Thermal Decomposition Process of Fireproof Sealant Measured with Thermogravimetric and Fourier Transform Infrared Spectroscopy Analysis and Estimated Using Shuffled Complex Evolution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. TG–FTIR Experiment
2.3. Kinetic Method
2.4. Shuffled Complex Evolution
3. Results and Discussion
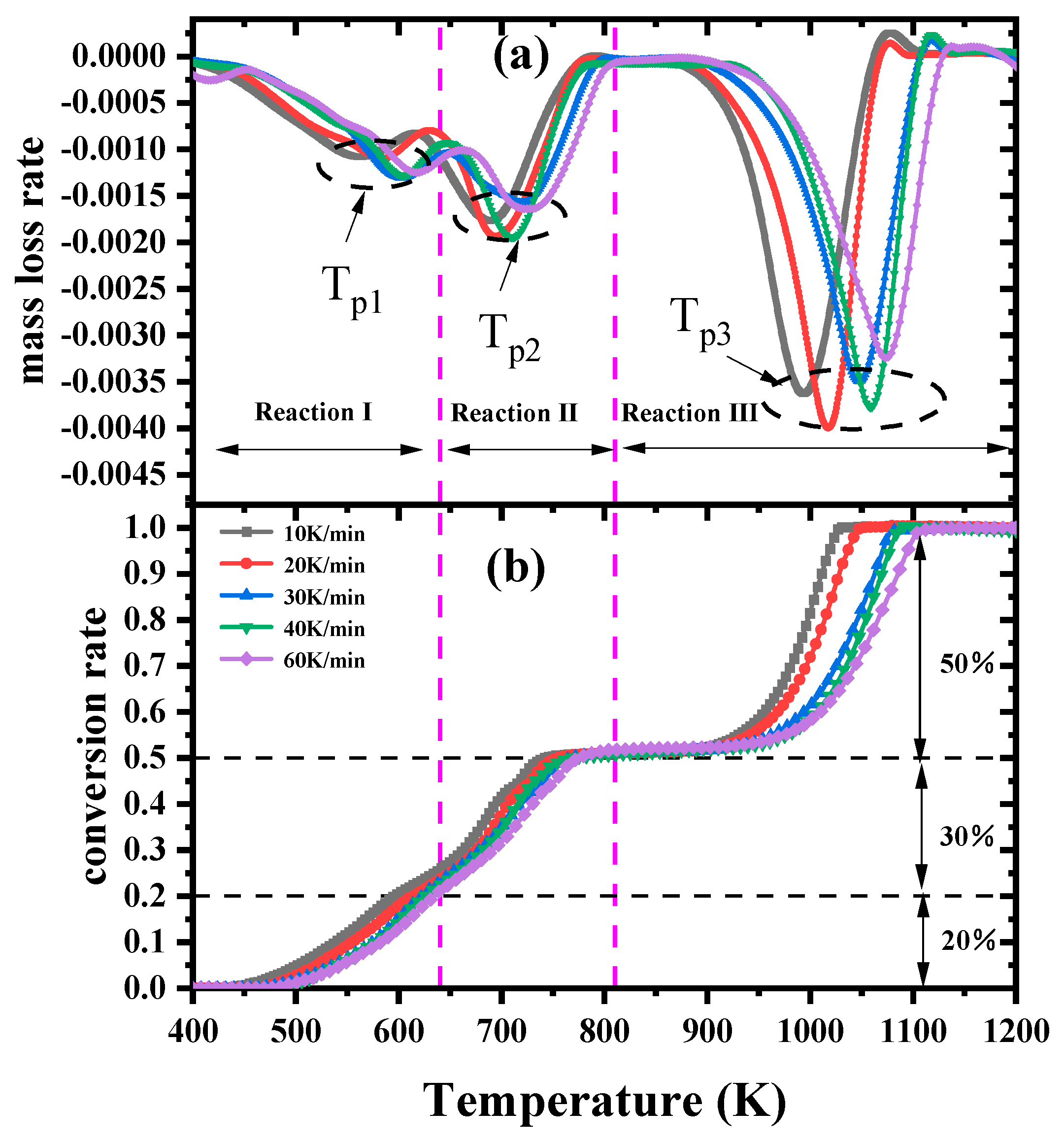
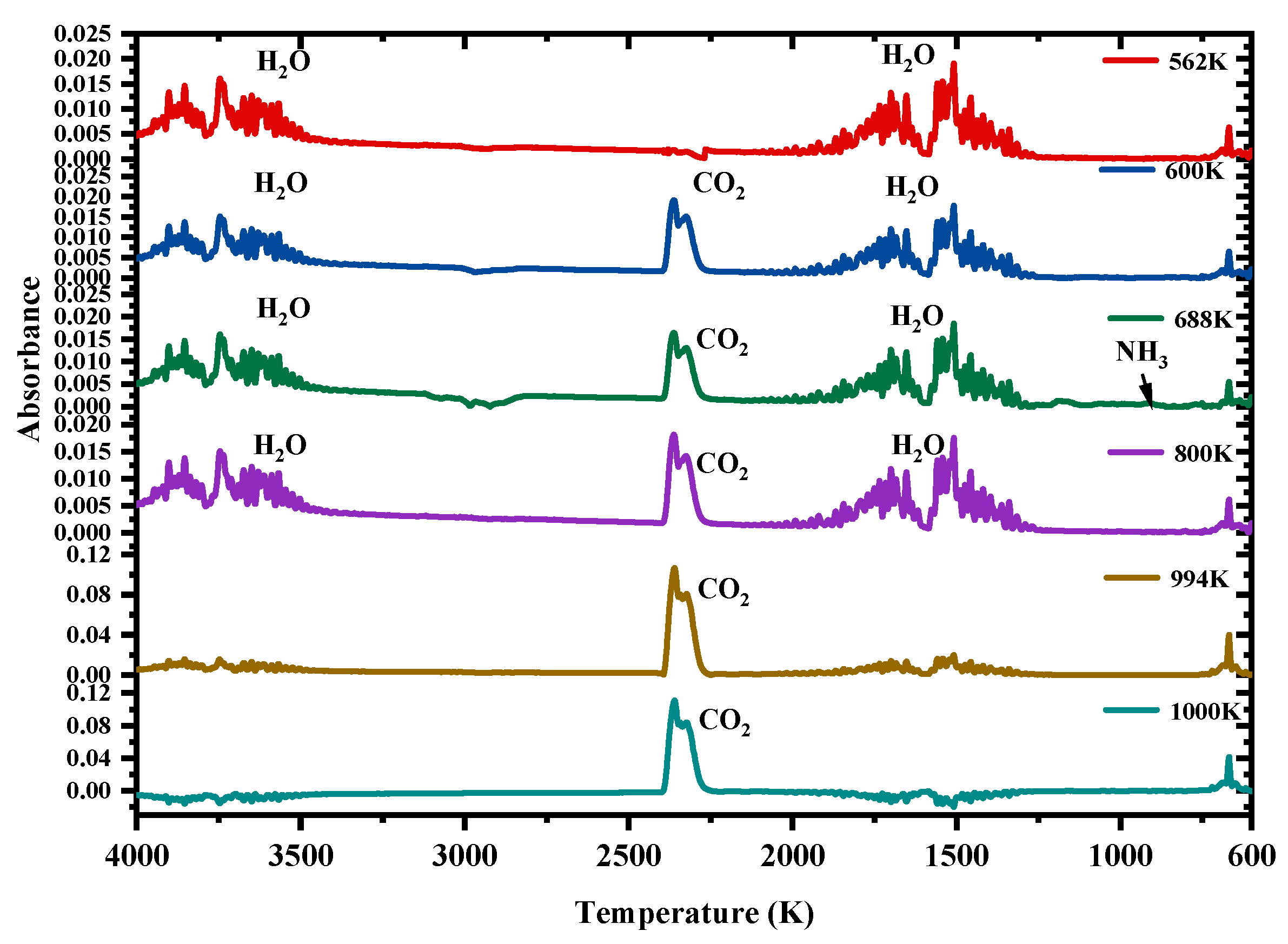
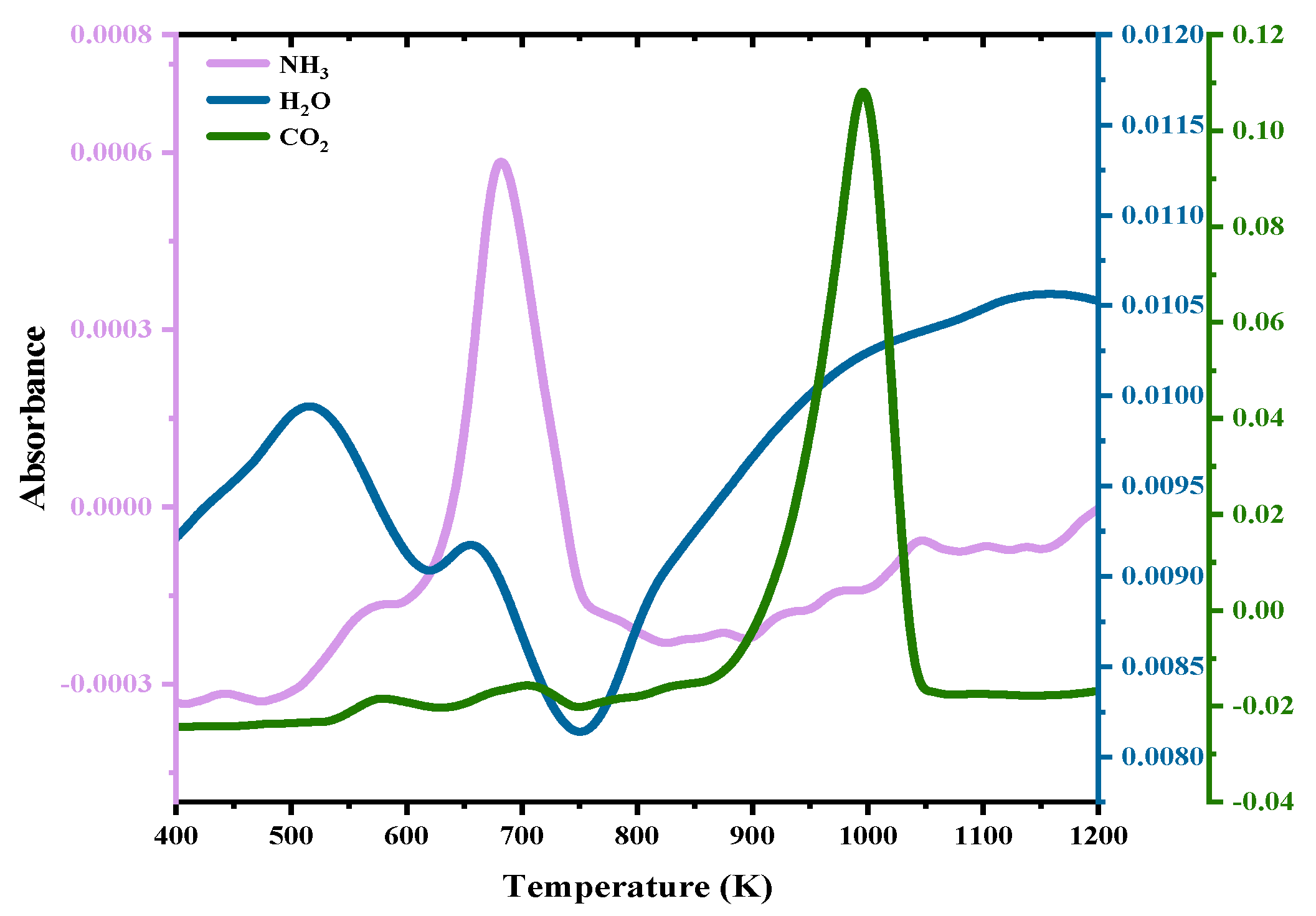
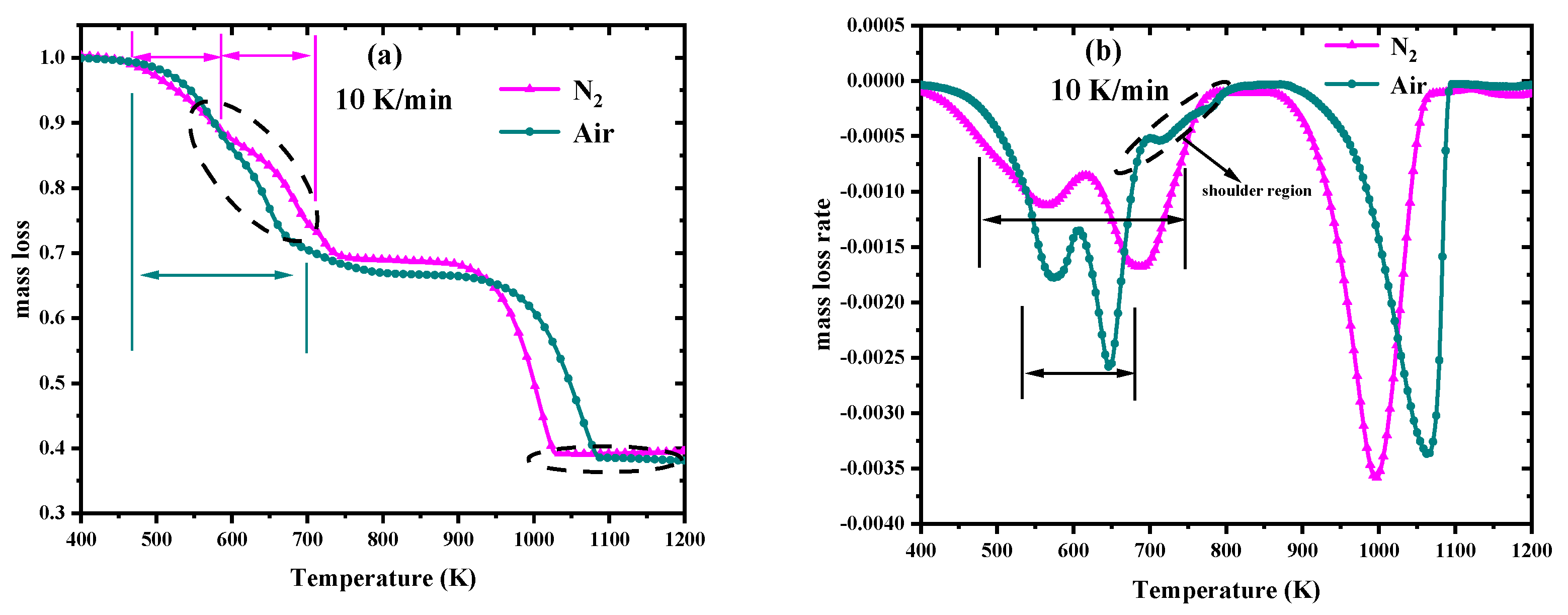
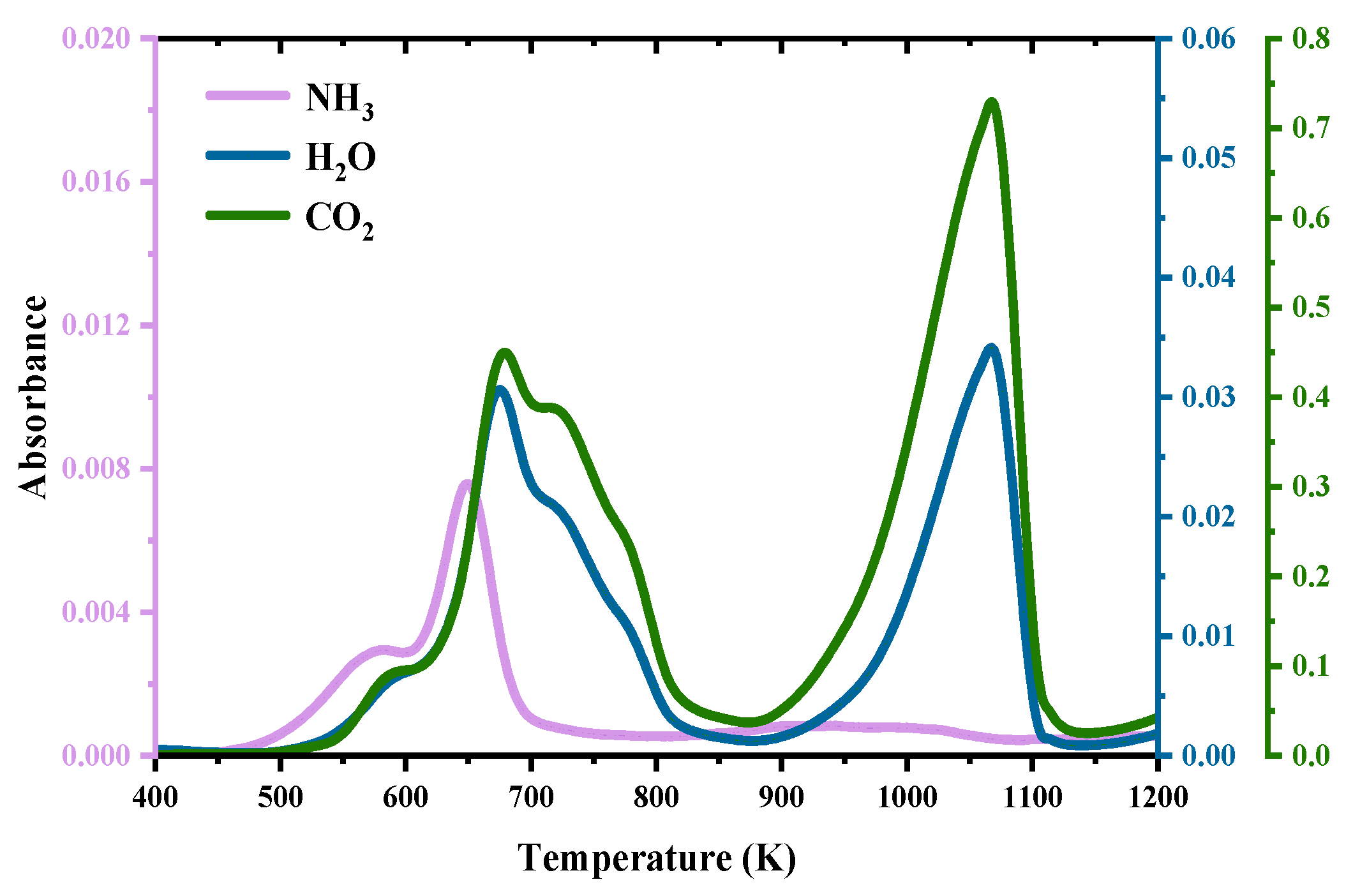
3.1. TG–FTIR Analysis
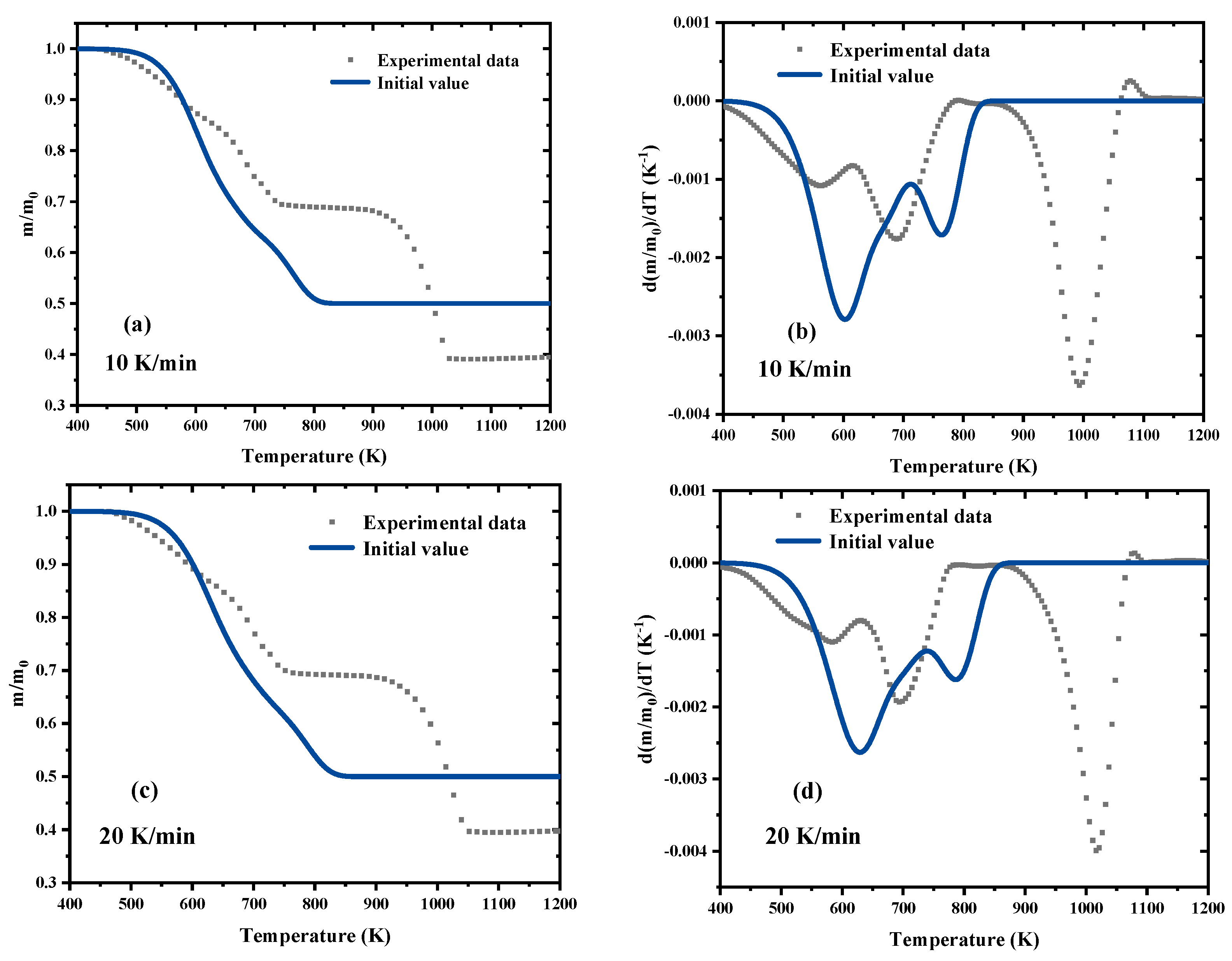
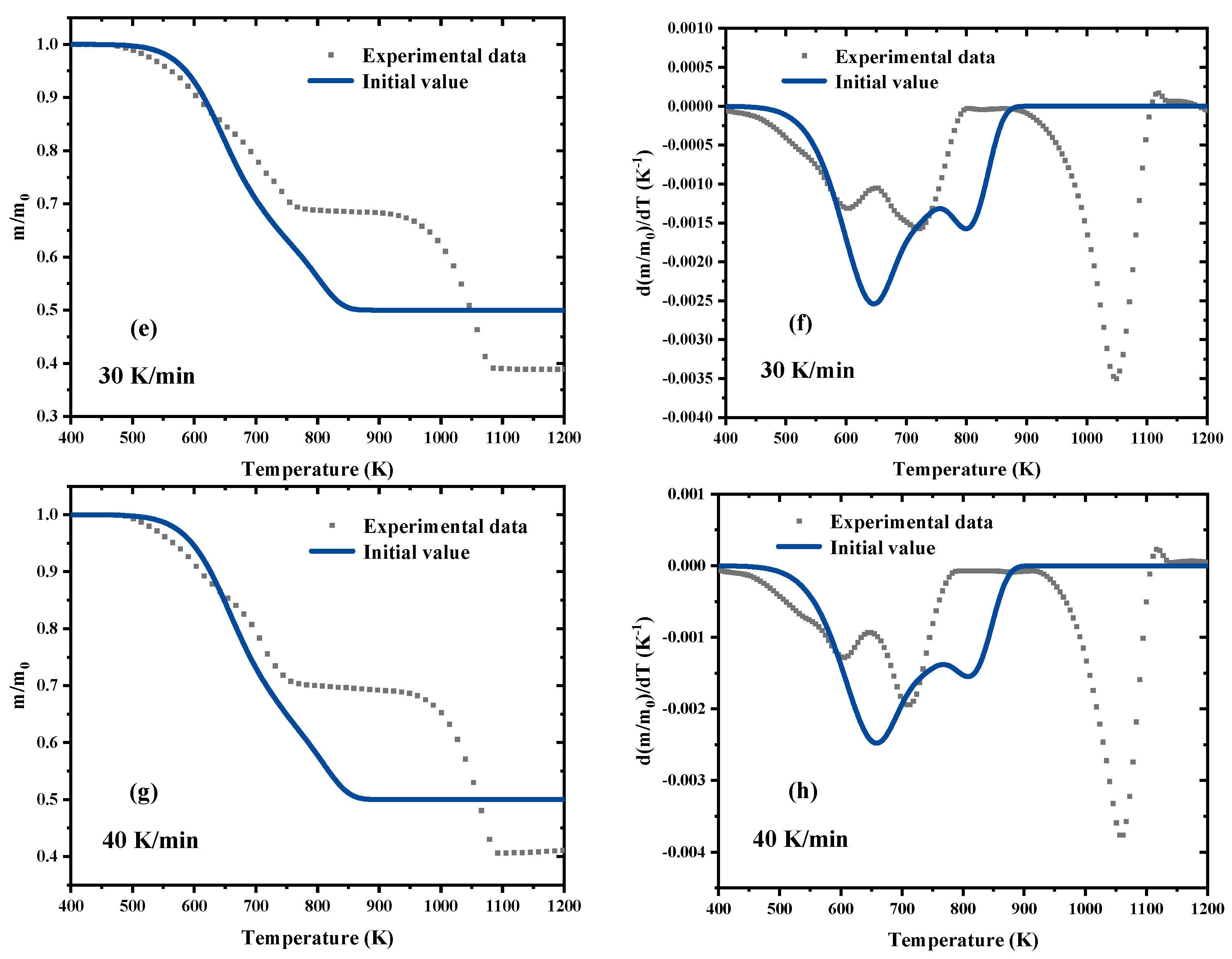
3.2. Kinetic Analysis Based on the Model-Free Methods
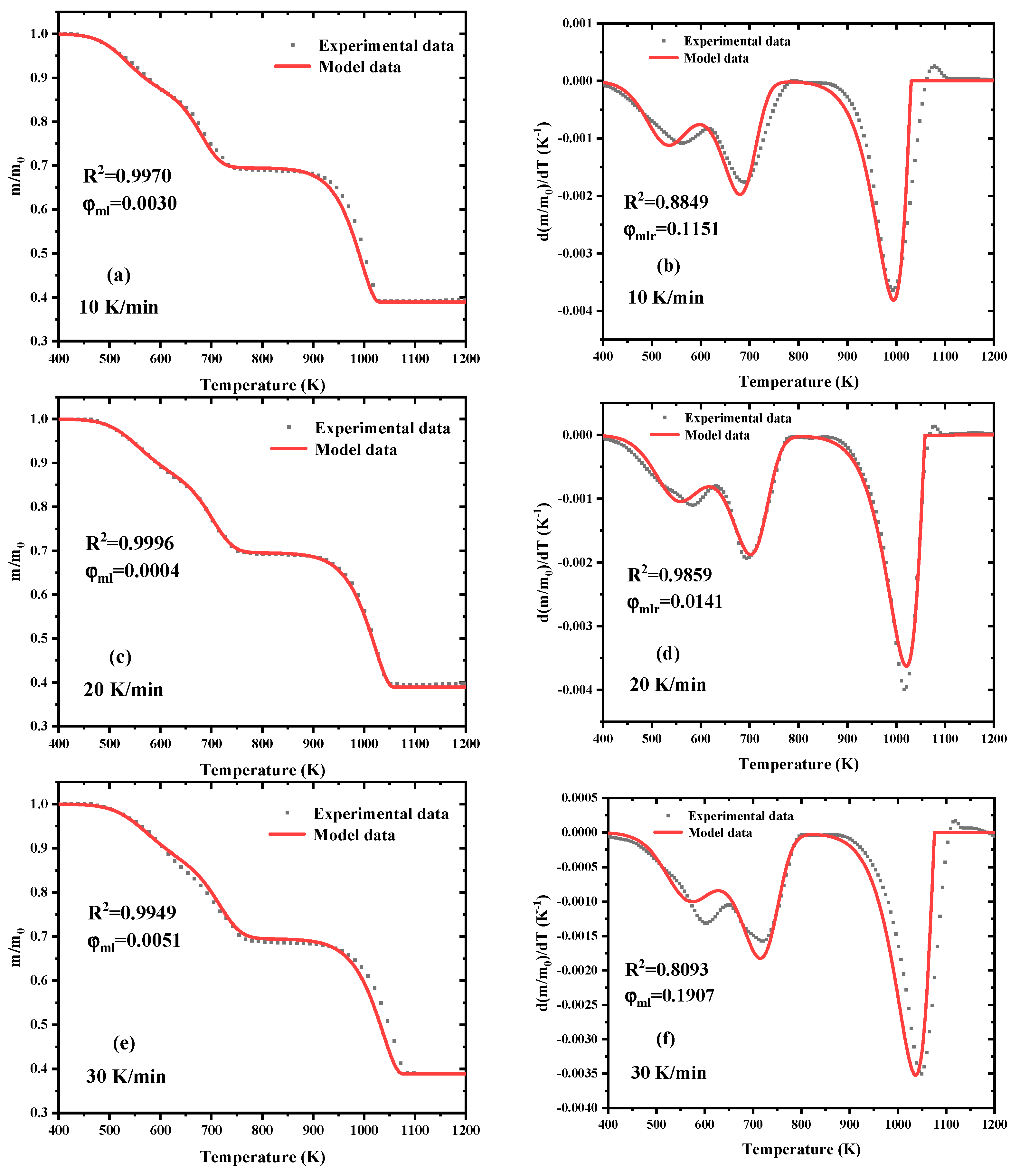
3.3. Parameters Optimization Process by SCE
3.4. Estimated Results at Extra Heating Rate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Higgins, E.; Taylor, M.; Francis, H. A Systemic Approach to Fire Prevention Support. Syst. Pract. Action Res. 2012, 25, 393–406. [Google Scholar] [CrossRef]
- Luo, W.Q.; Li, Z.; Luo, H.; Liu, Y.; Xia, G.; Zhu, H.; Zhou, J.; Yu, D.; Zhang, J.; Song, J.; et al. Preparation of Room Temperature Vulcanized Silicone Rubber Foam with Excellent Flame Retardancy. Scanning 2021, 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.E.; Zhou, Y. Discussion on fire-proof sealing technology and product. In Proceedings of the 2015 International Conference on Performance-Based Fire and Fire Protection Engineering (ICPFFPE 2015), Guangzhou, China, 5–6 December 2015; pp. 644–648. [Google Scholar]
- Klingler, W.W.; Bifulco, A.; Polisi, C.; Huang, Z.Y.; Gaan, S. Recyclable inherently flame-retardant thermosets: Chemistry, properties and applications. Compos. Part B Eng. 2023, 258, 24. [Google Scholar] [CrossRef]
