1. Introduction
A heat-treatment process (including post-annealing) is generally required to obtain sintered ceramic films with the desired properties, such as high wear resistance, chemical- and environmental-corrosion resistance, and high hardness [
1,
2,
3]. These sintering processes are performed at temperatures above 1400 °C in conventional furnaces under controlled environments, which results in long overall fabrication times [
4]. Furthermore, exposure to a high-temperature environment for several hours limits the choice of suitable substrate materials. The Al
2O
3 coatings with superior electrical and wear insulation are prepared using the typical methods, including the thermal spray, sol−gel, electrophoretic, and sputtering methods, as well as plasma electrolytic and electrolytic deposition in aqueous solutions [
5,
6,
7]. However, often poor bonding, easy cracking, thickness limitation, or the high cost of the abovementioned methods limit the coating application. Many alternative heat-treatment methods have been investigated, including excimer lasers, microwaves, and arc plasma methods, to overcome the disadvantages of the conventional thermal-sintering process [
8,
9,
10,
11]. Laser powder bed fusion is a technique that uses a high-power laser to fuse small particles of plastic, metal, or ceramic. However, the small spot size and single wavelength of the light, and most importantly, the high thermal stresses generated in the material, impede the widespread application of the technique. In microwave sintering for high-temperature applications, a thermal runaway develops in the sample and causes thermal instability during the sintering process.
Flash lamp annealing (FLA), or photonic sintering/curing (intense pulsed light), is one of the methods of thermal processing that utilizes ultrashort (0.1–10 ms) pulses from a xenon flash lamp (with a broadband of a 200–1000 nm spectrum) [
12]. The rapid transient heating of surfaces illuminated by the lamp offers extremely fast heating rates [
13,
14]. Because heat is generated within the surface/layer, this method allows the annealing of thin films, even on the temperature-sensitive substrate, without its damage.
The FLA-assisted sintering of only a few ceramics, such as scandia-stabilized zirconia films [
15], yttria-stabilized zirconia films [
16], hafnia-zirconia thin films [
17], and titanium dioxide samples [
18], has been demonstrated. However, the photonic processing of alumina is challenging due to its low absorption in the visible range because of a wide bandgap of 7–8 eV. We have demonstrated previously that, by mixing the precursor alumina particles with reddish-brown-colored iron oxide nanoparticles that were used initially for selective laser sintering [
19,
20], it was possible to boost the optical absorption of alumina precursors [
21]. Two questions, however, remained unanswered: (i) What pigments are the most effective for sintering alumina? and (ii) Can this approach be extended to other ceramic precursors? We intend to address these questions in the present study by admixing various pigments with red, brown, yellow, or black colors with the alumina dispersions. Commercially available pigments composed of metal oxides [
22] with maximum light absorption in the different wavelength regions were used for these purposes. The main novelty is that the interaction between a high-power flashlight and light-reflecting white alumina can be even more enhanced by adding the homogeneous doping of red-colored iron oxide nanoparticles. We identified red-colored Fe
2O
3 as the most efficient pigment, and we then applied it to zirconia, which is another ceramic material that is widely used for biomedical applications. We finally demonstrated FLA-sintered nanometer-thin ceramic zirconia coatings on a flexible metal foil, which fully withstood the annealing process.
2. Materials and Methods
To obtain thin and uniform layers, we utilized a bimodal mixture of the α-Al2O3 with an average particle size of 3 μm (micrometer Al2O3), submicron α-Al2O3 with a size of 200 nm (nanometer Al2O3), and the doping of 5 vol% FexOy red/brown/black/yellow pigments in DI water. Submicron α-Al2O3 Taimicron TM-DAR (Taimei Chemicals Co. LTD) and micron α-Al2O3 AA3 (Sumitomo, Chemical Co. LTD) were purchased as ceramic raw materials. Nano-FexOy additives (Bayferrox) were used as coloring agents (pigments). These included: iron oxide α-Fe2O3 Bayferrox Red 110 in the form of spherical nanoparticles with a 90 nm average particle size; iron oxide Fe3O4 Bayferrox Black 330 spherical nanoparticles with a 150 nm average size; iron and manganese oxide (Fe, Mn)2O3 Bayferrox Brown 645 T spherical nanoparticles with a 300 nm average size; iron hydroxide α-FeOOH Bayferrox Yellow 415 prismatic nanoparticles with a 200 nm × 300 nm average size. ZrO2 nanoparticles (TZ-0), with an average particle size of 40 nm, were purchased from Tosoh (Tosoh-Zirconia).
