Sonochemical Synthesis of Indium Nitride Nanoparticles and Photocatalytic Composites with Titania
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials and Methods of Characterization
2.2. Preparation of InN Nanoparticles
2.3. Decoration of TiO2 with InN Nanoparticles
2.4. Photocatalytic Tests on TiO2 and TiO2 Decorated onto InN Nanoparticles
3. Results and Discussion
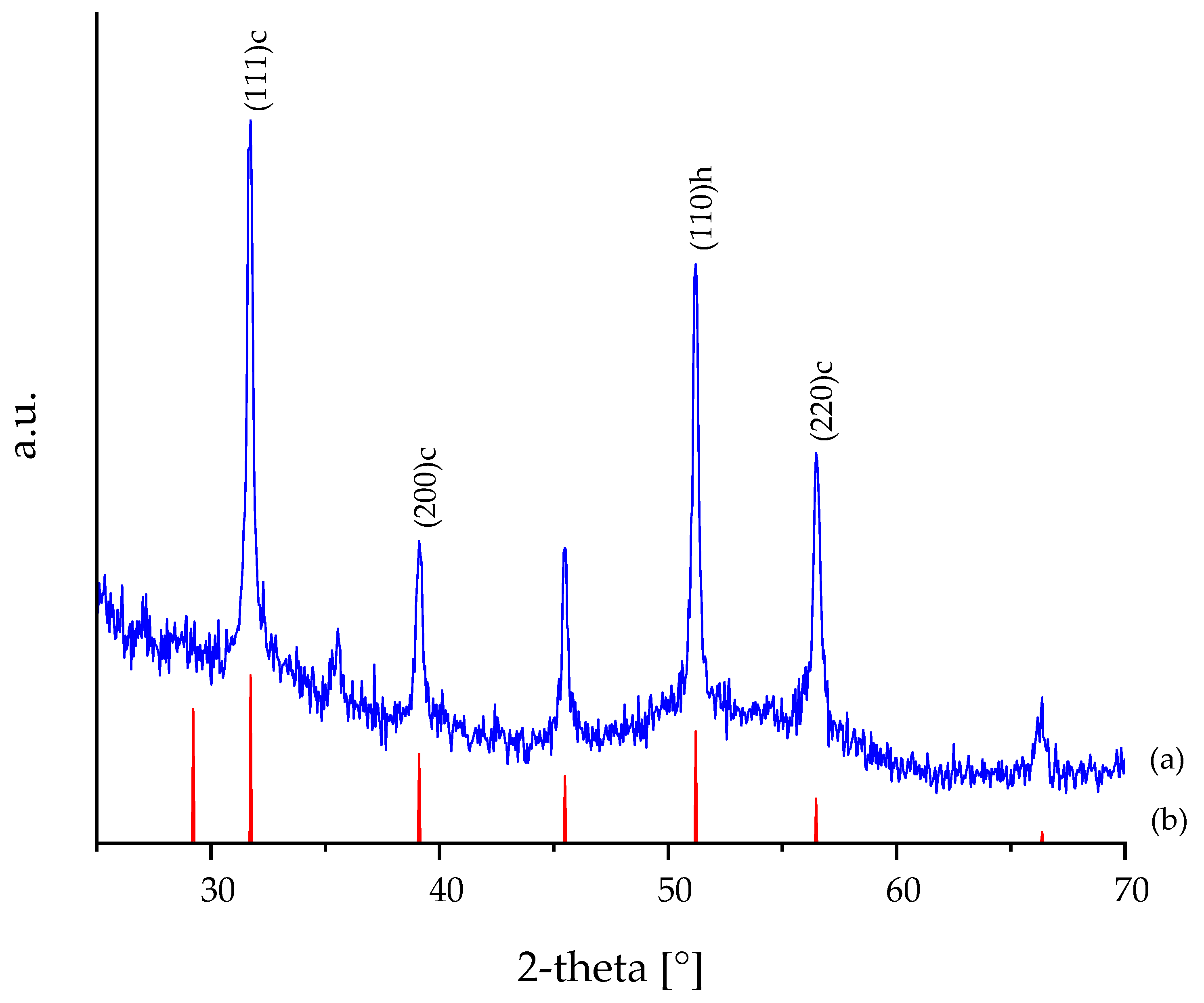
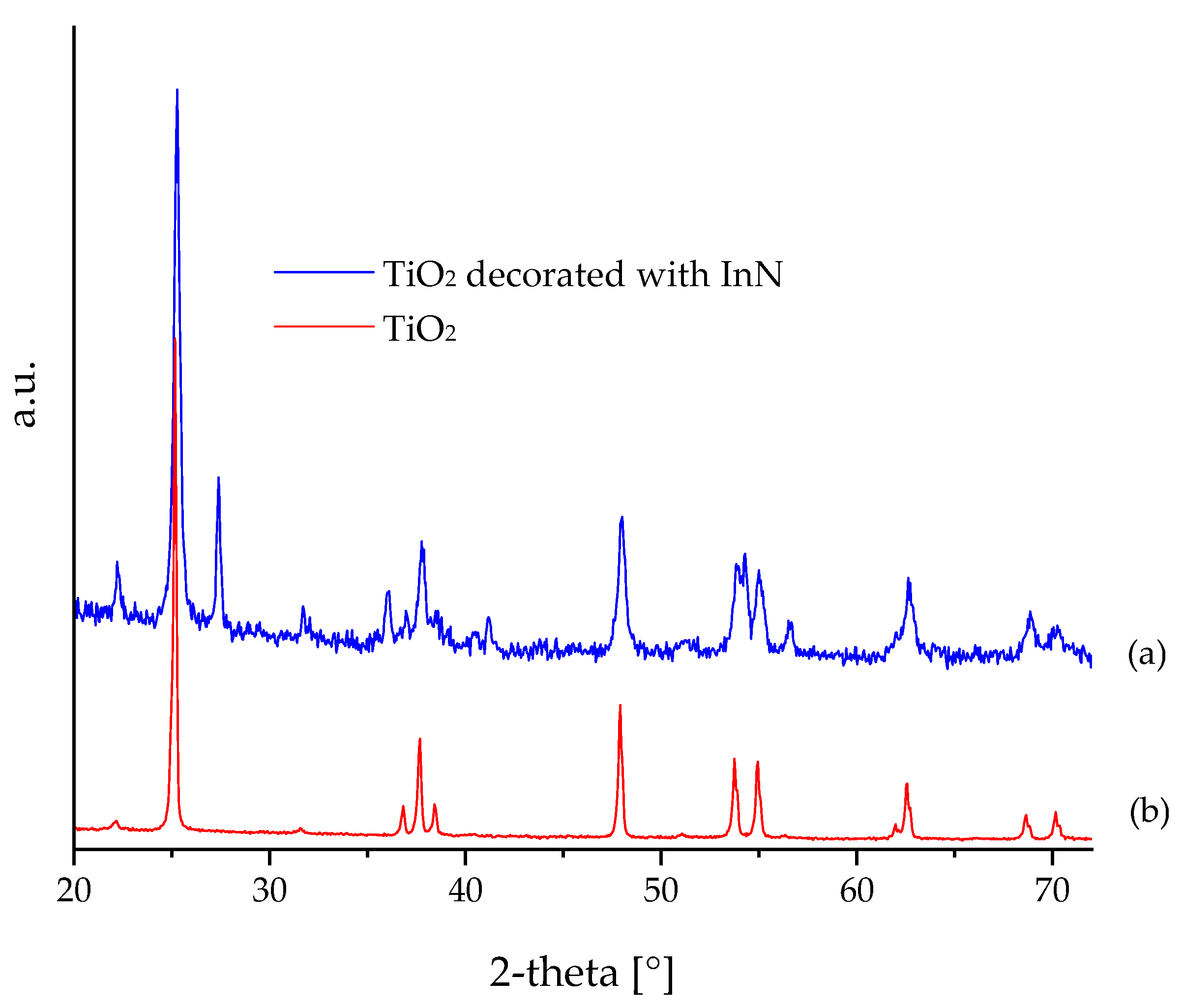
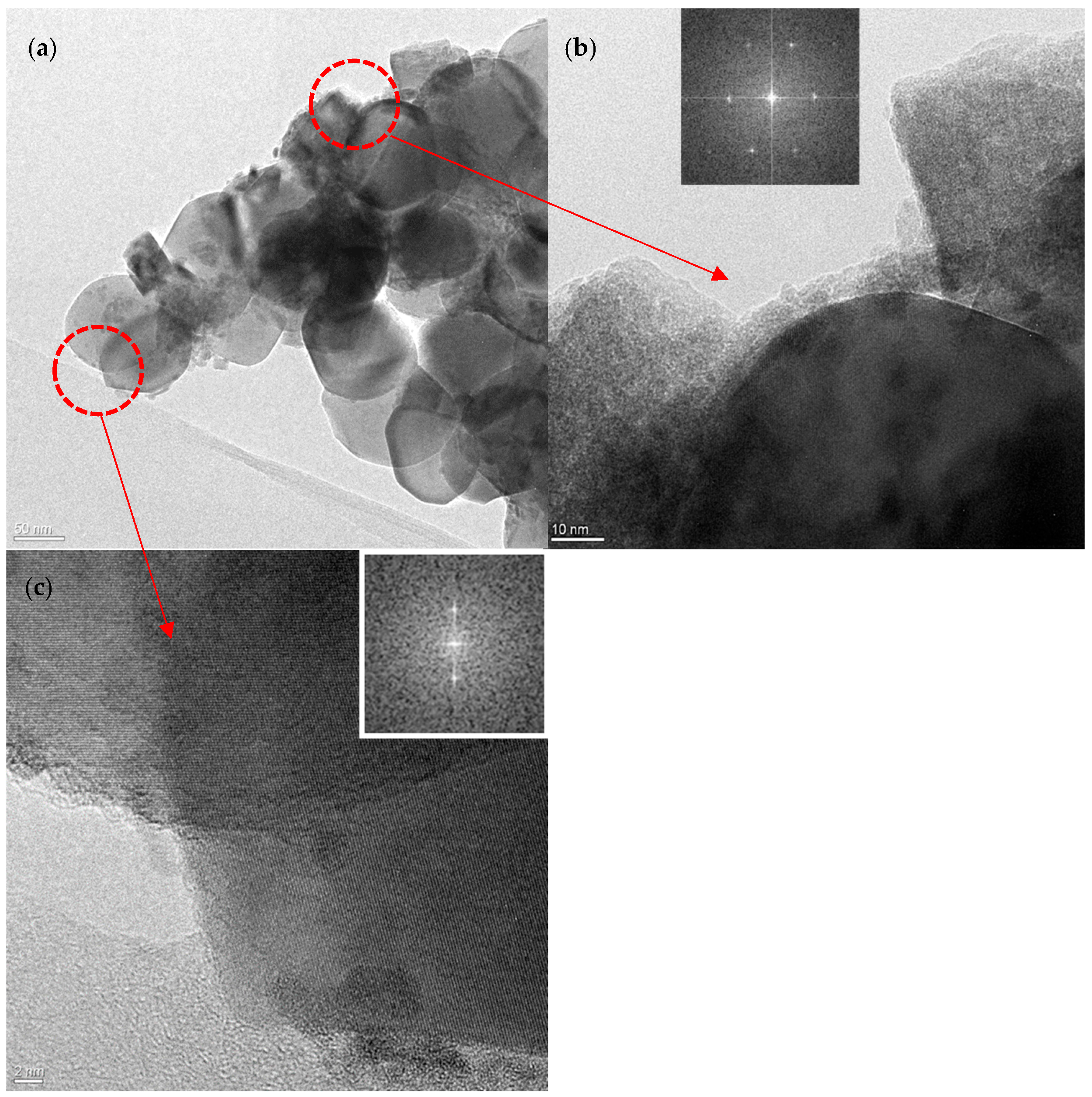
3.1. XRD and HR-TEM Analysis
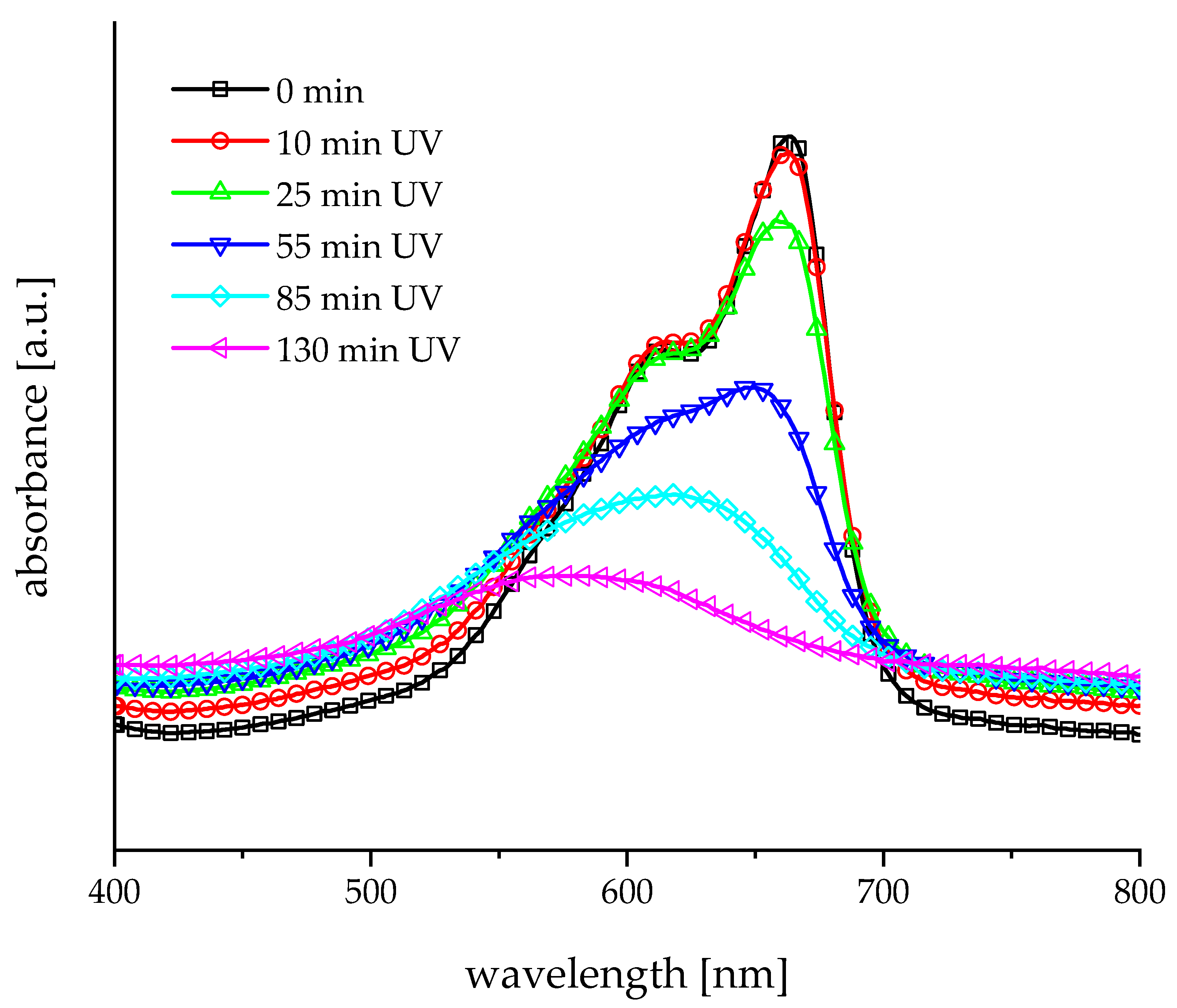
3.2. Photocatalytic Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, S.; Tian, H.; Luo, Y.; Yu, J.; Ren, C.; Sun, C.; Xu, Y.; Sun, M. First-principles calculations of aluminium nitride monolayer with chemical functionalization. Appl. Surf. Sci. 2019, 481, 1549–1553. [Google Scholar] [CrossRef]
- Bhuiyan, A.G.; Hashimoto, A.; Yamamoto, A. Indium nitride (InN): A review on growth, characterization, and properties. J. Appl. Phys. 2003, 94, 2779. [Google Scholar] [CrossRef]
- Polyakov, V.M.; Schwierz, F. Low-field electron mobility in wurtzite InN. Appl. Phys. Lett. 2006, 88, 032101. [Google Scholar] [CrossRef]
- Takai, S.; Lu, Y.; Oda, O.; Takeda, K.; Kondo, H.; Ishikawa, K.; Sekine, M.; Hori, M. Growth of InN films by radical-enhanced metal organic chemical vapor deposition at a low temperature of 200 °C. Jpn. J. Appl. Phys. 2017, 56, 06HE08. [Google Scholar] [CrossRef]
- Davydov, V.Y.; Klochikhin, A.A.; Seisyan, R.P.; Emtsev, V.V.; Ivanov, S.V.; Bechstedt, F.; Furthmuller, J.; Harima, H.; Mudrui, A.V.; Aderhold, J.; et al. Absorption and Emission of Hexagonal InN. Evidence of Narrow Fundamental Band Gap. Phys. Status Solidi (B) 2002, 229, R1–R3. [Google Scholar] [CrossRef]
