A Study of the Degradation Mechanism of Ladder-like Polyhedral Oligomeric Silsesquioxane via Fourier Transform Infrared Spectroscopy
Abstract
:1. Introduction
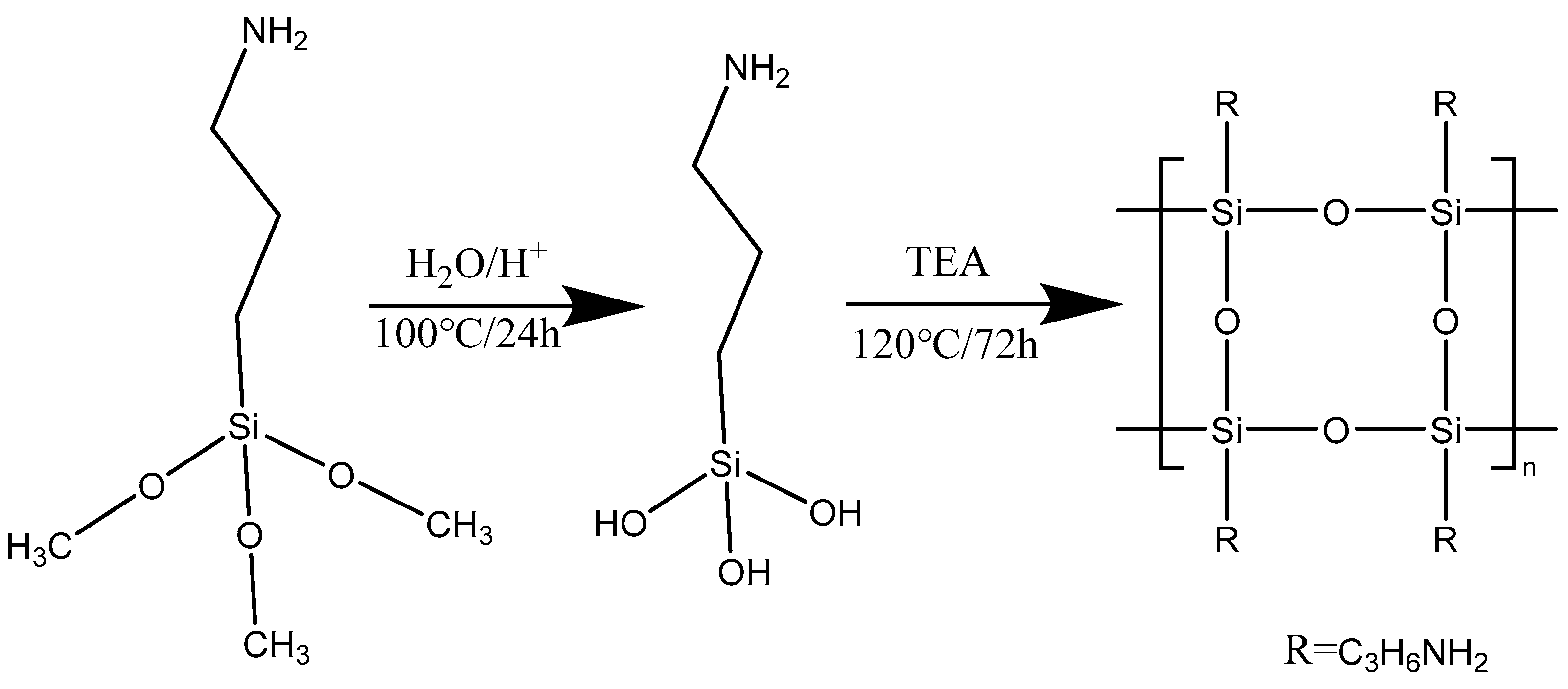
2. Materials and Methods
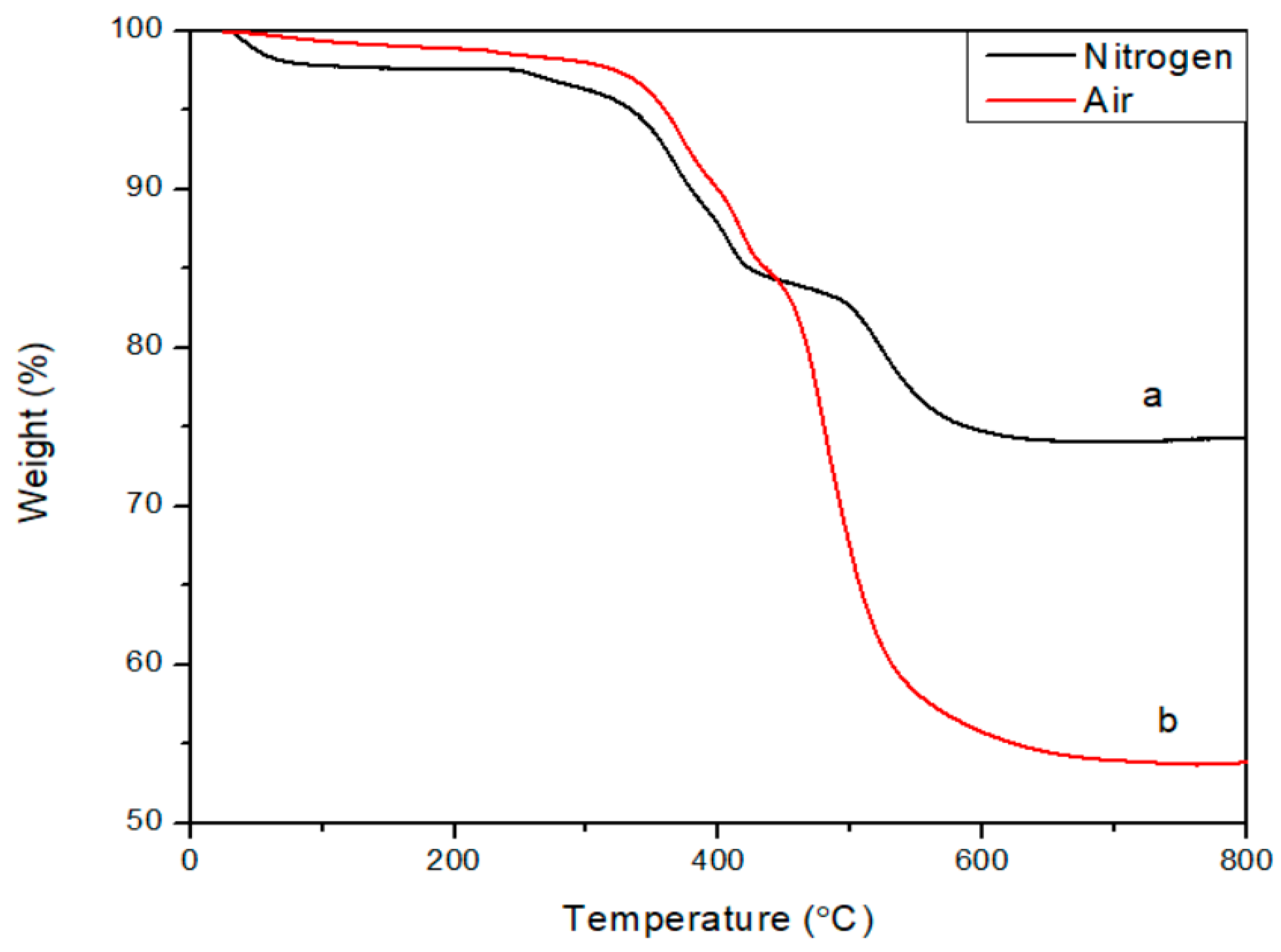
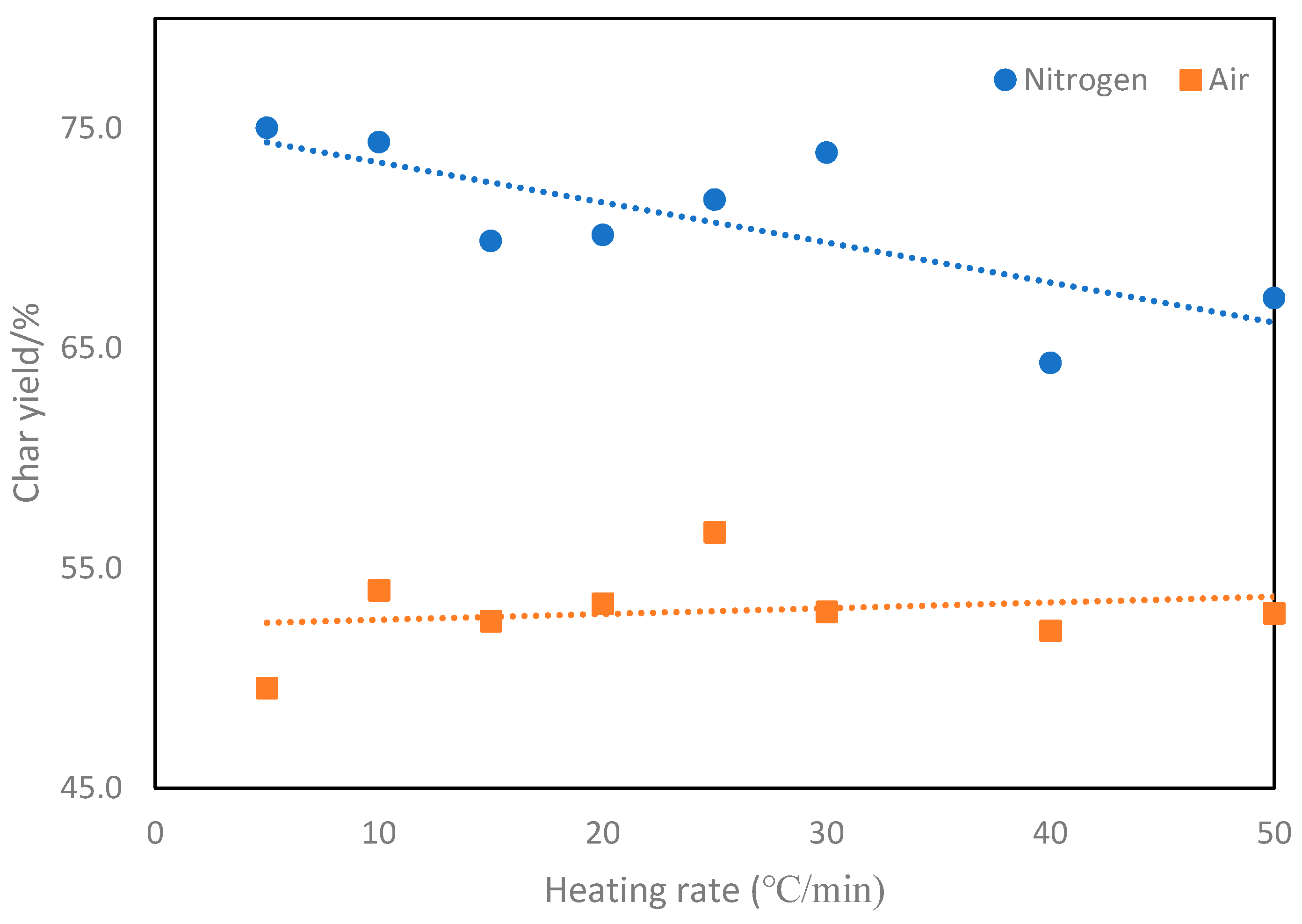
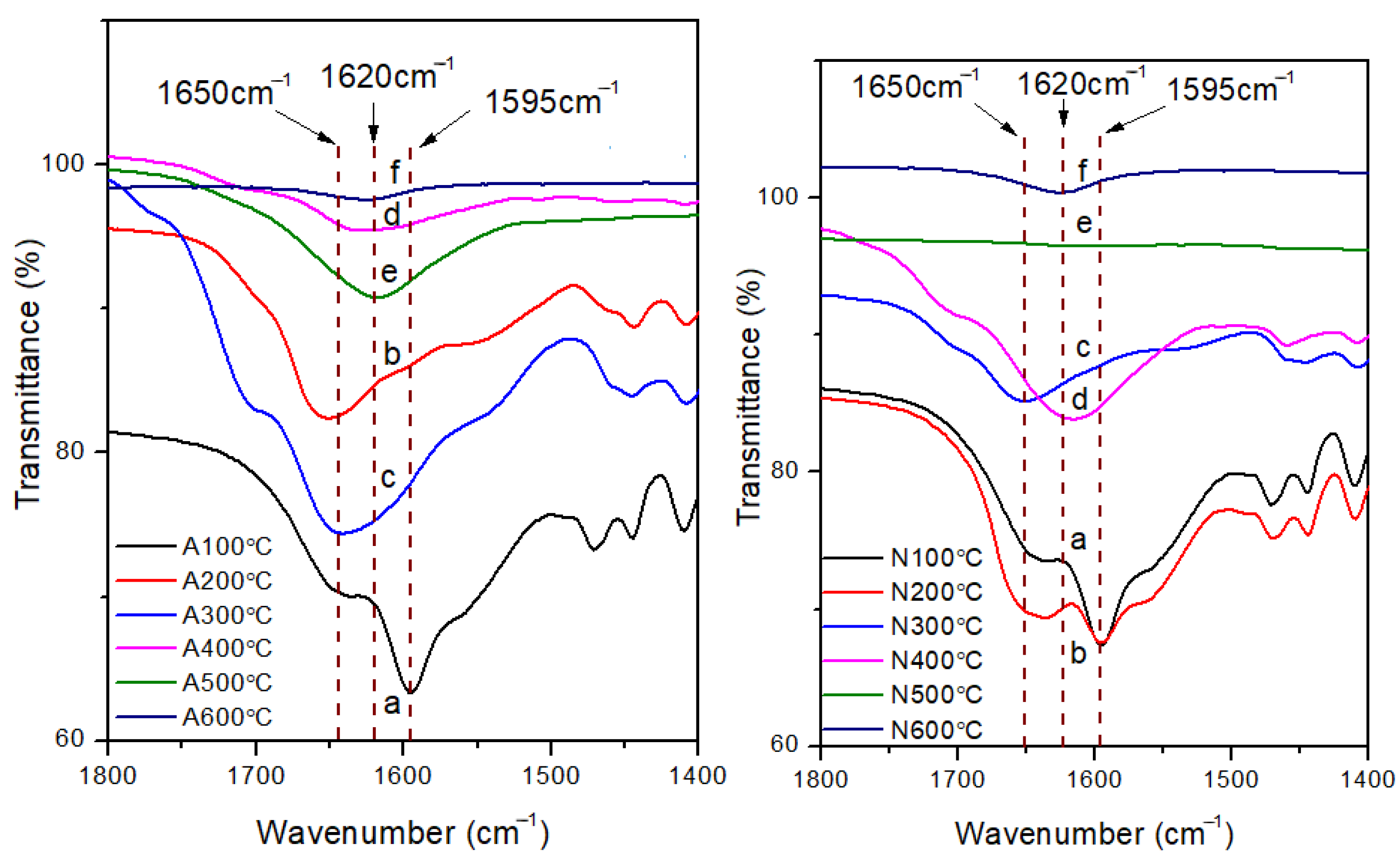
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kirby, R.; Mosurkal, R.; Li, L.; Kumar, J.; Soares, J.W. Polysiloxane-Based Organoclay Nanocomposites as Flame Retardants. Polym. Plast. Technol. Eng. 2013, 52, 1527–1534. [Google Scholar] [CrossRef]
- Lu, Z.; Feng, W.; Kang, X.; Wang, J.; Xu, H.; Wang, Y.; Liu, B.; Fang, X.; Ding, T. Synthesis of Siloxane-containing Benzoxazine and Its Synergistic Effect on Flame Retardancy of Polyoxymethylene. Polym. Adv. Technol. 2019, 30, 2686–2694. [Google Scholar] [CrossRef]
- Mosurkal, R.; Tucci, V.; Samuelson, L.A.; Smith, K.D.; Westmoreland, P.R.; Parmar, V.S.; Kumar, J.; Watterson, A.C. Novel Organo-Siloxane Copolymers for Flame Retardant Applications. In Advances in Silicones and Silicone-Modified Materials; American Chemical Society: Washington, DC, USA, 2010; pp. 157–165. [Google Scholar]