- de Buyl, F. Silicone sealants and structural adhesives. Int. J. Adhes. Adhes. 2001, 21, 411–422. [Google Scholar] [CrossRef]
- Zhao, G.; Chen, W.; Zhao, D.; Li, K. Mechanical Properties of Prefabricated Cold-Formed Steel Stud Wall Panels Sheathed with Fireproof Phenolic Boards under Out-of-Plane Loading. Buildings 2022, 12, 897. [Google Scholar] [CrossRef]
- Janeczek, K.; Kosyl, M.; Araźna, A.; Czaiński, M.; Lipiec, K.; Stęplewski, W.; Kościelski, M. Investigation of Thermal behaviour of Swelling Anti-Fire Composite Materials Identified with RFID Technology. Adv. Mater. Lett. 2022, 13, 1692–2202. [Google Scholar] [CrossRef]
- Zhong, Y.; Ding, Y.M.; Jiang, G.H.; Lu, K.H.; Li, C.H. Comparison of Artificial Neural Networks and kinetic inverse modeling to predict biomass pyrolysis behavior. J. Anal. Appl. Pyrolysis 2023, 169, 10. [Google Scholar] [CrossRef]
- Liu, H.Y.; Wang, Y.S.; Yan, H.; Fan, W.Y.; Song, F.; Yuan, I.V.; He, L. Research of the effect of compactness of fireproof mud plugging and proportion of backing material on fireproof performance. Case Stud. Therm. Eng. 2023, 41, 10. [Google Scholar] [CrossRef]
- Shen, J.J.; Ren, B.H.; Liu, W.T.; Gao, C.P.; Hu, Y.Y.; Cao, Q. Optimization of Exothermic, Foaming, and Mechanical Properties of Modified Polyurethane as Filling Material for Goaf Sealing Wall. Adv. Mater. Sci. Eng. 2020, 2020, 15. [Google Scholar] [CrossRef]
- Kao, S.F.; Wu, Y.S.; Huang, C.J. Combined Fireproof and Stuffing Materials Applied to Through-Floor Pipes. In Proceedings of the International Conference on Advances in Materials and Manufacturing Processes, Shenzhen, China, 6–8 November 2010; p. 1547. [Google Scholar]
- Font, R.; Fullana, A.; Caballero, J.A.; Candela, J.; García, A. Pyrolysis study of polyurethane. J. Anal. Appl. Pyrolysis 2001, 58, 63–77. [Google Scholar] [CrossRef]