- Wu, J.; Walukiewicz, W.; Yu, K.M.; Ager, J.W.; Haller, E.E. Unusual properties of the fundamental band gap of InN. Appl. Phys. Lett. 2002, 80, 3697. [Google Scholar] [CrossRef]
- Hori, M.; Kano, V.; Yamaguchi, T.; Saito, Y.; Araki, T.; Nanishi, Y.; Teraguchi, N.; Suzuki, A. Optical Proper-ties of InxGa1−xN with Entire Alloy Composition on InN Buffer Layer Grown by RF-MBE. Phys. Status Solidi (B) 2002, 234, 750–754. [Google Scholar] [CrossRef]
- Tansley, T.L.; Foley, C.P. Optical band gap of indium nitride. J. Appl. Phys. 1986, 59, 3241–3244. [Google Scholar] [CrossRef]
- Guo, Q.; Yoshida, A. Temperature Dependence of Band Gap Change in InN and AlN. Jpn. J. Appl. Phys. 1994, 33, 2453–2456. [Google Scholar] [CrossRef]
- Wu, J.; Walukiewicz, W.; Shan, W.; Yu, K.M.; Ager, J.W.; Li, S.X.; Haller, E.E.; Lu, H.; Schaff, W.J. Temperature dependence of the fundamental band gap of InN. J. Appl. Phys. 2003, 94, 4457. [Google Scholar] [CrossRef]
- Wu, J. When group-III nitrides go infrared: New properties and perspectives. J. Appl. Phys. 2009, 106, 011101. [Google Scholar] [CrossRef]
- Degheidy, A.; Elkenany, E. Electronic, optical, and mechanical properties of BN, AlN, and InN with zinc-blende structure under pressure. Chin. Phys. B 2017, 26, 086103. [Google Scholar] [CrossRef]
- Ahmad, U.; Aslam, S.; Mustafa, F.; Jamil, A.; Ashfaq Ahmad, M. Synthesis and characterization of InN quantum dots for optoelectronic applications. Opt. Int. J. Light Electron Opt. 2018, 173, 97–100. [Google Scholar] [CrossRef]
- Bai, Y.J.; Liu, Z.G.; Xu, X.G.; Cui, D.L.; Hao, X.P.; Feng, X.; Wang, Q.L. Preparation of InN nanocrystals by solvo-thermal method. J. Cryst. Growth 2002, 241, 189–192. [Google Scholar] [CrossRef]
