Burning Properties of Combined Glued Laminated Timber
Abstract
:1. Introduction
2. Materials and Methods
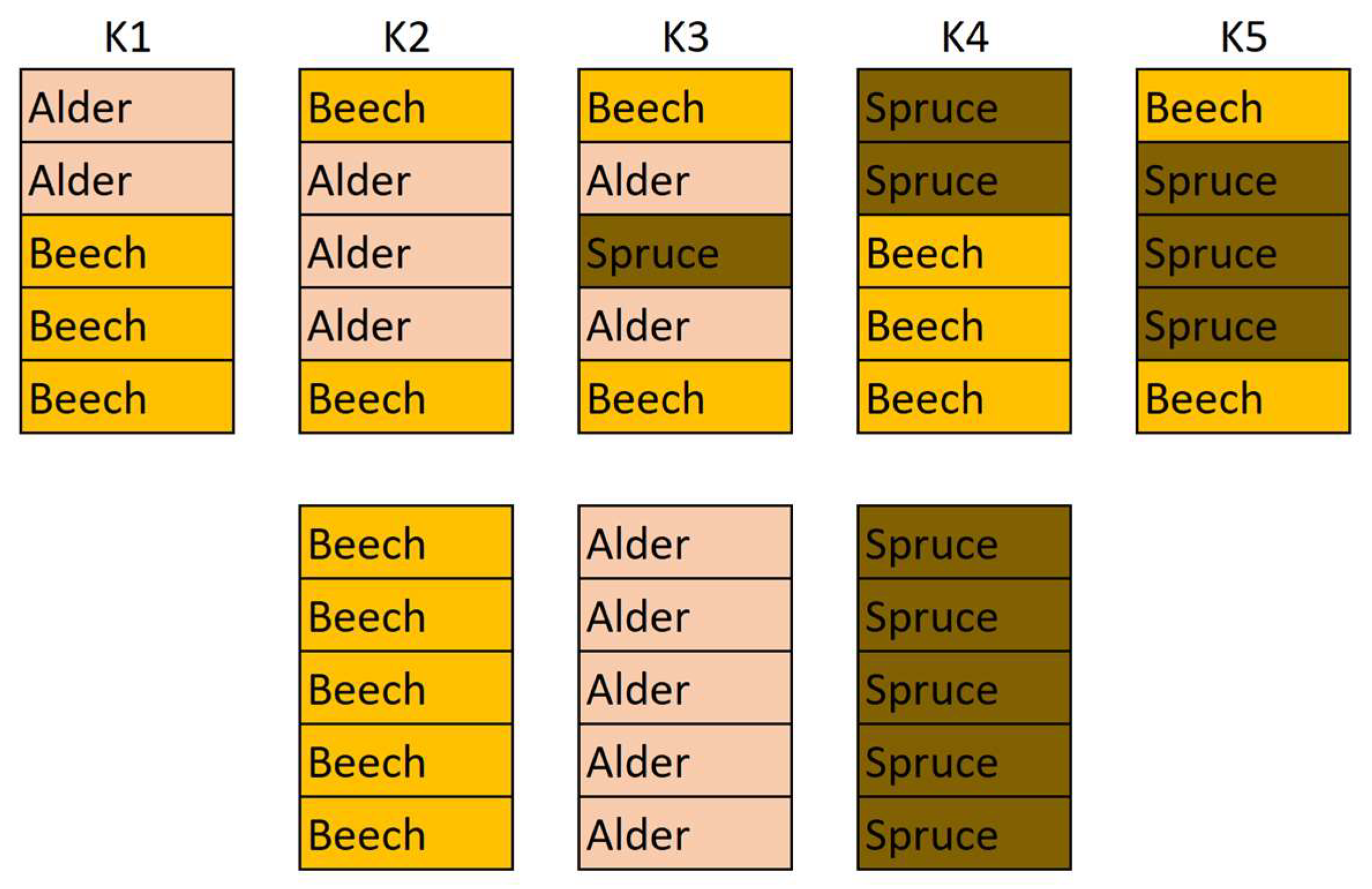
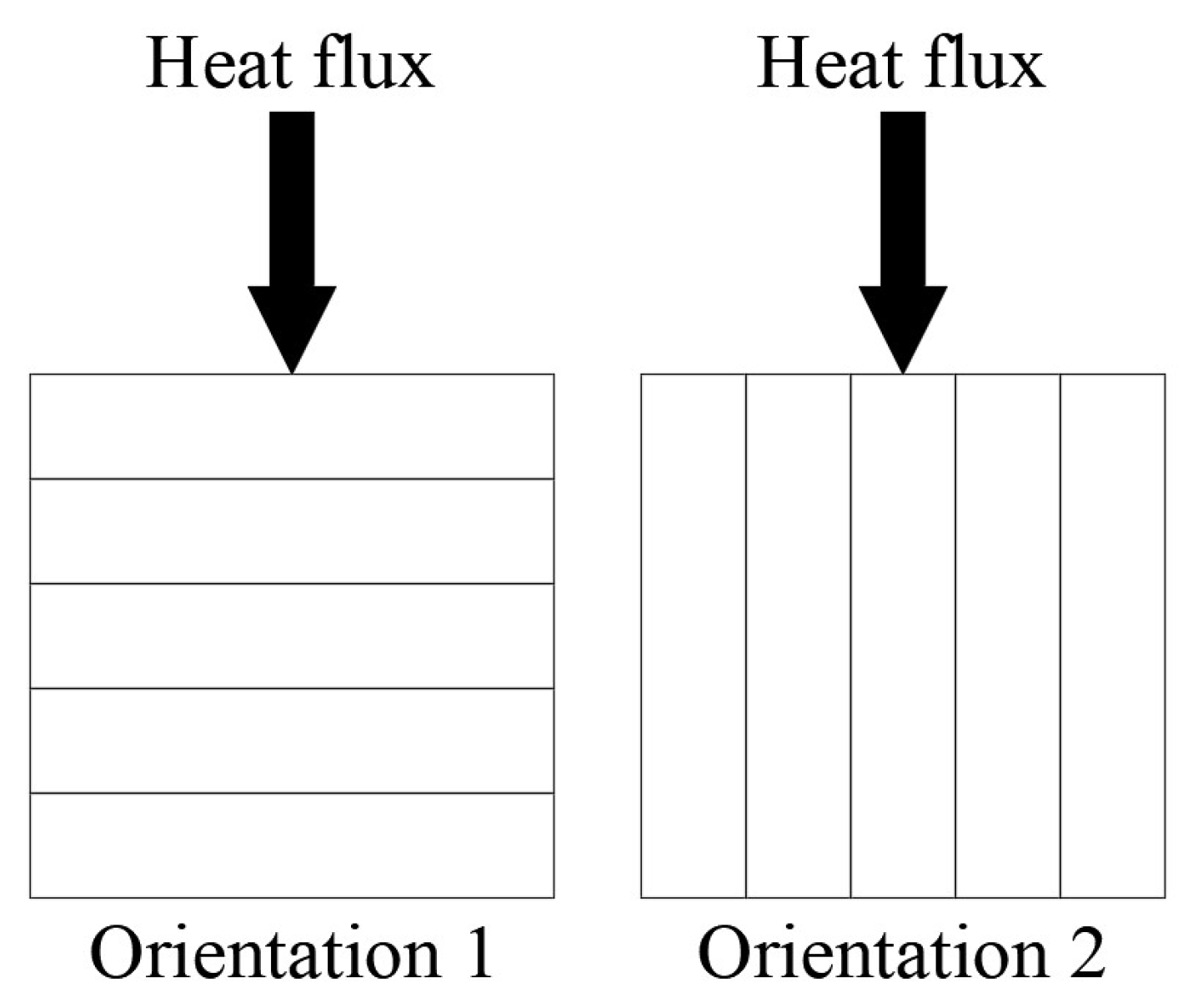
2.1. Sample Preparation
2.2. Experiment Design
- -
- Heat release rate (HRR) and its peak value (pHRR);
- -
- Mass loss rate (MLR);
- -
- Average rate of heat emission (ARHE) and its peak value (MARHE);
- -
- Effective heat of combustion (EHC).