- Krutof, A.; Hawboldt, K.A. Upgrading of biomass sourced pyrolysis oil review: Focus on co-pyrolysis and vapour upgrading during pyrolysis. Biomass Convers. Biorefin. 2018, 8, 775–787. [Google Scholar] [CrossRef]
- Rumphorst, M.P.; Ringel, H.D. Pyrolysis of Sewage-Sludge and Use of Pyrolysis Coke. J. Anal. Appl. Pyrolysis 1994, 28, 137–155. [Google Scholar] [CrossRef]
- Dubdub, I. Kinetics Study of Polypropylene Pyrolysis by Non-Isothermal Thermogravimetric Analysis. Materials 2023, 16, 584. [Google Scholar] [CrossRef] [PubMed]
- Jakic, M.; Vrandecic, N.S.; Klaric, I. Thermal degradation of poly(vinyl chloride)/poly(ethylene oxide) blends: Thermogravimetric analysis. Polym. Degrad. Stabil. 2013, 98, 1738–1743. [Google Scholar] [CrossRef]
- Gizynski, M.; Romelczyk-Baishya, B. Investigation of carbon fiber-reinforced thermoplastic polymers using thermogravimetric analysis. J. Thermoplast. Compos. Mater. 2021, 34, 126–140. [Google Scholar] [CrossRef]
- Mocanu, A.M.; Odochian, L.; Apostolescu, N.; Moldoveanu, C. TG–FTIR study on thermal degradation in air of some new diazoaminoderivatives. J. Therm. Anal. Calorim. 2010, 100, 615–622. [Google Scholar] [CrossRef]
- Souza, B.S.; Moreira, A.P.D.; Teixeira, A. TG–FTIR coupling to monitor the pyrolysis products from agricultural residues. J. Therm. Anal. Calorim. 2009, 97, 637–642. [Google Scholar] [CrossRef]
- Singh, R.K.; Ruj, B.; Sadhukhan, A.K.; Gupta, P. A TG–FTIR investigation on the co-pyrolysis of the waste HDPE, PP, PS and PET under high heating conditions. J. Energy Inst. 2020, 93, 1020–1035. [Google Scholar] [CrossRef]
- Xu, F.F.; Wang, B.; Yang, D.; Hao, J.H.; Qiao, Y.Y.; Tian, Y.Y. Thermal degradation of typical plastics under high heating rate conditions by TG–FTIR: Pyrolysis behaviors and kinetic analysis. Energy Conv. Manag. 2018, 171, 1106–1115. [Google Scholar] [CrossRef]