Particle dispersion. Ammonium citrate dibasic (98%, Sigma Aldrich Corp., St. Louis, MO, USA) was used as a surfactant to achieve a homogeneous dispersion of the particles in DI water. This dispersant was previously proven to be suitable for Fe
2O
3 and Al
2O
3 ceramic particles dispersed in water [
23]. The Fe
xO
y pigments were all initially dispersed using vibration milling for 20 min, with a vibrational frequency of 30 Hz (Retsch MM301, Retsch GmbH, Haan, Germany). All the powders (Fe
xO
y and Al
2O
3) were separately mixed with milling balls (1 mm diameter for TM-DAR, 5 mm diameter for AA3, and 0.5 mm diameter for Fe
xO
y) in three different bottles, with dispersing agent where needed, and DI water. Later, all the components were mixed to obtain a three-modal mixture, and an additional roll-milling step was applied for 24 h. A bimodal distribution of alumina particles was chosen in accordance with [
20,
23]. Additionally, 5 vol% of Fe
xO
y pigments with respect to the entire inorganic content was added to the dispersion. Photonic-sintering experiments were carried out for the coatings obtained with different amounts of the Fe
xO
y dopant; however, the concentration of nanoparticle pigments below 5 vol% was not sufficient for FLA-assisted sintering.
Figure S1a gives the zeta potentials of Fe
xO
y pigments dispersed in water as a function of the pH. The zeta-potential analysis was performed to obtain information about the surface electrostatic charges of α-Fe
2O
3, Fe
3O
4, (Fe, Mn)
2O
3, and α-FeOOH with the pH variation, and to estimate the amount of ammonium citrate to be added to the nanoparticle solutions. Ammonium citrate dibasic in the case of red α-Fe
2O
3 and brown (Fe, Mn)
2O
3 particles was not needed because it would have lowered the point of the zero-charge value at a 2.0 pH even more. The zeta-potential dependence on the amount of titrant (ammonium citrate) was measured only for the black Fe
3O
4 and α-FeOOH yellow-particle solutions (
Figure S1b).
Table S1 summarizes the absolute density, specific surface, and calculated BET average particle sizes for the best-performing red Al
2O
3: Fe
2O
3 slurry compounds;
Figure S2 shows the volume-based LD particle size distributions of the red Al
2O
3: Fe
2O
3 ceramic slurry components.
To achieve a uniform zirconia-based coating, a bimodal mixture of iron oxide α-Fe2O3 and ZrO2 nanoparticles was used. Both alumina-based and zirconia-based coatings were spin-coated with a 1500 rpm speed for 15 s on various substrates: on 1 mm-thick soda-lime glass to achieve 3 µm-thick (alumina) and on 30 µm-thick flexible stainless-steel foil to obtain 200 nm-thick (zirconia) coatings. The slurry solution was dried at 90 °C on a hot plate for 5 min after spin coating.
FLA and temperature-profile simulation. The FLA of the oxide ceramic alumina films doped with 5 vol% of FexOy red/brown/black/yellow pigments was performed in air with a photonic curing system (NovaCentrix PulseForge 1300). For the FLA process, samples were positioned 10 mm away from a Xe arc lamp, with the ceramic film side directed toward the incident light. One pulse had a 2500 μs envelope comprising five 400 μs long micropulses, with a 100 μs break after each micropulse. Repeating the FLA treatment led to the enlarging of the sintered film area. Therefore, consequent FLA pulses, using an 850 V lamp voltage with five-pulse repetition (with a total output exposure energy density of 125 J/cm2), were applied. SimPulse software was used to simulate the maximum temperature reached on the ceramic coating surface, as well as the temperature at the coating/substrate interface. For the simulations, the materials stacked 1 mm-thick SLG and 3 μm-thick alumina, or 200 nm zirconia coating. The correction of the coating absorptivity due to the presence of FexOy red/brown/black/yellow pigments was made according to each color case for alumina, and due to the Fe2O3 red pigments for zirconia.