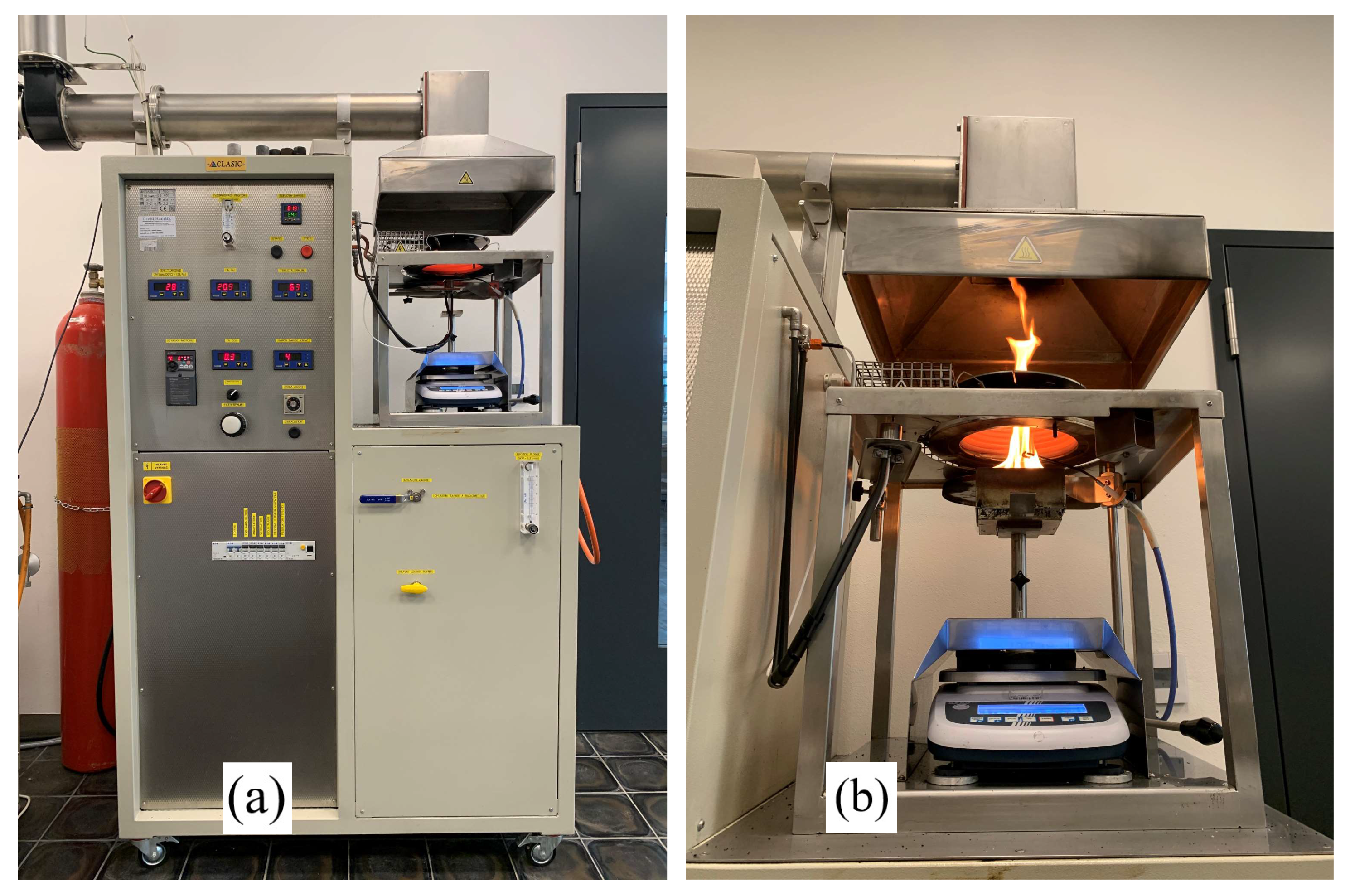
2.3. Calculations
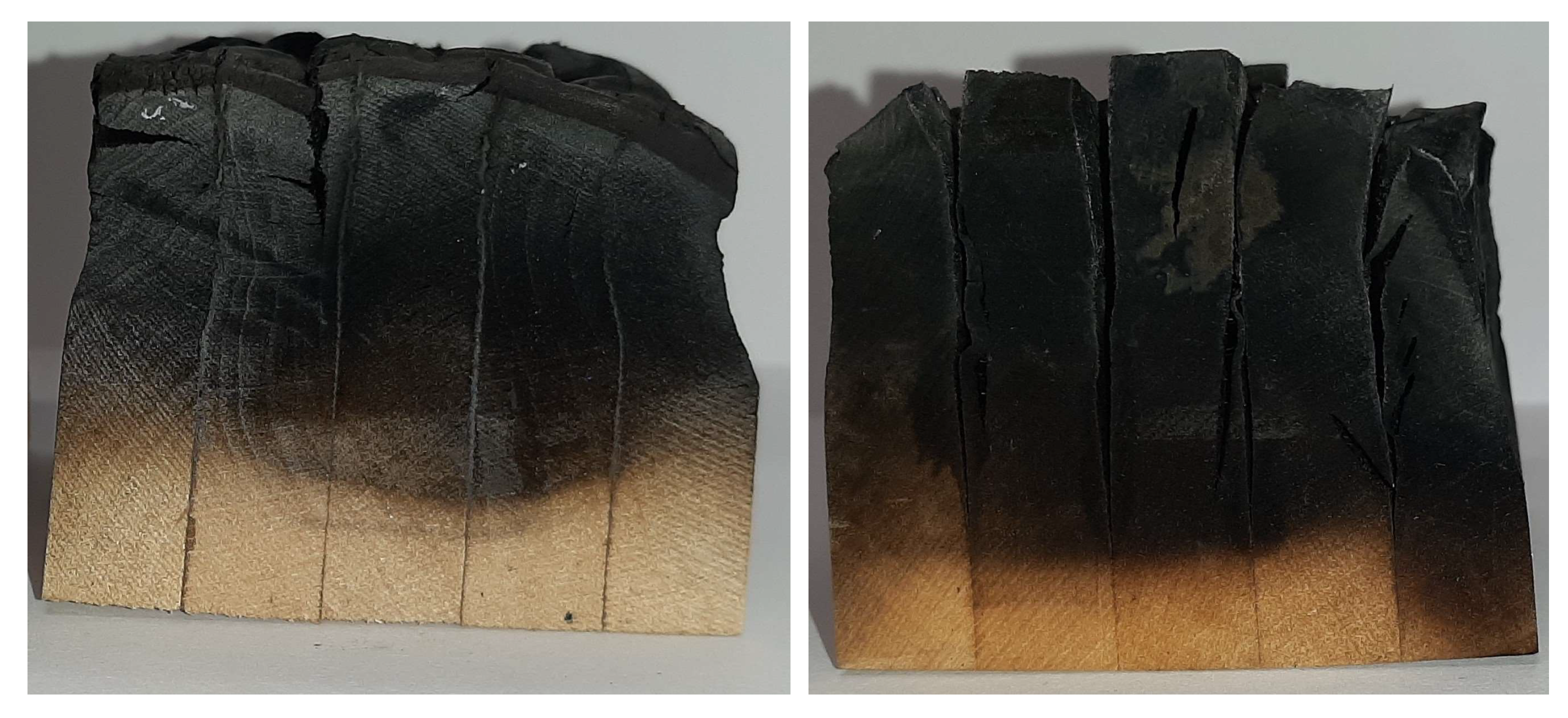
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barber, D. Tall Timber Buildings: What’s Next in Fire Safety? Fire Technol. 2015, 51, 1279–1284. [Google Scholar] [CrossRef]
- Drysdale, D. An Introduction to Fire Dynamics, 3rd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2011; ISBN 978-0-470-31903-1. [Google Scholar]
- Cuevas, J.; Torero, J.L.; Maluk, C. Flame extinction and burning behaviour of timber under varied oxygen concentrations. Fire Saf. J. 2021, 120, 103087. [Google Scholar] [CrossRef]
- Lin, S.; Qin, Y.; Huang, X.; Gollner, M. Use of pre-charred surfaces to improve fire performance of wood. Fire Saf. J. 2023, 136, 103745. [Google Scholar] [CrossRef]
- Morrisset, D.; Hadden, R.M.; Bartlett, A.I.; Law, A.; Emberley, R. Time dependent contribution of char oxidation and flame heat feedback on the mass loss rate of timber. Fire Saf. J. 2021, 120, 103058. [Google Scholar] [CrossRef]
- Gong, J.; Yang, L. A Review on Flaming Ignition of Solid Combustibles: Pyrolysis Kinetics, Experimental Methods and Modelling. Fire Technol. 2022. [Google Scholar] [CrossRef]
- Bartlett, A.I.; Hadden, R.M.; Bisby, L.A. A Review of Factors Affecting the Burning Behaviour of Wood for Application to Tall Timber Construction. Fire Technol. 2019, 55, 1–49. [Google Scholar] [CrossRef]
- Simms, D.L.; Law, M. The ignition of wet and dry wood by radiation. Combust. Flame 1967, 11, 377–388. [Google Scholar] [CrossRef]
