Influence of High Temperature on the Physical and Mechanical Properties of Porous Limestone from Baku (Azerbaijan)
Abstract
:1. Introduction
2. Materials and Methods
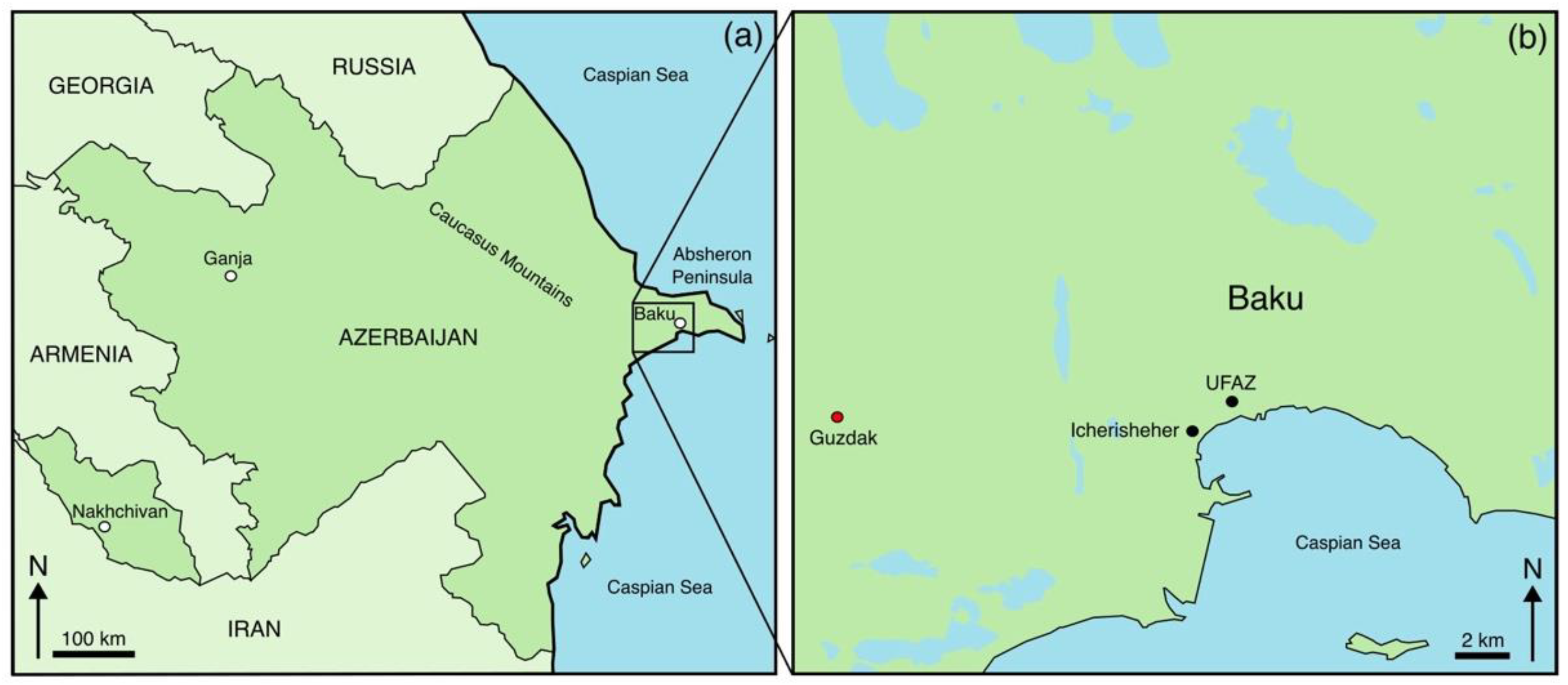
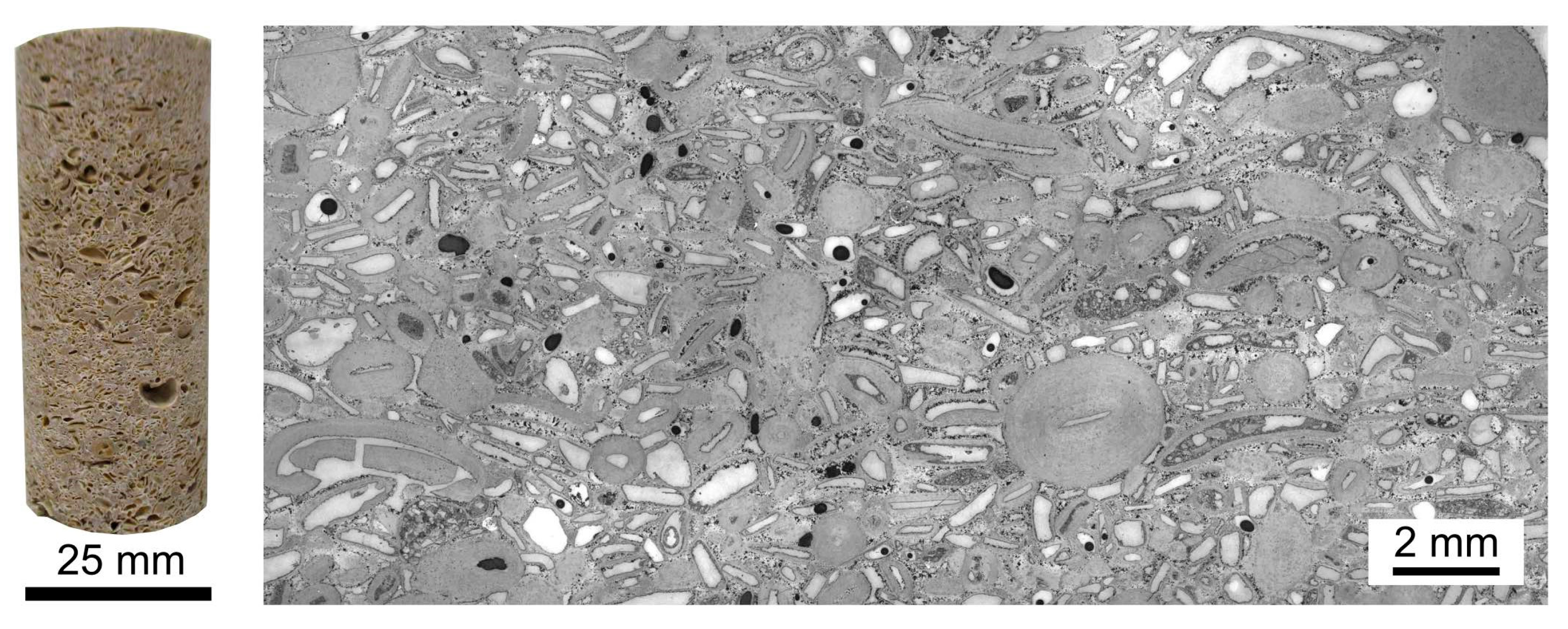
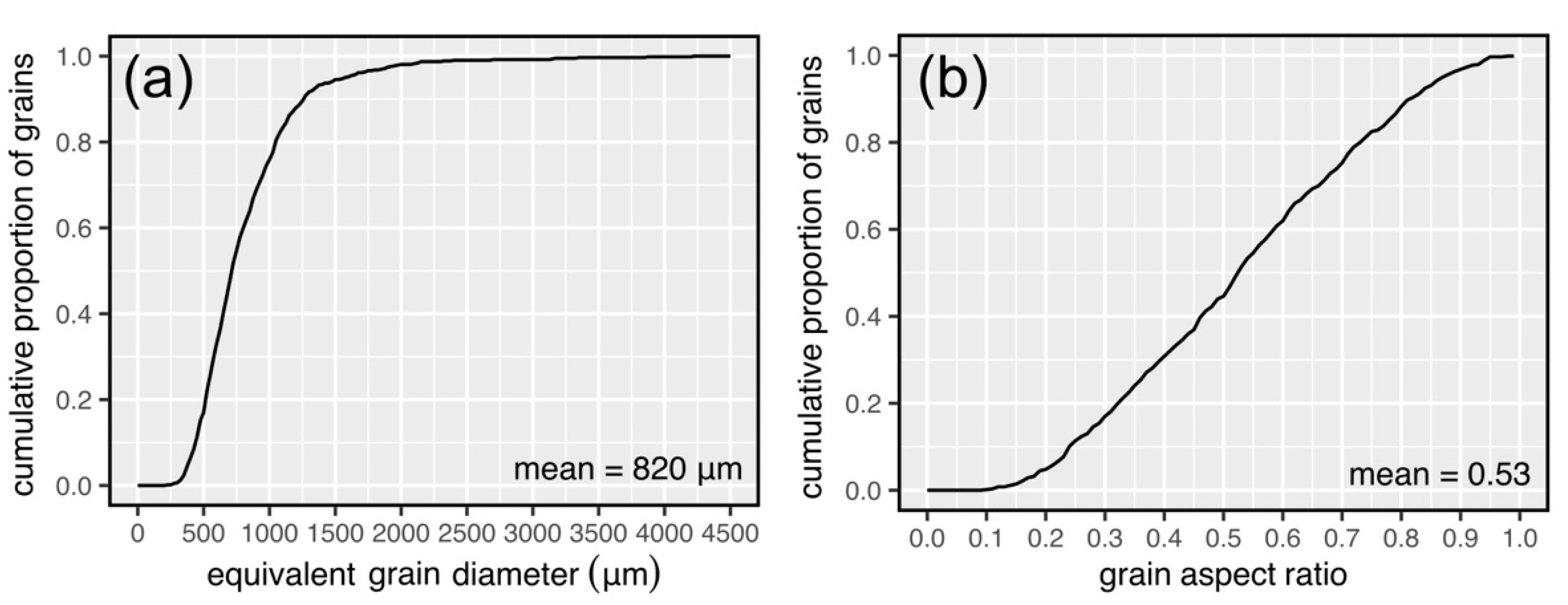
2.1. Sample Description and Microstructural Characterisation
2.2. Sample Preparation and Experimental Methods
2.2.1. Bulk Density and Connected-Porosity Measurements
2.2.2. Permeability Measurements
2.2.3. P-Wave Velocity Measurements
2.2.4. Thermal Property Measurements
2.2.5. Uniaxial Compressive Strength (UCS) and Young’s Modulus
3. Results
3.1. Physical Property Data for Intact Samples
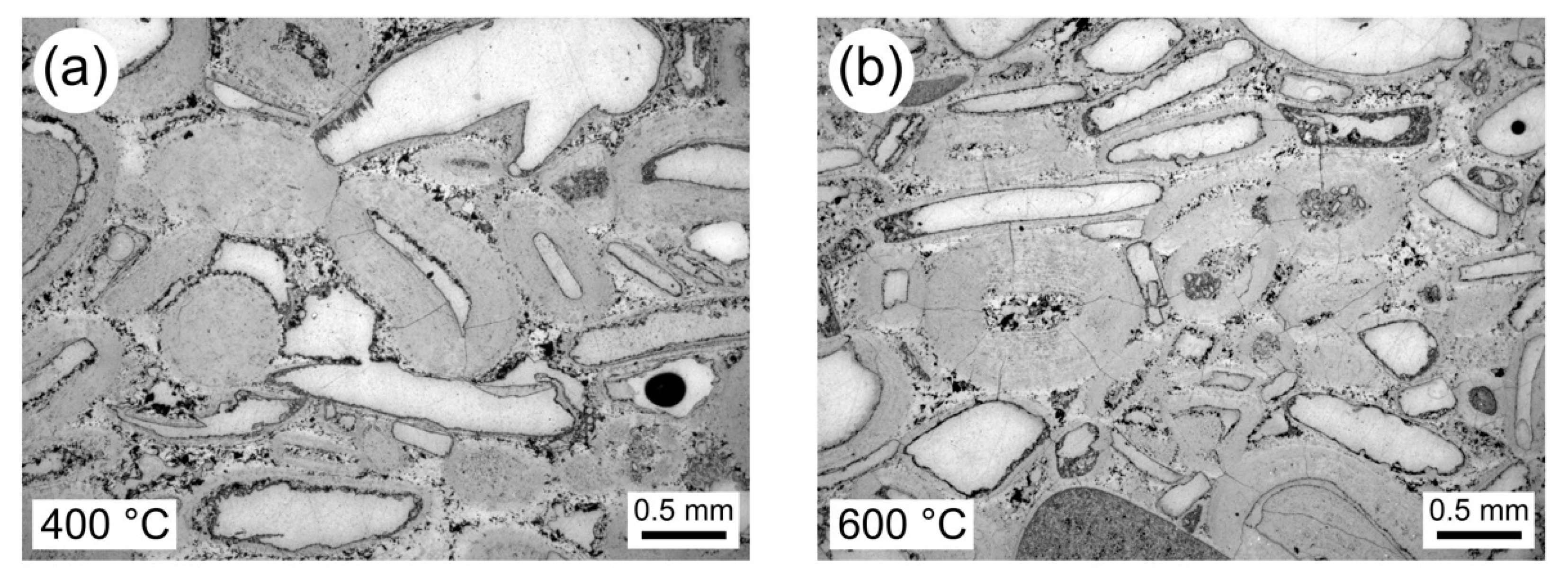
3.2. Thermal Stressing the Limestones
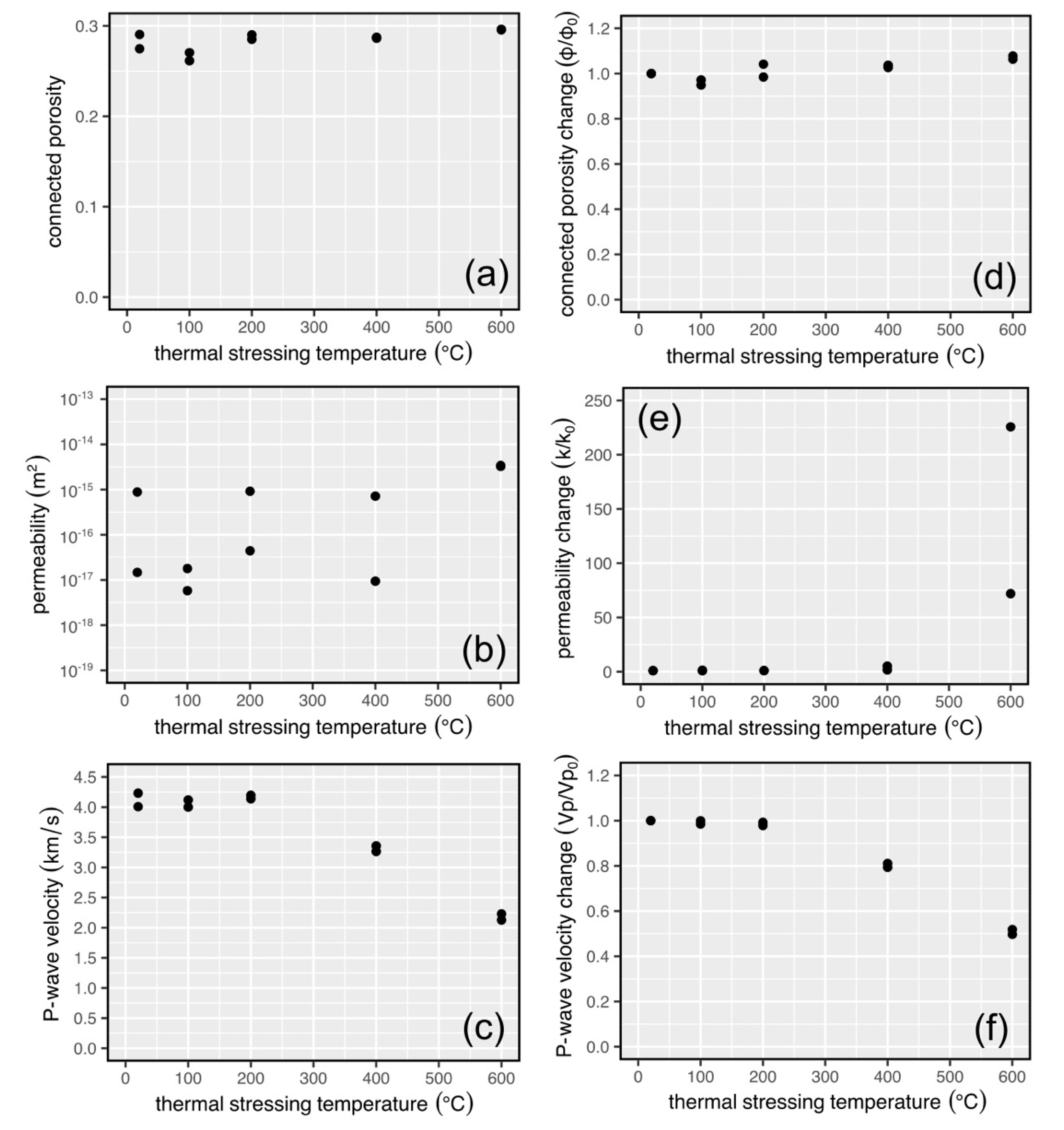
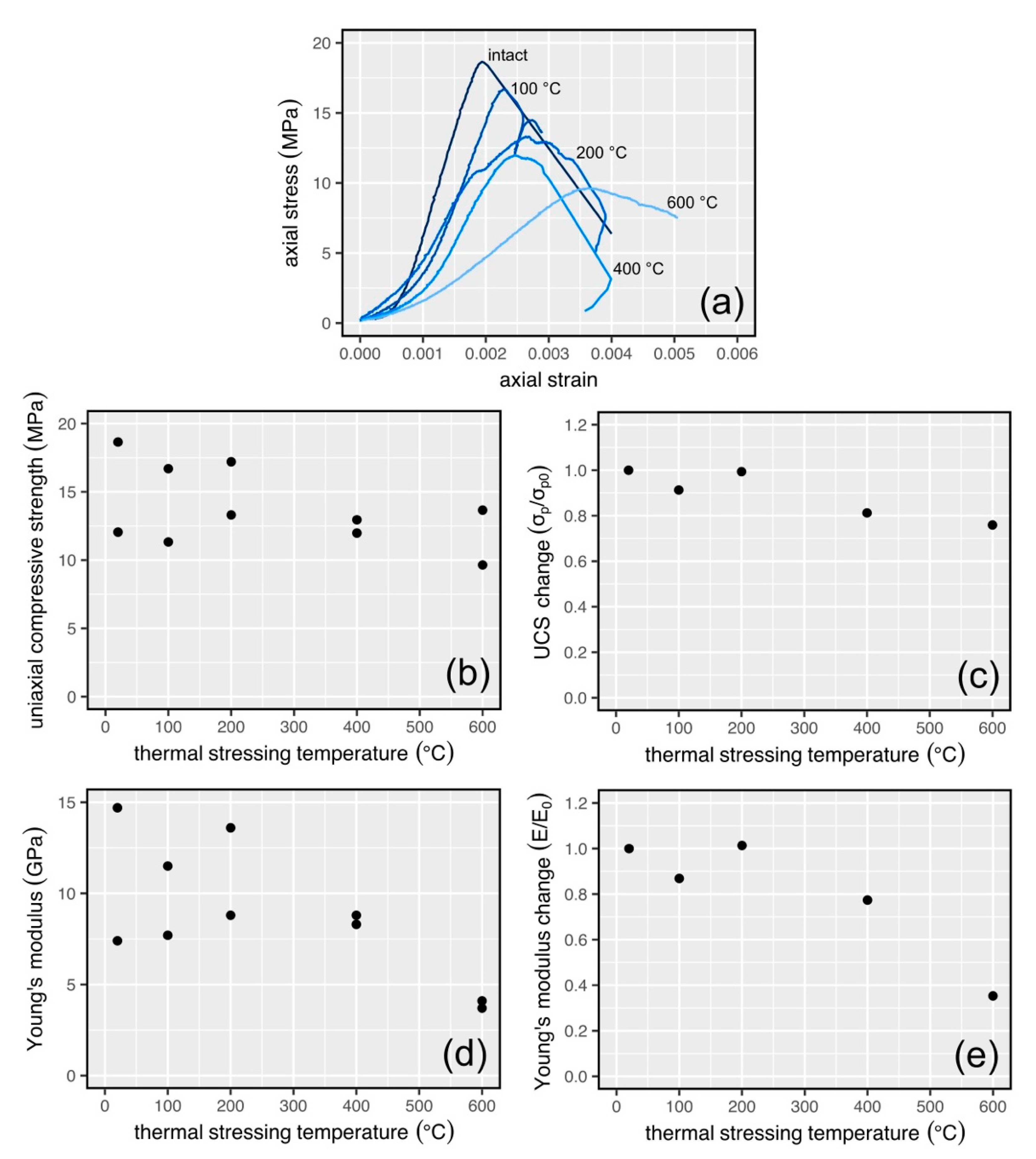
3.3. Physical Property Data for Thermally Stressed Samples
3.4. Mechanical Property Data for Thermally Stressed Samples
4. Discussion
4.1. Interpretation of the Physical and Mechanical Property Data
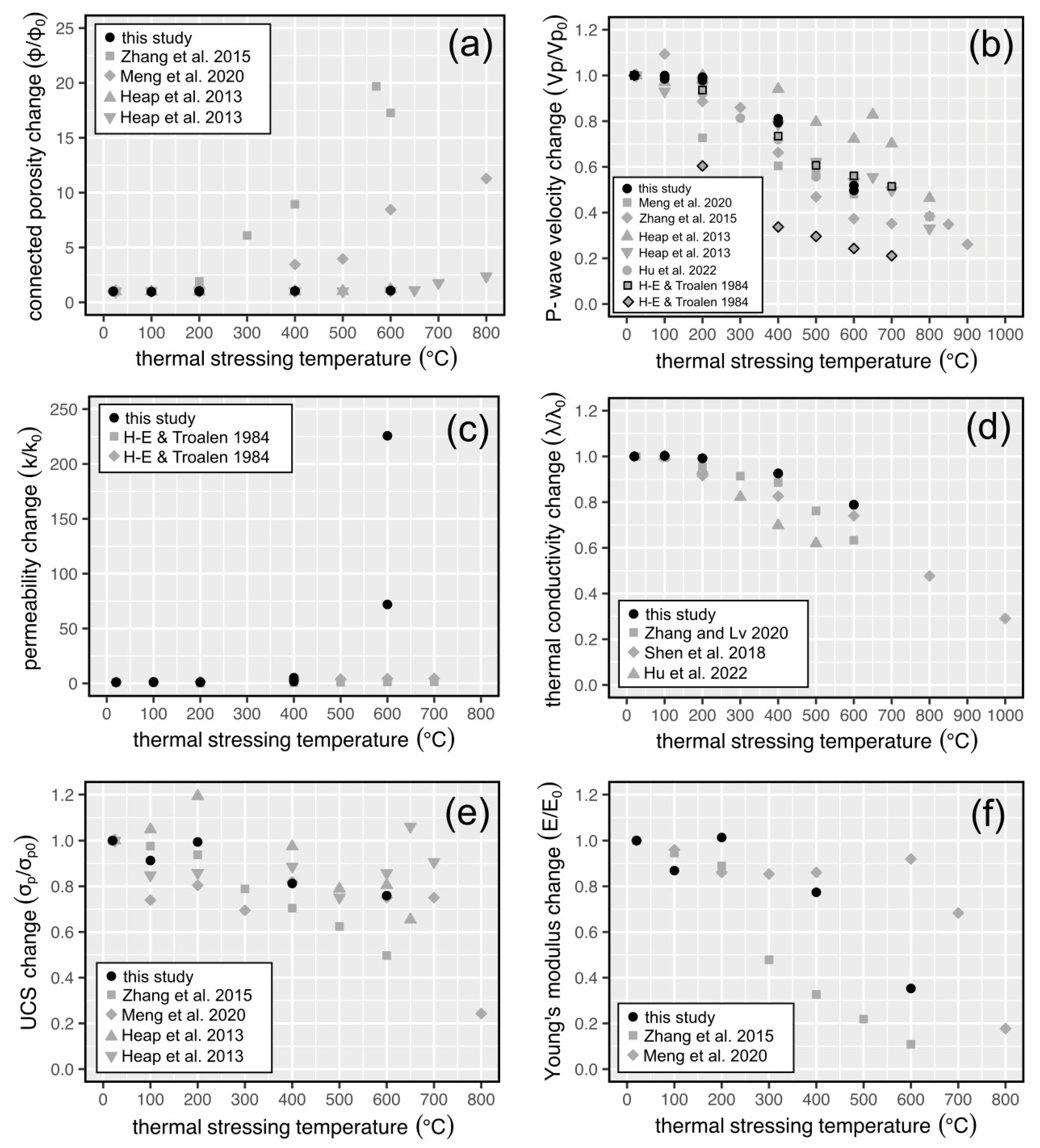
4.2. Comparisons with Previously Published Data
4.3. Implications for Building and Monument Restoration
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gauri, K.L.; Chowdhury, A.N.; Kulshreshtha, N.P.; Punuru, A.R. Geologic Features and Durability of Limestones at the Sphinx. Environ. Geol. Water Sci. 1990, 16, 57–62. [Google Scholar] [CrossRef]
- Fitzner, B.; Heinrichs, K.; La Bouchardiere, D. Limestone Weathering of Historical Monuments in Cairo, Egypt. Geol. Soc. Spec. Publ. 2002, 205, 217–239. [Google Scholar] [CrossRef]
- Jackson, J.; Priestley, K.; Allen, M.; Berberian, M. Active Tectonics of the South Caspian Basin. Geophys. J. Int. 2002, 148, 214–245. [Google Scholar] [CrossRef] [Green Version]
