An Anode-Supported Solid Oxide Fuel Cell (SOFC) Half-Cell Fabricated by Hybrid 3D Inkjet Printing and Laser Treatment
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussions
| Number of Layers | 10YSZ Content in the Paste, wt% | ||
|---|---|---|---|
| 15 | 25 | 35 | |
| Electrolyte Thickness, μm | |||
| 1 | Not formed | Not formed | 11.7 |
| 3 | Not formed | 11.6 | 16.0 |
| 5 | 8.2 | 14.1 | 23.7 |
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Filippov, S.P.; Yaroslavtsev, A.B. Hydrogen energy: Development prospects and materials. Russ. Chem. Rev. 2021, 90, 627–643. [Google Scholar] [CrossRef]
- Nicoletti, G.; Arcuri, N.; Nicoletti, G.; Bruno, R. A technical and environmental comparison between hydrogen and some fossil fuels. Energy Convers. Manag. 2015, 89, 205–213. [Google Scholar] [CrossRef]
- Chaubey, R.; Sahu, S.; James, O.O.; Maity, S. A review on development of industrial processes and emerging techniques for production of hydrogen from renewable and sustainable sources. Renew. Sustain. Energy Rev. 2013, 23, 443–462. [Google Scholar] [CrossRef]
- Golkhatmi, S.Z.; Asghar, M.I.; Lund, P.D. A review on solid oxide fuel cell durability: Latest progress, mechanisms, and study tools. Renew. Sustain. Energy Rev. 2022, 161, 112339. [Google Scholar] [CrossRef]
- Stambouli, A.B.; Traversa, E. Solid oxide fuel cells (SOFCs): A review of an environmentally clean and efficient source of energy. Renew. Sustain. Energy Rev. 2002, 6, 433–455. [Google Scholar] [CrossRef]
- Xu, Q.; Guo, Z.; Xia, L.; He, Q.; Li, Z.; Bello, I.T.; Zheng, K.; Ni, M. A comprehensive review of solid oxide fuel cells operating on various promising alternative fuels. Energy Convers. Manag. 2022, 253, 115175. [Google Scholar] [CrossRef]