Characterization methods. The morphologies and thicknesses of the layers, as well as the film compositions, were evaluated by scanning electron microscopy (SEM) and EDX on an FEI Quanta 650 SEM. Transmittance was measured with a UV–Vis spectrometer (Shimadzu UV-3600) from 250 to 1500 nm, taking air as the reference (baseline). X-ray diffraction patterns were measured on an X’Pert Pro in Bragg–Brentano geometry using Cu Kα1 radiation (λ = 1.5406 Å), scanning from 20° to 80° (2θ), with a step interval of 0.0167°. The size distribution of the dispersed particles in the water-based slurry was measured by laser diffraction (LS 13320, Beckman Coulter GmbH, Krefeld, Germany). The absolute densities of all the powders were measured by helium pycnometry (AccuPyc II 1340, Micromeritics, USA). BET measurements (SA 3100 Surface Area Analyzer, Beckman Coulter GmbH, Krefeld, Germany) allowed us to calculate the specific surface areas and BET average sizes of the particles.
3. Results and Discussion
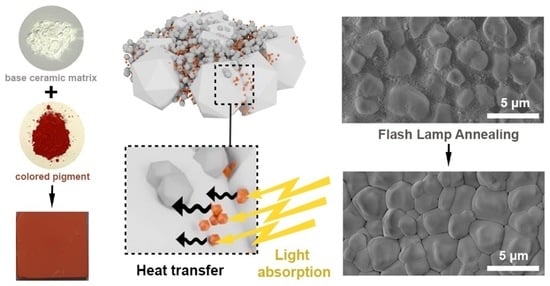
Figure 1 schematically describes the structures of the ceramic precursor and iron oxide pigments, with corresponding SEM images. To obtain thin and uniform layers, we utilized a bimodal mixture of the α-Al
2O
3, a submicron α-Al
2O
3 doping of red/brown/black/yellow Fe
xO
y pigments in DI water, as described in the “Materials and methods” section. The powdered additives (
Figure 1a) have intense colors, which are aimed to increase the overall absorption of the white-colored alumina precursor powder at the applied wavelength range (400–800 nm). A homogenous three-modal distribution was obtained with the nanometer Al
2O
3 and Fe
xO
y additives that filled the gaps between the micrometer Al
2O
3 particles (
Figure 1b). SEM images of the black/yellow/brown and red Fe
xO
y pigments (
Figure 1c) demonstrate that red α-Fe
2O
3, black Fe
3O
4, and brown (Fe, Mn)
2O
3 nanoparticles have a spherical shape, with average sizes of 90, 150, and 300 nm, respectively (material data specification), while yellow α-FeOOH particles, with a 200 nm × 300 nm size, have a prismatic elongated shape. Among the transition metals (Cr, Fe, Eu, Yb, Dy, Tb), the Fe dopant not only enhances sintering, but also improves the catalytic, magnetic, and optical properties of alumina due to the high surface density and the ion radius close to the Al [
24,
25]. By adding impurities, Fe
3+ ions enter the structure of alumina and significantly reduce the bandgap of the material.