- Long, J.; Shi, X.; Liu, B.; Lu, P.; Chen, L.; Wang, Y. Semi-Aromatic Polyamides Containing Siloxane Unit toward High Performance. Acta Polym. Sin. 2020, 51, 681–686. [Google Scholar]
- Song, R.; Chang, L.; Li, B. Flame Retardancy and Thermal Properties of Carboxyl-Containing Polysiloxane Derivatives in Polycarbonate. J. Appl. Polym. Sci. 2014, 131, 39814. [Google Scholar] [CrossRef]
- Xiao, S.; Akinyi, C.; Longun, J.; Iroh, J.O. Polyimide Copolymers and Nanocomposites: A Review of the Synergistic Effects of the Constituents on the Fire-Retardancy Behavior. Energies 2022, 15, 4014. [Google Scholar] [CrossRef]
- Zielecka, M.; Rabajczyk, A.; Pastuszka, Ł.; Jurecki, L. Flame Resistant Silicone-Containing Coating Materials. Coatings 2020, 10, 479. [Google Scholar] [CrossRef]
- Camino, B.; Camino, G. The Chemical Kinetics of the Polymer Combustion Allows for Inherent Fire Retardant Synergism. Polym. Degrad. Stab. 2019, 160, 142–147. [Google Scholar] [CrossRef]
- Hamdani, S.; Longuet, C.; Perrin, D.; Lopez-cuesta, J.M.; Ganachaud, F. Flame Retardancy of Silicone-Based Materials. Polym. Degrad. Stab. 2009, 94, 465–495. [Google Scholar] [CrossRef]
