Fire and Herbivory Interactively Suppress the Survival and Growth of Trees in an African Semiarid Savanna
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
2.2.1. Exclosure Plots
2.2.2. Fire Treatments
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Fire Temperatures
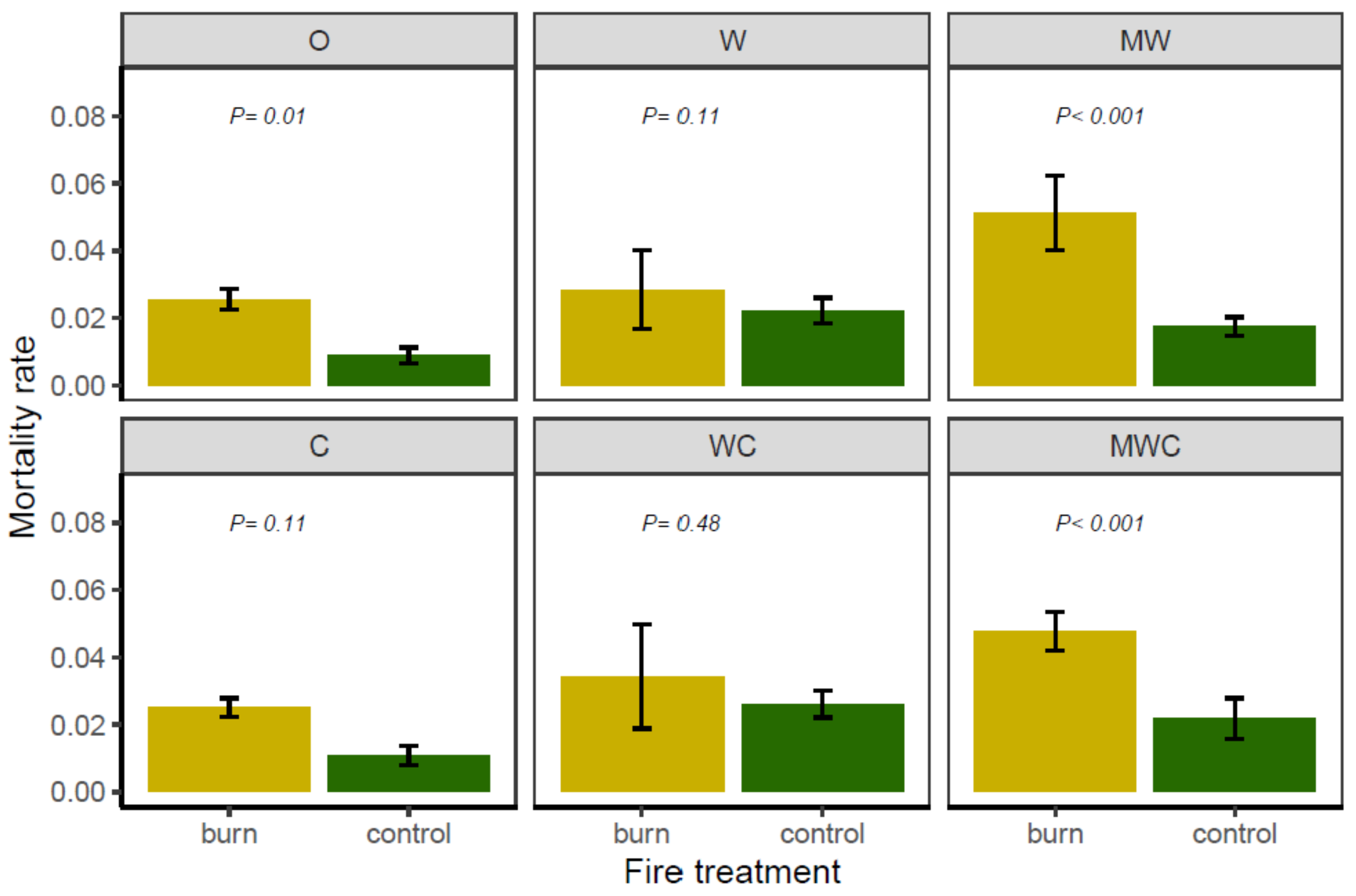
3.2. Effect of Herbivory Regime and Fire Treatment on Tree Mortality
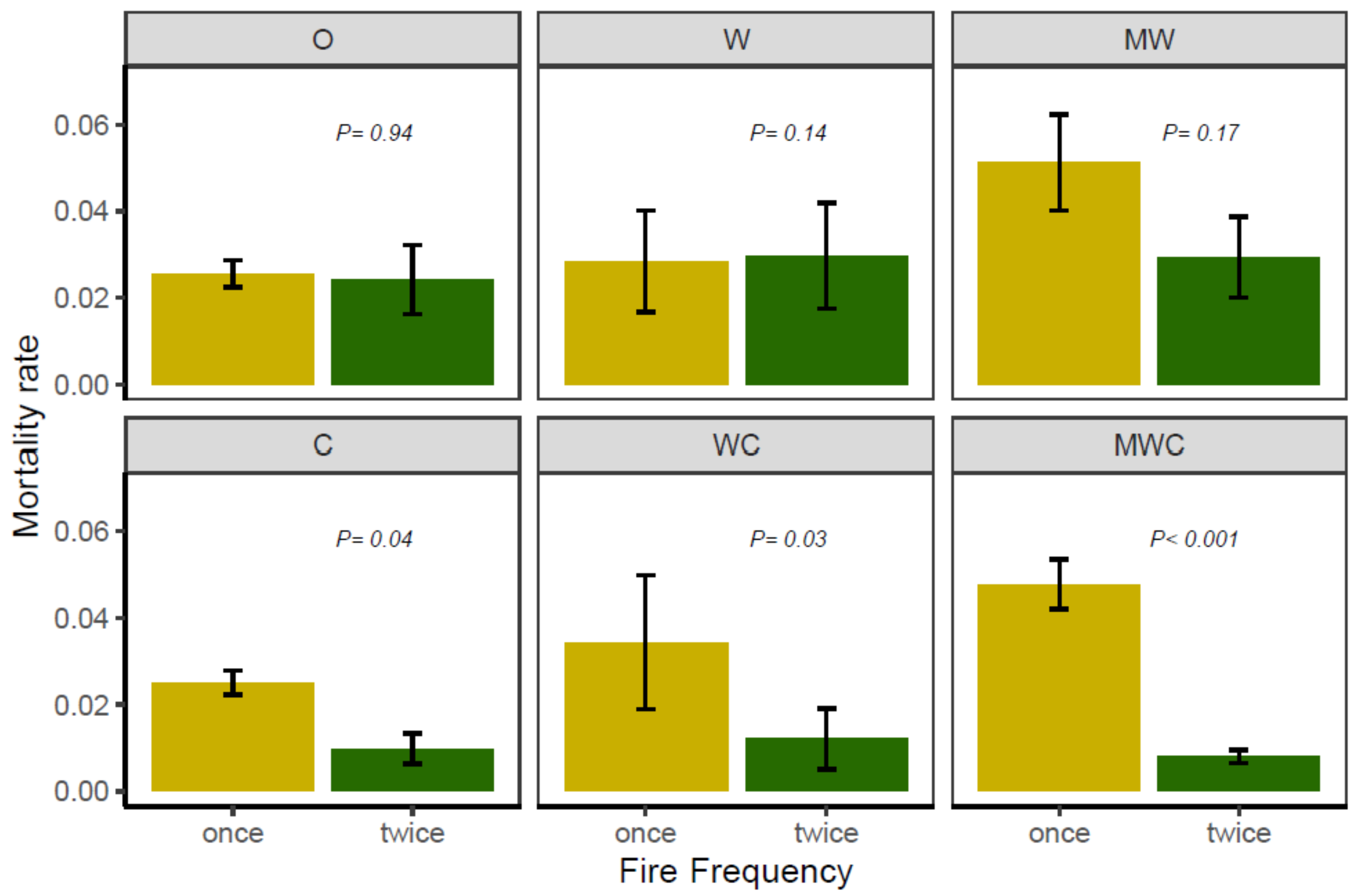
3.3. Effect of Herbivory Regime and Fire Frequency on Tree Mortality
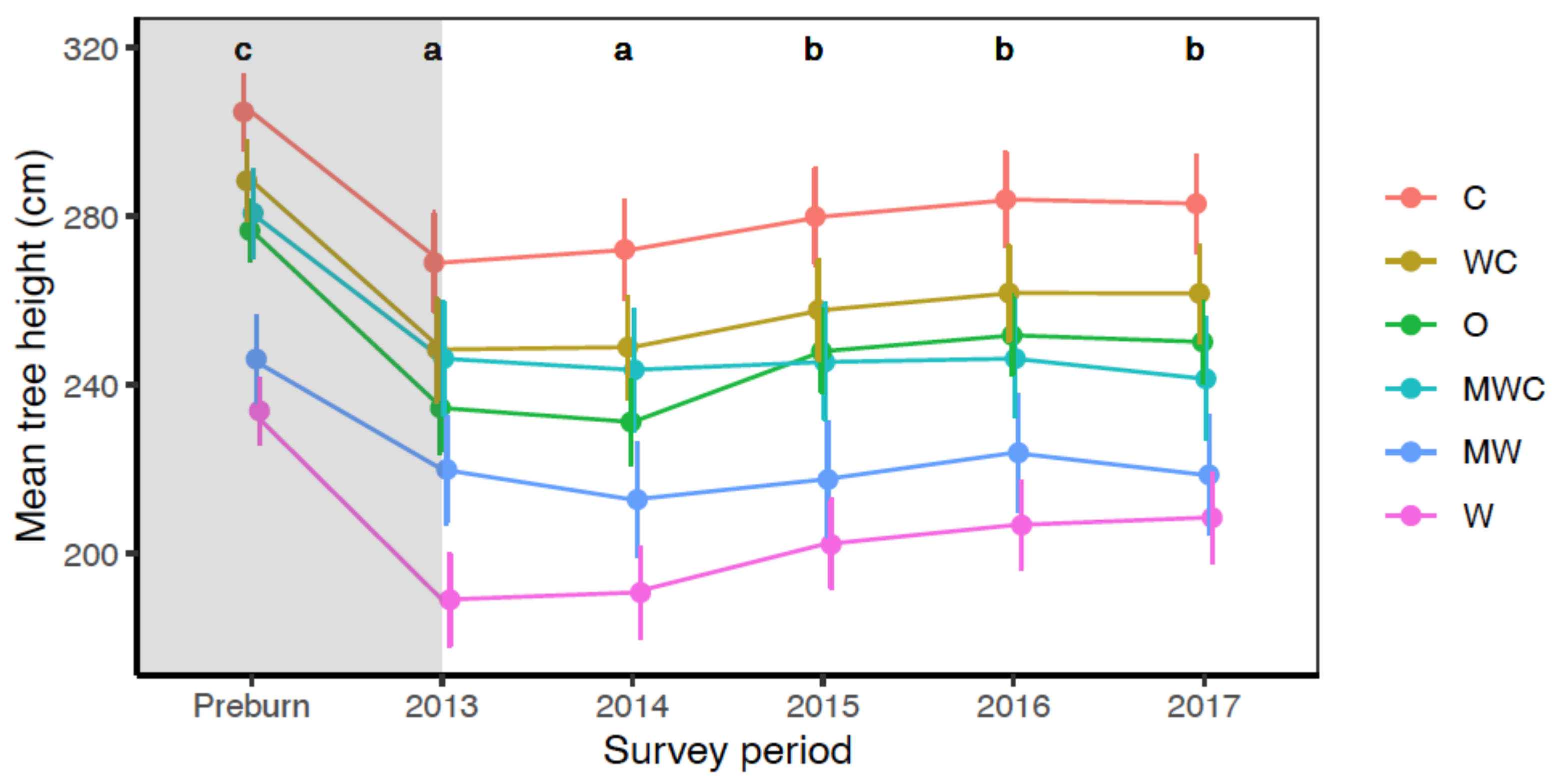
3.4. Effect of Fire on Tree Heights
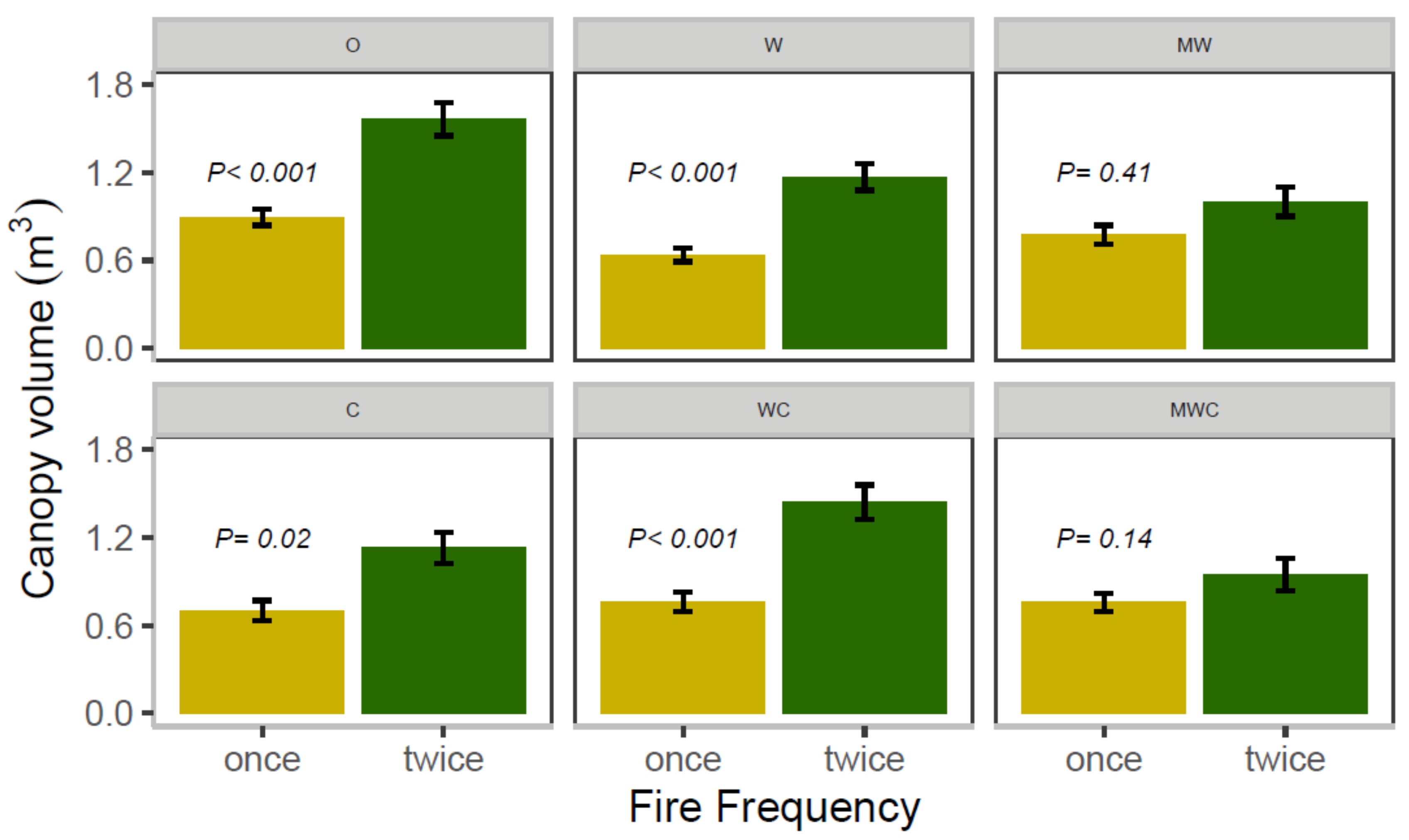
3.5. Sapling Canopy Volume
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sankaran, M.; Hanan, N.P.; Scholes, R.J.; Ratnam, J.; Augustine, D.J.; Cade, B.S.; Gignoux, J.; Higgins, S.I.; Le Roux, X.; Ludwig, F. Determinants of woody cover in African savannas. Nature 2005, 438, 846–849. [Google Scholar] [CrossRef]
- Archibald, S.; Hempson, G.P. Competing consumers: Contrasting the patterns and impacts of fire and mammalian herbivory in Africa. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bond, W.J.; Keeley, J.E. Fire as a global ‘herbivore’: The ecology and evolution of flammable ecosystems. Trends Ecol. Evol. 2005, 20, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Keeley, J.; Pausas, J.; Rundel, P.; Bond, W.; Bradstock, R. Fire as an evolutionary pressure shaping plant traits. Trends Plant Sci. 2011, 16, 406–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sankaran, M.; Ratnam, J.; Hanan, N. Woody cover in African savannas: The role of resources, fire and herbivory. Glob. Ecol. Biogeogr. 2008, 17, 236–245. [Google Scholar] [CrossRef]