- Yamamoto, A.; Tsujino, M.; Ohkubo, M.; Hashimoto, A. Metalorganic chemical vapor deposition growth of InN for InN/Si tandem solar cell. Sol. Energy Mater. Sol. Cells 1994, 35, 53. [Google Scholar] [CrossRef]
- Chen, Y. A Solution-Liquid-Solid Approach to Colloidal Indium Nitride Nanoparticles from Simple Alkylamide Precursors and Investigation of Its Mechanism. Master’s Thesis, Michigan State University, East Lansing, MI, USA, 2017. [Google Scholar] [CrossRef]
- Kasap, S.; Capper, P. (Eds.) Springer Handbook of Electronic and Photonic Materials; Springer Science &Business Media: New York, NY, USA, 2007; ISBN-13: 978-0-387-26059-4. [Google Scholar]
- Krames, M.R. Status and Future of High-Power Light-Emitting Diodes for Solid-State Lighting. J. Disp. Technol. 2007, 3, 160. [Google Scholar] [CrossRef]
- Nie, C.; Zhang, R.; Xie, Z.-L.; Xiu, X.-Q.; Liu, B.; Fu, D.-Y.; Liu, Q.-J.; Han, P.; Gu, S.-L.; Shi, Y.; et al. Synthesis of [100] Wurtzite InN Nanowires and [011] Zinc-Blende InN Nanorods. Chin. Phys. Lett. 2008, 25, 1780–1783. [Google Scholar] [CrossRef]
- Bhuiyan, A.G.; Yamamoto, A.; Hashimoto, A.; Ito, Y. High temperature growth of InN on GaP(1 1 1)B substrate using a new two-step growth method. J. Cryst. Growth 2002, 236, 59–65. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.; Xiao, H.; Zhang, X.; Hu, G.; Ran, J.; Wang, C.; Li, J.; Li, J.; Wang, Z. Growth temperature dependences of InN films grown by MOCVD. Appl. Surf. Sci. 2008, 255, 3149. [Google Scholar] [CrossRef]
- Chen, W.C.; Kuo, S.; Lai, F.; Lin, W.; Hsiao, C.; Tsai, D.P. Indium nitride epilayer prepared by UHV-plasma-assisted metalorganic molecule beam epitaxy. J. Vac. Sci. Technol. B 2011, 29, 051204. [Google Scholar] [CrossRef]