- Deshpande, G.; Rezac, M.E.; Irisawa, T. The Effect of Phenyl Content on the Degradation of Poly(Dimethyl Diphenyl) Siloxane Copolymers. Polym. Degrad. Stab. 2001, 74, 363–370. [Google Scholar] [CrossRef]
- Huang, J.; He, C.; Xiao, Y.; Mya, K.Y.; Dai, J.; Siow, Y.P. Polyimide/POSS Nanocomposites: Interfacial Interaction, Thermal Properties and Mechanical Properties. Polymer 2003, 44, 4491–4499. [Google Scholar] [CrossRef]
- Yan, L.; Fu, L.; Chen, Y.; Tian, H.; Xiang, A.; Rajulu, A.V. Improved Thermal Stability and Flame Resistance of Flexible Polyimide Foams by Vermiculite Reinforcement. J. Appl. Polym. Sci. 2017, 134, 44828. [Google Scholar] [CrossRef]
- Faghihi, K.; Hajibeygi, M.; Shabanian, M. Polyimide–Silver Nanocomposite Containing Phosphine Oxide Moieties in the Main Chain: Synthesis and Properties. Chin. Chem. Lett. 2010, 21, 1387–1390. [Google Scholar] [CrossRef]
- Seckin, T.; Köytepe, S. Synthesis and Characterization of Borax-Polyimide for Flame Retardant Applications. Macromol. Symp. 2010, 296, 575–582. [Google Scholar] [CrossRef]