- Higgins, S.I.; Bond, W.J.; Trollope, W.S.W. Fire, Resprouting and Variability: A Recipe for Grass-Tree Coexistence in Savanna. J. Ecol. 2000, 88, 213–229. [Google Scholar] [CrossRef]
- Sankaran, M.; Augustine, D.J.; Ratnam, J. Native ungulates of diverse body sizes collectively regulate long-term woody plant demography and structure of a semi-arid savanna. J. Ecol. 2013, 101, 1389–1399. [Google Scholar] [CrossRef]
- Staver, A.C.; Bond, W.J. Is there a ‘browse trap’? Dynamics of herbivore impacts on trees and grasses in an African savanna. J. Ecol. 2014, 102, 595–602. [Google Scholar] [CrossRef]
- Bond, W.J.; Woodward, F.I.; Midgley, G.F. The global distribution of ecosystems in a world without fire. New Phytol. 2005, 165, 525–538. [Google Scholar] [CrossRef]
- Van Langevelde, F.; Van De Vijver, C.A.D.M.; Kumar, L.; Van De Koppel, J.; De Ridder, N.; Van Andel, J.; Skidmore, A.K.; Hearne, J.W.; Stroosnijder, L.; Bond, W.J.; et al. Effects of fire and herbivory on the stability of savanna ecosystems. Ecology 2003, 84, 337–350. [Google Scholar] [CrossRef]
- Bond, W.J.; Archibald, S. Confronting complexity: Fire policy choices in South African savanna parks. Int. J. Wildland Fire 2003, 12, 381. [Google Scholar] [CrossRef]
- Reid, R. Savannas of Our Birth: People, Wildlife, and Change in East Africa; University of California Press: Berkeley, CA, USA, 2012; ISBN 978-0-520-95407-6. [Google Scholar]
- Reinhardt, E.D.; Dickinson, M.B. First-Order Fire Effects Models for Land Management: Overview and Issues. Fire Ecol. 2010, 6, 131–142. [Google Scholar] [CrossRef]
- Ryan, K.; Elliot, W.J. Chapter 9: Fire effects and soil erosion models. In Wildland Fire in Ecosystems: Effects of Fire on Soils and Water; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Washington, DC, USA, 2005. [Google Scholar]