- Singh, M.; Zappa, D.; Comini, E. Solid oxide fuel cell: Decade of progress, future perspectives and challenges. Int. J. Hydrogen Energy 2021, 46, 27643–27674. [Google Scholar] [CrossRef]
- Baharuddin, N.A.; Muchtar, A.; Sulong, A.B.; Abdullah, H. Fabrication methods for planar solid oxide fuel cells: A review. Adv. Mat. Res. 2013, 662, 396–401. [Google Scholar] [CrossRef]
- Jang, I.; Kelsall, G.H. Fabrication of 3D NiO-YSZ structures for enhanced performance of solid oxide fuel cells and electrolysers. Electrochem. Commun. 2022, 137, 107260. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, X.; Lu, Z.; Liu, Z.; Ge, X.; Xu, J.; Xin, X.; Sha, X.; Su, W. A study of the process parameters for yttria-stabilized zirconia electrolyte films prepared by screen-printing. J. Power Sources 2006, 160, 1065–1073. [Google Scholar] [CrossRef]
- Zhu, X.; Lü, Z.; Wei, B.; Huang, X.; Zhang, Y.; Su, W. A symmetrical solid oxide fuel cell prepared by dry-pressing and impregnating methods. J. Power Sources 2011, 196, 729–733. [Google Scholar] [CrossRef]
- Meng, G.; Song, H.; Xia, C.; Liu, X.; Peng, D. Novel CVD techniques for Micro-and IT-SOFC fabrication. Fuel Cells 2004, 4, 48–55. [Google Scholar] [CrossRef]
- Pederson, L.R.; Singh, P.; Zhou, X.D. Application of vacuum deposition methods to solid oxide fuel cells. Vacuum 2006, 80, 1066–1083. [Google Scholar] [CrossRef]
- Minary-Jolandan, M. Formidable Challenges in Additive Manufacturing of Solid Oxide Electrolyzers (SOECs) and Solid Oxide Fuel Cells (SOFCs) for Electrolytic Hydrogen Economy toward Global Decarbonization. Ceramics 2022, 5, 761–779. [Google Scholar] [CrossRef]