- Fina, A.; Abbenhuis, H.C.L.; Tabuani, D.; Camino, G. Metal Functionalized POSS as Fire Retardants in Polypropylene. Polym. Degrad. Stab. 2006, 91, 2275–2281. [Google Scholar] [CrossRef]
- Scott, D.W.; Scott, B.D.W. Thermal Rearrangement of Branched-Chain Methylpolysiloxanes1 Branched-Chain Methylpolysiloxanes. J. Am. Chem. Soc. 1946, 68, 356–358. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Kuo, S.W. Functional Polyimide/Polyhedral Oligomeric Silsesquioxane Nanocomposites. Polymers 2019, 11, 26. [Google Scholar] [CrossRef]
- Turgut, G.; Dogan, M.; Tayfun, U.; Ozkoc, G. The Effects of POSS Particles on the Flame Retardancy of Intumescent Polypropylene Composites and the Structure-Property Relationship. Polym. Degrad. Stab. 2018, 149, 96–111. [Google Scholar] [CrossRef]
- Devaraju, S.; Vengatesan, M.R.; Selvi, M.; Kumar, A.A.; Alagar, M. Synthesis and Characterization of Bisphenol-A Ether Diamine-Based Polyimide POSS Nanocomposites for Low K Dielectric and Flame-Retardant Applications. High. Perform. Polym. 2012, 24, 85–96. [Google Scholar] [CrossRef]
- Jothibasu, S.; Premkumar, S.; Alagar, M.; Hamerton, I. Synthesis and Characterization of a POSS-Maleimide Precursor for Hybrid Nanocomposites. High. Perform. Polym. 2008, 20, 67–85. [Google Scholar] [CrossRef]
