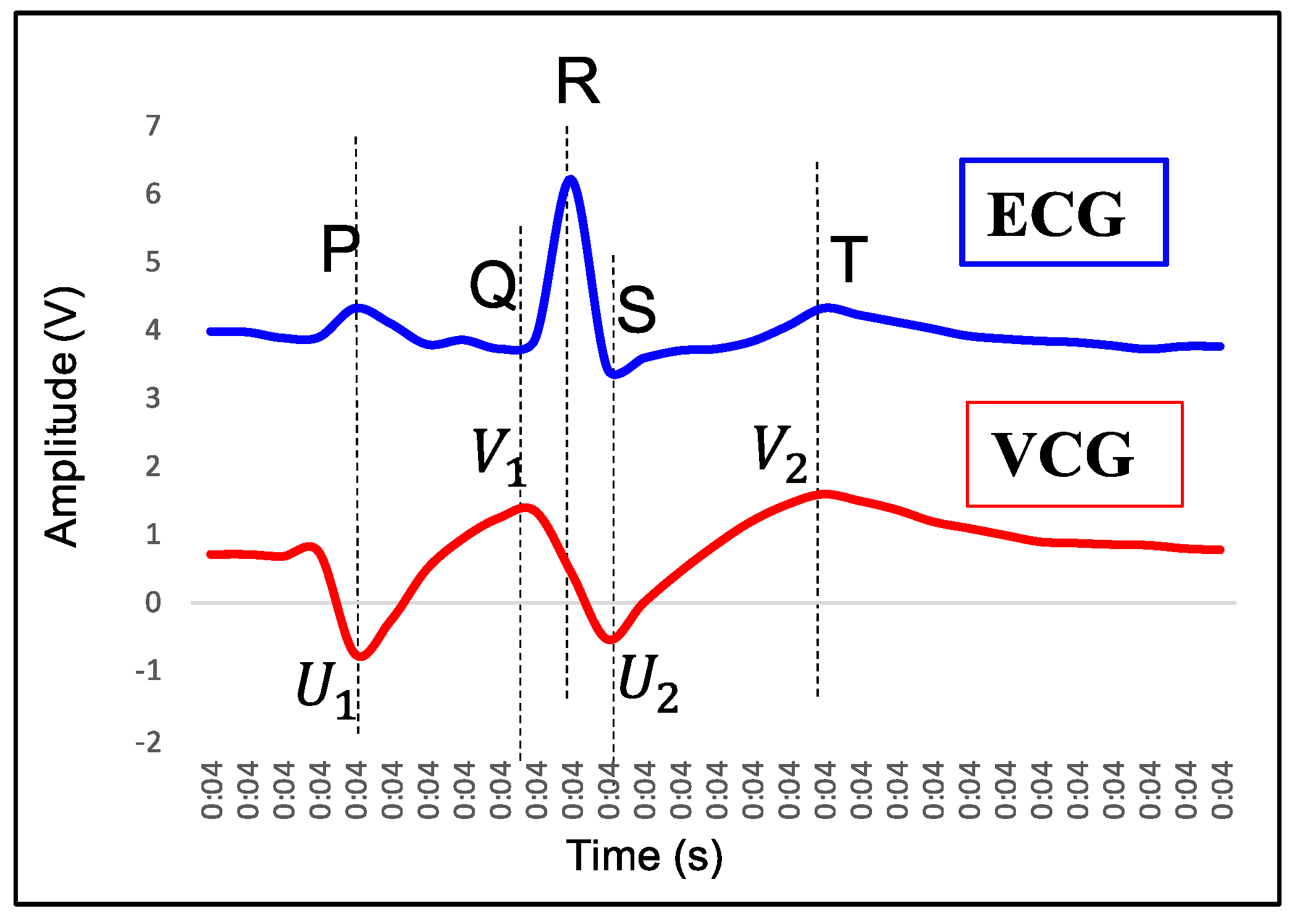
- P wave: The P wave represents the depolarization of the left and right atrium. It corresponds to atrial contraction. The point of local maximum in the ECG for the P wave corresponds to the point of the first local minima in the VCG, as shown in Figure 24. The opposite nature of the electrical and mechanical signal is due to atrial contractions [41];
- 2.
- PR interval: During the PR interval, the electrical pulse moves from atria to ventricles. The PR interval in the ECG has a duration of approximately 120–200 ms. The contractions of ventricles generate vibration patterns that are observable in the VCG plot. When the ventricle contracts, a dominant negative deflection is generated. Thus, it is possible to distinguish between a heartbeat and a beat drop. The atrial contractions result in positive deflections. The PR interval in the ECG is mapped to the interval between the first local minima and the second zero crossing of the VCG signal;
- 3.
- QRS complex: The point Q in the ECG corresponds to the point of first local maximum , while the point of minimum S in the ECG corresponds to the point of second local minima in the vibrocardiogram signal, as shown in Figure 25. The R wave in the ECG corresponds to the zero crossing of the VCG signal [42]. The zero crossing is a point which is located at the center of and . It is located exactly at the center of the first maxima and the second minima of the VCG signal.
- 4.
- ST segment: The T wave represents ventricular repolarization. It appears as a small wave after the QRS complex. The ST segment starts at the end of the S wave and ends at the beginning of the T wave. The ST segment is an isoelectric line that represents the time between depolarization and repolarization of the ventricles. The T wave in the ECG corresponds to the wave of the VCG, as shown in Figure 26;
- 5.
- QT interval: The QT interval begins at the start of the QRS complex and finishes at the end of the T wave. The QT interval in the ECG is mapped to the interval of the VCG. It represents the time taken for the ventricles to depolarize and then repolarize [43].