- Lomberg, M.; Boldrin, P.; Tariq, F.; Offer, G.; Wu, B.; Brandon, N.P. Additive manufacturing for solid oxide cell electrode fabrication. ECS Trans. 2015, 68, 2119–2127. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Wang, Y.; McMurtrey, M.D.; Jerred, N.D.; Liou, F.; Li, J. Additive manufacturing for energy: A review. Appl. Energy 2021, 282, 116041. [Google Scholar] [CrossRef]
- Rasaki, S.A.; Liu, C.; Lao, C.; Zhang, H.; Chen, Z. The innovative contribution of additive manufacturing towards revolutionizing fuel cell fabrication for clean energy generation: A comprehensive review. Renew. Sustain. Energy Rev. 2021, 148, 111369. [Google Scholar] [CrossRef]
- Sobolev, A.; Stein, P.; Borodianskiy, K. Synthesis and characterization of NiO colloidal ink solution for printing components of solid oxide fuel cells anodes. Ceram. Int. 2020, 46, 25260–25265. [Google Scholar] [CrossRef]
- Rahumi, O.; Sobolev, A.; Rath, M.K.; Borodianskiy, K. Nanostructured engineering of nickel cermet anode for solid oxide fuel cell using inkjet printing. J. Eur. Ceram. Soc. 2021, 41, 4528–4536. [Google Scholar] [CrossRef]
- Tomov, R.I.; Fakeeh, A.; Krishnan, V.V.; Balasubramanian, K.; Kumar, V.R.; Glowacki, B.A. Direct ceramic inkjet printing and infiltration of functional coatings for metal supported SOFC. ECS Trans. 2015, 68, 2491–2497. [Google Scholar] [CrossRef]
- Pham, T.T.; Tu, H.P.; Dao, T.D.; To, T.D.; Doan, D.C.T.; Dang, M.C. Fabrication of an anode functional layer for an electrolyte-supported solid oxide fuel cell using electrohydrodynamic jet printing. Adv. Nat. Sci. Nanosci. Nanotechnol. 2019, 10, 015004. [Google Scholar] [CrossRef]