- Ding, Y.M.; Jiang, G.H.; Fukumoto, K.; Zhao, M.Q.; Zhang, X.T.; Wang, C.J.; Li, C.H. Experimental and numerical simulation of multi-component combustion of typical no-charring material. Energy 2023, 262, 9. [Google Scholar] [CrossRef]
- Kajda-Szczesniak, M.; Czop, M. Comparison of Pyrolysis and Combustion Processes of Vinyl Floor Panels Using Thermogravimetric Analysis (TG–FTIR) in Terms of the Circular Economy. Energies 2022, 15, 1516. [Google Scholar] [CrossRef]
- Miskolczi, N.; Tomasek, S. Investigation of Pyrolysis Behavior of Sewage Sludge by Thermogravimetric Analysis Coupled with Fourier Transform Infrared Spectrometry Using Different Heating Rates. Energies 2022, 15, 5116. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Shang, F.J.; Huang, Y.B.; Zhang, J.; Guo, Y.; He, L.X.; Wang, L.F.; Zhu, T.Y.; Liu, R.; Liu, P.; et al. Pyrolysis characteristics, reaction mechanisms and gas emission of organic fireproof plugging materials by TG–FTIR-MS. J. Therm. Anal. Calorim. 2023, 148, 12751–12760. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Wight, C.A. Isothermal and non-isothermal kinetics of thermally stimulated reactions of solids. Int. Rev. Phys. Chem. 1998, 17, 407–433. [Google Scholar] [CrossRef]
- Ding, Y.M.; Wang, C.J.; Chaos, M.; Chen, R.Y.; Lu, S.X. Estimation of beech pyrolysis kinetic parameters by Shuffled Complex Evolution. Bioresour. Technol. 2016, 200, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Mariani, V.C.; Luvizotto, L.G.J.; Guerra, F.A.; Coelho, L.D. A hybrid shuffled complex evolution approach based on differential evolution for unconstrained optimization. Appl. Math. Comput. 2011, 217, 5822–5829. [Google Scholar] [CrossRef]