- Sensenig, R.L.; Demment, M.W.; Laca, E.A. Allometric scaling predicts preferences for burned patches in a guild of East African grazers. Ecology 2010, 91, 2898–2907. [Google Scholar] [CrossRef] [PubMed]
- Kimuyu, D.M.; Sensenig, R.L.; Riginos, C.; Veblen, K.E.; Young, T.P. Native and domestic browsers and grazers reduce fuels, fire temperatures, and acacia ant mortality in an African savanna. Ecol. Appl. 2014, 24, 741–749. [Google Scholar] [CrossRef]
- LaMalfa, E.M.; Kimuyu, D.M.; Sensenig, R.L.; Young, T.P.; Riginos, C.; Veblen, K.E. Tree resprout dynamics following fire depend on herbivory by wild ungulate herbivores. J. Ecol. 2019, 107, 2493–2502. [Google Scholar] [CrossRef]
- Sankaran, M.; Ratnam, J.; Hanan, N.P. Tree-grass coexistence in savannas revisited-insights from an examination of assumptions and mechanisms invoked in existing models. Ecol. Lett. 2004, 7, 480–490. [Google Scholar] [CrossRef]
- Midgley, J.J.; Sawe, T.; Abanyam, P.; Hintsa, K.; Gacheru, P. Spinescent East African savannah acacias also have thick bark, suggesting they evolved under both an intense fire and herbivory regime. Afr. J. Ecol. 2016, 54, 118–120. [Google Scholar] [CrossRef]
- Young, T.; Isbell, L. Sex Differences in Giraffe Feeding Ecology: Energetic and Social Constraints. Ethology 1991, 87, 79–89. [Google Scholar] [CrossRef]
- Young, T.P.; Okello, B.D.; Kinyua, D.; Palmer, T.M. KLEE: A long-term multi-species herbivore exclusion experiment in Laikipia, Kenya. Afr. J. Range Forage Sci. 1997, 14, 94–102. [Google Scholar] [CrossRef]
- Du Toit, J.T.; Olff, H. Generalities in grazing and browsing ecology: Using across-guild comparisons to control contingencies. Oecologia 2014, 174, 1075–1083. [Google Scholar] [CrossRef]