- Emberley, R.; Inghelbrecht, A.; Yu, Z.; Torero, J.L. Self-extinction of timber. Proc. Combust. Inst. 2017, 36, 3055–3062. [Google Scholar] [CrossRef]
- Di Blasi, C. Modeling chemical and physical processes of wood and biomass pyrolysis. Prog. Energy Combust. Sci. 2008, 34, 47–90. [Google Scholar] [CrossRef]
- Senneca, O.; Cerciello, F. Kinetics of combustion of lignocellulosic biomass: Recent research and critical issues. Fuel 2023, 347, 128310. [Google Scholar] [CrossRef]
- Di Blasi, C.; Branca, C.; Santoro, A.; Gonzalez Hernandez, E. Pyrolytic behavior and products of some wood varieties. Combust. Flame 2001, 124, 165–177. [Google Scholar] [CrossRef]
- Radmanović, K.; Đukić, I.; Pervan, S. Specific Heat Capacity of Wood. Drv. Ind. 2014, 65, 151–157. [Google Scholar] [CrossRef]
- Pečenko, R.; Svensson, S.; Hozjan, T. Modelling heat and moisture transfer in timber exposed to fire. Int. J. Heat Mass Transf. 2015, 87, 598–605. [Google Scholar] [CrossRef]
- Friquin, K.L. Material properties and external factors influencing the charring rate of solid wood and glue-laminated timber. Fire Mater. 2011, 35, 303–327. [Google Scholar] [CrossRef]
- Kurt, Ş.; Korkmaz, M. Effects of Moisture and Direction of Grain on the Thermal Conductivity and Mechanical Properties of Black Alder and Scots Pine. Kastamonu Üniversitesi Orman Fakültesi Derg. 2022, 22, 33–39. [Google Scholar] [CrossRef]
- CALCULLA-Specific Heat of Substances Table [WWW Document]. Available online: https://calculla.com/specific_heat_table (accessed on 2 January 2024).