- Leu, C.M.; Chang, Y.T.; Wei, K.H. Synthesis and Dielectric Properties of Polyimide-Tethered Polyhedral Oligomeric Silsesquioxane (POSS) Nanocomposites via Poss-Diamine. Macromolecules 2003, 36, 9122–9127. [Google Scholar] [CrossRef]
- Kuo, S.W.; Chang, F.C. POSS Related Polymer Nanocomposites. Prog. Polym. Sci. 2011, 36, 1649–1696. [Google Scholar] [CrossRef]
- Wu, H.; Zeng, B.; Chen, J.; Wu, T.; Li, Y.; Liu, Y.; Dai, L. An Intramolecular Hybrid of Metal Polyhedral Oligomeric Silsesquioxanes with Special Titanium-Embedded Cage Structure and Flame Retardant Functionality. Chem. Eng. J. 2019, 374, 1304–1316. [Google Scholar] [CrossRef]
- Zhai, C.; Xin, F.; Cai, L.; Chen, Y.; Qian, L. Flame Retardancy and Pyrolysis Behavior of an Epoxy Resin Composite Flame-retarded by Diphenylphosphinyl-POSS. Polym. Eng. Sci. 2020, 60, 3024–3035. [Google Scholar] [CrossRef]
- Safarikova, B.; Kalendova, A.; Habrova, V.; Zatloukalova, S.; Machovsky, M. Synergistic Effect between Polyhedral Oligomeric Silsesquioxane and Flame Retardants. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2014; pp. 106–109. [Google Scholar] [CrossRef]