- Liu, H.R.; Chen, B.; Wang, C.J. Pyrolysis kinetics study of biomass waste using Shuffled Complex Evolution algorithm. Fuel Process. Technol. 2020, 208, 8. [Google Scholar] [CrossRef]
- Zhang, W.L.; Zhang, J.; Ding, Y.M.; Zhou, R.; Mao, S.H. The accuracy of multiple methods for estimating the reaction order of representative thermoplastic polymers waste for energy utilization. Energy 2022, 239, 8. [Google Scholar] [CrossRef]
- Zhang, X.D.; Xu, M.; Sun, R.F.; Sun, L. Study on biomass pyrolysis kinetics. In Proceedings of the 50th ASME Turbo-Expo 2005, Reno, NV, USA, 6–9 June 2005; pp. 401–405. [Google Scholar]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Gil, M.V.; Casal, D.; Pevida, C.; Pis, J.J.; Rubiera, F. Thermal behaviour and kinetics of coal/biomass blends during co-combustion. Bioresour. Technol. 2010, 101, 5601–5608. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.Y.; Sorooshian, S.; Gupta, V.K. Optimal Use of the SCE-UA Global Optimization Method for Calibrating Watershed Models. J. Hydrol. 1994, 158, 265–284. [Google Scholar] [CrossRef]
- Duan, Q.Y.; Gupta, V.K.; Sorooshian, S. Shuffled Complex Evolution Approach for Effective and Efficient Global Minimization. J. Optim. Theory Appl. 1993, 76, 501–521. [Google Scholar] [CrossRef]
- Chaos, M.; Khan, M.; Krishnamoorthy, N.; de Ris, J.; Dorofeev, S. FPA bench-scale flammability tests and extraction of solid fuel properties for fire models. In Proceedings of the 6th International Seminar on Fire and Explosion Hazards (ISFEH6), Leeds, UK, 11–16 April 2011; pp. 995–1007. [Google Scholar]
- Chaos, M.; Khan, M.; Krishnamoorthy, N.; De Ris, J.; Dorofeev, S. Bench-scale flammability experiments: Determination of material properties using pyrolysis models for use in CFD fire simulations. In Proceedings of the 12th International Fire Science and Engineering Conference, Interflam2010, Nottingham, UK, 5–7 July 2010; pp. 697–708. [Google Scholar]