- Flity, H.; Jannot, Y.; Terrei, L.; Lardet, P.; Schick, V.; Acem, Z.; Parent, G. Thermal conductivity parallel and perpendicular to fibers direction and heat capacity measurements of eight wood species up to 160 °C. Int. J. Therm. Sci. 2024, 195, 108661. [Google Scholar] [CrossRef]
- Morrisset, D.; Santamaria, S.; Hadden, R.; Emberley, R. Implications of data smoothing on experimental mass loss rates. Fire Saf. J. 2022, 131, 103611. [Google Scholar] [CrossRef]
- Utiskul, Y.P.; Quintiere, J.G. An application of mass loss rate model with fuel response effects in fully-developed compartment fires. Fire Saf. Sci. 2008, 9, 827–838. [Google Scholar] [CrossRef]
- Lowden, L.A.; Hull, T.R. Flammability behaviour of wood and a review of the methods for its reduction. Fire Sci. Rev. 2013, 2, 4. [Google Scholar] [CrossRef]
- Babrauskas, V. Heat Release Rates. In SFPE Handbook of Fire Protection Engineering; Hurley, M.J., Gottuk, D., Hall, J.R., Harada, K., Kuligowski, E., Puchovsky, M., Torero, J., Watts, J.M., Wieczorek, C., Eds.; Springer: New York, NY, USA, 2016; pp. 799–904. [Google Scholar] [CrossRef]
- White, R.H.; Dietenberger, M.A. Fire safety of wood construction. In Wood Handbook—Wood as an Engineering Material; Department of Agriculture, Forest Service, Forest Products Laboratory: Madison, WI, USA, 2010. [Google Scholar]
- Schmid, J.; Just, A.; Klippel, M.; Fragiacomo, M. The Reduced Cross-Section Method for Evaluation of the Fire Resistance of Timber Members: Discussion and Determination of the Zero-Strength Layer. Fire Technol. 2015, 51, 1285–1309. [Google Scholar] [CrossRef]
- Bowyer, J.; Bratkovich, S.; Howe, J.; Fernholz, K.; Frank, M.; Hanessian, S.; Groot, H. Modern Tall Wood Buildings: Opportunities for Innovation; Dovetail Partners, Inc.: Minneapolis, MN, USA, 2016. [Google Scholar]
- Xing, Z.; Wang, Y.; Zhang, J.; Ma, H. Comparative study on fire resistance and zero strength layer thickness of CLT floor under natural fire and standard fire. Constr. Build. Mater. 2021, 302, 124368. [Google Scholar] [CrossRef]
- Uzelac Glavinić, I.; Boko, I.; Torić, N.; Lovrić Vranković, J. Application of hardwood for glued laminated timber in Europe. J. Croat. Assoc. Civil Eng. 2020, 72, 607–616. [Google Scholar] [CrossRef]