Water-based slurries with four different-colored (red α-Fe
2O
3, black Fe
3O
4, brown (Fe, Mn)
2O
3, and yellow α-FeOOH) additives were prepared with the use of ammonia citrate dibasic, as shown in
Figure 2a. The spin-coating of these slurries resulted in colored coatings on glass with different color shades and intensities (
Figure 2b). From the photographs, the most intense color is observed for the red and brown pigments, while yellow coatings, and especially black coatings, exhibit low coloration. It was proven that the powder properties, such as the transparency, particle size, and concentration, are the most important factors that affect the color intensity of the powder [
26]. Thus, having the same vol% of all four pigments, the smaller particles (red: 90 nm and brown: 150 nm) provide the highest color intensity. Due to the homogeneity of the spin-coated layers on glass, it was possible to characterize the optical properties. Because the emission spectrum of the xenon lamp (
Figure 2c) has a broad peak in the visible region (400–800 nm), the absorption obtained by the Fe
xO
y pigment was sufficient for the subsequent FLA. It can be seen in
Figure 2c that the highest absorption in the peak region of the FLA lamp spectra (from 400 to 500 nm) was obtained in the case of the red α-Fe
2O
3 pigment, which was in the range of 88%, while the absorption was only 60%, 41%, and 22% for the brown-, yellow-, and black-colored layers, respectively. The colored pigments play the key role in the sintering process of ceramic-based films by FLA because these particles are necessary to generate enough light absorption, which is followed by a rapid heat transfer from the Fe
xO
y pigments to the base alumina particles (
Figure 2d). At this point, it was expected that the highest sintering activity would be achieved for the red Al
2O
3: Fe
2O
3 coating.
After the spin-coating and drying of the colored precursor films, FLA was performed in air. The temperature reached in the films upon the given FLA parameters was simulated by using SimPulse software (
Figure 3a). According to the simulated temperature profile (
Figure 3a), a maximum annealing temperature of 1850 °C was reached for the red Al
2O
3: Fe
2O
3 ceramic film, while maximum temperatures below 1200 °C, 500 °C, and 400 °C were reached for the brown Al
2O
3: (Fe, Mn)
2O
3, yellow Al
2O
3: FeOOH, and black Al
2O
3: Fe
3O
4 ceramic films, respectively. The SEM images (
Figure 3b) illustrate the morphology changes in the films, which occurred as a result of the photonic sintering. Only the red Al
2O
3: Fe
2O
3 ceramic film experienced a final-stage sintering, and the morphology of the homogeneous dispersed initial mixture of the micrometer and nanometer-sized α-Al
2O
3 particles with various pigments changed to a sintered-grain structure. In the case of brown Al
2O
3: (Fe, Mn)
2O
3 particles, only the initiation of sintering with a starting sintering neck formation was observed. For the black Al
2O
3: Fe
3O
4 and yellow Al
2O
3: α-FeOOH particles, no sintering was detected, which can be explained by the lower optical absorption, which was confirmed by the optical measurements of the slurries on glass and, therefore, the lower temperatures reached on the surface.
We focus on the red Al
2O
3: Fe
2O
3 layers because it was possible to obtain uniformly sintered coatings in this case. The BET specific surface area and density of Bayferrox Red 110 (α-Fe
2O
3) are 15.2 m
2/g and 5.0 g/cm
3, respectively, from the data specification, and so the calculated BET average particle diameter is 104 nm. The phase compositions of these films were analyzed by XRD in a grazing incidence mode before and after FLA (
Figure 4a). Both patterns match well with the diffraction pattern of the trigonal α-Al
2O
3: reflexes at 26, 35, 38, 43, 52, 57, 61, 66, 68, and 77° correspond to the reference pattern ICSD 52648. The reflexes at approximately 24, 33, and 41 and 50, 54, and 62° (marked with red arrows) for the unprocessed layer correspond to α-Fe
2O
3 and are not present in the patterns collected from the FLA-processed layer, which indicates the melting of the light-absorbing iron oxide. Both corundum (α-Al
2O
3) and hematite (Fe
2O
3) belong to the same space group (R
c), with lattice parameters of a = 4.760 Å and c = 12.993 Å, and a = 5.039 Å and c = 13.740 Å, respectively, and they can form solid solutions. It can be observed that all the main peak positions slightly shifted to a lower 2θ degree (by 1°) from
Figure S3, which indicates the cell-volume increase. The obtained results are in accordance with the abovementioned crystallographic data and can be explained by the implementation of Fe ions within the corundum lattice [
23], which can also be seen from the EDX elemental map collected from the sample after FLA (
Figure S5). In addition, it can be observed that the main Al
2O
3 peaks at 26, 35, 38, 43, 52, 57, and 68° have higher peak intensities (maximum peak heights), as well as lower FHWM values (narrow peak shapes) for the pattern obtained after FLA, compared with the one before the FLA. This may be due to the crystallite shape and size change caused by the FLA—from bimodal alumina particles before the FLA to the sintered alumina grains [
27].
