An Experimental Study on the Transportation Characteristics of Perfluoro(2-methyl-3-pentanone) in a Straight Pipe
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Pressures and Temperatures in the Discharge System
3.2. Transportation Characteristics of C6F12O in the System
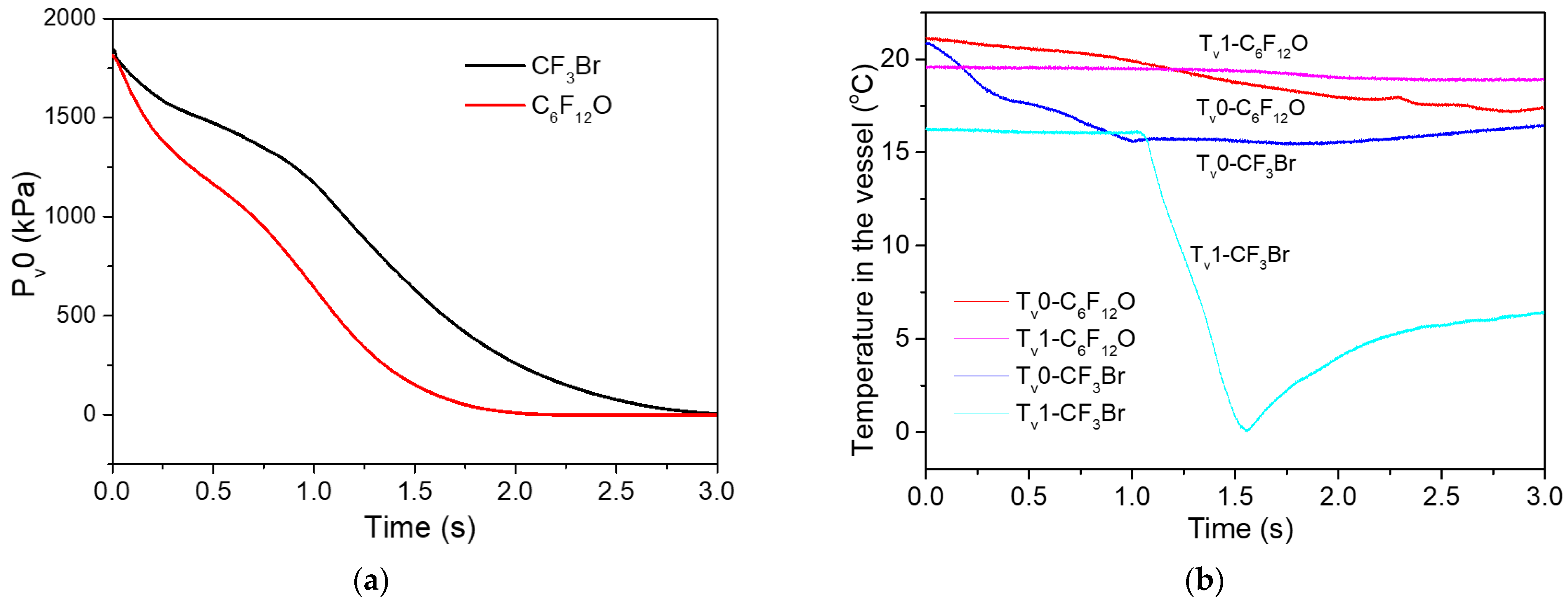
3.2.1. Variations in the Vessel
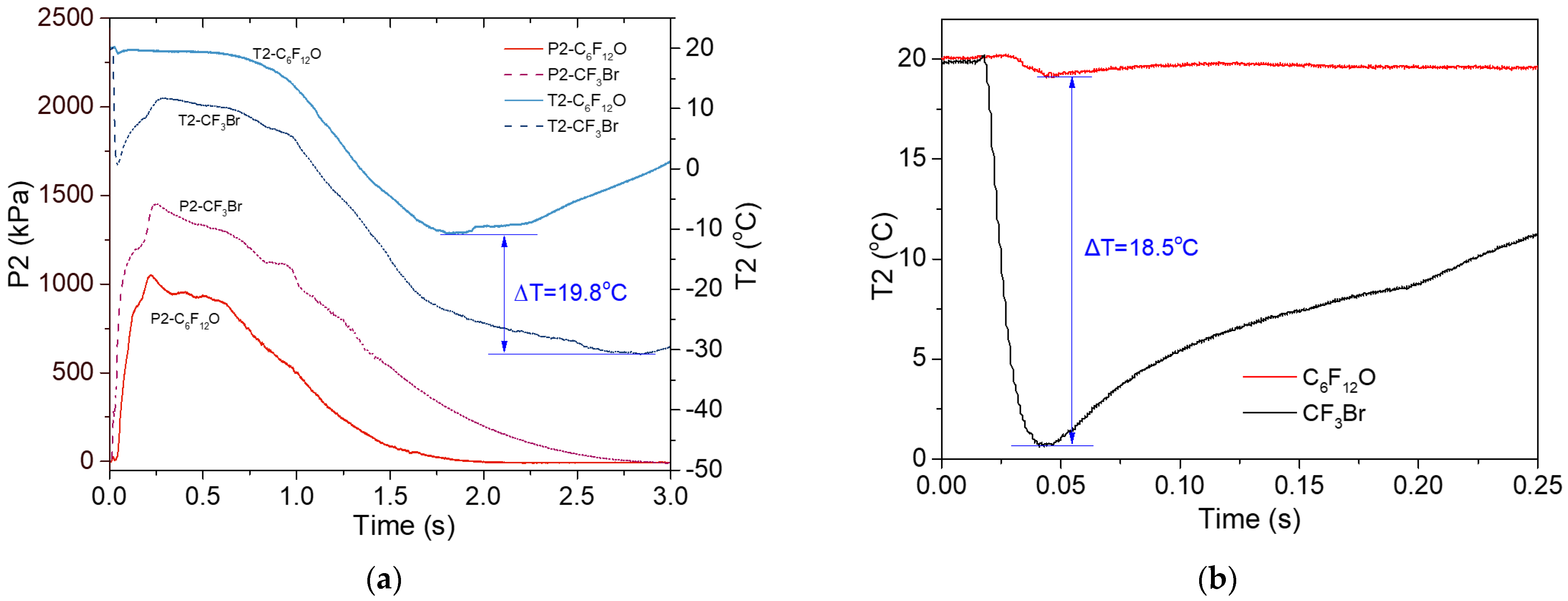
3.2.2. Variations in the Pipe
3.3. Effect of the Filling Pressure on the Flow Behaviors of C6F12O
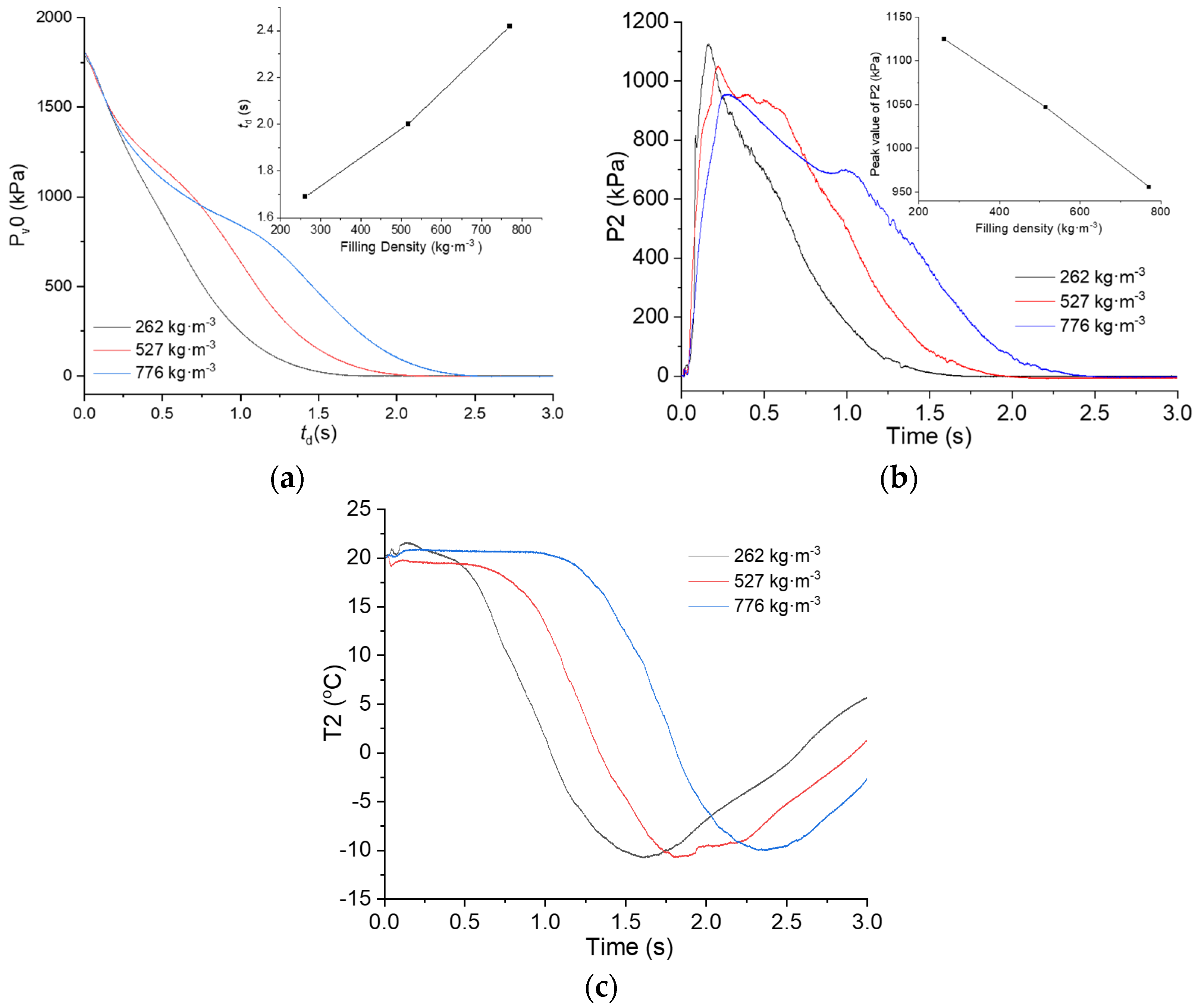
3.4. Effect of the Filling Density on the Flow Behaviors of C6F12O
4. Conclusions
- Under the driving of pressurized nitrogen, the C6F12O was sprayed out of the pipe very rapidly. In the process of just a few seconds, the agent in the pipe experienced complex changes within the three stages of “gas, gas-liquid mixture and gas”. The intermediate stage exhibited characteristics of gas-liquid two-phase flow, which is where the fluid was dominated by liquid C6F12O together with a small amount of vapor and nitrogen;