- Fu, S.P.; Lin, T.J.; Su, W.S.; Shieh, C.Y.; Chen, Y.F.; Chang, C.A.; Chen, N.C.; Chang, P.H. Influence of hydrogenation on surface morphologies, transport, and optical properties of InN epifilms. J. Appl. Phys. 2006, 99, 126102. [Google Scholar] [CrossRef]
- Fujiwara, K.; Ishii, A.; Ohta, J.; Fujioka, H.; Oshima, M. Experimental and theoretical investigation on the structural properties of InN grown on sapphire. Thin Solid Films 2004, 112, 464–465. [Google Scholar] [CrossRef]
- Nakamura, S.; Harada, Y.; Seno, M. Novel metalorganic chemical vapor deposition system for GaN growth. Appl. Phys. Lett. 1991, 58, 2021. [Google Scholar] [CrossRef]
- Kubota, K.; Kobayashi, Y.; Fujimoto, K. Preparation and properties of III–V nitride thin films. J. Appl. Phys. 1989, 66, 2984. [Google Scholar] [CrossRef]
- Ilhom, S.; Mohammad, A.; Shukla, D.; Grasso, J.; Willis, B.; Okyay, A.; Biyikli, N. Elucidating the role of nitrogen plasma composition in the low-temperature self-limiting growth of indium nitride thin films. RSC Adv. 2020, 10, 27357–27368. [Google Scholar] [CrossRef]
- Johnson, M.C.; Lee, C.J.; Bourret-Courchesne, E.D.; Konsek, S.L.; Aloni, S. Growth and morphology of 0.80eV photoemitting indium nitride nanowires. Appl. Phys. Lett. 2004, 85, 5670. [Google Scholar] [CrossRef]
- Hsieh, J.C.; Yun, D.S.; Hu, E.; Belcher, A.M. Ambient pressure, low-temperature synthesis and characterization of colloidal InN nanocrystals. J. Mater. Chem. 2010, 20, 1435–1437. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.; Chang, H.; Hsu, T.; Hsiao, C.; Kei, C.; Kuo, S.; Chyi, J. Optical properties of indium nitride nanorods prepared by chemical-beam epitaxy. Nanotechnology 2006, 17, 3930–3932. [Google Scholar] [CrossRef]