- Moncrieff, G.R.; Chamaillé-Jammes, S.; Higgins, S.I.; O’Hara, R.B.; Bond, W.J. Tree allometries reflect a lifetime of herbivory in an African savanna. Ecology 2011, 92, 2310–2315. [Google Scholar] [CrossRef] [PubMed]
- Sensenig, R.L.; Kimuyu, D.K.; Ruiz Guajardo, J.C.; Veblen, K.E.; Riginos, C.; Young, T.P. Fire disturbance disrupts an acacia ant–plant mutualism in favor of a subordinate ant species. Ecology 2017, 98, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.M.; Kimuyu, D.; Veblen, K.E.; Sensenig, R.L.; LaMalfa, E.; Young, T.P. Synergistic effects of long-term herbivory and previous fire on fine-scale heterogeneity of prescribed grassland burns. Ecology 2021, 102, e03270. [Google Scholar] [CrossRef] [PubMed]
- Porensky, L.M.; Wittman, S.E.; Riginos, C.; Young, T.P. Herbivory and drought interact to enhance spatial patterning and diversity in a savanna understory. Oecologia 2013, 173, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Kimuyu, D.M.; Veblen, K.E.; Riginos, C.; Chira, R.M.; Githaiga, J.M.; Young, T.P. Influence of cattle on browsing and grazing wildlife varies with rainfall and presence of megaherbivores. Ecol. Appl. 2017, 27, 786–798. [Google Scholar] [CrossRef]
- Young, T.P.; Kimuyu, D.N.; LaMalfa, E.M.; Werner, C.M.; Jones, C.; Masudi, P.; Ang’ila, R.; Sensenig, R.L. Effects of large mammalian herbivory, previous fire, and year of burn on fire behavior in an African savanna. Ecosphere 2022, 13, e3980. [Google Scholar] [CrossRef]
- Condit, R.; Ashton, P.S.; Manokaran, N.; LaFrankie, J.V.; Hubbell, S.P.; Foster, R.B. Dynamics of the forest communities at Pasoh and Barro Colorado: Comparing two 50–ha plots. Phil. Trans. R. Soc. Lond. B 1999, 354, 1739–1748. [Google Scholar] [CrossRef] [Green Version]