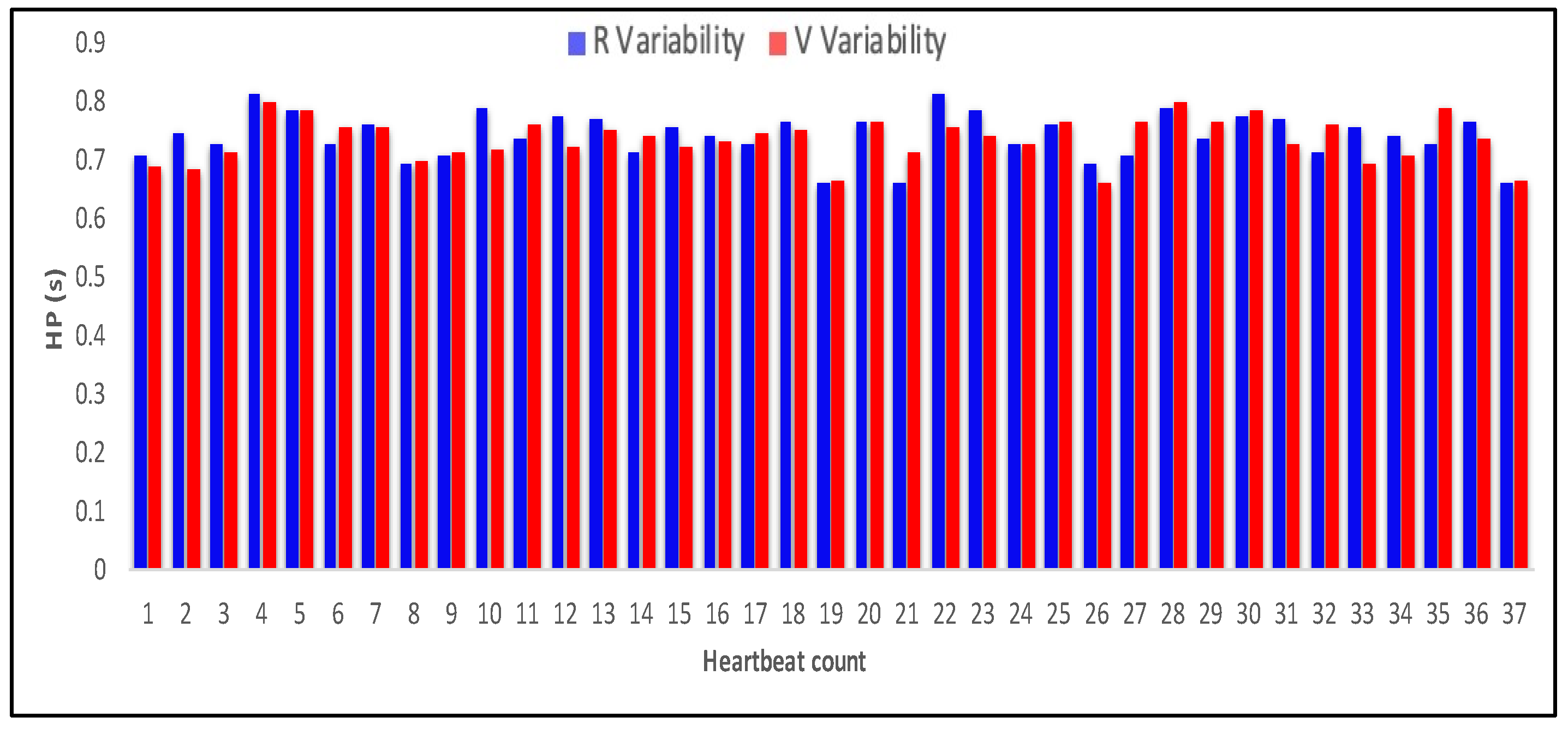
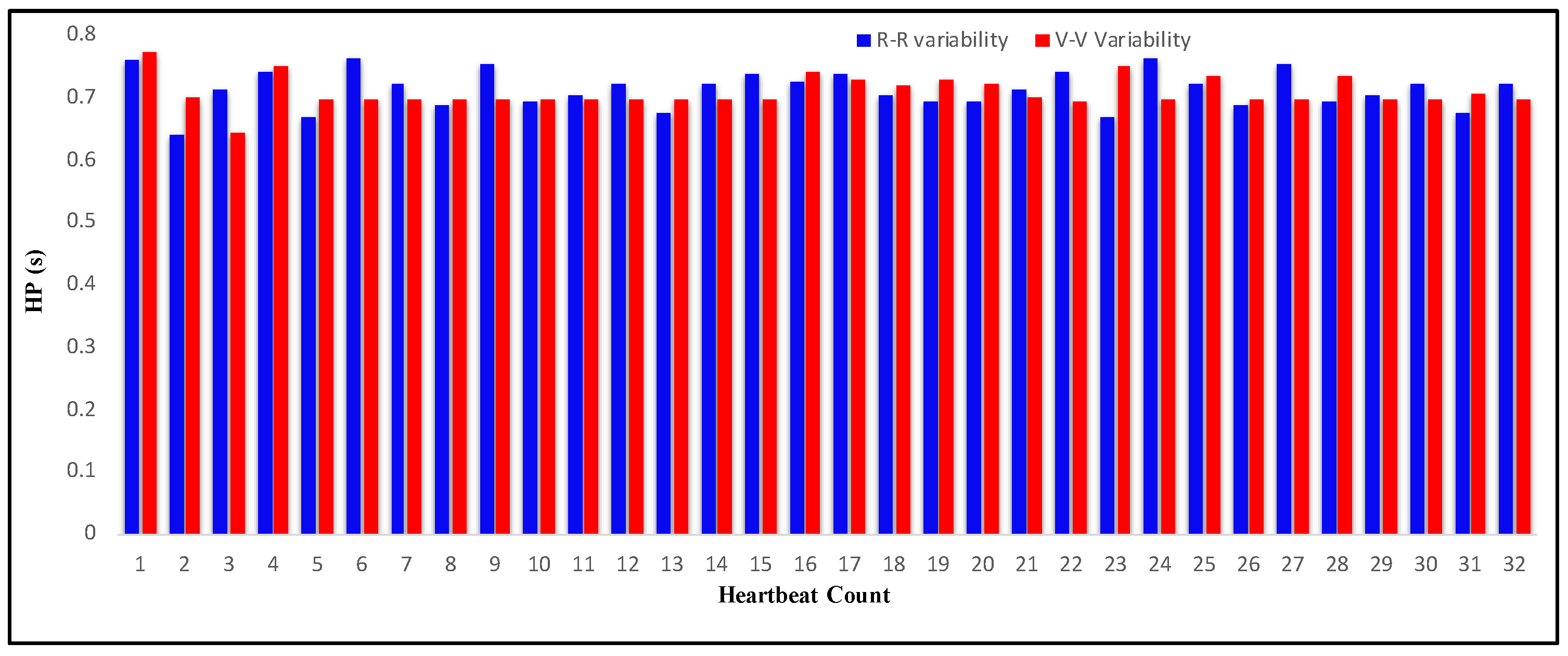
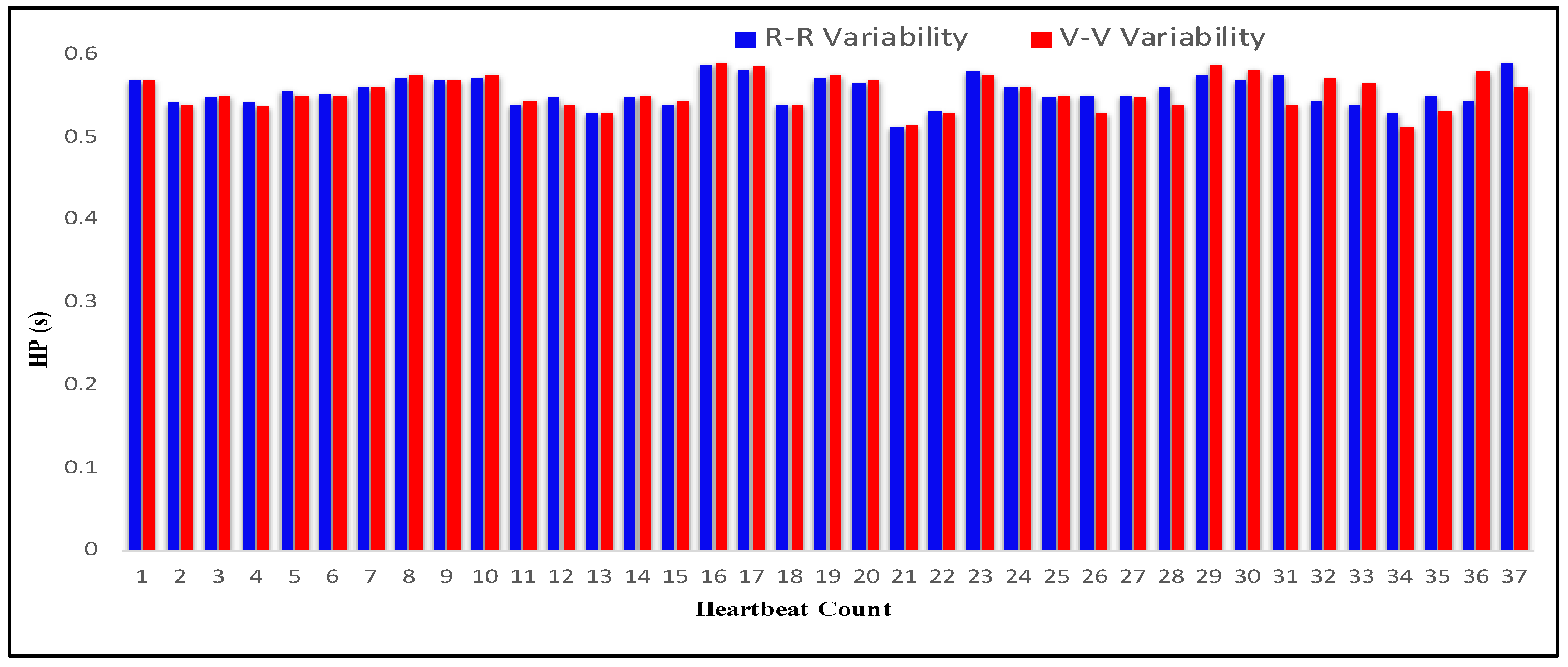
4.3. Measurement of HRV
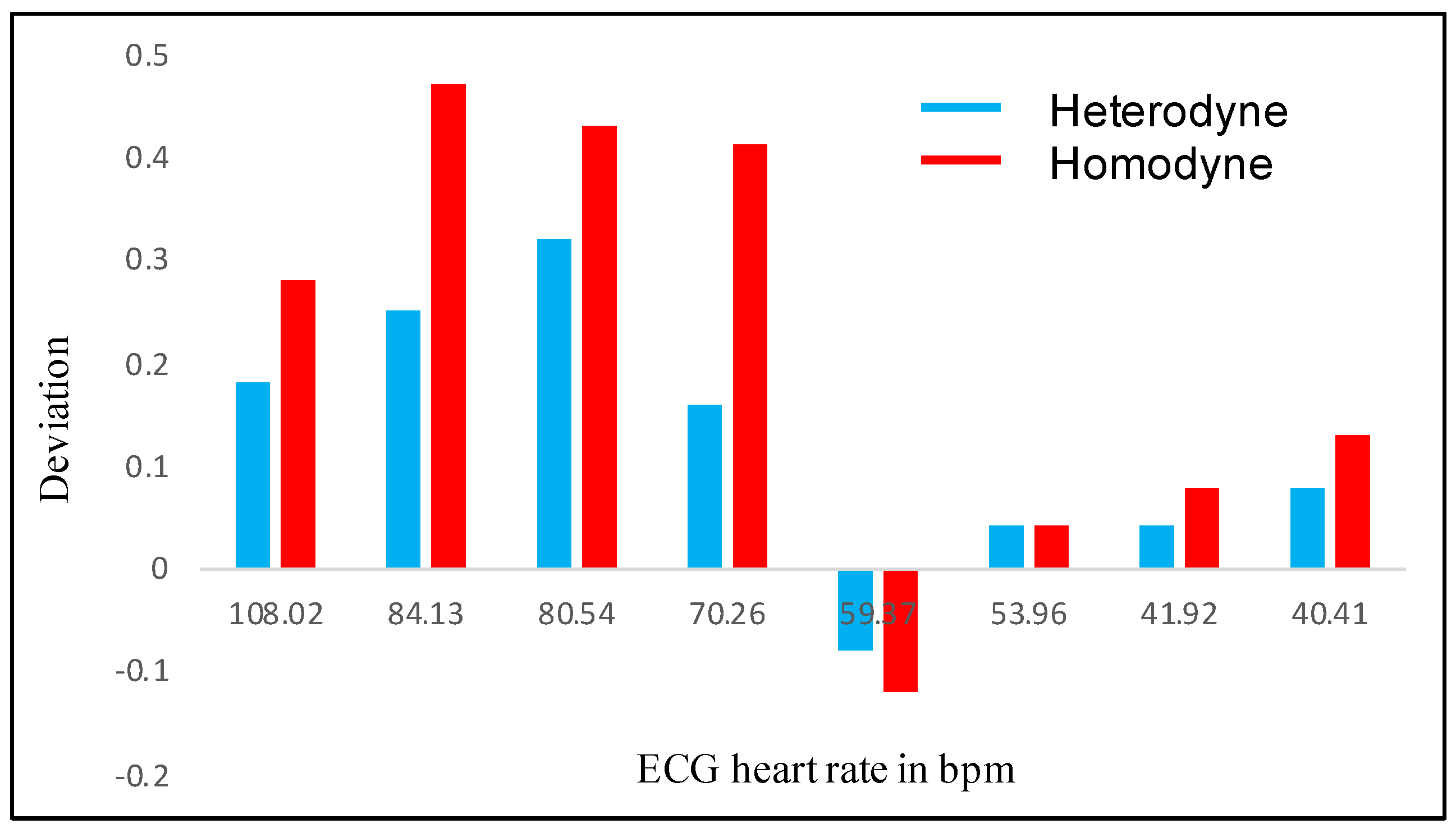
4.4. Comparison of Results of Optical Homodyne and Heterodyne Technique
5. Discussion
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tanzi, L.; Vezzetti, E.; Moreno, R.; Aprato, A.; Audisio, A.; Massè, A. Hierarchical fracture classification of proximal femur X-Ray images using a multi-stage deep learning approach. Eur. J. Radiol. 2020, 133, 109373. [Google Scholar] [CrossRef]
- El-Saadawy, H.; Tantawi, M.; Shedeed, H.A.; Tolba, M.F. Deep Learning Method for Bone Abnormality Detection Using Multi-View X-rays, International Conference on Artificial Intelligence and Computer Vision; Springer: Cham, Switzerland, 2021; pp. 46–55. [Google Scholar]
- Schroeder, E.B.; Liao, D.; Chambless, L.E.; Prineas, R.J.; Evans, G.W.; Heiss, G. Hypertension, Blood Pressure, and Heart Rate Variability. Hypertension 2003, 42, 1106–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mejía-Mejía, E.; Budidha, K.; Abay, T.Y.; May, J.M.; Kyriacou, P.A. Heart Rate Variability (HRV) and Pulse Rate Variability (PRV) for the Assessment of Autonomic Responses. Front. Physiol. 2020, 11, 779. [Google Scholar] [CrossRef] [PubMed]
- Sessa, F.; Anna, V.; Messina, G.; Cibelli, G.; Monda, V.; Marsala, G.; Ruberto, M.; Biondi, A.; Cascio, O.; Bertozzi, G.; et al. Heart rate variability as predictive factor for sudden cardiac death. Aging 2018, 10, 166–177. [Google Scholar] [CrossRef] [Green Version]
- Dekker, J.M.; Crow, R.S.; Folsom, A.R.; Hannan, P.J.; Liao, D.; Swenne, C.A.; Schouten, E.G. Low Heart Rate Variability in a 2-Minute Rhythm Strip Predicts Risk of Coronary Heart Disease and Mortality from Several Causes. Circulation 2000, 102, 1239–1244. [Google Scholar] [CrossRef]
- Jacobs, S.F. Optical heterodyne (coherent) detection. Am. J. Phys. 1988, 56, 235–245. [Google Scholar] [CrossRef]
- Koukoulas, T.; Theobald, P.; Robinson, S.P.; Hayman, G.; Moss, B. Particle velocity measurements using heterodyne interferometry and Doppler shift demodulation for absolute calibration of hydrophones. Proc. Meet. Acoust. 2012, 17, 070022. [Google Scholar] [CrossRef]
- Koukoulas, T.; Robinson, S.; Rajagopal, S.; Zeqiri, B. A comparison between heterodyne and homodyne interferometry to realise the SI unit of acoustic pressure in water. Metrologia 2016, 53, 891–898. [Google Scholar] [CrossRef]