- Khashan, K.S.; Taha, J.M.; Abbas, S.F. Fabrication and Properties of InN NPs/Si as a photodetector. Energy Procedia 2017, 119, 656–661. [Google Scholar] [CrossRef]
- Chirico, P.; Hector, A.L. Solvothermal Synthesis of Gallium and Indium Nitrides Using Lithium Amide. Z. Naturforschung B 2010, 65, 1051–1057. [Google Scholar] [CrossRef]
- Li, B.; Xie, Y.; Huang, J.; Liu, Y.; Qian, Y. A novel method for the preparation of III-V semiconductors: Sonochemical synthesis of InP nanocrystalls. Ultrason. Sonochem. 2001, 8, 331–334. [Google Scholar] [CrossRef]
- Gedanken, A. Using sonochemistry for the fabrication of nanomaterials. Ultrason. Sonochem. 2004, 11, 47–55. [Google Scholar] [CrossRef]
- Gedanken, A. Using Sonochemical Methods for the Preparation of Mesoporous Materials and for the Deposition of Catalysts into the Mesopores. Chem. Eur. J. 2001, 7, 4546–4552. [Google Scholar] [CrossRef]
- Livage, J. Amorphous transition metal oxides. J. Phys. Colloq. 1981, 42, C4–C981. [Google Scholar] [CrossRef]
- Landau, M.V.; Vradman, L.; Herskowitz, M.; Koltypin, Y.; Gedanken, A. Ultrasonically Controlled Deposition–Precipitation. J. Catal. 2001, 201, 22–36. [Google Scholar] [CrossRef]
- Perkas, N.; Wang, Y.; Koltypin, Y.; Gedanken, A.; Chandrasekaran, S. Mesoporous iron–titania catalyst for cyclohexane oxidation. Chem. Commun. 2001, 11, 988–989. [Google Scholar] [CrossRef]
- Ramesh, S.; Koltypin, Y.; Prozorov, R.; Gedanken, A. Sonochemical Deposition and Characterization of Nanophasic Amorphous Nickel on Silica Microspheres. Chem. Mater. 1997, 9, 546. [Google Scholar] [CrossRef]