- Chaos, M.; Khan, M.M.; Krishnamoorthy, N.; de Ris, J.L.; Dorofeev, S.B. Evaluation of optimization schemes and determination of solid fuel properties for CFD fire models using bench-scale pyrolysis tests. Proc. Combust. Inst. 2011, 33, 2599–2606. [Google Scholar] [CrossRef]
- Gopalakrishnan, K.; Kim, S. Global Optimization of Pavement Structural Parameters during Back-Calculation Using Hybrid Shuffled Complex Evolution Algorithm. J. Comput. Civil. Eng. 2010, 24, 441–451. [Google Scholar] [CrossRef]
- Gomes, R.C.M.; Vitorino, M.A.; Corrêa, M.B.D.; Fernandes, D.A.; Wang, R.X. Shuffled Complex Evolution on Photovoltaic Parameter Extraction: A Comparative Analysis. IEEE Trans. Sustain. Energy 2017, 8, 805–815. [Google Scholar] [CrossRef]
- Ding, Y.M.; Fukumoto, K.; Ezekoye, O.A.; Lu, S.X.; Wang, C.J.; Li, C.H. Experimental and numerical simulation of multi-component combustion of typical charring material. Combust. Flame 2020, 211, 417–429. [Google Scholar] [CrossRef]
- Luo, F.B.; Wu, K.; Lu, M.G.; Nie, S.B.; Li, X.Y.; Guan, X.X. Thermal degradation and flame retardancy of microencapsulated ammonium polyphosphate in rigid polyurethane foam. J. Therm. Anal. Calorim. 2015, 120, 1327–1335. [Google Scholar] [CrossRef]
- Yi, D.Q.; Yang, R.J. Ammonium Polyphosphate/Montmorillonite Nanocompounds in Polypropylene. J. Appl. Polym. Sci. 2010, 118, 834–840. [Google Scholar] [CrossRef]
- Huang, Z.; Ruan, B.; Wu, J.; Ma, N.; Jiang, T.; Tsai, F.C. High-efficiency ammonium polyphosphate intumescent encapsulated polypropylene flame retardant. J. Appl. Polym. Sci. 2021, 138, 11. [Google Scholar] [CrossRef]