- Babayev, G.; Ismail-Zadeh, A.; Le Moüel, J.L. Scenario-Based Earthquake Hazard and Risk Assessment for Baku (Azerbaijan). Nat. Hazards Earth Syst. Sci. 2010, 10, 2697–2712. [Google Scholar] [CrossRef] [Green Version]
- Scawthorn, C.; Eidinger, J.M.; Schiff, A.J. Fire Following Earthquake; Technical Council on Lifeline Earthquake Engineering Monograph, No. 26; American Society of Civil Engineers Press: Reston, VA, USA, 2005. [Google Scholar]
- Zhang, W.; Qian, H.; Sun, Q.; Chen, Y. Experimental Study of the Effect of High Temperature on Primary Wave Velocity and Microstructure of Limestone. Environ. Earth Sci. 2015, 74, 5739–5748. [Google Scholar] [CrossRef]
- Meng, Q.B.; Wang, C.K.; Liu, J.F.; Zhang, M.W.; Lu, M.M.; Wu, Y. Physical and Micro-Structural Characteristics of Limestone after High Temperature Exposure. Bull. Eng. Geol. Environ. 2020, 79, 1259–1274. [Google Scholar] [CrossRef]
- Heap, M.J.; Mollo, S.; Vinciguerra, S.; Lavallée, Y.; Hess, K.U.; Dingwell, D.B.; Baud, P.; Iezzi, G. Thermal Weakening of the Carbonate Basement under Mt. Etna Volcano (Italy): Implications for Volcano Instability. J. Volcanol. Geotherm. Res. 2013, 250, 42–60. [Google Scholar] [CrossRef] [Green Version]
- Lion, M.; Skoczylas, F.; Ledésert, B. Effects of Heating on the Hydraulic and Poroelastic Properties of Bourgogne Limestone. Int. J. Rock Mech. Min. Sci. 2005, 42, 508–520. [Google Scholar] [CrossRef]
- Homand-Etienne, F.; Troalen, J.P. Behaviour of Granites and Limestones Subjected to Slow and Homogeneous Temperature Changes. Eng. Geol. 1984, 20, 219–233. [Google Scholar] [CrossRef]
- Hu, J.; Xie, H.; Gao, M.; Li, C.; Sun, Q. Damage Mechanism and Heat Transfer Characteristics of Limestone after Thermal Shock Cycle Treatments Based on Geothermal Development. Int. J. Rock Mech. Min. Sci. 2022, 160, 105269. [Google Scholar] [CrossRef]