- Stucchi, M.; Bianchi, C.L.; Pirola, C.; Vitali, S.; Cerrato, G.; Morandi, S.; Argirusis, C.; Sourkouni, G.; Sakkas, P.M.; Capucci, V. Surface decoration of commercial micro-sized TiO2 by means of high energy ultrasound: A way to enhance its photocatalytic activity under visible light. Appl. Catal. B Environ. 2015, 178, 124–132. [Google Scholar] [CrossRef]
- Stucchi, M.; Bianchi, C.L.; Argirusis, C.; Pifferi, V.; Cerrato, G.; Boffito, D.C. Ultrasound assisted synthesis of Ag-decorated TiO2 active in visible light. Ultrason. Sonochem. 2018, 40, 282–288. [Google Scholar] [CrossRef]
- Xie, Y.; Ali, G.; Yoo, S.H.; Cho, S.O. Sonication-Assisted Synthesis of CdS Quantum-Dot-Sensitized TiO2 Nanotube Arrays with Enhanced Photoelectrochemical and Photocatalytic Activity. ACS Appl. Mater. Interfaces 2010, 2, 2910–2914. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical evidence for the mechanism of the primary stage of photosynthesis Bull. Chem. Soc. Jpn. 1971, 44, 1148. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37. [Google Scholar] [CrossRef] [PubMed]
- Matthews, R.W.; McEvoy, S.R. Photocatalytic degradation of phenol in the presence of near-UV illuminated titanium dioxide. J. Photochem. Photobiol. A Chem. 1992, 64, 231. [Google Scholar] [CrossRef]
- Muruganandham, M.; Swaminathan, M. Photocatalytic decolourisation and degradation of Reactive Orange 4 by TiO2-UV process. Dye. Pigment. 2006, 68, 133. [Google Scholar] [CrossRef]
- Alberci, R.M.; Jardim, W.F. Photocatalytic destruction of VOCs in the gas-phase using titanium dioxide. Appl. Catal. B Environ. 1997, 14, 55. [Google Scholar] [CrossRef]
- Zhu, X.; Xu, H.; Bi, C.; Song, H.; Zhou, G.; Zhong, K.; Yang, J.; Yi, J.; Xu, H.; Wang, X. Piezo-photocatalysis for efficient charge separation to promote CO2 photoreduction in nanoclusters. Ultrason. Sonochem. 2023, 101, 106653. [Google Scholar] [CrossRef] [PubMed]