- Smithson, M.; Verkuilen, J. A better lemon squeezer? Maximum-likelihood regression with beta-distributed dependent variables. Psychol. Methods 2006, 11, 54–71. [Google Scholar] [CrossRef] [Green Version]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Mächler, M.; Bolker, B.M. Modeling zero-inflated count data with glmmTMB. BioRxiv 2017, 132753. [Google Scholar] [CrossRef] [Green Version]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Soft. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Fox, J.S.; Weisberg, S. An R Companion to Applied Regression; The R Journal: Indianapolis, IN, USA, 2019. [Google Scholar]
- Russel, L.; Buerkner, P.; Herve, M.; Love, J.; Riebl, H.; Singmann, H. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. CRAN. 2020. Available online: https://cran.r-project.org/web/packages/emmeans/index.ht (accessed on 26 June 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.r-project.org/ (accessed on 17 August 2018).
- Pringle, R.M.; Kimuyu, D.M.; Sensenig, R.L.; Palmer, T.M.; Riginos, C.; Veblen, K.E.; Young, T.P. Synergistic effects of fire and elephants on arboreal animals in an African savanna. J. Anim. Ecol. 2015, 84, 1637–1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Archibald, S. African grazing lawns—how fire, rainfall, and grazer numbers interact to affect grass community states. J. Wildl. Manag. 2008, 72, 492–501. [Google Scholar] [CrossRef]
- Odadi, W.O.; Kimuyu, D.M.; Sensenig, R.L.; Veblen, K.E.; Riginos, C.; Young, T.P. Fire-induced negative nutritional outcomes for cattle when sharing habitat with native ungulates in an African savanna. J. Appl. Ecol. 2017, 54, 935–944. [Google Scholar] [CrossRef] [Green Version]