- Kılıç, Ö. The Influence of High Temperatures on Limestone P-Wave Velocity and Schmidt Hammer Strength. Int. J. Rock Mech. Min. Sci. 2006, 43, 980–986. [Google Scholar] [CrossRef]
- Zhang, W.; Lv, C. Effects of Mineral Content on Limestone Properties with Exposure to Different Temperatures. J. Pet. Sci. Eng. 2020, 188, 106941. [Google Scholar] [CrossRef]
- Shen, Y.; Yang, Y.; Yang, G.; Hou, X.; Ye, W.; You, Z.; Xi, J. Damage Characteristics and Thermo-Physical Properties Changes of Limestone and Sandstone during Thermal Treatment from −30 °C to 1000 °C. Heat Mass Transf. Stoffuebertragung 2018, 54, 3389–3407. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, Q.; Zhu, S.; Wang, B. Experimental Study on Mechanical and Porous Characteristics of Limestone Affected by High Temperature. Appl. Therm. Eng. 2017, 110, 356–362. [Google Scholar] [CrossRef]
- Mao, X.B.; Zhang, L.Y.; Li, T.Z.; Liu, H.S. Properties of Failure Mode and Thermal Damage for Limestone at High Temperature. Min. Sci. Technol. 2009, 19, 290–294. [Google Scholar] [CrossRef]
- Meng, Q.; Zhang, M.; Han, L.; Pu, H.; Chen, Y. Experimental Research on the Influence of Loading Rate on the Mechanical Properties of Limestone in a High-Temperature State. Bull. Eng. Geol. Environ. 2019, 78, 3479–3492. [Google Scholar] [CrossRef]
- Castagna, A.; Ougier-Simonin, A.; Benson, P.M.; Browning, J.; Walker, R.J.; Fazio, M.; Vinciguerra, S. Thermal Damage and Pore Pressure Effects of the Brittle-Ductile Transition in Comiso Limestone. J. Geophys. Res. Solid Earth 2018, 123, 7644–7660. [Google Scholar] [CrossRef] [Green Version]
- Bakker, R.R.; Violay, M.E.S.; Benson, P.M.; Vinciguerra, S.C. Ductile Flow in Sub-Volcanic Carbonate Basement as the Main Control for Edifice Stability: New Experimental Insights. Earth Planet. Sci. Lett. 2015, 430, 533–541. [Google Scholar] [CrossRef] [Green Version]