- Bourbigot, S.; Duquesne, S.; Fontaine, G.; Bellayer, S.; Turf, T.; Samyn, F. Characterization and Reaction to Fire of Polymer Nanocomposites with and without Conventional Flame Retardants. Mol. Cryst. Liq. Cryst. 2008, 486, 325–1367. [Google Scholar] [CrossRef]
- Xue, M.; Zhang, X.; Wu, Z.; Wang, H.; Ding, X.; Tian, X. Preparation and Flame Retardancy of Polyurethane/POSS Nanocomposites. Chin. J. Chem. Phys. 2013, 26, 445–450. [Google Scholar] [CrossRef]
- Handke, M.; Handke, B.; Kowalewska, A.; Jastrzębski, W. New Polysilsesquioxane Materials of Ladder-like Structure. J. Mol. Struct. 2009, 924–926, 254–263. [Google Scholar] [CrossRef]
- Feng, Y.; Qi, S.; Wu, Z.; Wang, X.; Yang, X.; Wu, D. Preparation and Characterization of Polyimide/Ladder like Polysiloxane Hybrid Films. Mater. Lett. 2010, 64, 2710–2713. [Google Scholar] [CrossRef]
- Shi, H.; Yang, J.; You, M.; Li, Z.; He, C. Polyhedral Oligomeric Silsesquioxanes (POSS)-Based Hybrid Soft Gels: Molecular Design, Material Advantages, and Emerging Applications. ACS Mater. Lett. 2020, 2, 296–316. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Kuo, S.