- Derby, B. Inkjet printing ceramics: From drops to solid. J. Eur. Ceram. Soc. 2011, 31, 2543–2550. [Google Scholar] [CrossRef]
- Seo, H.; Iwai, H.; Kishimoto, M.; Ding, C.; Saito, M.; Yoshida, H. Microextrusion printing for increasing electrode–electrolyte interface in anode-supported solid oxide fuel cells. J. Power Sources 2020, 450, 227682. [Google Scholar] [CrossRef]
- Seo, H.; Kishimoto, M.; Ding, C.; Iwai, H.; Saito, M.; Yoshida, H. Improvement in the Electrochemical Performance of Anode-supported Solid Oxide Fuel Cells by Meso-and Nanoscale Structural Modifications. Fuel Cells 2020, 20, 570–579. [Google Scholar] [CrossRef]
- Simonenko, T.L.; Simonenko, N.P.; Gorobtsov, P.Y.; Simonenko, E.P.; Kuznetsov, N.T. Microextrusion Printing of Multilayer Hierarchically Organized Planar Nanostructures Based on NiO, (CeO2)0.8(Sm2O3)0.2 and La0.6Sr0.4Co0.2Fe0.8O3−δ. Micromachines 2022, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Komissarenko, D.A.; Sokolov, P.S.; Evstigneeva, A.D.; Slyusar, I.V.; Nartov, A.S.; Volkov, P.A.; Lyskov, N.V.; Evdokimov, P.V.; Putlayev, V.I.; Dosovitsky, A.E. DLP 3D printing of scandia-stabilized zirconia ceramics. J. Eur. Ceram. Soc. 2021, 41, 684–690. [Google Scholar] [CrossRef]
- Jia, K.; Zheng, L.; Liu, W.; Zhang, J.; Yu, F.; Meng, X.; Li, C.; Sunarso, J.; Yang, N. A new and simple way to prepare monolithic solid oxide fuel cell stack by stereolithography 3D printing technology using 8 mol% yttria stabilized zirconia photocurable slurry. J. Eur. Ceram. Soc. 2022, 42, 4275–4285. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, J.; Yu, F.; Zhang, W.; Meng, X.; Yang, N.; Liu, S. A novel fabrication of yttria-stabilized-zirconia dense electrolyte for solid oxide fuel cells by 3D printing technique. Int. J. Hydrogen Energy 2019, 44, 6182–6191. [Google Scholar] [CrossRef]