- Farquharson, J.I.; Heap, M.J.; Lavallée, Y.; Varley, N.R.; Baud, P. Evidence for the Development of Permeability Anisotropy in Lava Domes and Volcanic Conduits. J. Volcanol. Geotherm. Res. 2016, 323, 163–185. [Google Scholar] [CrossRef]
- Heap, M.J.; Kennedy, B.M. Exploring the Scale-Dependent Permeability of Fractured Andesite. Earth Planet. Sci. Lett. 2016, 447, 139–150. [Google Scholar] [CrossRef]
- Heap, M.J.; Kushnir, A.R.L.; Gilg, H.A.; Wadsworth, F.B.; Reuschlé, T.; Baud, P. Microstructural and Petrophysical Properties of the Permo-Triassic Sandstones (Buntsandstein) from the Soultz-Sous-Forêts Geothermal Site (France). Geotherm. Energy 2017, 5, 26. [Google Scholar] [CrossRef] [Green Version]
- Heap, M.J.; Lavallée, Y.; Petrakova, L.; Baud, P.; Reuschlé, T.; Varley, N.R.; Dingwell, D.B. Microstructural Controls on the Physical and Mechanical Properties of Edifice-Forming Andesites at Volcán de Colima, Mexico M. J. Geophys. Res. Solid Earth 2014, 119, 2925–2963. [Google Scholar] [CrossRef]
- Gustafsson, S.E. Transient plane source techniques for thermal conductivity and thermal diffusivity measurements of solid materials. Rev. Sci. Instrum. 1991, 62, 797–804. [Google Scholar] [CrossRef]
- Heap, M.J.; Kushnir, A.R.L.; Vasseur, J.; Wadsworth, F.B.; Harlé, P.; Baud, P.; Kennedy, B.M.; Troll, V.R.; Deegan, F.M. The Thermal Properties of Porous Andesite. J. Volcanol. Geotherm. Res. 2020, 398, 106901. [Google Scholar] [CrossRef]
- Heap, M.J.; Jessop, D.E.; Wadsworth, F.B.; Rosas-Carbajal, M.; Komorowski, J.C.; Gilg, H.A.; Aron, N.; Buscetti, M.; Gential, L.; Goupil, M.; et al. The Thermal Properties of Hydrothermally Altered Andesites from La Soufrière de Guadeloupe (Eastern Caribbean). J. Volcanol. Geotherm. Res. 2022, 421, 107444. [Google Scholar] [CrossRef]