- Wang, S.; Ding, P.; Lin, S.; Huang, X.; Usmani, A. Deformation of wood slice in fire: Interactions between heterogeneous chemistry and thermomechanical stress. Proc. Combust. Inst. 2021, 38, 5081–5090. [Google Scholar] [CrossRef]
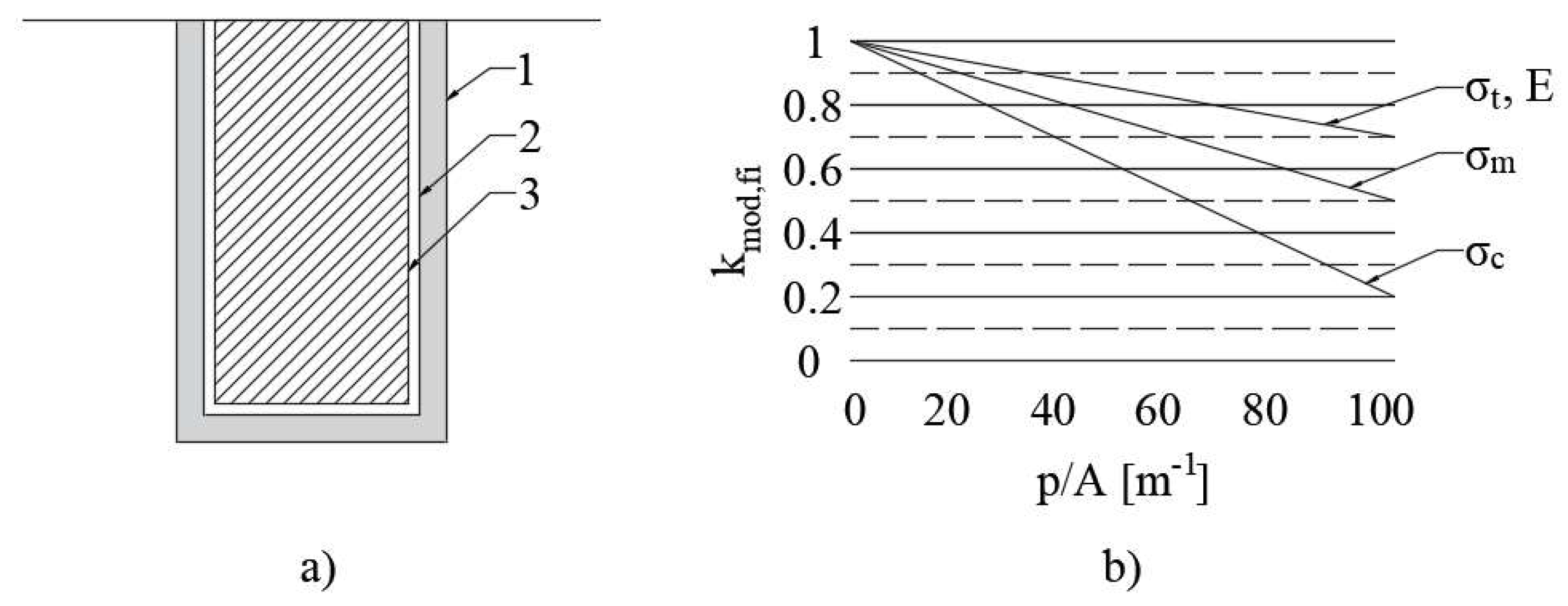
- ČSN EN 1995-1-2; Eurocode 5: Design of Timber Structures-Part 1–2: General-Structural Fire Design. Czech Standardization Agency: Prague, Czech Republic, 2006.
- Ohnesorge, D.; Henning, M.; Becker, G. Bedeutung von Laubholz bei der Brettschichtholzherstellung. Holztechnologie 2009, 6, 47–49. [Google Scholar]
- Li, J.; Stoliarov, S.I. Measurement of kinetics and thermodynamics of the thermal degradation for charring polymers. Polym. Degrad. Stab. 2013, 106, 2–15. [Google Scholar] [CrossRef]
- Richter, K.; Pizzi, A.P.; Despres, A. Thermal stability of structural one-component polyurethane adhesives for wood-Structure-property relationship. J. Appl. Polym. Sci. 2006, 102, 5698–5707. [Google Scholar] [CrossRef]
- Clauß, S.; Gabriel, J.; Karbach, A.; Matner, M.; Niemz, P. Influence of the adhesive formulation on the mechanical properties and bonding performance of polyurethane prepolymers. Holzforschung 2011, 65, 835–844. [Google Scholar] [CrossRef]
- Frangi, A.; Fontana, M.; Mischler, A. Shear behaviour of bond lines in glued laminated timber beams at high temperatures. Wood Sci. Technol. 2004, 38, 119–126. [Google Scholar] [CrossRef]
- Brandon, D.; Östman, B. Fire Safety Challenges of Tall Wood Buildings–Phase 2: Task 1-Literature Review; Fire Protection Research Foundation: Quincy, MA, USA, 2016. [Google Scholar]
- Spearpoint, M.J.; Quintiere, J.G. Predicting the burning of wood using an integral model. Combust. Flame 2000, 123, 308–325. [Google Scholar] [CrossRef]
- Kytka, T.; Gašparík, M.; Sahula, L.; Karami, E.; Teterin, D.; Das, S.; Novák, D.; Sarvašová Kvietková, M. Bending characteristics of glued laminated timber depending on the alternating effects of freezing and heating. Constr. Build. Mater. 2022, 350, 128916. [Google Scholar] [CrossRef]
- ISO 5660-1; Reaction-to-Fire Tests-Heat Release, Smoke Production and Mass Loss Rate-Part 1: Heat Release Rate (Cone Calorimeter Method) and Smoke Production Rate (Dynamic Measurement). International Organization for Standardization: Geneva, Switzerland, 2015.