- Li, W.; Ghazanfari, A.; McMillen, D.; Leu, M.C.; Hilmas, G.E.; Watts, J. Characterization of zirconia specimens fabricated by ceramic on-demand extrusion. Ceram. Int. 2018, 44, 12245–12252. [Google Scholar] [CrossRef]
- Ghazanfari, A.; Li, W.; Leu, M.C.; Watts, J.L.; Hilmas, G.E. Additive manufacturing and mechanical characterization of high density fully stabilized zirconia. Ceram. Int. 2017, 43, 6082–6088. [Google Scholar] [CrossRef] [Green Version]
- Varghese, G.; Moral, M.; Castro-García, M.; López-López, J.J.; Marín-Rueda, J.R.; Yagüe-Alcaraz, V.; Hernández-Afonso, L.; Ruiz-Morales, J.C.; Canales-Vázquez, J. Fabrication and characterisation of ceramics via low-cost DLP 3D printing. Bol. Soc. Esp. Ceram. Vidr. 2018, 57, 9–18. [Google Scholar] [CrossRef]
- Esposito, V.; Gadea, C.; Hjelm, J.; Marani, D.; Hu, Q.; Agersted, K.; Ramousse, S.; Jensen, S.H. Fabrication of thin yttria-stabilized-zirconia dense electrolyte layers by inkjet printing for high performing solid oxide fuel cells. J. Power Sources 2015, 273, 89–95. [Google Scholar] [CrossRef]
- Ahmed, M.; Jones, T.D.A.; Abdolvand, A. Ink formulation and printing of electrolyte for a doped ceria solid oxide fuel cell. Mater. Lett. 2022, 327, 132999. [Google Scholar] [CrossRef]
- Merino, R.I.; Laguna-Bercero, M.A.; Lahoz, R.; Larrea, Á.; Oliete, P.B.; Orera, A.; Pena, J.I.; Sanjuán, M.L.; Sola, D. Laser processing of ceramic materials for electrochemical and high temperature energy applications. Bol. Soc. Esp. Ceram. Vidr. 2022, 61, 19–39. [Google Scholar] [CrossRef]