- Fredrich, J.T.; Wong, T. Micromechanics of Thermally Induced Cracking in Three Crustal Rocks. J. Geophys. Res. Solid Earth 1986, 91, 12743–12764. [Google Scholar] [CrossRef]
- Jones, C.; Keaney, G.; Meredith, P.G.; Murrell, S.A.F. Acoustic Emission and Fluid Permeability Measurements on Thermally Cracked Rocks. Phys. Chem. Earth 1997, 22, 13–17. [Google Scholar] [CrossRef]
- Nara, Y.; Meredith, P.G.; Yoneda, T.; Kaneko, K. Influence of Macro-Fractures and Micro-Fractures on Permeability and Elastic Wave Velocities in Basalt at Elevated Pressure. Tectonophysics 2011, 503, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Vinciguerra, S.; Trovato, C.; Meredith, P.G.; Benson, P.M. Relating Seismic Velocities, Thermal Cracking and Permeability in Mt. Etna and Iceland Basalts. Int. J. Rock Mech. Min. Sci. 2005, 42, 900–910. [Google Scholar] [CrossRef] [Green Version]
- Kant, M.A.; Ammann, J.; Rossi, E.; Madonna, C.; Höser, D.; Rudolf von Rohr, P. Thermal Properties of Central Aare Granite for Temperatures up to 500 °C: Irreversible Changes Due to Thermal Crack Formation. Geophys. Res. Lett. 2017, 44, 771–776. [Google Scholar] [CrossRef]
- David, E.C.; Brantut, N.; Schubnel, A.; Zimmerman, R.W. Sliding Crack Model for Nonlinearity and Hysteresis in the Uniaxial Stress-Strain Curve of Rock. Int. J. Rock Mech. Min. Sci. 2012, 52, 9–17. [Google Scholar] [CrossRef]
- Griffiths, L.; Heap, M.J.; Baud, P.; Schmittbuhl, J. Quantification of Microcrack Characteristics and Implications for Stiffness and Strength of Granite. Int. J. Rock Mech. Min. Sci. 2017, 100, 138–150. [Google Scholar] [CrossRef]
- Samtani, M.; Dollimore, D.; Alexander, K.S. Comparison of Dolomite Decomposition Kinetics with Related Carbonates and the Effect of Procedural Variables on Its Kinetic Parameters. Thermochim. Acta 2002, 392–393, 135–145. [Google Scholar] [CrossRef]