- Pinotti, M.; Paone, N.; Santos, F.A.; Tomasini, E.P. Carotid artery pulse wave measured by a laser vibrometer. In Proceedings of the Third International Conference on Vibration Measurements by Laser Techniques: Advances and Applications, Ancona, Italy, 16–19 June 1998; Volume 3411, pp. 611–616. [Google Scholar]
- Morbiducci, U.; Scalise, L.; De Melis, M.; Grigioni, M. Optical Vibrocardiography: A Novel Tool for the Optical Monitoring of Cardiac Activity. Ann. Biomed. Eng. 2006, 35, 45–58. [Google Scholar] [CrossRef]
- Scalise, L.; Cosoli, G.; Casacanditella, L.; Casaccia, S.; Rohrbaugh, J. The measurement of blood pressure without contact: An LDV-based technique. In Proceedings of the 2017 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Rochester, MN, USA, 7–10 May 2017; pp. 245–250. [Google Scholar]
- Scalise, L.; Morbiducci, U.; De Melis, M. A laser Doppler approach to cardiac motion monitoring: Effects of surface and measurement position. In Proceedings of the Seventh International Conference on Vibration Measurements by Laser Techniques: Advances and Applications, Ancona, Italy, 19–22 June 2006; Volume 6345, p. 63450. [Google Scholar]
- De Melis, M.; Grigioni, M.; Morbiducci, U.; Scalise, L. Optical Monitoring of Heartbeat, Modelling in Medicine and Biology; WIT Press: Southampton, UK, 2005; pp. 181–190. [Google Scholar]
- Donati, S.; Falzoni, L.; Merlo, S. A PC-interfaced, compact laser-diode feedback interferometer for displacement measurements. IEEE Trans. Instrum. Meas. 1996, 45, 942–947. [Google Scholar] [CrossRef]
- Roos, P.A.; Stephens, M.; Wieman, C.E. Laser vibrometer based on optical-feedback-induced frequency modulation of a single-mode laser diode. Appl. Opt. 1996, 35, 6754–6761. [Google Scholar] [CrossRef] [Green Version]
- Merlo, S.; Donati, S. Reconstruction of displacement waveforms with a single-channel laser-diode feedback interferometer. IEEE J. Quantum Electron. 1997, 33, 527–531. [Google Scholar] [CrossRef]
- Beheim, G.; Fritsch, K. Range finding using frequency-modulated laser diode. Appl. Opt. 1986, 25, 1439–1442. [Google Scholar] [CrossRef]
- Shinohara, S.; Yoshida, H.; Ikeda, H.; Nishide, K.; Sumi, M. Compact and high-precision range finder with wide dynamic range and its application. IEEE Trans. Instrum. Meas. 1992, 41, 40–44. [Google Scholar] [CrossRef]
- De Groot, P.J.; Gallatin, G.M.; Macomber, S.H. Ranging and velocimetry signal generation in a backscatter-modulated laser diode. Appl. Opt. 1988, 27, 4475–4480. [Google Scholar] [CrossRef]