- Arora, I.; Chawla, H.; Chandra, A.; Sagadevan, S.; Garg, S. Advances in the strategies for enhancing the photocatalytic activity of TiO2: Conversion from UV-light active to visible-light active photocatalyst. Inorg. Chem. Commun. 2022, 143, 109700. [Google Scholar] [CrossRef]
- Anandan, S.; Ikuma, Y.; Niwa, K. An Overview of Semi-Conductor Photocatalysis: Modification of TiO2 Nanomaterials. Solid State Phenom. 2010, 162, 239–260. [Google Scholar] [CrossRef]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef] [PubMed]
- Obata, H.; Koizumi, M. Photochemical Reactions between Methylene Blue and Tri-, Di- and Monomethylamine. I. Bull. Chem. Soc. Jpn. 1957, 30, 136–142. [Google Scholar] [CrossRef]
- Obata, H.; Kogasaka, K.; Koizumi, M. Photochemical Reactions between Methylene Blue and Tri-, Di- and Monomethylamine. III. The Behavior of Methylene Blue in the Presence of Oxygen. Bull. Chem. Soc. Jpn. 1959, 32, 125. [Google Scholar] [CrossRef]
- Usui, Y.; Obata, H.; Koizumi, M. Photoreduction of methylene blue by visible light in the aqueous solution containing certain kinds of inorganic salts. I. General features of the reaction. Bull. Chem. Soc. Jpn. 1961, 34, 1049–1056. [Google Scholar] [CrossRef]
- Tichapondwa, S.M.; Newman, J.P.; Kubheka, O. Effect of TiO2 phase on the photocatalytic degradation of methylene blue dye. Phys. Chem. Earth Parts A/B/C 2020, 118–119, 102900. [Google Scholar] [CrossRef]
- Mills, A.; Wang, J. Photobleaching of methylene blue sensitised by TiO2: An ambiguous system? J. Photochem. Photobiol. A Chem. 1999, 127, 123–134. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B Environ. 2001, 31, 145. [Google Scholar] [CrossRef]