- Pellegrini, A.F.A.; Pringle, R.M.; Govender, N.; Hedin, L.O. Woody plant biomass and carbon exchange depend on elephant-fire interactions across a productivity gradient in African savanna. J. Ecol. 2017, 105, 111–121. [Google Scholar] [CrossRef]
- Staver, A.C.; Bond, W.J.; Stock, W.D.; van Rensburg, S.J.; Waldram, M.S. Browsing and Fire Interact to Suppress Tree Density in an African Savanna. Ecol. Appl. 2009, 19, 1909–1919. [Google Scholar] [CrossRef]
- Young, T.P.; Francombe, C. Growth and yield estimates in natural stands of leleshwa (Tarconanthus camphoratus). For. Ecol. Manag. 1991, 41, 309–321. [Google Scholar] [CrossRef]
- Grady, J.M.; Hoffmann, W.A. Caught in a fire trap: Recurring fire creates stable size equilibria in woody resprouters. Ecology 2012, 93, 2052–2060. [Google Scholar] [CrossRef] [Green Version]
- Schafer, J.L.; Just, M.G. Size Dependency of Post-Disturbance Recovery of Multi-Stemmed Resprouting Trees. PLoS ONE 2014, 9, e105600. [Google Scholar] [CrossRef]
- Schutz, A.E.N.; Bond, W.J.; Cramer, M.D. Juggling carbon: Allocation patterns of a dominant tree in a fire-prone savanna. Oecologia 2009, 160, 235–246. [Google Scholar] [CrossRef]
- Nolan, R.H.; Mitchell, P.J.; Bradstock, R.A.; Lane, P.N.J. Structural adjustments in resprouting trees drive differences in post-fire transpiration. Tree Physiol. 2014, 34, 123–136. [Google Scholar] [CrossRef]
- Shannon, G.; Thaker, M.; Vanak, A.T.; Page, B.R.; Grant, R.; Slotow, R. Relative Impacts of Elephant and Fire on Large Trees in a Savanna Ecosystem. Ecosystems 2011, 14, 1372–1381. [Google Scholar] [CrossRef]
- Vanak, A.T.; Shannon, G.; Thaker, M.; Page, B.; Grant, R.; Slotow, R. Biocomplexity in large tree mortality: Interactions between elephant, fire and landscape in an African savanna. Ecography 2012, 35, 315–321. [Google Scholar] [CrossRef]