- Upon the release of C6F12O, the pressure in the vessel went steadily downward until it reached zero, while the vessel temperature just showed a minor drop of several degrees Celsius. The pipeline pressure plots exhibited an asymmetric hump shape, which contained three stages of “rapid increase, gentle decrease and continuous decrease”. In the first two stages, the piping temperature remained stable. However, a big drop of about 30 °C in the piping temperature occurred in the third stage of gas discharge;
- In comparison to that of the CF3Br released under similar conditions, the temperature and pressure curves of C6F12O exhibited similar trajectories, but with much lower varying amplitudes. Moreover, such differences can be mainly ascribed to their different vapor pressures and boiling points;
- With other conditions being the same, raising the filling pressure in the vessel of the C6F12O reduced the discharge duration and the pipeline temperatures. Increasing the filling density extended the discharge duration, but showed little influence on the pipeline temperatures.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xing, H.; Lu, S.; Yang, H.; Zhang, H. Review on Research Progress of C6F12O as a Fire Extinguishing Agent. Fire 2022, 5, 50. [Google Scholar] [CrossRef]
- 3M Company. 3MTM Novec1230TM Fire Protection Fluid; 3M Company: Saint Paul, MN, USA, 2020. [Google Scholar]
- Mclinden, M.O.; RPerkins, A.; Lemmon, E.W.; Fortin, T.J. Thermodynamic Properties of 1,1,1,2,2,4,5,5,5-Nonafluoro-4-(trifluoromethyl)-3-pentanone: Vapor Pressure, (p, ρ, T) Behavior, and Speed of Sound Measurements, and an Equation of State. J. Chem. Eng. Data 2015, 60, 3646–3659. [Google Scholar] [CrossRef]
- Kim, A.; Crampton, G.; Kanabus-Kaminska, M. Performance of Novec1230 in Electronic Facility Fire Protection; NRCC-53526; National Research Council Canada: Montreal, QC, Canada, 2010.