- Özdemir, Ş.K.; Ito, S.; Shinohara, S.; Yoshida, H.; Sumi, M. Correlation-based speckle velocimeter with self-mixing interference in a semiconductor laser diode. Appl. Opt. 1999, 38, 6859–6865. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Grattan, K.; Palmer, A.; Boyle, W.J.O. Self-mixing interference inside a single-mode diode laser for optical sensing applications. J. Light. Technol. 1994, 12, 1577–1587. [Google Scholar] [CrossRef]
- Cosoli, G.; Casacanditella, L.; Tomasini, E.; Scalise, L. Evaluation of Heart Rate Variability by means of Laser Doppler Vibrometry measurements. J. Phys. Conf. Ser. 2015, 658, 012002. [Google Scholar] [CrossRef]
- Donati, S. Developing self-mixing interferometry for instrumentation and measurements. Laser Photonics-Rev. 2012, 6, 393–417. [Google Scholar] [CrossRef]
- Perchoux, J.; Quotb, A.; Atashkhooei, R.; Azcona, F.J.; Ramírez-Miquet, E.E.; Bernal, O.; Jha, A.; Luna-Arriaga, A.; Yanez, C.; Caum, J.; et al. Current Developments on Optical Feedback Interferometry as an All-Optical Sensor for Biomedical Applications. Sensors 2016, 16, 694. [Google Scholar] [CrossRef]
- Taimre, T.; Nikolić, M.; Bertling, K.; Lim, Y.L.; Bosch, T.; Rakić, A.D. Laser feedback interferometry: A tutorial on the self-mixing effect for coherent sensing. Adv. Opt. Photonics 2015, 7, 570–631. [Google Scholar] [CrossRef]
- Hisatake, S.; Kitahara, G.; Ajito, K.; Fukada, Y.; Yoshimoto, N.; Nagatsuma, T. Phase-Sensitive Terahertz Self-Heterodyne System Based on Photodiode and Low-Temperature-Grown GaAs Photoconductor at 1.55 μm. IEEE Sens. J. 2012, 13, 31–36. [Google Scholar] [CrossRef]
- Otsuka, K. Self-Mixing Thin-Slice Solid-State Laser Metrology. Sensors 2011, 11, 2195–2245. [Google Scholar] [CrossRef] [PubMed]
- Mohr, T.; Breuer, S.; Blömer, D.; Simonetta, M.; Patel, S.; Schlosser, M.; Deninger, A.; Birkl, G.; Giuliani, G.; Elsäßer, W. Terahertz homodyne self-mixing transmission spectroscopy. Appl. Phys. Lett. 2015, 106, 061111. [Google Scholar] [CrossRef] [Green Version]
- Donati, S.; Rossi, D.; Norgia, M. Single Channel Self-Mixing Interferometer Measures Simultaneously Displacement and Tilt and Yaw Angles of a Reflective Target. IEEE J. Quantum Electron. 2015, 51, 1–8. [Google Scholar] [CrossRef]
- Bernal, O.D.; Zabit, U.; Bosch, T. Study of Laser Feedback Phase Under Self-Mixing Leading to Improved Phase Unwrapping for Vibration Sensing. IEEE Sens. J. 2013, 13, 4962–4971. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Yu, Y.; Xi, J.; Guo, Q. Simultaneous measurement of vibration and parameters of a semiconductor laser using self-mixing interferometry. Appl. Opt. 2014, 53, 4256–4263. [Google Scholar] [CrossRef] [Green Version]