-W. Progress in the Self-Assembly of Organic/Inorganic Polyhedral Oligomeric Silsesquioxane (POSS) Hybrids. Soft Matter 2022, 18, 5535–5561. [Google Scholar] [CrossRef]
- Feng, L.; Iroh, J.O. Synthesis, Characterization and Dynamic Mechanical Properties of Polyimide-Polyurea-Polysilsesquioxane Block Terpolymers. J. Chem. Its Appl. 2022, 1, 1–9. [Google Scholar] [CrossRef]
- Wen-Chang Liaw, K.-P.C. Preparation and Properties of Poly(Imide Siloxane) Segmented Copolymer/Silica Hybrid Nanocomposites. J. Appl. Polym. Sci. 2007, 105, 809–820. [Google Scholar] [CrossRef]
- Xiao, S.; Iroh, J.O. Novel Polyimide-Block-Poly(Dimethyl Siloxane) Copolymers: Effect of Time on the Synthesis and Thermal Properties. High. Perform. Polym. 2021, 34, 095400832110404. [Google Scholar] [CrossRef]
- Choi, S.-S.; Lee, A.S.; Hwang, S.S.; Baek, K.-Y. Structural Control of Fully Condensed Polysilsesquioxanes: Ladderlike vs. Cage Structured Polyphenylsilsesquioxanes. Macromolecules 2015, 48, 6063–6070. [Google Scholar] [CrossRef]


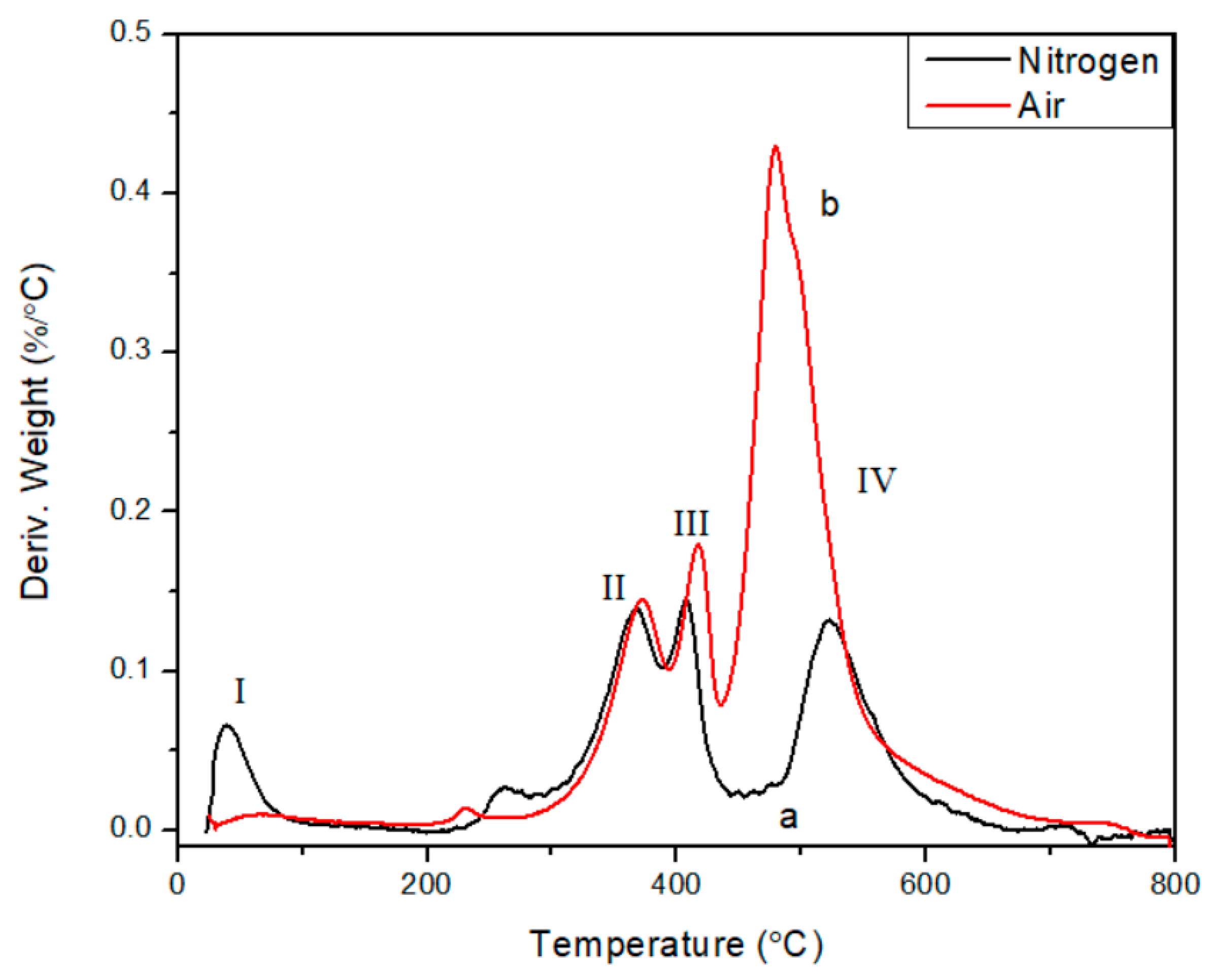
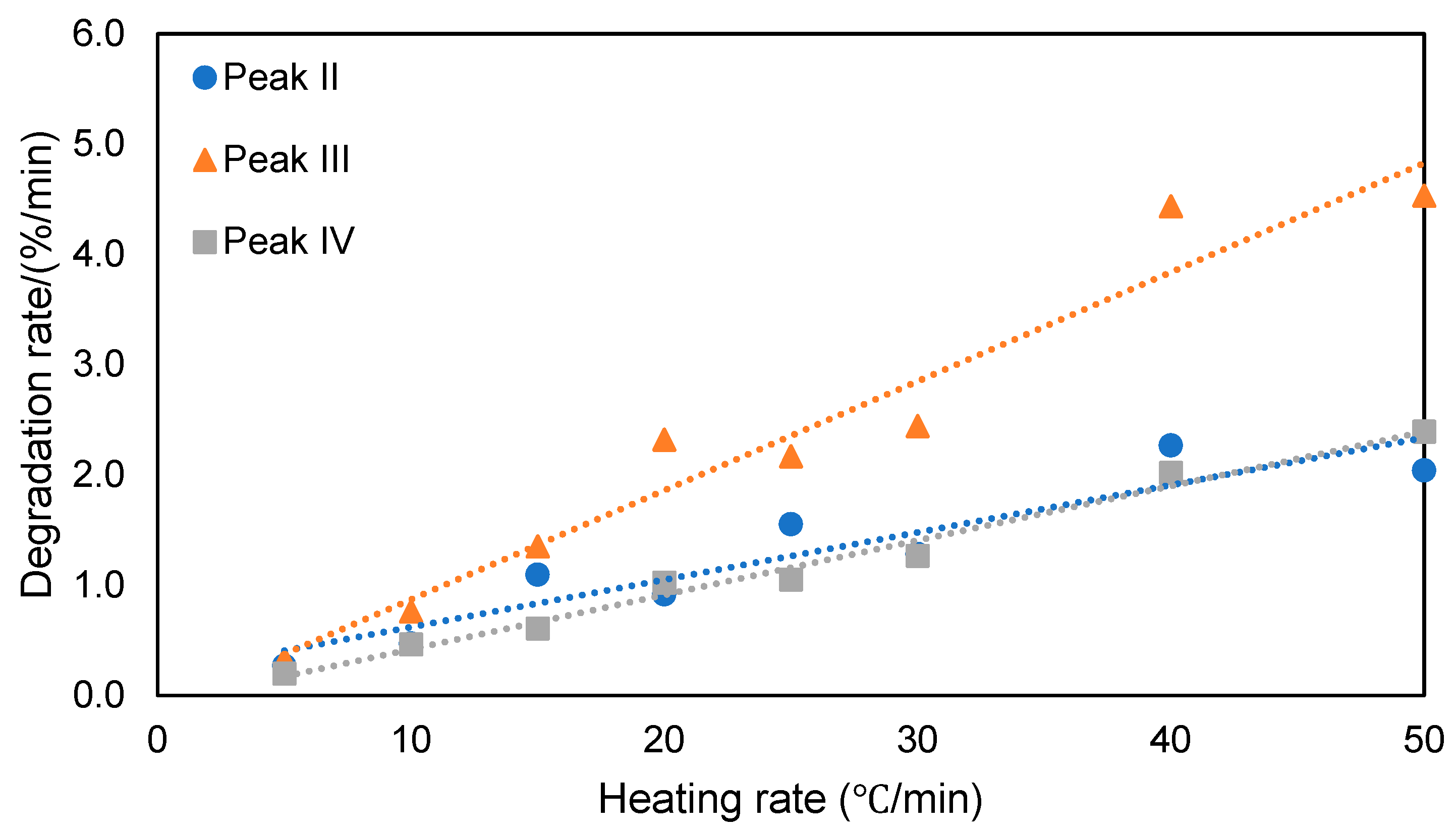

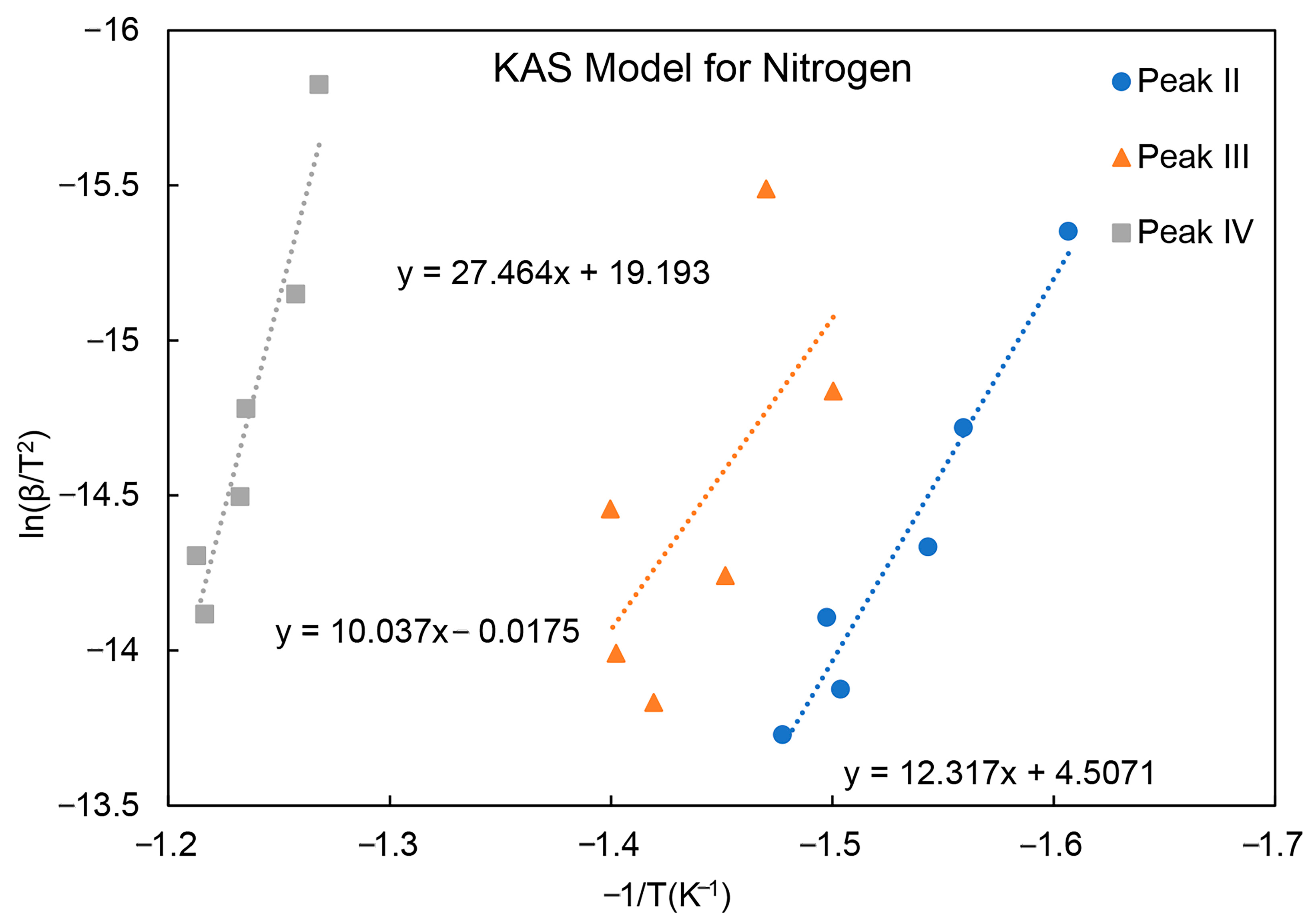