- Mollo, S.; Vinciguerra, S.; Iezzi, G.; Iarocci, A.; Scarlato, P.; Heap, M.J.; Dingwell, D.B. Volcanic Edifice Weakening via Devolatilization Reactions. Geophys. J. Int. 2011, 186, 1073–1077. [Google Scholar] [CrossRef] [Green Version]
- Steiner, S.; Lothenbach, B.; Proske, T.; Borgschulte, A.; Winnefeld, F. Effect of Relative Humidity on the Carbonation Rate of Portlandite, Calcium Silicate Hydrates and Ettringite. Cem. Concr. Res. 2020, 135, 106116. [Google Scholar] [CrossRef]
- Baud, P.; Exner, U.; Lommatzsch, M.; Reuschlé, T.; Wong, T.F. Mechanical Behavior, Failure Mode, and Transport Properties in a Porous Carbonate. J. Geophys. Res. Solid Earth 2017, 122, 7363–7387. [Google Scholar] [CrossRef]
- Chakrabarti, B.; Yates, T.; Lewry, A. Effect of Fire Damage on Natural Stonework in Buildings. Constr. Build. Mater. 1996, 10, 539–544. [Google Scholar] [CrossRef]
- Ozguven, A.; Ozcelik, Y. Investigation of Some Property Changes of Natural Building Stones Exposed to Fire and High Heat. Constr. Build. Mater. 2013, 38, 813–821. [Google Scholar] [CrossRef]
- González-Gómez, W.S.; Quintana, P.; May-Pat, A.; Avilés, F.; May-Crespo, J.; Alvarado-Gil, J.J. Thermal Effects on the Physical Properties of Limestones from the Yucatan Peninsula. Int. J. Rock Mech. Min. Sci. 2015, 75, 182–189. [Google Scholar] [CrossRef]
- Heap, M.J.; Lavallée, Y.; Laumann, A.; Hess, K.U.; Meredith, P.G.; Dingwell, D.B. How tough is tuff in the event of fire? Geology 2012, 40, 311–314. [Google Scholar] [CrossRef]
- Heap, M.J.; Kushnir, A.; Griffiths, L.; Wadsworth, F.; Marmoni, G.M.; Fiorucci, M.; Martino, S.; Baud, P.; Gilg, H.A.; Reuschlé, T. Fire resistance of the Mt. Epomeo Green Tuff, a widely-used building stone on Ischia Island (Italy). Volcanica 2018, 1, 33–48. [Google Scholar] [CrossRef] [Green Version]