| Herbivore Treatment | 2013 | 2018 | |
|---|---|---|---|
| First | First | Reburns | |
| C | 180 | 211 | 166 |
| O | 168 | 212 | 211 |
| W | 191 | 182 | 164 |
| MW | 178 | 157 | 170 |
| WC | 158 | 147 | 109 |
| MWC | 154 | 140 | 136 |
| Mean temperature | 172 ± 5.7 (SE) | 175 ± 13.0 (SE) | 159 ± 14.1 (SE) |
| Response Variable | Fixed Effects | Type II Wald χ2 | p Value |
|---|---|---|---|
| Mortality | Herbivore | 20.88 | 0.001 |
| Fire | 22.02 | <0.001 | |
| Herbivore × Fire | 33.48 | <0.001 | |
| Mortality | Frequency | 7.60 | 0.006 |
| Herbivore | 18.82 | 0.002 | |
| Frequency × Herbivore | 20.29 | 0.001 | |
| Tree height in 2017 | Herbivore | 12.96 | 0.024 |
| Survey period | 457.99 | <0.001 | |
| Canopy volume | Original height | 231.57 | <0.001 |
| Frequency | 56.36 | <0.001 | |
| Herbivore | 9.70 | 0.084 | |
| Frequency × Herbivore | 14.15 | 0.015 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngugi, M.W.; Kimuyu, D.M.; Sensenig, R.L.; Odadi, W.O.; Kiboi, S.K.; Omari, J.K.; Young, T.P. Fire and Herbivory Interactively Suppress the Survival and Growth of Trees in an African Semiarid Savanna. Fire 2022, 5, 169. https://doi.org/10.3390/fire5050169
Ngugi MW, Kimuyu DM, Sensenig RL, Odadi WO, Kiboi SK, Omari JK, Young TP. Fire and Herbivory Interactively Suppress the Survival and Growth of Trees in an African Semiarid Savanna. Fire. 2022; 5(5):169. https://doi.org/10.3390/fire5050169
Chicago/Turabian StyleNgugi, Mary W., Duncan M. Kimuyu, Ryan L. Sensenig, Wilfred O. Odadi, Samuel K. Kiboi, Joyce K. Omari, and Truman P. Young. 2022. "Fire and Herbivory Interactively Suppress the Survival and Growth of Trees in an African Semiarid Savanna" Fire 5, no. 5: 169. https://doi.org/10.3390/fire5050169