- Kvitek, O.; Hendrych, R.; Kolská, Z.; Švorčík, V. Grafting of Gold Nanoparticles on Glass Using Sputtered Gold Interlayers. J. Chem. 2014, 2014, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Dean, P.; Lim, Y.L.; Valavanis, A.; Kliese, R.; Nikolić, M.; Khanna, S.P.; Lachab, M.; Indjin, D.; Ikonić, Z.; Harrison, P.; et al. Terahertz imaging through self-mixing in a quantum cascade laser. Opt. Lett. 2011, 36, 2587–2589. [Google Scholar] [CrossRef] [Green Version]
- ISOMET. Available online: https://www.isomet.com/acousto_optics.html (accessed on 1 July 2021).
- Capelli, G.; Bollati, C.; Giuliani, G. Non-contact monitoring of heartbeat using optical laser diode vibrocardiography. In Proceedings of the 2011 International Workshop on BioPhotonics, Parma, Italy, 8–10 June 2011; pp. 1–3. [Google Scholar]
- ANALOG DEVICES. Available online: https://www.analog.com/media/en/technical-documentation/data-sheets/ad8232.pdf (accessed on 1 July 2021).
- Villegas, A.; McEneaney, D.; Escalona, O. Arm-ECG Wireless Sensor System for Wearable Long-Term Surveillance of Heart Arrhythmias. Electronics 2019, 8, 1300. [Google Scholar] [CrossRef] [Green Version]
- Donati, S.; Giuliani, G.; Merlo, S. Laser diode feedback interferometer for measurement of displacements without ambiguity. IEEE J. Quantum Electron. 1995, 31, 113–119. [Google Scholar] [CrossRef]
- Donati, S.; Norgia, M. Self-Mixing Interferometry for Biomedical Signals Sensing. IEEE J. Sel. Top. Quantum Electron. 2013, 20, 104–111. [Google Scholar] [CrossRef]
- De Mul, F.F.M.; Van Spijker, J.; Van Der Plas, D.; Greve, J.; Aarnoudse, J.G.; Smits, T.M. Mini laser-Doppler (blood) flow monitor with diode laser source and detection integrated in the probe. Appl. Opt. 1984, 23, 2970–2973. [Google Scholar] [CrossRef]
- Hast, J.; Myllylä, R.; Sorvoja, H.; Miettinen, J. Arterial pulse shape measurement using self-mixing effect in a diode laser. Quantum Electron. 2002, 32, 975–980. [Google Scholar] [CrossRef]
- Arasanz, A.; Azcona, F.; Royo, S.; Jha, A.; Pladellorens, J. A new method for the acquisition of arterial pulse wave using self-mixing interferometry. Opt. Laser Technol. 2014, 63, 98–104. [Google Scholar] [CrossRef] [Green Version]
- Scalise, L.; Morbiducci, U. Non-contact cardiac monitoring from carotid artery using optical vibrocardiography. Med. Eng. Phys. 2008, 30, 490–497. [Google Scholar] [CrossRef]
- Riva, C.; Ross, B.; Benedek, G.B. Laser Doppler measurements of blood flow in capillary tubes and retinal arteries. Investig. Ophthalmol. 1972, 11, 936–944. [Google Scholar]
- Norgia, M.; Donati, S.; D’Alessandro, D. Interferometric measurements of displacement on a diffusing target by a speckle tracking technique. IEEE J. Quantum Electron. 2001, 37, 800–806. [Google Scholar] [CrossRef]