| Sample | Bulk Sample Density (kg/m3) | Connected Porosity | Permeability (m2) | P-Wave Velocity (km/s) | Thermal Conductivity (W/mK) | Thermal Diffusivity (mm2/s) | Specific Heat Capacity (kJ/kgK) |
|---|---|---|---|---|---|---|---|
| BL1 | 1951.2 | 0.27 | 1.47 × 10−17 | 4.23 | 1.329 ± 0.076 | 1.094 ± 0.067 | 0.622 ± 0.068 |
| BL2 | 1927.2 | 0.29 | 8.83 × 10−16 | 4.01 | 1.329 ± 0.076 | 1.094 ± 0.067 | 0.630 ± 0.068 |
| BL3 | 1953.1 | 0.27 | 6.39 × 10−18 | 4.18 | 1.267 ± 0.160 | 0.992 ± 0.139 | 0.654 ± 0.173 |
| BL4 | 1947.8 | 0.28 | 1.30 × 10−17 | 4.00 | 1.267 ± 0.160 | 0.992 ± 0.139 | 0.656 ± 0.173 |
| BL5 | 1940.2 | 0.28 | 8.22 × 10−16 | 4.23 | 1.188 ± 0.109 | 0.670 ± 0.103 | 0.914 ± 0.060 |
| BL6 | 1949.6 | 0.29 | 4.55 × 10−17 | 4.23 | 1.188 ± 0.109 | 0.670 ± 0.103 | 0.909 ± 0.060 |
| BL7 | 1946.7 | 0.28 | 4.37 × 10−16 | 4.12 | 1.329 ± 0.032 | 0.893 ± 0.088 | 0.764 ± 0.073 |
| BL8 | 1952.7 | 0.28 | 1.83 × 10−18 | 4.14 | 1.329 ± 0.032 | 0.893 ± 0.088 | 0.762 ± 0.073 |
| BL9 | 1960.1 | 0.27 | 4.74 × 10−17 | 4.48 | 1.374 ± 0.019 | 0.852 ± 0.027 | 0.823 ± 0.016 |
| BL10 | 1944.2 | 0.28 | 1.44 × 10−17 | 4.10 | 1.374 ± 0.019 | 0.852 ± 0.027 | 0.830 ± 0.016 |
| BL11 | 1957.1 | 0.28 | 3.82 × 10−16 | 4.41 | 1.308 ± 0.056 | 1.026 ± 0.099 | 0.652 ± 0.080 |
| BL12 | 1954.9 | 0.28 | 3.89 × 10−17 | 4.23 | 1.308 ± 0.056 | 1.026 ± 0.099 | 0.652 ± 0.080 |
| Sample | Bulk Sample Density (kg/m3) | Connected Porosity | Permeability (m2) | P-Wave Velocity (km/s) | Thermal Conductivity (W/mK) | Thermal Diffusivity (mm2/s) | Specific Heat Capacity (kJ/kgK) |
|---|---|---|---|---|---|---|---|
| BL1 | 1951.2 | 0.27 | 1.47 × 10−17 | 4.23 | 1.329 ± 0.076 | 1.094 ± 0.067 | 0.622 ± 0.068 |
| BL2 | 1927.2 | 0.29 | 8.83 × 10−16 | 4.01 | 1.329 ± 0.076 | 1.094 ± 0.067 | 0.630 ± 0.068 |
| BL3 (100 °C) | 1960.6 | 0.26 | 5.81 × 10−18 | 4.12 | 1.271 ± 0.126 | 1.029 ± 0.112 | 0.630 ± 0.110 |