- Malbakhova, I.; Bagishev, A.; Vorobyev, A.; Borisenko, T.; Logutenko, O.; Titkov, A. Fabrication of NiO/YSZ-Based Anodes for Solid Oxide Fuel Cells by Hybrid 3D Inkjet Printing and Laser Treatment. Ceramics 2022, 5, 1115–1127. [Google Scholar] [CrossRef]
- Bagishev, A.S.; Mal’bakhova, I.M.; Vorob’ev, A.M.; Borisenko, T.A.; Asmed’yanova, A.D.; Titkov, A.I.; Nemudryi, A.P. Layer-by-Layer Formation of the NiO/CGO Composite Anode for SOFC by 3D Inkjet Printing Combined with Laser Treatment. Russ. J. Electrochem. 2022, 58, 600–605. [Google Scholar] [CrossRef]
- Ye, G.; Ju, F.; Lin, C.; Gopalan, S.; Pal, U.; Seccombe, D.A. Low-cost single-step co-firing technique for SOFC manufacturing. ECS Proc. Vol. 2005, 2005, 451–458. [Google Scholar] [CrossRef]
- Gunduz, I.E.; McClain, M.S.; Cattani, P.; Chiu, G.T.-C.; Rhoads, J.F.; Son, S.F. 3D printing of extremely viscous materials using ultrasonic vibrations. Addit. Manuf. 2018, 22, 98–103. [Google Scholar] [CrossRef]
- Licari, J.J.; Swanson, D.W. Adhesives Technology for Electronic Applications: Materials, Processing, Reliability; William Andrew: Norwich, NY, USA, 2011. [Google Scholar]
- Bazilevskii, A.V.; Meyer, J.D.; Rozhkov, A.N. Dynamics and breakup of pulse microjets of polymeric liquids. Fluid Dyn. 2005, 40, 376–392. [Google Scholar] [CrossRef]
- Hutchings, I.M.; Martin, G.D.; Hoath, S.D. High speed imaging and analysis of jet and drop formation. J. Imaging Sci. Technol. 2007, 51, 438–444. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, X.; Zhang, H.; Xue, Q.; Xu, H.; Wang, L.; Feng, Z. The effect of powder grain size on the microstructure and electrical properties of 8 mol% Y2O3-stabilized ZrO2. RSC Adv. 2017, 7, 39153–39159. [Google Scholar] [CrossRef] [Green Version]