- Jin, E.; Chung, Y.J. Fire risk assessment of cypress wood coated with metal oxide and metal silicate flame retardant using cone calorimeter. J. Fire Sci. 2020, 38, 504–521. [Google Scholar] [CrossRef]
- Repič, R.; Pondelak, A.; Kržišnik, D.; Humar, M.; Knez, N.; Knez, F.; Škapin, A.S. Environmentally friendly protection of European beech against fire and fungal decay using a combination of thermal modification and mineralisation. Wood Mater. Sci. Eng. 2023, 1–12. [Google Scholar] [CrossRef]
- Martinka, J.; Hroncová, E.; Chrebet, T.; Balog, K. The influence of spruce wood heat treatment on its thermal stability and burning process. Eur. J. Wood Wood Prod. 2014, 72, 477–486. [Google Scholar] [CrossRef]
- Čermák, P.; Dejmal, A.; Paschová, Z.; Kymäläinen, M.; Dömény, J.; Brabec, M.; Hess, D.; Rautkari, L. One-sided surface charring of beech wood. J. Mater. Sci. 2019, 54, 9497–9506. [Google Scholar] [CrossRef]
- MacLeod, C.E.; Law, A.; Hadden, R.M. Quantifying the heat release from char oxidation in timber. Fire Saf. J. 2023, 138, 103793. [Google Scholar] [CrossRef]
- Nakrani, D.; Tiwari, M.K.; Wani, T.; Srivastava, G. Characterization of combustion of hardwood and softwood through experimental and computer simulations. J. Therm. Anal. Calorim. 2023, 148, 7727–7745. [Google Scholar] [CrossRef]
- Shapchenkova, O.; Loskutov, S.; Aniskina, A.; Börcsök, Z.; Pásztory, Z. Thermal characterization of wood of nine European tree species: Thermogravimetry and differential scanning calorimetry in an air atmosphere. Eur. J. Wood Wood Prod. 2022, 80, 409–417. [Google Scholar] [CrossRef]
- Richter, F.; Atreya, A.; Kotsovinos, P.; Rein, G. The effect of chemical composition on the charring of wood across scales. Proc. Combust. Inst. 2019, 37, 4053–4061. [Google Scholar] [CrossRef]
- Yang, T.H.; Wang, S.Y.; Tsai, M.J.; Lin, C.Y. The charring depth and charring rate of glued laminated timber after a standard fire exposure test. Build. Environ. 2009, 44, 231–236. [Google Scholar] [CrossRef]
- Na, B.; Pizzi, A.; Delmotte, L.; Lu, X. One-component polyurethane adhesives for green wood gluing: Structure and temperature-dependent creep. J. Appl. Polym. Sci. 2005, 96, 1231–1243. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Webster, D.C. Thermal stability and flame retardancy of polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Bernaczyk, A.; Wagenführ, A.; Terfloth, C.; Lincke, J.; Krystofiak, T.; Niemz, P. Investigations into the Influence of Temperature on the Tensile Shear Strength of Various Adhesives. Materials 2023, 16, 6173. [Google Scholar] [CrossRef]
- Okuni, I.M.; Bradford, T.E. Modelling of Elevated Temperature Performance of Adhesives Used in Cross Laminated Timber: An Application of ANSYS Mechanical 2020 R1 Structural Analysis Software. Environ. Sci. Proc. 2020, 3, 46. [Google Scholar] [CrossRef]
- Hartig, J.; Haller, P. Investigations on multifunctional timber elements impregnated with paraffinic phase change materials. In Proceedings of the World Conference on Timber Engineering (WCTE 2023), Oslo, Norway, 19–22 June 2023. [Google Scholar] [CrossRef]
- Li, K.; Hostikka, S.; Dai, P.; Li, Y.; Zhang, H.; Ji, J. Charring shrinkage and cracking of fir during pyrolysis in an inert atmosphere and at different ambient pressures. Proc. Combust. Inst. 2017, 36, 3185–3194. [Google Scholar] [CrossRef]
- Brandon, D.; Dagenais, C. Fire Safety Challenges of Tall Wood Buildings–Phase 2: Task 5–Experimental Study of Delamination of Cross Laminated Timber (CLT) in Fire; National Fire Protection Association: Quincy, MA, USA, 2017. [Google Scholar]
- Ji, J.; Cheng, Y.; Yang, L.; Guo, Z.; Fan, W. An Integral Model for Wood Auto-ignition under Variable Heat Flux. J. Fire Sci. 2006, 24, 413–425. [Google Scholar] [CrossRef]