- Giuliani, G.; Bozzi-Pietra, S.; Donati, S. Self-mixing laser diode vibrometer. Meas. Sci. Technol. 2002, 14, 24–32. [Google Scholar] [CrossRef]
- Zabit, U.; Bernal, O.D.; Bosch, T. Self-Mixing Laser Sensor for Large Displacements: Signal Recovery in the Presence of Speckle. IEEE Sens. J. 2013, 13, 824–831. [Google Scholar] [CrossRef] [Green Version]
- Nikolić, M.; Jovanović, D.P.; Lim, Y.L.; Bertling, K.; Taimre, T.; Rakić, A.D. Approach to frequency estimation in self-mixing interferometry: Multiple signal classification. Appl. Opt. 2013, 52, 3345–3350. [Google Scholar] [CrossRef] [PubMed]
- Cosoli, G.; Casacanditella, L.; Tomasini, E.P.; Scalise, L. The non-contact measure of the heart rate variability by laser Doppler vibrometry: Comparison with electrocardiography. Meas. Sci. Technol. 2016, 27, 065701. [Google Scholar] [CrossRef]
- Kim, H.-G.; Cheon, E.-J.; Bai, D.-S.; Lee, Y.H.; Koo, B.-H. Stress and Heart Rate Variability: A Meta-Analysis and Review of the Literature. Psychiatry Investig. 2018, 15, 235–245. [Google Scholar] [CrossRef] [Green Version]
- Giuliani, G.; Norgia, M.; Donati, S.; Bosch, T. Laser diode self-mixing technique for sensing applications. J. Opt. A Pure Appl. Opt. 2002, 4, S283–S294. [Google Scholar] [CrossRef] [Green Version]
- De Melis, M.; Morbiducci, U.; Scalise, L. Identification of cardiac events by Optical Vibrocardiograpy: Comparison with Phonocardiography. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 23–26 August 2007; Volume 2007, pp. 2956–2959. [Google Scholar]
- Rawankar, A.; Urakawa, J.; Shimizu, H.; You, Y.; Terunuma, N.; Aryshev, A.; Honda, Y. Design studies on compact four mirror laser resonator with mode-locked pulsed laser for 5 μm laser wire. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2013, 700, 145–152. [Google Scholar] [CrossRef]
- You, Y.; Urakawa, J.; Rawankar, A.; Aryshev, A.; Shimizu, H.; Honda, Y.; Yan, L.; Huang, W.; Tang, C. Measurement of beam waist for an optical cavity based on Gouy phase. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2012, 694, 6–10. [Google Scholar] [CrossRef]
- Aarathy, E.R.; Rawankar, A.; Kumar, N.S. Measurement of Parameters of Frequency-Locked Two-Mirror Laser Resonator. In Lecture Notes in Electrical Engineering; Springer: Singapore, 2018; Volume 472, pp. 277–286. [Google Scholar]
- Rawankar, A.A.; Terunuma, N.; Urakawa, J.; Akagi, T.; Aryshev, A.S.; Honda, Y.; Jehanno, D. Pulsed green laser wire system for effective inverse Compton scattering. In Proceedings of the IBIC 2014—3rd International Beam Instrumentation Conference, Monterey, CA, USA, 14–18 September 2014. [Google Scholar]