- Lenormand, P.; Caravaca, D.; Laberty-Robert, C.; Ansart, F. Thick films of YSZ electrolytes by dip-coating process. J. Eur. Ceram. Soc. 2005, 25, 2643–2646. [Google Scholar] [CrossRef] [Green Version]
- Cebollero, J.A.; Laguna-Bercero, M.A.; Lahoz, R.; Silva, J.; Moreno, R.; Larrea, A. Optimization of laser-patterned YSZ-LSM composite cathode-electrolyte interfaces for solid oxide fuel cells. J. Eur. Ceram. Soc. 2019, 39, 3466–3474. [Google Scholar] [CrossRef] [Green Version]
- Ishiyama, T.; Kishimoto, H.; Yamaji, K.; Horita, T.; Yokokawa, H. Calculation of ohmic resistance increase induced by phase transformation of zirconia electrolyte in SOFC cell by Raman spectroscopy. ECS Trans. 2019, 91, 809–813. [Google Scholar] [CrossRef]
- Dunyushkina, L. Solid oxide fuel cells with a thin film electrolyte: A review on manufacturing technologies and electrochemical characteristics. J. Electrochem. Sci. Technol. 2022, 1, 1–25. [Google Scholar] [CrossRef]
- Yoon, D.; Lee, J.J.; Park, H.G.; Hyun, S.H. NiO/YSZ–YSZ nanocomposite functional layer for high performance solid oxide fuel cell anodes. J. Electrochem. Soc. 2010, 157, B455. [Google Scholar] [CrossRef]
- Bi, L.; Fabbri, E.; Traversa, E. Effect of anode functional layer on the performance of proton-conducting solid oxide fuel cells (SOFCs). Electrochem. Commun. 2012, 16, 37–40. [Google Scholar] [CrossRef]
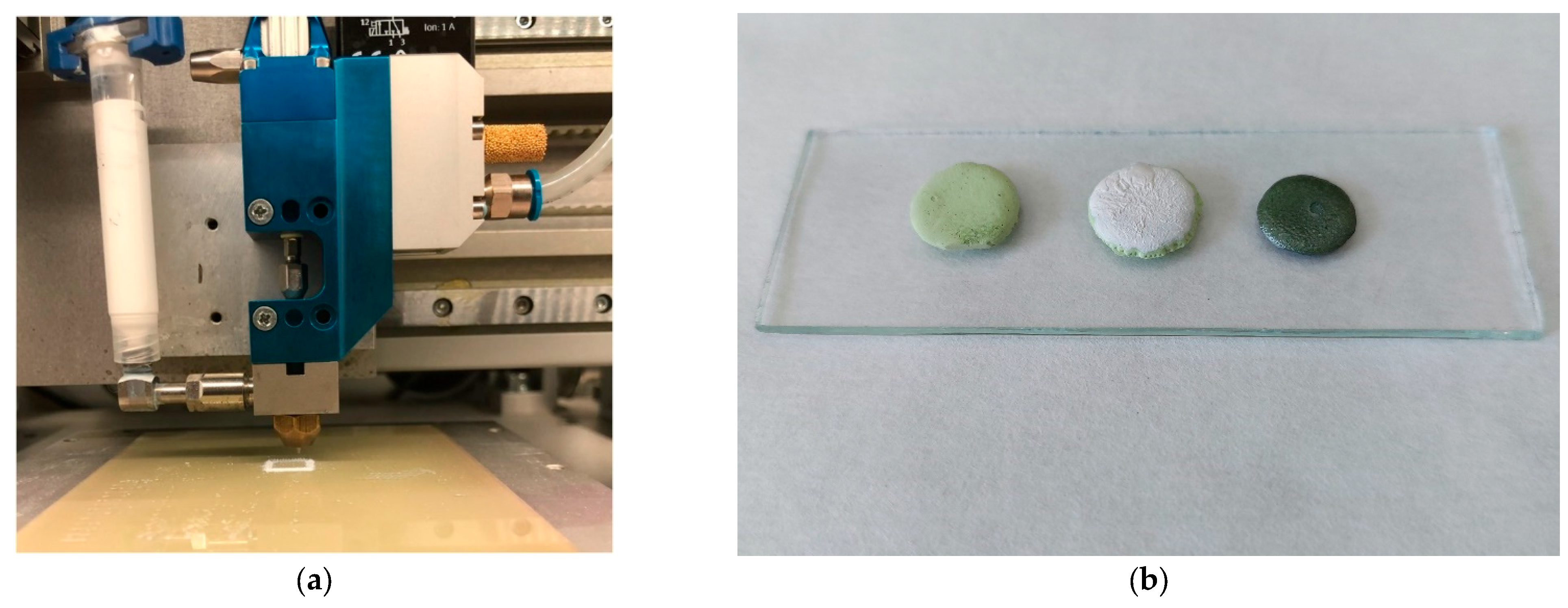
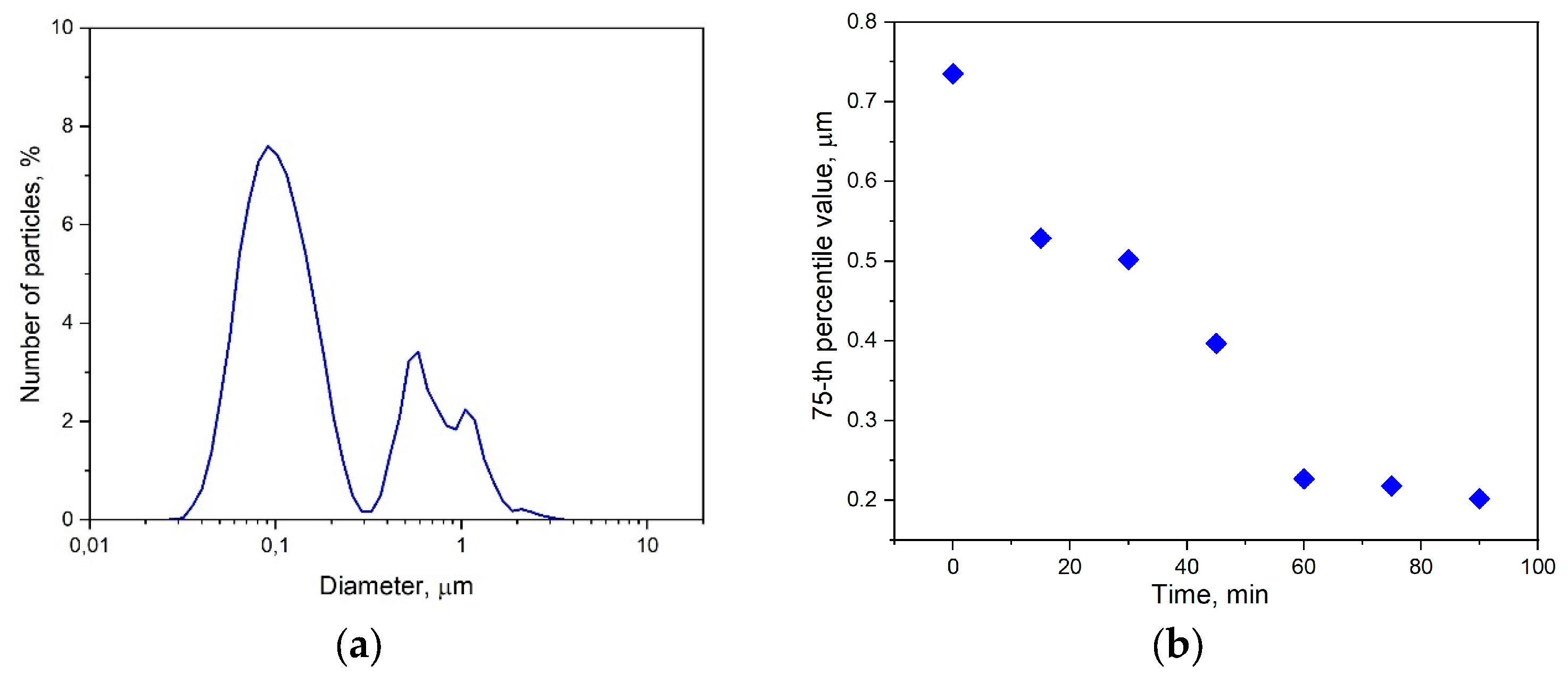

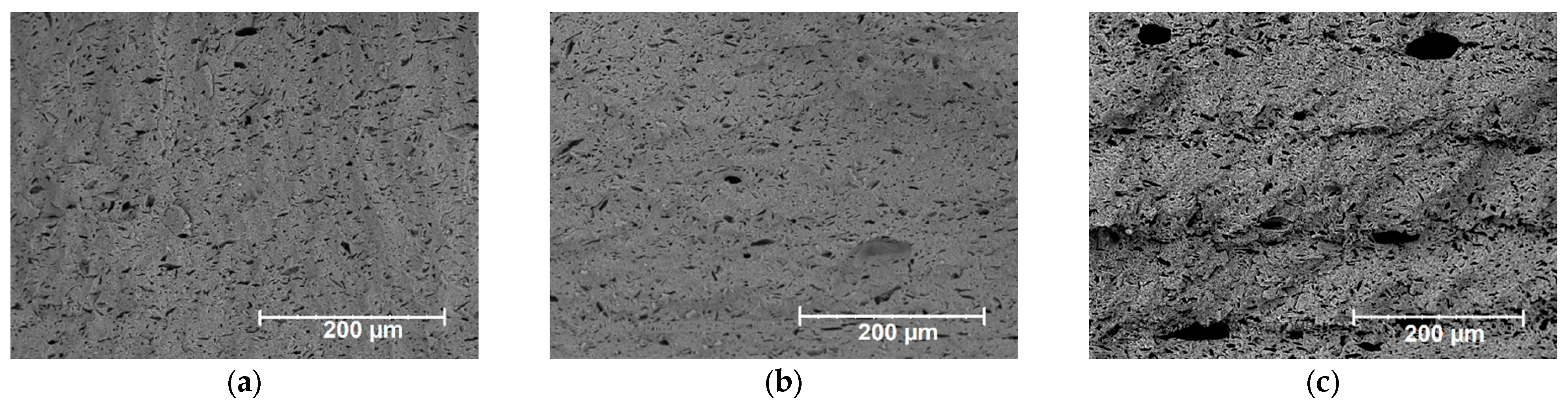
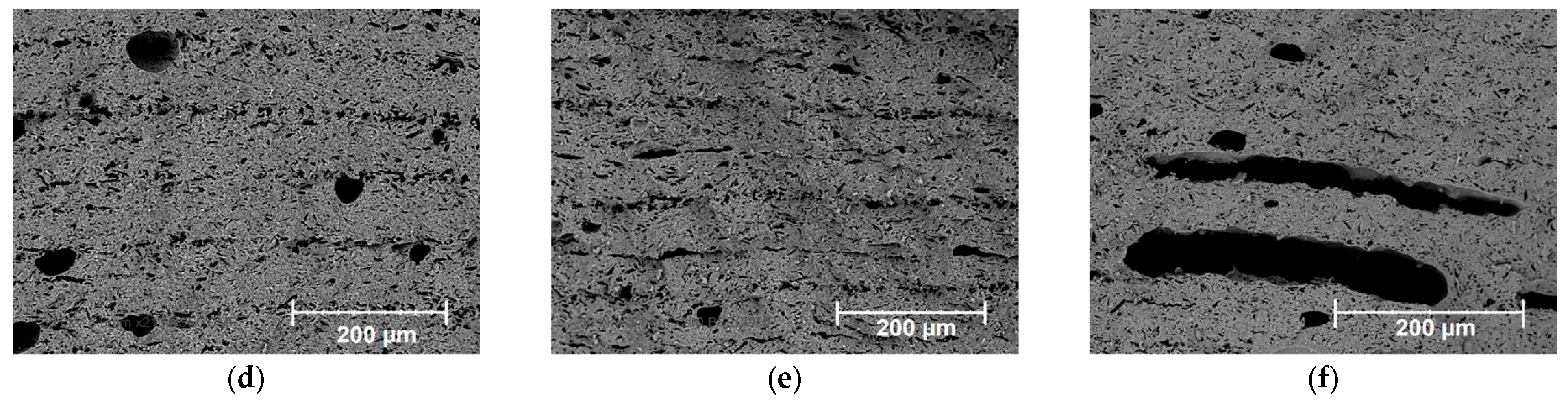