- Samyn, F.; Bourbigot, S.; Duquesne, S.; Delobel, R. Effect of zinc borate on the thermal degradation of ammonium polyphosphate. Thermochim. Acta 2007, 456, 134–144. [Google Scholar] [CrossRef]
- Yu, S.W.; Xiao, S.J.; Zhao, Z.W.; Huo, X.W.; Wei, J.F. Microencapsulated ammonium polyphosphate by polyurethane with segment of dipentaerythritol and its application in flame retardant polypropylene. Chin. J. Chem. Eng. 2019, 27, 1735–1743. [Google Scholar] [CrossRef]
- Drevelle, C.; Duquesne, S.; Le Bras, M.; Lefebvre, J.; Delobel, R.; Castrovinci, A.; Magniez, C.; Vouters, M. Influence of ammonium polyphosphate on the mechanism of thermal degradation of an acrylic binder resin. J. Appl. Polym. Sci. 2004, 94, 717–729. [Google Scholar] [CrossRef]
- He, J.J.; Jiang, L.; Sun, J.H.; Lo, S.M. Thermal degradation study of pure rigid polyurethane in oxidative and non-oxidative atmospheres. J. Anal. Appl. Pyrolysis 2016, 120, 269–283. [Google Scholar] [CrossRef]


| Reaction | Conversion Rate | Friedman | KAS | Starink | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ea | R2 | lnA | Ea | R2 | lnA | Ea | R2 | lnA | ||
| I | 0.1 | 89.64 | 0.97 | 18.21 | 73.45 | 0.93 | 1.77 | 73.76 | 0.93 | 2.66 |
| 0.2 | 160.05 | 0.92 | 29.69 | 126.36 | 0.95 | 10.80 | 126.66 | 0.95 | 11.70 | |
| Average | 124.84 | 23.95 | 99.91 | 6.29 | 100.21 | 7.18 | ||||
| II | 0.3 | 235.62 | 0.95 | 40.29 | 240.70 | 0.92 | 28.61 | 240.95 | 0.92 | 29.52 |
| 0.4 | 188.28 | 0.74 | 30.16 | 183.12 | 0.96 | 17.26 | 183.44 | 0.96 | 18.18 | |
| 0.5 | 164.21 | 0.79 | 23.07 | 183.75 | 0.98 | 15.59 | 184.10 | 0.98 | 16.51 | |
| Average | 196.03 | 31.17 | 202.52 | 20.49 | 202.83 | 21.40 | ||||
| III | 0.6 | 177.69 | 0.90 | 19.44 | 187.75 | 0.91 | 9.18 | 188.25 | 0.91 | 10.12 |
| 0.7 | 171.25 | 0.89 | 18.52 | 181.82 | 0.91 | 8.06 | 182.35 | 0.91 | 9.00 | |
| 0.8 | 165.18 | 0.86 | 17.57 | 176.65 | 0.90 | 7.34 | 177.19 | 0.90 | 8.29 | |