- Gu, S.; Fu, B.; Dodbiba, G.; Fujita, T.; Fang, B. A sustainable approach to separate and recover indium and tin from spent indium–tin oxide targets. RSC Adv. 2017, 7, 52017. [Google Scholar] [CrossRef]
- Stucchi, M.; Bianchi, C.L.; Pirola, C.; Cerrato, G.; Morandi, S.; Argirusis, C.; Capucci, V. Copper NPs decorated titania: A novel synthesis by high energy US with a study of the photocatalytic activity under visible light. Ultrason. Sonochem. 2016, 31, 295–301. [Google Scholar] [CrossRef]
- Fujii, M.; Kawai, T.; Kawai, S. Photocatalytic activity and the energy levels of electrons in a semiconductor particle under irradiation. Chem. Phys. Lett. 1984, 106, 83–98. [Google Scholar] [CrossRef]
- Bedja, I.; Kamat, P.V. Capped Semiconductor Colloids. Synthesis and Photoelectrochemical Behavior of TiO2-Capped SnO2 Nanocrystallites. J. Phys. Chem. 1995, 99, 9182–9188. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paraskevopoulou, A.; Pandis, P.; Argirusis, C.; Sourkouni, G. Sonochemical Synthesis of Indium Nitride Nanoparticles and Photocatalytic Composites with Titania. Ceramics 2024, 7, 478-490. https://doi.org/10.3390/ceramics7020031
Paraskevopoulou A, Pandis P, Argirusis C, Sourkouni G. Sonochemical Synthesis of Indium Nitride Nanoparticles and Photocatalytic Composites with Titania. Ceramics. 2024; 7(2):478-490. https://doi.org/10.3390/ceramics7020031
Chicago/Turabian StyleParaskevopoulou, Aikaterina, Pavlos Pandis, Christos Argirusis, and Georgia Sourkouni. 2024. "Sonochemical Synthesis of Indium Nitride Nanoparticles and Photocatalytic Composites with Titania" Ceramics 7, no. 2: 478-490. https://doi.org/10.3390/ceramics7020031