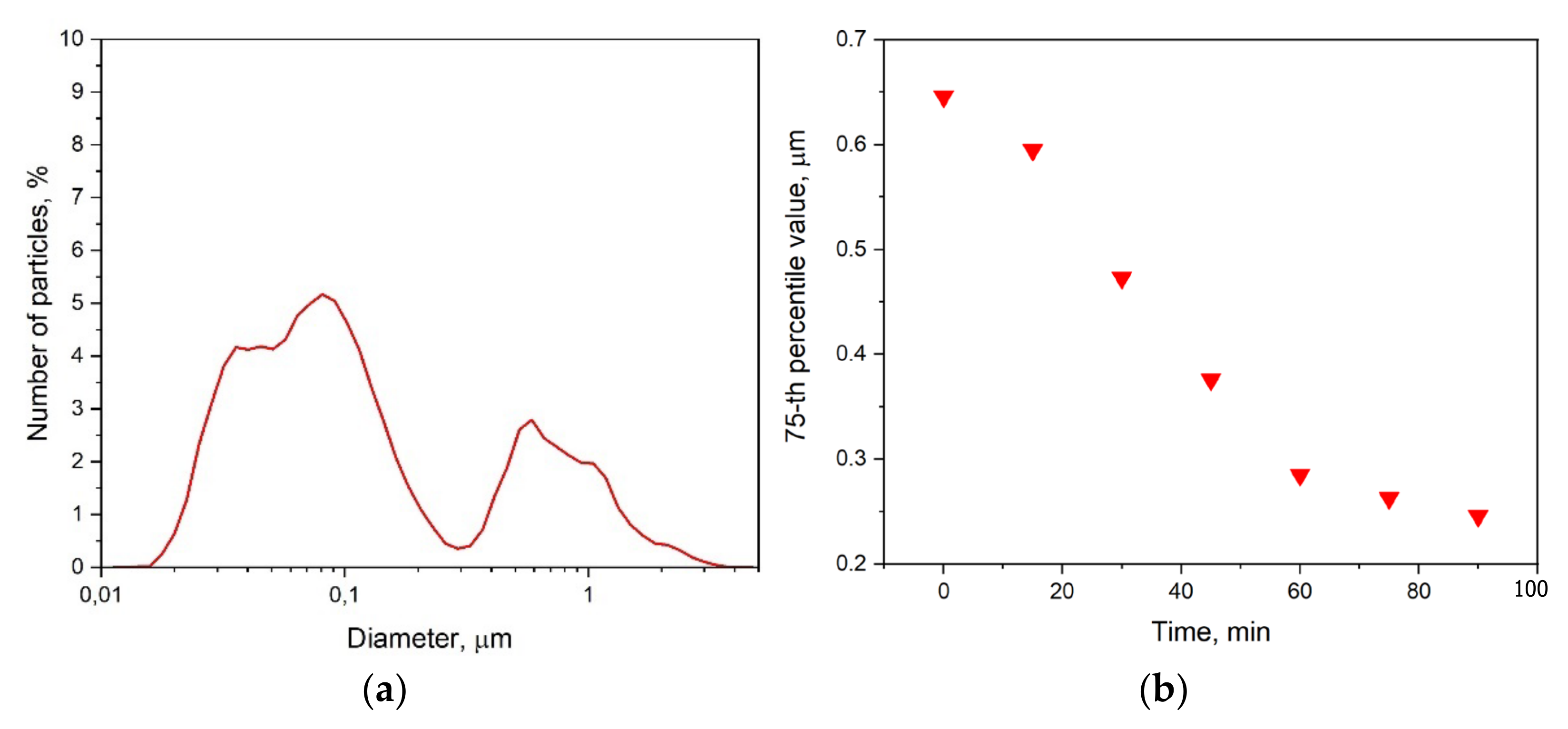
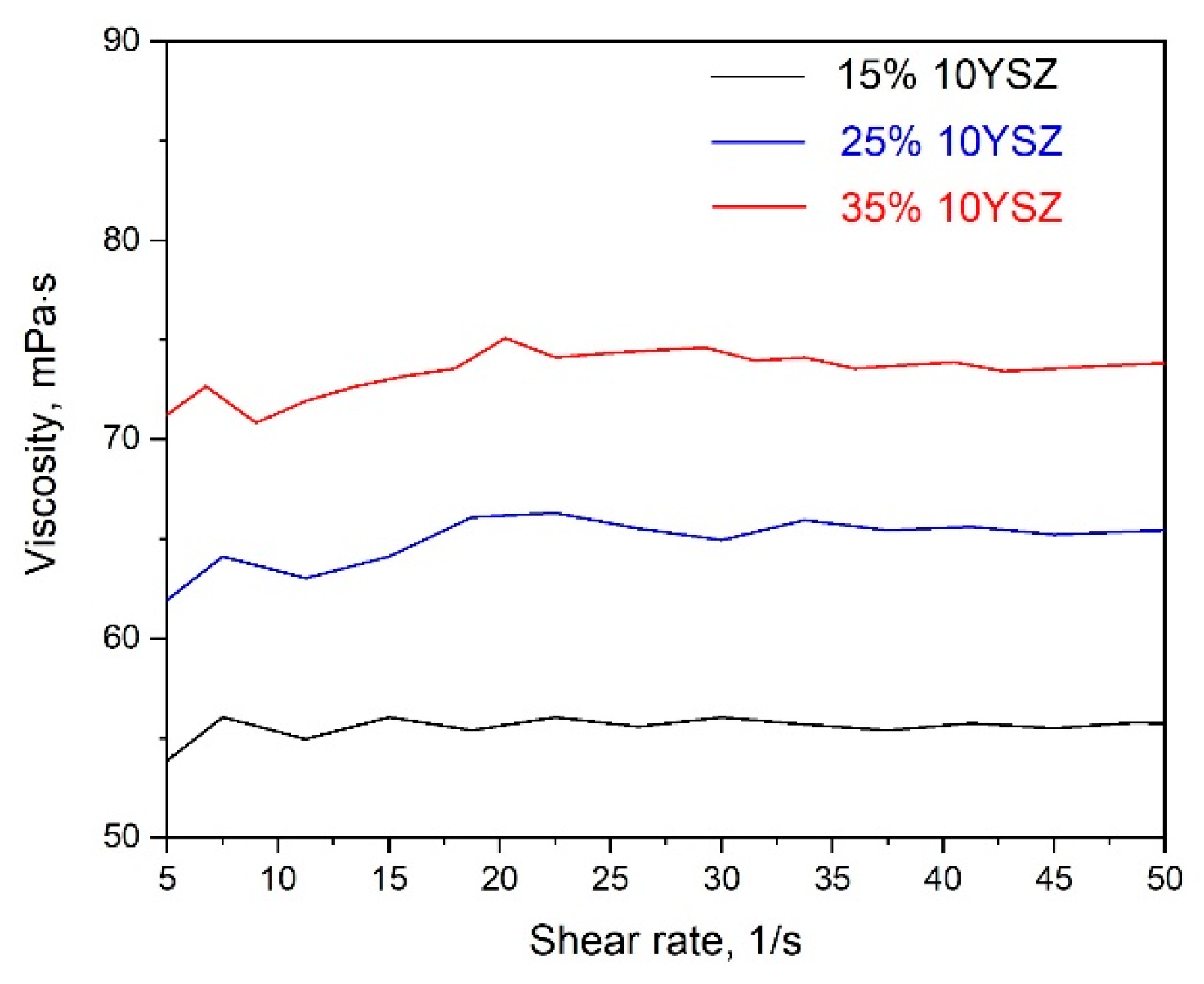


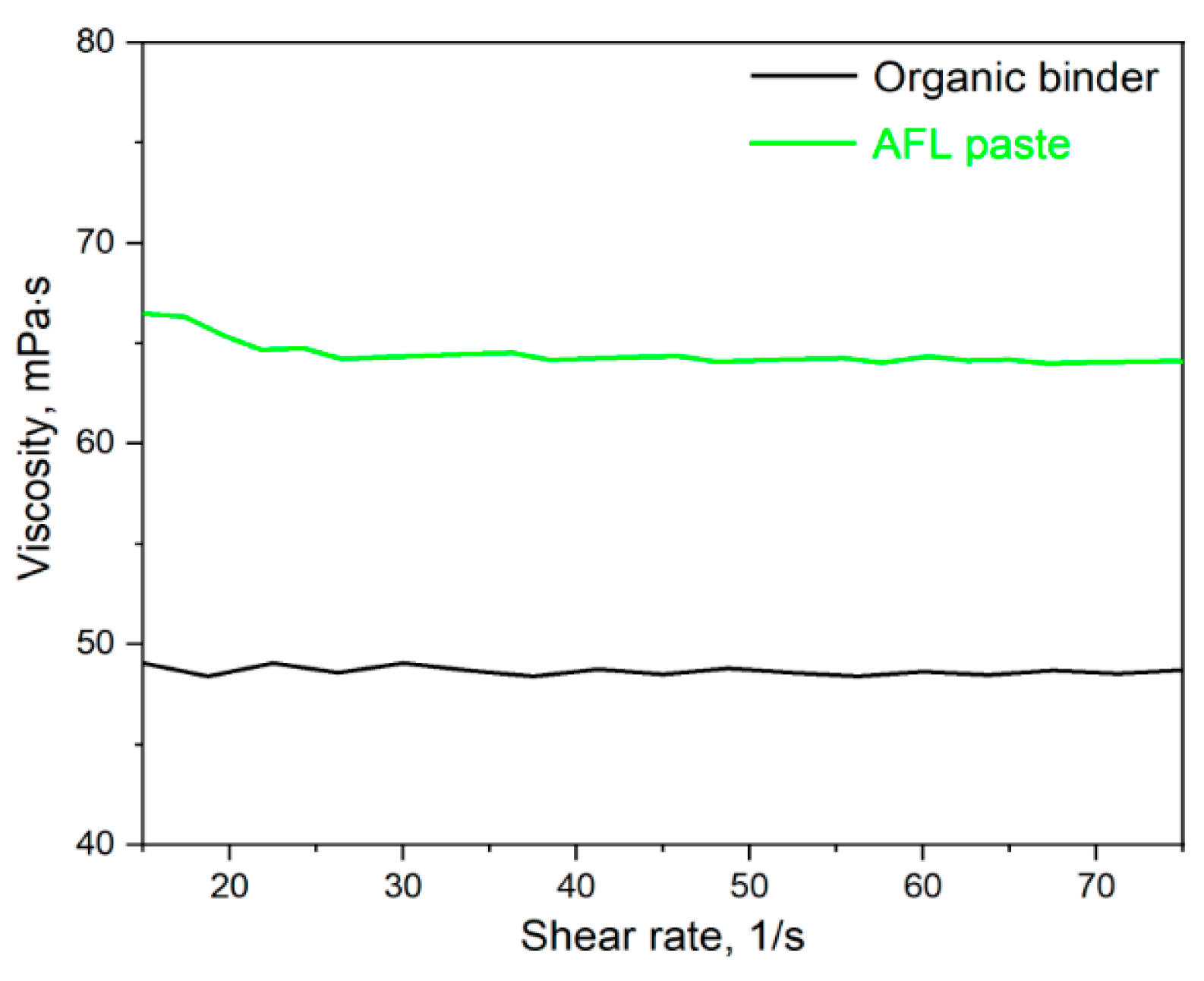
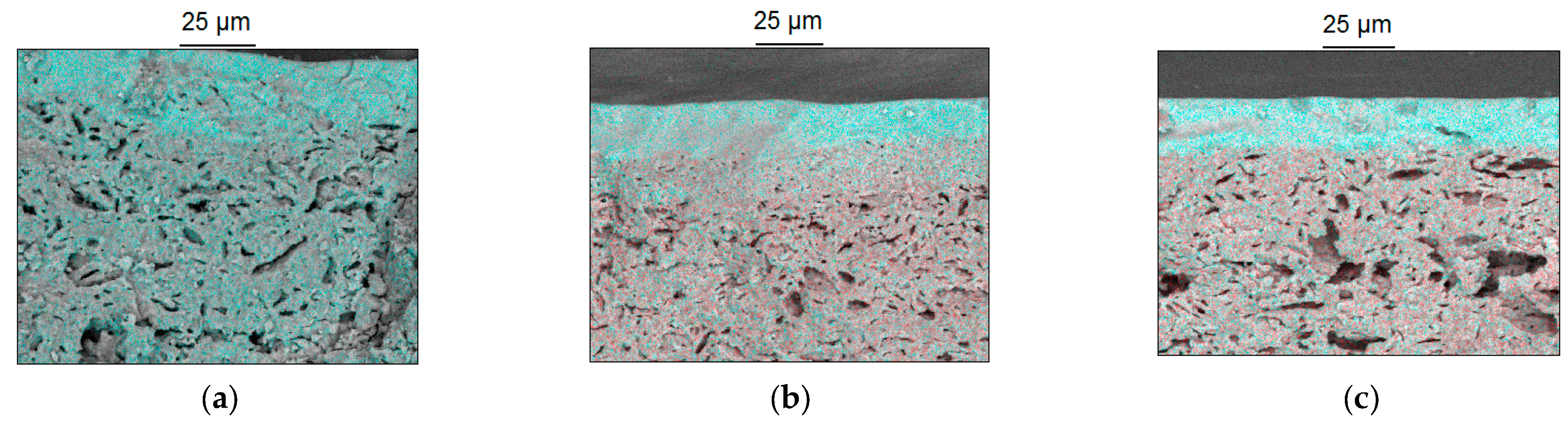
| Paste Composition | Thixotropy Index (TI) |
|---|---|
| Organic binder | 1.01 |
| Paste without pore former | 1.06 |
| Paste with pore former | 1.24 |
| Parameter | Value |
|---|---|
| Nozzle diameter | 250 μm |
| Nozzle inlet pressure | 2.1 atm |
| Nozzle raster | 0.4 mm |
| Laser raster | 0.091 mm |
| Exposure Values, J/cm2 | Porosity, % |
|---|---|
| 0 | 12.6 |
| 3.1 | 14.1 |
| 18.1 | 19.8 |
| 25.6 | 22.5 |
| 36.7 | 29.9 |
| 51.0 | 33.4 (taking into account the large cavities) |
| 12.9 (not taking into account the large cavities) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malbakhova, I.; Bagishev, A.; Vorobyev, A.; Borisenko, T.; Logutenko, O.; Lapushkina, E.; Titkov, A. An Anode-Supported Solid Oxide Fuel Cell (SOFC) Half-Cell Fabricated by Hybrid 3D Inkjet Printing and Laser Treatment. Ceramics 2023, 6, 1384-1396. https://doi.org/10.3390/ceramics6030085
Malbakhova I, Bagishev A, Vorobyev A, Borisenko T, Logutenko O, Lapushkina E, Titkov A. An Anode-Supported Solid Oxide Fuel Cell (SOFC) Half-Cell Fabricated by Hybrid 3D Inkjet Printing and Laser Treatment. Ceramics. 2023; 6(3):1384-1396. https://doi.org/10.3390/ceramics6030085
Chicago/Turabian StyleMalbakhova, Inna, Artem Bagishev, Alexander Vorobyev, Tatiana Borisenko, Olga Logutenko, Elizaveta Lapushkina, and Alexander Titkov. 2023. "An Anode-Supported Solid Oxide Fuel Cell (SOFC) Half-Cell Fabricated by Hybrid 3D Inkjet Printing and Laser Treatment" Ceramics 6, no. 3: 1384-1396. https://doi.org/10.3390/ceramics6030085