- Pagliaro, J.L.; Linteris, G.T. Hydrocarbon Flame Inhibition by C6F12O (Novec 1230): Unstretched Burning Velocity Measurements and Predictions. Fire Saf. J. 2017, 87, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, X.; Tian, S. Insight into the Compatibility Between C6F12O and Metal Materials: Experiment and Theory. IEEE Access. 2018, 6, 58154–58160. [Google Scholar] [CrossRef]
- Zhang, X.; Lan, J.; Tian, S.; Rao, X.; Li, X.; Yuan, Z.; Jin, X.; Gao, S.; Zhang, X. Study of Compatibility between Eco-friendly Insulating Medium C6F12O and Sealing Material EPDM. J. Mol. Struct. 2021, 1244, 130949. [Google Scholar] [CrossRef]
- Ditch, B.D.; Rivers, P.E.; Thomas, S.D. Thermal decomposition product testing with C6F-ketone. In Proceedings of the Halon Options Technical Working Conference (HOTWC), Albuquerque, NM, USA, 24–26 April 2001; pp. 349–354. [Google Scholar]
- Environmental Protection Agency. Protection of Stratospheric Ozone: Listing of substitutes for Ozone-depleting Substances, Final Rule and Proposed Rule; United State Environmental Protection Agency: Washington, DC, USA, 2003.
- ISO 14520-1; Gaseous Fire Extinguishing Systems-Physical Properties and System Design, Part 5: FK-5-1-12 Extinguishant. ISO: Geneva, Switzerland, 2023.
- National Fire Protection Association (NFPA). Standard on Clean Agent Fire Extinguishing Systems; National Fire Protection Association (NFPA): Quincy, MA, USA, 2001. [Google Scholar]
- Womeldorf, B.; Mitchell, M.; Grosshandler, M. Selection of a Simulant of CF3Br for Use in Engine Nacelle Certification Tests. In Proceedings of the Halon Options Technical Working Conference (HOTWC), Albuquerque, NM, USA, 9–11 May 1995; pp. 197–210. [Google Scholar]
- Fan, W.; Gao, Z.; Wang, D.; Yang, Y.; Gao, Y. Study on the Flow Characteristics of Fire Extinguishing Agent FK-5-1-12 in the Pipeline Release Process. Fire Mater. 2022, 46, 376–387. [Google Scholar] [CrossRef]
- Williamson, H.V. Halon 1301 flow in pipelines. Fire Technol. 1976, 12, 18–32. [Google Scholar] [CrossRef]
- Elliott, D.G.; Garrison, P.W.; Klein, G.A.; Moran, K.M. Flow of Nitrogen Pressurized Halon 1301 in Fire Extinguishing Systems; JPL Publication: Pasadena, CA, USA, 1984.
- Kim, J.; Baek, B.; Lee, J. Numerical analysis of flow characteristics of fire extinguishing agents in aircraft fire extinguishing systems. J. Mech. Sci. Technol. 2009, 23, 1877–1884. [Google Scholar] [CrossRef]
- Jin, J.; Pan, R.; Li, Q.; Chen, R. Effects of Release Pressure on the Transportation Characteristics in Pipeline and the Diffusion Behaviors in Enclosure Space of Typical Gas Extinguishing Agent. J. Loss Prevent. Proc. 2021, 70, 104441. [Google Scholar] [CrossRef]
- Bromotrifluoromethane. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C75638&Mask=4 (accessed on 6 December 2022).