Figure S4 additionally shows the Al 2p, Fe 2p, and O 1s XPS peaks of the red Al
2O
3: Fe
2O
3 layer samples before and after the FLA. The results show that the Al 2p peak was at 71.8 eV in the case of Al
2O
3: Fe
2O
3 before FLA, whereas it was shifted to a lower binding energy (71.0 eV) for the sample sintered by FLA; the same trend was observed for the O 1s peak. The shift in the Al 2p peak towards a lower binding energy after the FLA is mainly attributed to the decrease in the coordination number and amount of Al
3+ ions in the film. Similar results were observed for AlO
x thin films annealed at higher temperatures [
28]. Various SEM images (
Figure 4b) confirm the formation of the well-defined grains, which reach a size of 1–5 μm after grain growth via sintering. The α-Fe
2O
3 nanoparticles are assumed to initiate grain growth by local melting after FLA due to their effective light absorption and heat conduction. It can also be seen from the cross-sectional SEM that the grains are merged between each other, with no gaps or pinholes.
To check whether the selected red a-Fe
2O
3 pigments can be employed for the FLA of other refractory oxide ceramics, a thin film of a red ZrO
2: Fe
2O
3 bimodal mixture composed of nanometer-sized ZrO
2 and α-Fe
2O
3 additives (
Figure 5a) was deposited onto a glass slide. The SEM morphological characterization of the layer before FLA confirms a homogeneous distribution of the nanometer-sized particles in the layer, with a thickness of around 200 nm (
Figure 5b). The optical absorption spectrum of the as-prepared red ceramic film exhibits a high absorption of the Xe-lamp spectra: from 40 to 70% in a 400–550 nm wavelength range (
Figure 5c). The red ZrO
2: Fe
2O
3 coating contained much pigment, and the layer thickness was only 200 nm, and so it was not possible to sinter the layers on glass because they were destroyed during the FLA. The temperature reached at the interface of the coating and the glass was simulated to be more than 900 °C. The low softening point of the glass, as well as the thermal-expansion mismatch with the ceramic layer, resulted in the unfortunate damaging and delaminating of the top layer of the glass due to the heat accumulation at this interface. Therefore, we changed the substrate to stainless steel for the FLA-assisted sintering of the thin red ZrO
2: Fe
2O
3 coating. The stainless steel has a higher thermal conductivity and is therefore able to withstand a high temperature at the interface. Simulated temperature profiles on the surface of the ceramic layer during the FLA process confirmed the high temperature reached by the absorption onto the flexible stainless-steel foil (thickness of only 30 μm) (
Figure 5d). A zirconia-based coating was sintered with FLA (
Figure 5e).
Figure S6 contains the XRD patterns of the red ZrO
2: Fe
2O
3 ceramic film before and after FLA. In the initial state, when pure ZrO
2 was mixed with Fe
2O
3, the highly crystalline monoclinic phase was observed. The phase transition of ZrO
2, and the appearance of the tetragonal phase dominating over the monoclinic, have been observed after FLA. This effect was reported previously for a series of samples annealed at temperatures up to 1100 °C [
29]. The XPS measurements of both red ZrO
2:Fe
2O
3 layers before and after FLA are shown in
Figure S7. The Zr 3d
5/2 binding energies of tetragonal (dominated after FLA) and monoclinic (before FLA) films differ by 3.6 eV. In the O 1s region, this difference is 3.1 eV. The Zr 3d and O 1s binding energies depend on the exact preparation parameters (i.e., temperatures during deposition and annealing, and film thickness). It was demonstrated earlier that, by annealing at 920 °C, the film can be transformed into the tetragonal structure, which is attributed to the reduction in the film (i.e., the formation of oxygen vacancies, which stabilize the tetragonal phase [
30]). The transformation of the monoclinic phase of ZrO
2 to tetragonal broadens its applications in devices such as solid oxide fuel cells, which operate over a broad temperature range.