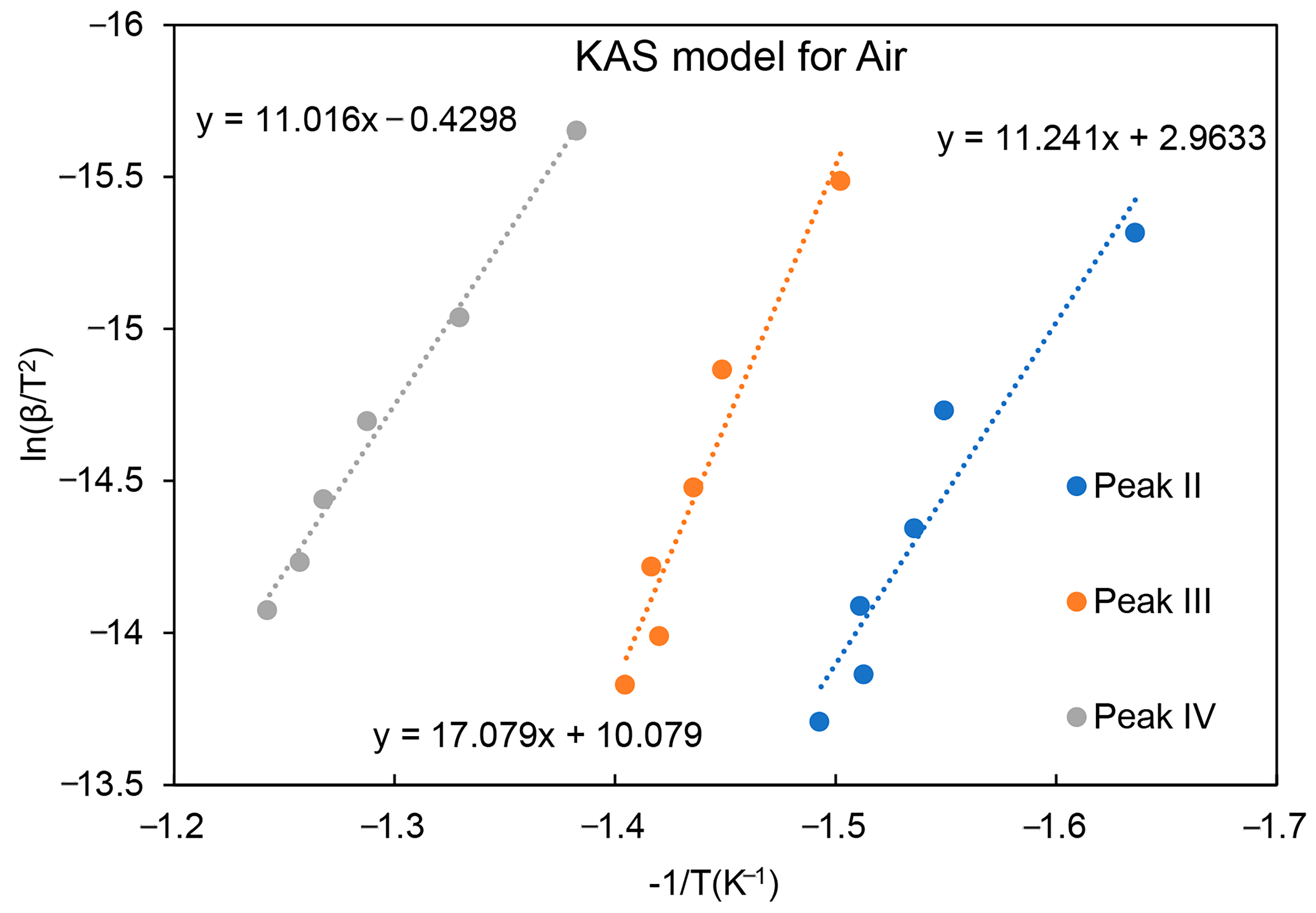
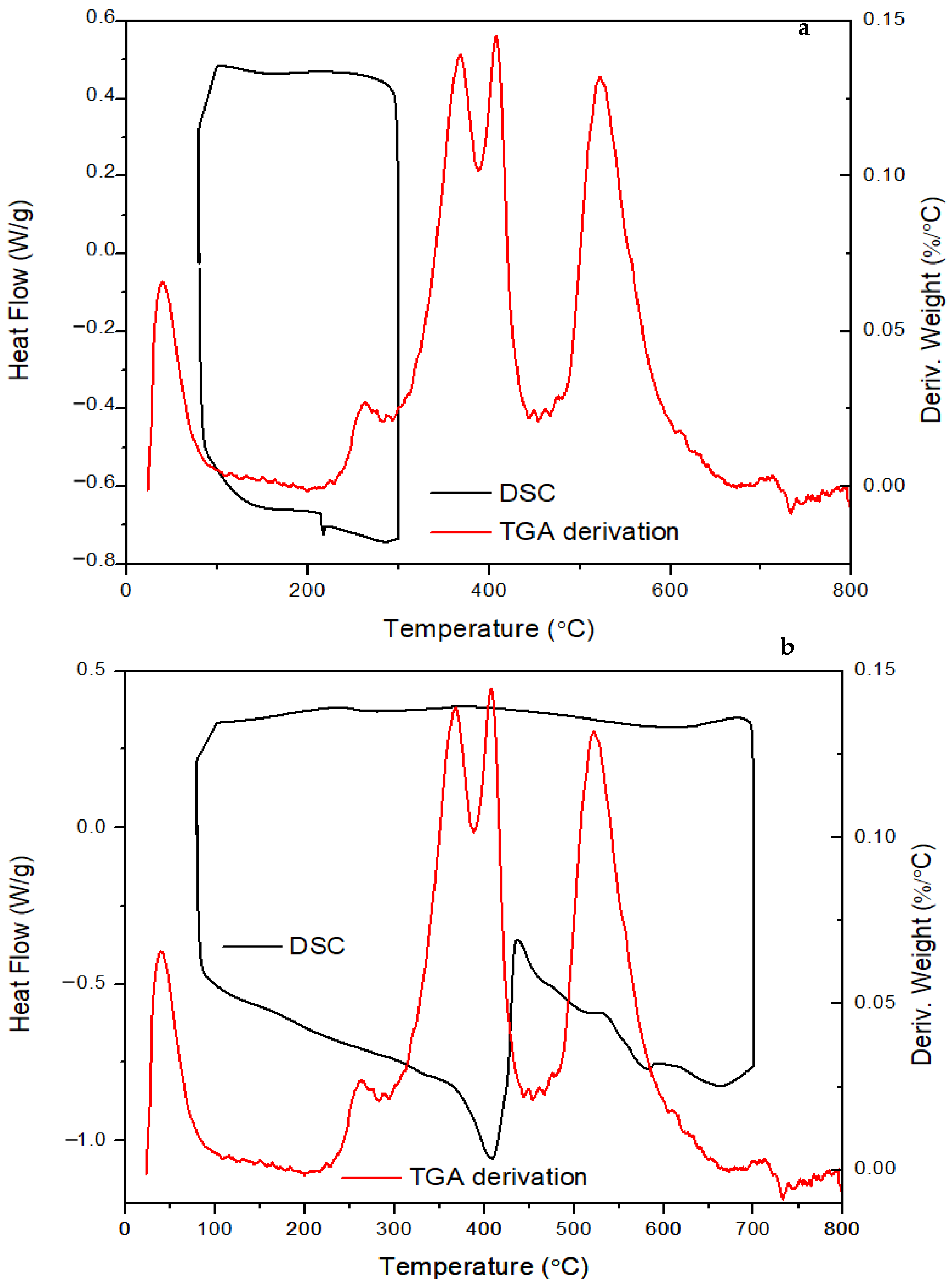

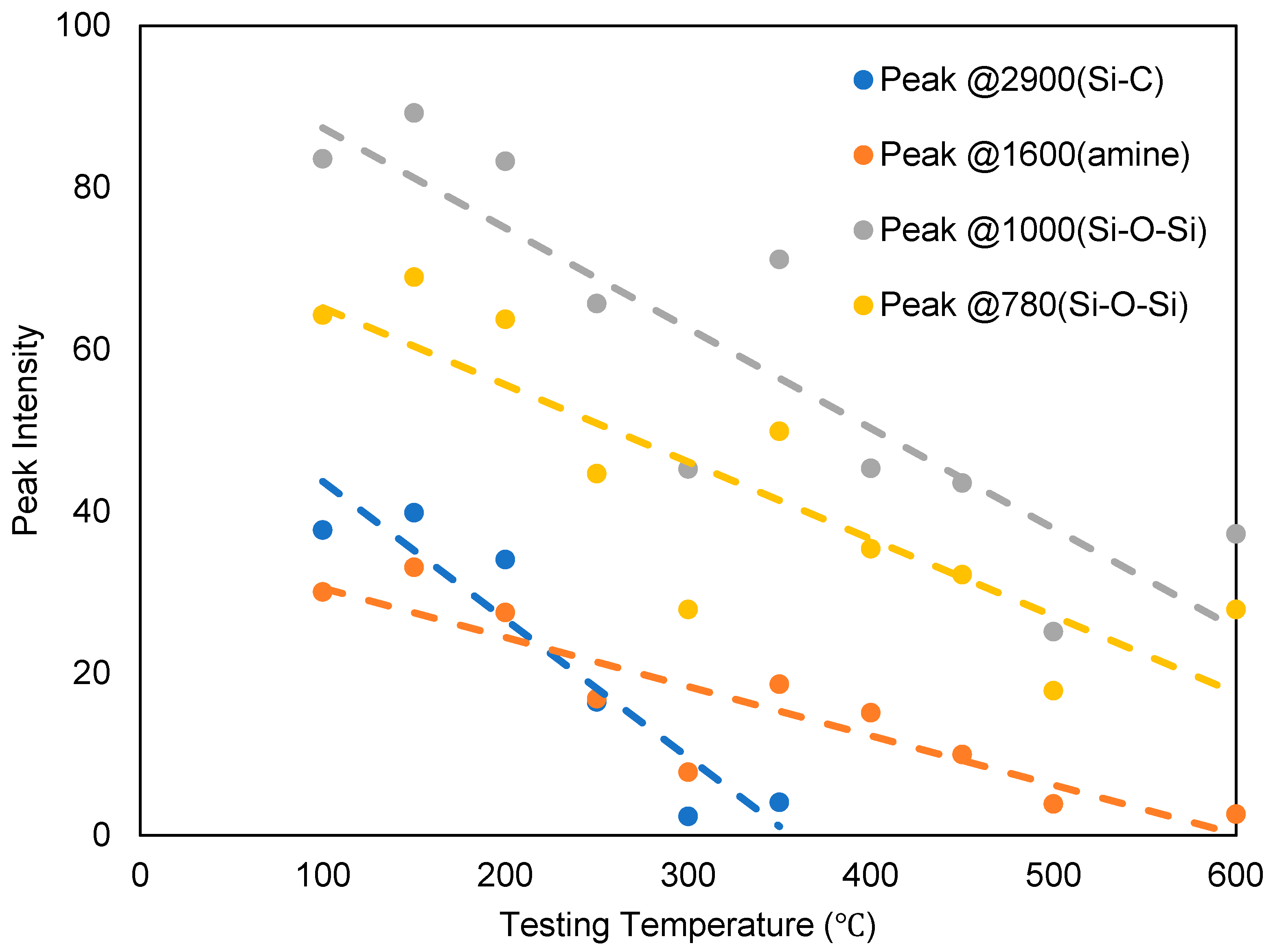
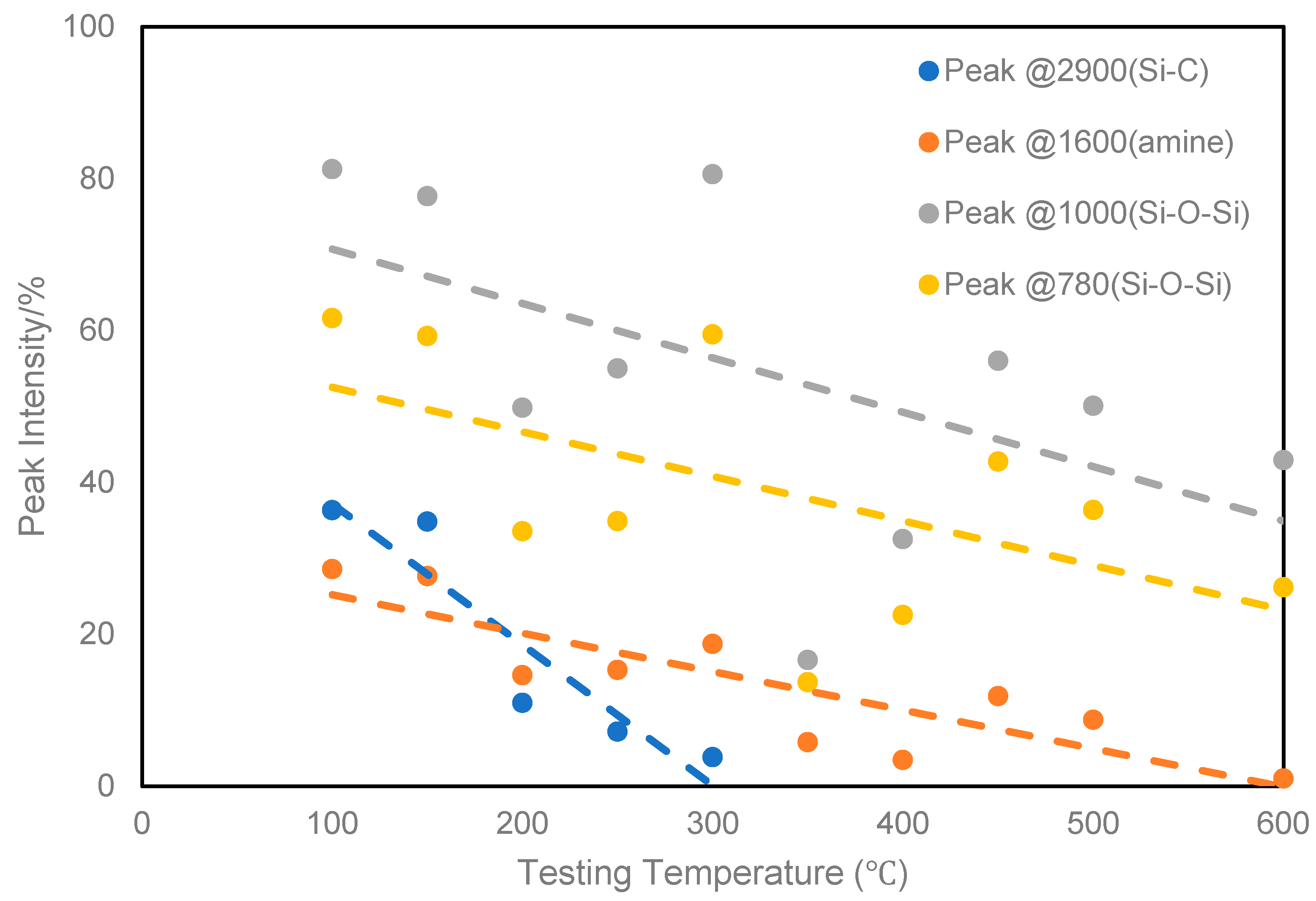
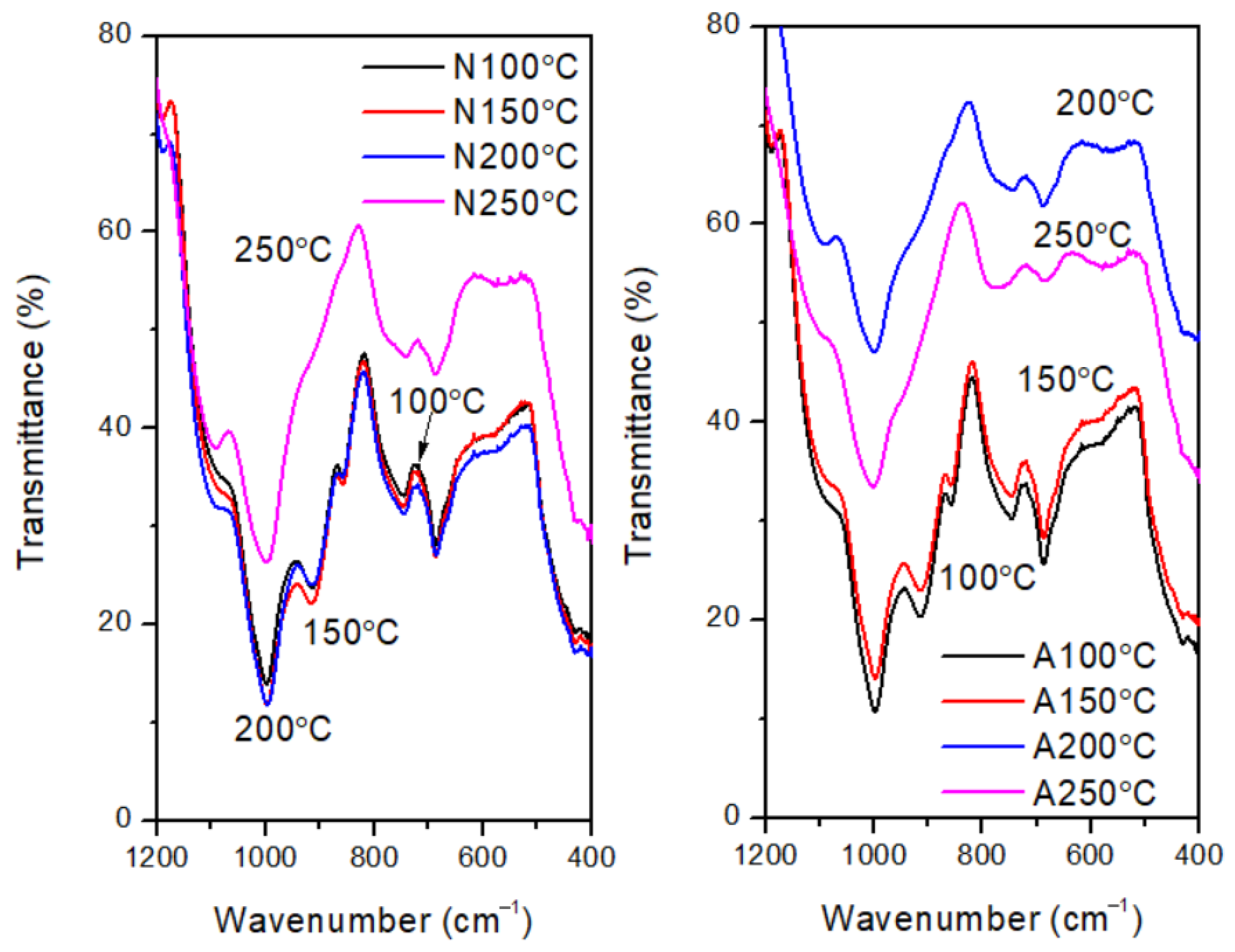
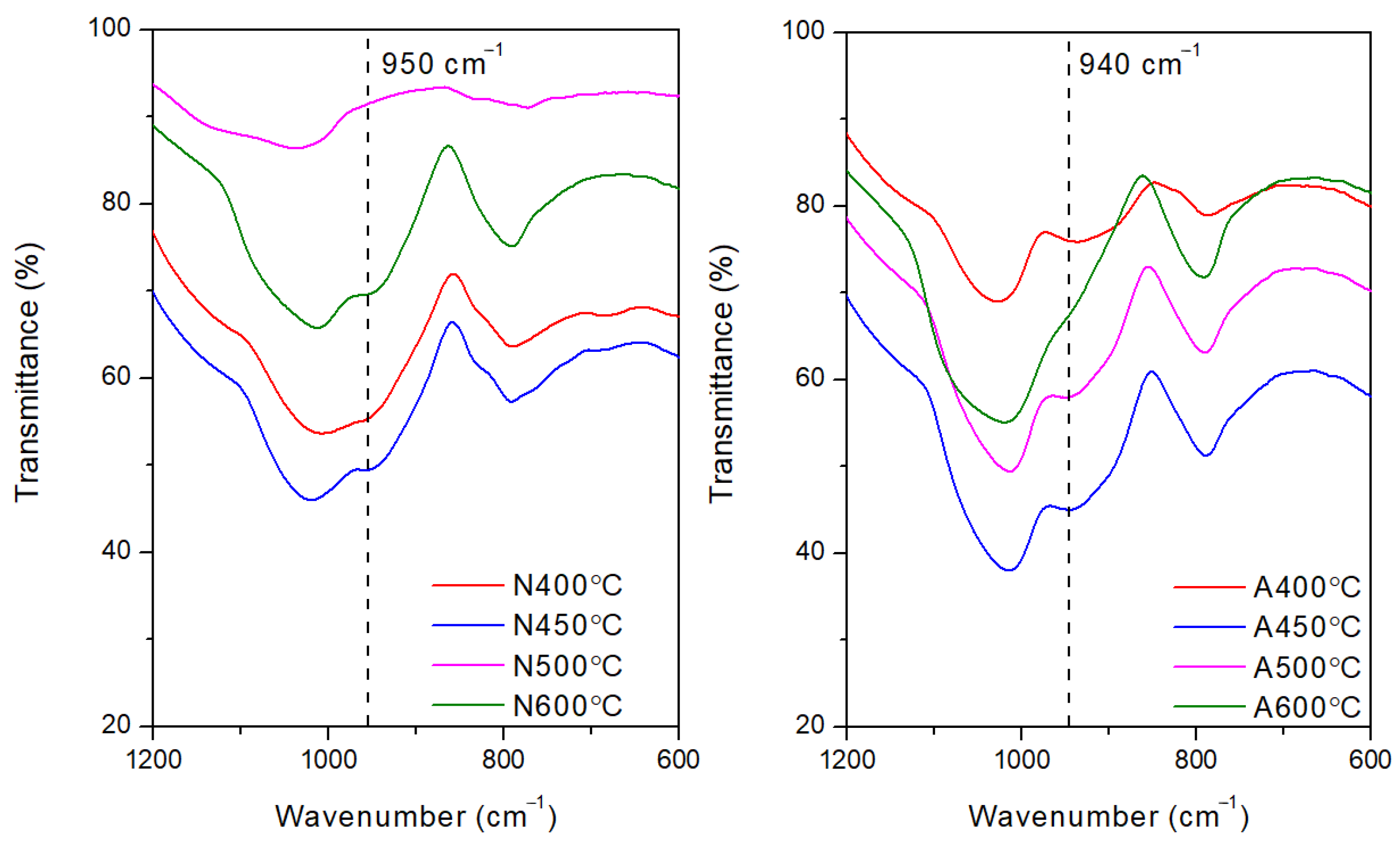

| Activation Energy/(kJ/mol) | Peak II | Peak III | Peak IV |
|---|---|---|---|
| Nitrogen | 100 | 82 | 223 |
| Air | 94 | 142 | 92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, S.; Cui, X.; Iroh, J.O. A Study of the Degradation Mechanism of Ladder-like Polyhedral Oligomeric Silsesquioxane via Fourier Transform Infrared Spectroscopy. Fire 2023, 6, 429. https://doi.org/10.3390/fire6110429
Xiao S, Cui X, Iroh JO. A Study of the Degradation Mechanism of Ladder-like Polyhedral Oligomeric Silsesquioxane via Fourier Transform Infrared Spectroscopy. Fire. 2023; 6(11):429. https://doi.org/10.3390/fire6110429
Chicago/Turabian StyleXiao, Shengdong, Xuemei Cui, and Jude O. Iroh. 2023. "A Study of the Degradation Mechanism of Ladder-like Polyhedral Oligomeric Silsesquioxane via Fourier Transform Infrared Spectroscopy" Fire 6, no. 11: 429. https://doi.org/10.3390/fire6110429