- Tuzla, K.; Palmer, T.; Chen, J.C.; Sundaram, R.K.; Yeung, W.S. Development of Computer Program for Fire Suppression Fluid Flow, Report Number ITF 2000-1, Vols. I and II; Institute of Thermo-Fluid Engineering & Science, Lehigh University: Bethlehem, PA, USA, 2000. [Google Scholar]
- Jin, J.; Hua, M.; Yan, P.; Zhang, H.; Li, Q.; Pan, X. The Transport and Diffusion Characteristics of Superheated Fire Extinguish Agent Released via Different Nozzles in a Confined Space. Saf. Sci. 2020, 129, 104787. [Google Scholar] [CrossRef]
- Singh, J.; Zerpa, L.E.; Partington, B.; Gamboa, J. Effect of Nozzle Geometry on Critical-subcritical Flow Transitions. Heliyon 2019, 5, e01273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
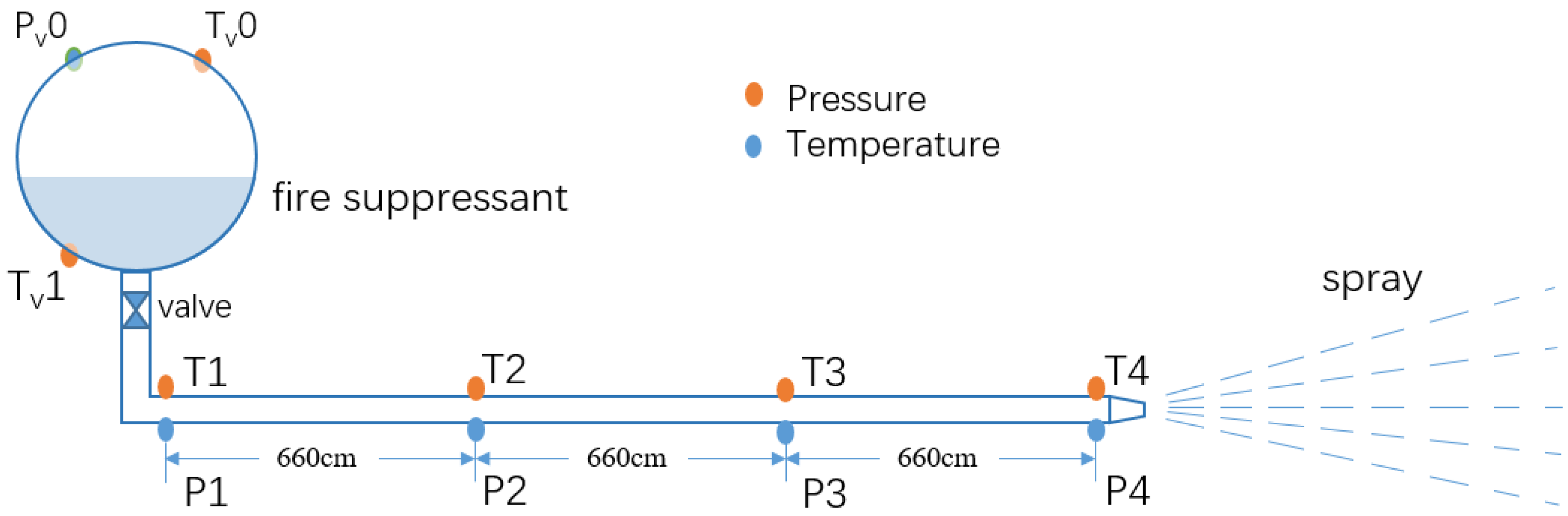


| Test Sequence | Agent | Agent Mass (kg) | Filling Density (kg·m−3) | Filling Pressure (kPa) |
|---|---|---|---|---|
| 1 | C6F12O | 2.01 | 517 | 1822 |
| 2 | CF3Br | 2.02 | 519 | 1846 |
| 3 | C6F12O | 2.12 | 545 | 1910 |
| 4 | C6F12O | 2.08 | 535 | 2540 |
| 5 | C6F12O | 2.14 | 550 | 3230 |
| 6 | C6F12O | 1.02 | 262 | 1799 |
| 7 | C6F12O | 2.05 | 527 | 1812 |
| 8 | C6F12O | 3.02 | 776 | 1813 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, X.; Chen, Y.; Huang, Q.; Zhao, C.; Li, S.; Huang, J.; Wang, J. An Experimental Study on the Transportation Characteristics of Perfluoro(2-methyl-3-pentanone) in a Straight Pipe. Fire 2023, 6, 156. https://doi.org/10.3390/fire6040156
Ni X, Chen Y, Huang Q, Zhao C, Li S, Huang J, Wang J. An Experimental Study on the Transportation Characteristics of Perfluoro(2-methyl-3-pentanone) in a Straight Pipe. Fire. 2023; 6(4):156. https://doi.org/10.3390/fire6040156
Chicago/Turabian StyleNi, Xiaomin, Ye Chen, Qiurui Huang, Chenxi Zhao, Songyang Li, Jiahui Huang, and Jian Wang. 2023. "An Experimental Study on the Transportation Characteristics of Perfluoro(2-methyl-3-pentanone) in a Straight Pipe" Fire 6, no. 4: 156. https://doi.org/10.3390/fire6040156