| BL4 (100 °C) | 1949.3 | 0.27 | 1.79 × 10−17 | 4.00 | 1.271 ± 0.126 | 1.029 ± 0.112 | 0.634 ± 0.110 |
| BL5 (200 °C) | 1942.5 | 0.29 | 9.18 × 10−16 | 4.14 | 1.178 ± 0.108 | 0.678 ± 0.135 | 0.895 ± 0.101 |
| BL6 (200 °C) | 1955.3 | 0.29 | 4.42 × 10−17 | 4.20 | 1.178 ± 0.108 | 0.678 ± 0.135 | 0.889 ± 0.101 |
| BL7 (400 °C) | 1940.1 | 0.29 | 7.16 × 10−16 | 3.27 | 1.230 ± 0.099 | 0.899 ± 0.045 | 0.705 ± 0.073 |
| BL8 (400 °C) | 1946.8 | 0.29 | 9.42 × 10−18 | 3.36 | 1.230 ± 0.099 | 0.899 ± 0.045 | 0.703 ± 0.073 |
| BL9 (600 °C) | 1921.0 | 0.30 | 3.41 × 10−15 | 2.23 | 1.084 ± 0.025 | 0.746 ± 0.023 | 0.756 ± 0.028 |
| BL10 (600 °C) | 1943.5 | 0.30 | 3.25 × 10−15 | 2.13 | 1.084 ± 0.025 | 0.746 ± 0.023 | 0.747 ± 0.028 |
| Sample | Young’s Modulus (GPa) | Uniaxial Compressive Strength (MPa) |
|---|---|---|
| BL1 | 14.7 | 18.6 |
| BL2 | 7.4 | 12.0 |
| BL3 | 11.5 | 16.7 |
| BL4 | 7.7 | 11.3 |
| BL5 | 13.6 | 17.2 |
| BL6 | 8.8 | 13.3 |
| BL7 | 8.8 | 12.0 |
| BL8 | 8.3 | 13.0 |
| BL9 | 4.1 | 13.7 |
| BL10 | 3.7 | 9.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jodry, C.; Heap, M.J.; Bayramov, K.; Alizada, G.; Rustamova, S.; Nabiyeva, S. Influence of High Temperature on the Physical and Mechanical Properties of Porous Limestone from Baku (Azerbaijan). Fire 2023, 6, 263. https://doi.org/10.3390/fire6070263
Jodry C, Heap MJ, Bayramov K, Alizada G, Rustamova S, Nabiyeva S. Influence of High Temperature on the Physical and Mechanical Properties of Porous Limestone from Baku (Azerbaijan). Fire. 2023; 6(7):263. https://doi.org/10.3390/fire6070263
Chicago/Turabian StyleJodry, Clara, Michael J. Heap, Kamal Bayramov, Gunel Alizada, Sona Rustamova, and Sevinj Nabiyeva. 2023. "Influence of High Temperature on the Physical and Mechanical Properties of Porous Limestone from Baku (Azerbaijan)" Fire 6, no. 7: 263. https://doi.org/10.3390/fire6070263