| 0.9 | 172.01 | 0.89 | 17.99 | 172.28 | 0.89 | 6.90 | 172.84 | 0.89 | 7.84 | |
| Average | 171.54 | 18.38 | 179.63 | 7.87 | 180.15 | 8.81 | ||||
| Parameters | Initial Values | Search Range | Optimized Values |
|---|---|---|---|
| YAPP,0 | 0.30 | [0.00, 0.40] | 0.19 |
| ln(ZAPP) | 12.47 | [2.49, 24.94] | 14.70 |
| Ea,APP | 108.32 | [21.64, 216.64] | 113.47 |
| nAPP | 1.00 | [0.00, 5.00] | 1.09 |
| νAPP | 0.50 | [0.00, 1.00] | 0.11 |
| YMEL,0 | 0.30 | [0.00, 0.40] | 0.39 |
| ln(ZMEL) | 24.35 | [4.87, 48.70] | 19.52 |
| Ea,MEL | 200.46 | [40.09, 400.92] | 206.35 |
| nMEL | 1.00 | [0.00, 5.00] | 0.62 |
| νMEL | 0.50 | [0.00, 1.00] | 0.23 |
| ln(ZAA) | 11.69 | [2.34, 23.38] | 9.36 |
| Ea,AA | 171.54 | [34.31, 343.08] | 65.68 |
| nAA | 1.00 | [0.00, 5.00] | 1.88 |
| νAA | 0.50 | [0.00, 1.00] | 0.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Xu, X.; Zhang, J.; Zhong, Y.; Li, X.; Ding, Y. Thermal Decomposition Process of Fireproof Sealant Measured with Thermogravimetric and Fourier Transform Infrared Spectroscopy Analysis and Estimated Using Shuffled Complex Evolution. Fire 2024, 7, 25. https://doi.org/10.3390/fire7010025
Liu W, Xu X, Zhang J, Zhong Y, Li X, Ding Y. Thermal Decomposition Process of Fireproof Sealant Measured with Thermogravimetric and Fourier Transform Infrared Spectroscopy Analysis and Estimated Using Shuffled Complex Evolution. Fire. 2024; 7(1):25. https://doi.org/10.3390/fire7010025
Chicago/Turabian StyleLiu, Wei, Xinrong Xu, Jiaqing Zhang, Yu Zhong, Xiang Li, and Yanming Ding. 2024. "Thermal Decomposition Process of Fireproof Sealant Measured with Thermogravimetric and Fourier Transform Infrared Spectroscopy Analysis and Estimated Using Shuffled Complex Evolution" Fire 7, no. 1: 25. https://doi.org/10.3390/fire7010025