- Donati, S.; Norgia, M. Overview of self-mixing interferometer applications to mechanical engineering. Opt. Eng. 2018, 57, 051506. [Google Scholar] [CrossRef]

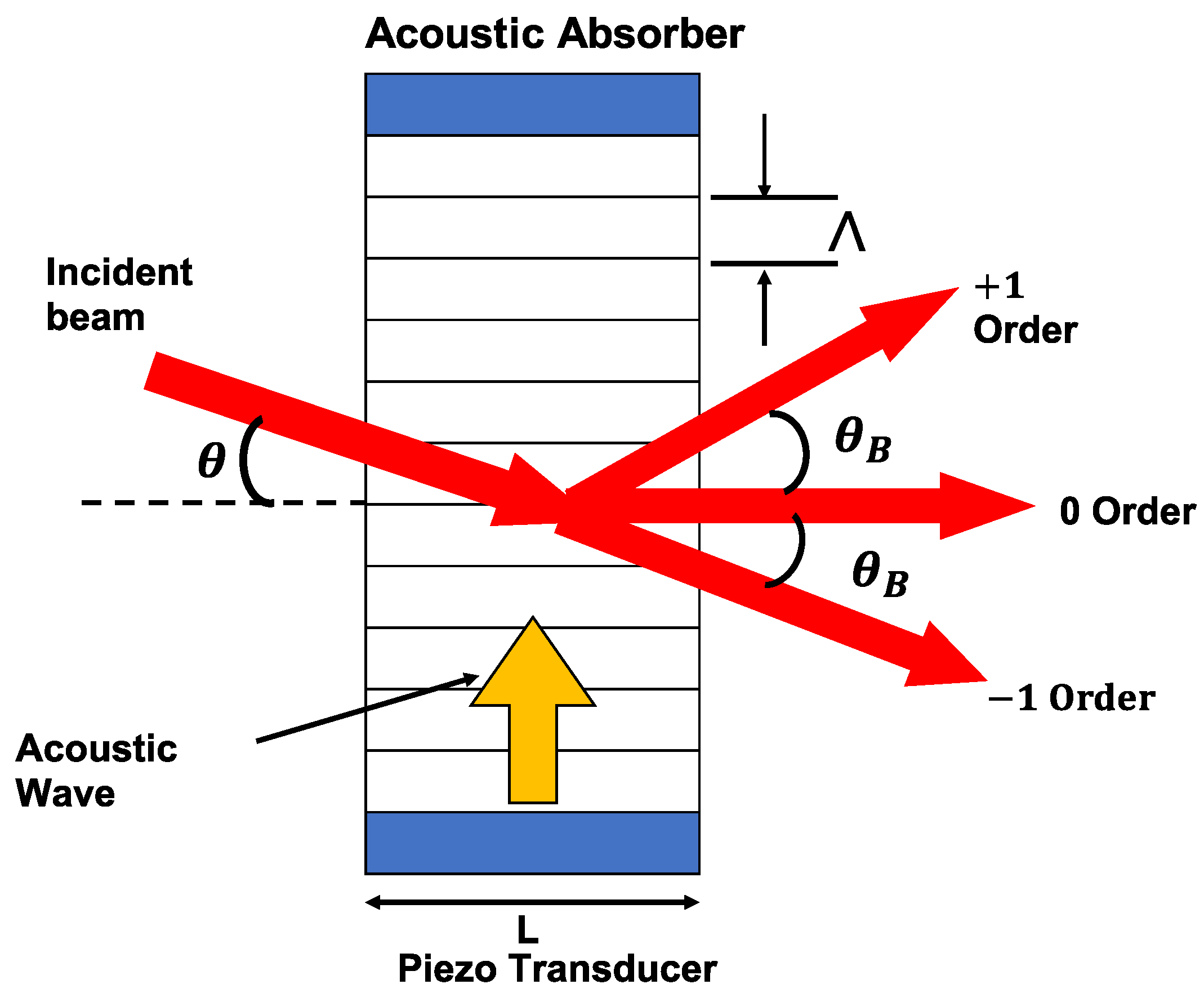




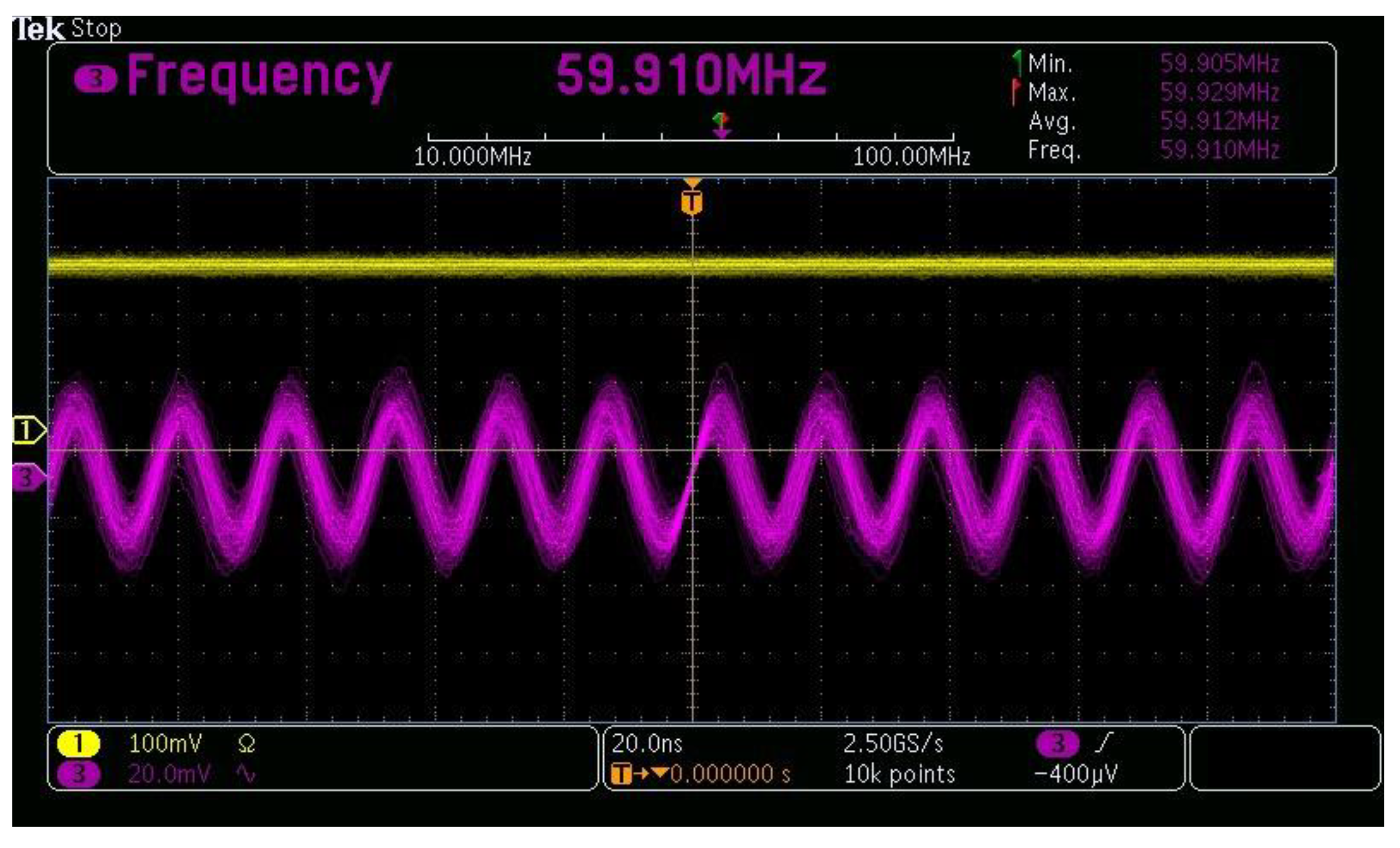
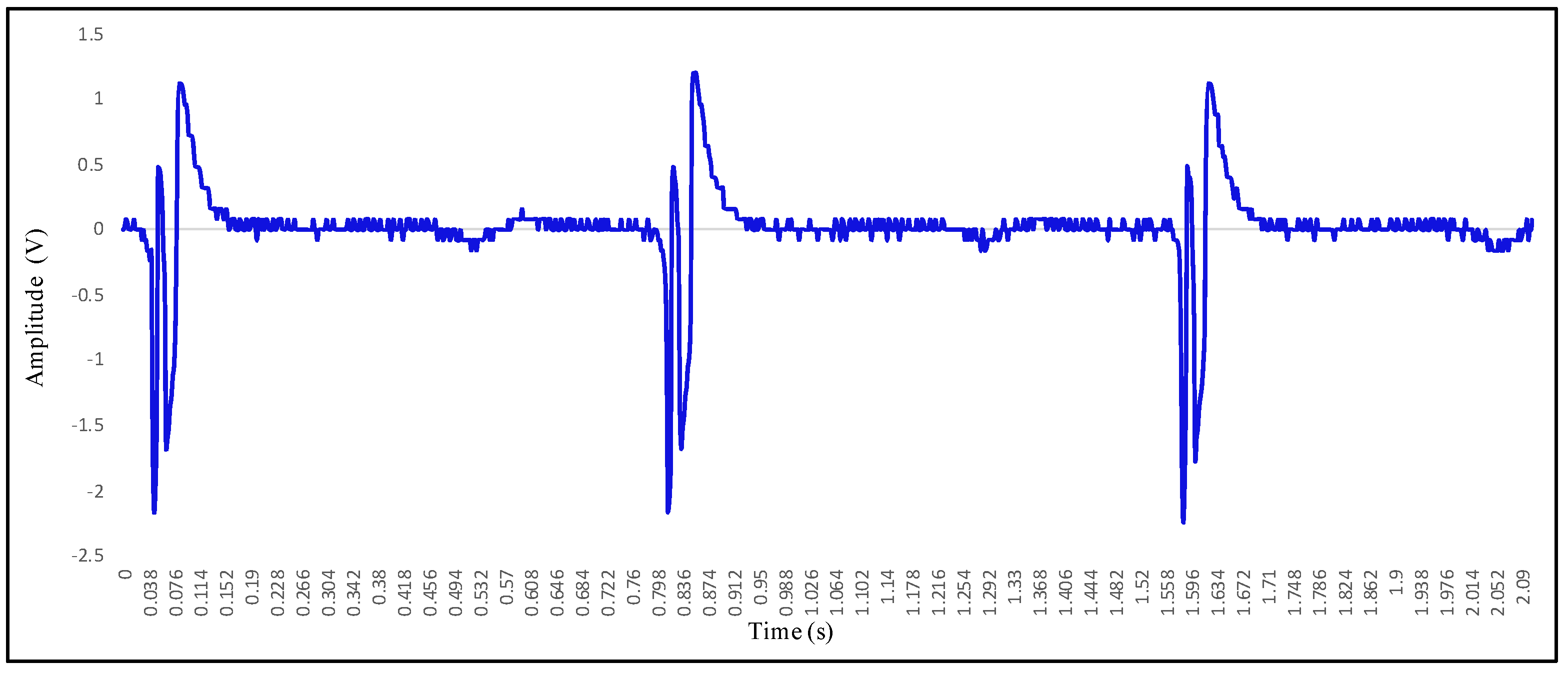









| Parameter | Specification |
|---|---|
| Material | Lead molybdate |
| Active aperture | 2 mm |
| Center frequency | 60 MHz |
| Center wavelength | 660 nm |
| Parameter | Specification |
|---|---|
| RF signal generator voltage | 12 V |
| RF signal generator output frequency | 70 MHz–200 MHz |
| Output power RF generator | 10 dBm |
| RF amplifier supply voltage | 12 V DC |
| RF amplifier gain | 32 dB |
| Subject Number | Gender | Age (Years) | Weight (kg) | Height (m) | Body Mass Index (BMI) (kg·m−2) |
|---|---|---|---|---|---|
| 1 | M | 20 | 61 | 1.71 | 20.86 |
| 2 | M | 20 | 59 | 1.69 | 20.65 |
| 3 | M | 22 | 64 | 1.73 | 21.38 |
| 4 | M | 22 | 66 | 1.75 | 21.55 |
| 5 | M | 25 | 68 | 1.79 | 21.22 |
| 6 | M | 25 | 64 | 1.75 | 20.89 |
| 7 | M | 30 | 65 | 1.70 | 22.49 |
| 8 | M | 32 | 67 | 1.84 | 19.78 |
| 9 | M | 38 | 69 | 1.77 | 22.02 |
| 10 | M | 40 | 70 | 1.76 | 22.59 |
| 11 | F | 20 | 55 | 1.76 | 17.75 |
| 12 | F | 20 | 53 | 1.71 | 18.12 |
| 13 | F | 22 | 57 | 1.74 | 18.82 |
| 14 | F | 25 | 64 | 1.78 | 20.20 |
| 15 | F | 25 | 58 | 1.73 | 19.37 |
| 16 | F | 28 | 68 | 1.80 | 20.98 |
| 17 | F | 30 | 64 | 1.78 | 20.19 |
| 18 | F | 32 | 63 | 1.70 | 21.79 |
| 19 | F | 38 | 67 | 1.75 | 21.87 |
| 20 | F | 40 | 69 | 1.65 | 25.34 |
| Heartbeat Count | R Period (s) | R-R Variability (s) | U Period (s) | V Period (s) | V-V Variability (s) |
|---|---|---|---|---|---|
| 1 | 0.256836 | - | 0.23564 | 0.27564 | - |
| 2 | 1.283203 | 1.026367 | 1.207031 | 1.247031 | 0.971391 |
| 3 | 2.301758 | 1.018555 | 2.2177735 | 2.2577735 | 1.0107425 |
| 4 | 3.321289 | 1.019531 | 3.2285155 | 3.2685155 | 1.010742 |
| 5 | 4.28125 | 0.959961 | 4.25 | 4.29 | 1.0214845 |
| 6 | 5.303711 | 1.022461 | 5.2714845 | 5.3114845 | 1.0214845 |
| 7 | 6.291992 | 0.988281 | 6.2929685 | 6.3329685 | 1.021484 |
| 8 | 7.303711 | 1.011719 | 7.314453 | 7.354453 | 1.0214845 |
| 9 | 8.256836 | 0.953125 | 8.324453 | 8.364453 | 1.01 |
| 10 | 9.303711 | 1.046875 | 9.364453 | 9.404453 | 1.04 |
| 11 | 10.2978515 | 0.9941405 | 10.324453 | 10.364453 | 0.96 |
| 12 | 11.3427735 | 1.044922 | 11.2914453 | 11.3314453 | 0.9669923 |
| 13 | 12.2714845 | 0.928711 | 12.3214453 | 12.3614453 | 1.03 |