- Čermák, P.; Mikita, T.; Kadavý, J.; Trnka, M. Evaluating Recent and Future Climatic Suitability for the Cultivation of Norway Spruce in the Czech Republic in Comparison with Observed Tree Cover Loss between 2001 and 2020. Forests 2021, 12, 1687. [Google Scholar] [CrossRef]
- Babrauskas, V.; Peacock, R.D. Heat release rate: The single most important variable in fire hazard. Fire Saf. J. 1992, 18, 255–272. [Google Scholar] [CrossRef]
- Chow, W.K. Concerns on estimating heat release rate of design fires in fire engineering approach. Int. J. Eng. Perform.-Based Fire Codes 2012, 11, 11–19. [Google Scholar]
- Chow, C.L.; Chow, W.K. Heat release rate of accidental fire in a supertall building residential flat. Build. Environ. 2010, 45, 1632–1640. [Google Scholar] [CrossRef]
- Tang, Z.; Yue, K.; Lu, D.; Shi, X.; Chu, Y.; Tian, Z.; Lu, W. Experimental investigation into fire performance of mixed species glulam beams under three-side fire exposure. Eur. J. Wood Wood Prod. 2022, 80, 235–245. [Google Scholar] [CrossRef]
- Yang, T.-H.; Wang, S.-Y.; Tsai, M.-J.; Lin, C.-Y. Temperature distribution within glued laminated timber during a standard fire exposure test. Mater. Des. 2009, 30, 518–525. [Google Scholar] [CrossRef]





| Density [kg/m3] | Thermal Conductivity at 20 °C (L/T) [W/mK] | Specific Heat Capacity at 20 °C [J/kgK] | Source | |
|---|---|---|---|---|
| Alder | 492 | 0.273/0.166 | 1400 | [16,17] |
| Beech | 710 | 0.515/0.127 | 1213 | [18] |
| Spruce | 471 | 0.352/0.0956 | 1244 | [18] |
| Combination-Adhesive-Orientation | TTI [s] | pHRR [kW/m2] | Time at pHRR [s] | EHC [MJ/kg] | MARHE [kW/m2] |
|---|---|---|---|---|---|
| Beech-PUR-1 | 123 | 53.6 | 425 | 3.2 | 42.6 |
| Beech-PUR-2 | 99 | 59.2 | 400 | 3.0 | 49.4 |
| Beech-RPF-1 | 95 | 58.6 | 620 | 3.2 | 46.0 |
| Beech-RPF-2 | 118 | 60.0 | 115 | 3.2 | 41.6 |
| Alder-PUR-1 | 88 | 39.5 | 640 | 3.1 | 34.3 |
| Alder-PUR-2 | 74 | 73.4 | 505 | 4.4 | 55.1 |
| Alder-RPF-1 | 113 | 43.9 | 605 | 3.1 | 34.6 |
| Alder-RPF-2 | 91 | 53.5 | 470 | 3.6 | 42.4 |
| Spruce-PUR-1 | 93 | 56.6 | 125 | 2.5 | 25.2 |
| Spruce-PUR-2 | 80 | 47.5 | 105 | 3.0 | 31.1 |
| Spruce-RPF-1 | 107 | 38.1 | 135 | 3.3 | 30.9 |
| Spruce-RPF-2 | 103 | 44.2 | 120 | 3.1 | 28.5 |
| K1-PUR-1 | 106 | 48.9 | 130 | 2.6 | 34.2 |
| K1-PUR-2 | 92 | 74.6 | 685 | 3.4 | 57.7 |
| K1-RPF-1 | 95 | 51.1 | 345 | 3.3 | 39.8 |
| K1-RPF-2 | 96 | 68.4 | 770 | 4.4 | 55.8 |
| K2-PUR-1 | 98 | 69.5 | 610 | 3.5 | 43.6 |
| K2-PUR-2 | 83 | 63.9 | 585 | 3.6 | 51.0 |
| K2-RPF-1 | 104 | 70.1 | 615 | 3.4 | 44.9 |
| K2-RPF-2 | 101 | 53.7 | 580 | 3.5 | 43.2 |
| K3-PUR-1 | 92 | 73.7 | 650 | 3.4 | 46.7 |
| K3-PUR-2 | 99 | 54.4 | 530 | 3.1 | 43.9 |
| K3-RPF-1 | 103 | 69.5 | 600 | 2.8 | 41.9 |
| K3-RPF-2 | 104 | 44.8 | 525 | 3.0 | 36.9 |
| K4-PUR-1 | 108 | 49.7 | 130 | 2.9 | 36.6 |
| K4-PUR-2 | 121 | 52.0 | 450 | 2.9 | 41.0 |
| K4-RPF-1 | 97 | 47.8 | 800 | 3.4 | 39.8 |
| K4-RPF-2 | 110 | 50.4 | 130 | 2.4 | 33.8 |
| K5-PUR-1 | 91 | 66.6 | 630 | 2.9 | 45.6 |
| K5-PUR-2 | 125 | 54.5 | 525 | 3.0 | 37.1 |
| K5-RPF-1 | 108 | 70.8 | 570 | 3.5 | 43.6 |
| K5-RPF-2 | 109 | 46.1 | 305 | 3.5 | 39.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kytka, T.; Gašparík, M.; Novák, D.; Sahula, L.; Karami, E.; Das, S. Burning Properties of Combined Glued Laminated Timber. Fire 2024, 7, 30. https://doi.org/10.3390/fire7010030
Kytka T, Gašparík M, Novák D, Sahula L, Karami E, Das S. Burning Properties of Combined Glued Laminated Timber. Fire. 2024; 7(1):30. https://doi.org/10.3390/fire7010030
Chicago/Turabian StyleKytka, Tomáš, Miroslav Gašparík, David Novák, Lukáš Sahula, Elham Karami, and Sumanta Das. 2024. "Burning Properties of Combined Glued Laminated Timber" Fire 7, no. 1: 30. https://doi.org/10.3390/fire7010030