| 14 | 13.276367 | 1.0048825 | 13.314453 | 13.354453 | 0.9930077 |
| 15 | 14.256836 | 0.980469 | 14.314453 | 14.354453 | 1 |
| 16 | 15.321289 | 1.064453 | 15.3231445 | 15.3631445 | 1.00869153 |
| 17 | 16.41289 | 1.091601 | 16.354453 | 16.394453 | 1.03130847 |
| 18 | 17.361289 | 0.948399 | 17.384453 | 17.424453 | 1.03 |
| 19 | 18.381289 | 1.02 | 18.384453 | 18.424453 | 1 |
| 20 | 19.5289 | 1.147611 | 19.434453 | 19.474453 | 1.05 |
| Subject No. | R-R Variability ECG (ms) | V-V Variability Heterodyne (ms) | V-V Variability Homodyne (ms) | HR in bpm (ECG) | HR in bpm (Heterodyne) | HR in bpm (Homodyne) |
|---|---|---|---|---|---|---|
| 1 | 555 ± 3 | 554.5 ± 3 | 554 ± 3 | 108.02 | 108.20 | 108.30 |
| 15 | 713 ± 5 | 711 ± 3 | 709 ± 5 | 84.13 | 84.38 | 84.60 |
| 12 | 745 ± 6 | 742 ± 3 | 741 ± 6 | 80.54 | 80.86 | 80.97 |
| 16 | 854 ± 8 | 852 ± 4 | 849 ± 5 | 70.26 | 70.42 | 70.67 |
| 5 | 1010 ± 6 | 1012 ± 4 | 1012 ± 4 | 59.37 | 59.29 | 59.25 |
| 7 | 1112 ± 6 | 1111 ± 6 | 1111 ± 6 | 53.96 | 54.00 | 54.00 |
| 19 | 1431 ± 10 | 1430 ± 8 | 1429 ± 8 | 41.92 | 41.96 | 42.00 |
| 10 | 1484 ± 17 | 1482 ± 8 | 1480 ± 15 | 40.41 | 40.54 | 40.68 |
| Subject Number | HR in bpm (ECG) | HR in bpm (Heterodyne) | HR in bpm (Homodyne) | Deviation in bpm (Heterodyne) | Deviation in bpm (Homodyne) |
|---|---|---|---|---|---|
| 1 | 108.02 | 108.2 | 108.3 | 0.18 | 0.28 |
| 15 | 84.13 | 84.38 | 84.6 | 0.25 | 0.47 |
| 12 | 80.54 | 80.86 | 80.97 | 0.32 | 0.43 |
| 16 | 70.26 | 70.42 | 70.67 | 0.16 | 0.41 |
| 5 | 59.37 | 59.29 | 59.25 | −0.08 | −0.12 |
| 7 | 53.96 | 54 | 54 | 0.04 | 0.04 |
| 19 | 41.92 | 41.96 | 42 | 0.04 | 0.08 |
| 10 | 40.41 | 40.49 | 40.54 | 0.08 | 0.13 |
| Subject No. | Measurement Site | HR in bpm (Homodyne) | HR in bpm (Heterodyne) |
|---|---|---|---|
| 5 | Coronary Artery | 59.29 | 59.25 |
| 5 | Radial Artery | 60.12 | 60.04 |
| 15 | Coronary Artery | 84.38 | 84.6 |
| 15 | Radial Artery | 85.22 | 84.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gondane, J.; Panse, M.S. Development of an Optical System for Non-Contact Type Measurement of Heart Rate and Heart Rate Variability. Appl. Syst. Innov. 2021, 4, 48. https://doi.org/10.3390/asi4030048
Gondane J, Panse MS. Development of an Optical System for Non-Contact Type Measurement of Heart Rate and Heart Rate Variability. Applied System Innovation. 2021; 4(3):48. https://doi.org/10.3390/asi4030048
Chicago/Turabian StyleGondane, Jyoti, and Meena S. Panse. 2021. "Development of an Optical System for Non-Contact Type Measurement of Heart Rate and Heart Rate Variability" Applied System Innovation 4, no. 3: 48. https://doi.org/10.3390/asi4030048





