Shear at Fluid-Fluid Interfaces Affects the Surface Topologies of Alginate Microfibers
Abstract
:1. Introduction
2. Experiments
2.1. Creation of the Microfluidic Device and Solutions
2.2. Fabrication of Alginate Microfibers
2.3. Viscosity Measurements
2.4. SEM Imaging
2.5. Profilometry Analysis
3. Results
3.1. Viscosity Measurements
3.2. Surface Topology
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McNamara, M.C.; Sharifi, F.; Okuzono, J.; Montazami, R.; Hashemi, N.N. Microfluidic Manufacturing of Alginate Fibers with Encapsulated Astrocyte Cells. ACS Appl. Bio Mater. 2019, 2, 1603–1613. [Google Scholar] [CrossRef]
- Sharifi, F.; Sooriyarachchi, A.C.; Altural, H.; Montazami, R.; Rylander, M.N.; Hashemi, N.N. Fiber Based Approaches as Medicine Delivery Systems. ACS Biomater. Sci. Eng. 2016, 2, 1411–1434. [Google Scholar] [CrossRef]
- McNamara, M.C.; Sharifi, F.; Wrede, A.H.; Kimlinger, D.F.; Thomas, D.-G.; Vander Wiel, J.B.; Chen, Y.; Montazami, R.; Hashemi, N.N. Microfibers as Physiologically Relevant Platforms for Creation of 3D Cell Cultures. Macromol. Biosci. 2017, 17, 1700279. [Google Scholar] [CrossRef]
- Shekarforoush, E.; Mendes, A.C.; Baj, V.; Beeren, S.R.; Chronakis, I.S. Electrospun Phospholipid Fibers as Micro-Encapsulation and Antioxidant Matrices. Molecules 2016, 22, 1708. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Huang, K.-S.; Yang, C.-H.; Wang, C.-Y.; Yang, Y.-S.; Hsu, H.C.; Liao, Y.-J.; Tsai, C.-W. Microfluidic Synthesis of Microfibers for Magnetic-Responsive Controlled Drug Release and Cell Culture. PLoS ONE 2012, 7, e33184. [Google Scholar] [CrossRef]
- Sharifi, F.; Patel, B.B.; Dzuilko, A.K.; Montazami, R.; Sakaguchi, D.S.; Hashemi, N.N. Polycaprolactone Microfibrous Scaffolds to Navigate Neural Stem Cells. Biomacromolecules 2016, 17, 3287–3297. [Google Scholar] [CrossRef] [Green Version]
- Patel, B.B.; Sharifi, F.; Stroud, D.P.; McNamara, M.C.; Hashemi, N.N.; Sakaguchi, D.S. 3D Microfibrous Scaffolds Selectively Promotes Proliferation and Glial Differentiation of Adult Neural Stem Cells: A Platform to Tune Cellular Behavior in Neural Tissue Engineering. Macromol. Biosci. 2018, 17, 1800236. [Google Scholar]
- Ikeda, K.; Nagata, S.; Okitsu, T.; Takeuchi, S. Cell fiber-based three-dimensional culture system for highly efficient expansion of human induced pluripotent stem cells. Sci. Rep. 2107, 7, 2850. [Google Scholar] [CrossRef]
- Higashi, K.; Ogawa, M.; Fujimoto, K.; Onoe, H.; Miki, N. Hollow Hydrogel Microfiber Encapsulating Microorganisms for Mass-Cultivation in Open Systems. Micromachines 2017, 8, 176. [Google Scholar] [CrossRef]
- Antoni, D.; Burckel, H.; Josset, E.; Noel, G. Three-Dimensional Cell Culture: A Breakthrough in Vivo. Int. J. Mol. Sci. 2015, 16, 5517–5527. [Google Scholar] [CrossRef]
- Groff, K.; Bachli, E.; Lansdowne, M.; Capaldo, T. Review of Evidence of Environmental Impacts of Animal Research and Testing. Environments 2014, 1, 14–30. [Google Scholar] [CrossRef] [Green Version]
- Kang, E.; Choi, Y.Y.; Chae, S.-K.; Moon, J.-H.; Chang, J.-Y.; Lee, S.-H. Microfluidic Spinning of Flat Alginate Fibers with Grooves for Cell-Aligning Scaffolds. Adv. Mater. 2012, 24, 4271–4277. [Google Scholar] [CrossRef]
- Sun, T.; Li, X.; Shi, Q.; Wang, H.; Huang, Q.; Fukuda, T. Microfluidic spun alginate hydrogel microfbiers and their application in tissue engineering. Gels 2018, 4, 38. [Google Scholar] [CrossRef]
- Caplin, J.D.; Granados, N.G.; James, M.R.; Montazami, R.; Hashemi, N.N. Microfluidic Organ-on-a-Chip Technology for Advancement of Drug Development and Toxicology. Adv. Healthc. Mater. 2015, 4, 1426–1450. [Google Scholar] [CrossRef] [Green Version]
- Hashemi, N.; Lackore, J.M.; Sharifi, F.; Goodrich, P.J.; Winchell, M.L.; Hashemi, N.N. A paper-based microbial fuel cell operating under continuous flow condition. Technology 2016, 4, 98–103. [Google Scholar] [CrossRef] [Green Version]
- Pemathilaka, R.L.; Caplin, J.D.; Aykar, S.S.; Montazami, R.; Hashemi, N.N. Placenta-on-a-Chip: In Vitro Study of Caffeine Transport across Placental Barrier Using Liquid Chromatography Mass Spectrometry. Glob. Chall. 2019, 3, 180112. [Google Scholar] [CrossRef]
- Sechi, D.; Greer, B.; Johnson, J.; Hashemi, N.N. Three-Dimensional Paper-Based Microfluidic Device for Assays of Protein and Glucose in Urine. Anal. Chem. 2013, 85, 10733–10737. [Google Scholar] [CrossRef] [Green Version]
- Acar, H.; Cinar, S.; Thunga, M.; Kessler, M.R.; Hashemi, N.N.; Montazami, R. Study of Physically Transient Insulating Materials as a Potential Platform for Transient Electronics and Bioelectronics. Adv. Funct. Mater. 2014, 24, 4136–4143. [Google Scholar] [CrossRef]
- Sharifi, F.; Bai, Z.; Montazami, R.; Hashemi, N.N. Mechanical and physical properties of poly(vinyl alcohol) microfibers fabricated by a microfluidic approach. RSC Adv. 2016, 6, 55343–55353. [Google Scholar] [CrossRef] [Green Version]
- Bai, Z.; Mendoza Reyes, J.M.; Montazami, R.; Hashemi, N.N. On-chip development of hydrogel microfibers from round to square/ribbon shape. J. Mater. Chem. A 2014, 2, 4878–4884. [Google Scholar] [CrossRef] [Green Version]
- Lu, M.; Sharifi, F.; Hashemi, N.N.; Montazami, R. Fluid-Induced Alignment of Carbon Nanofibers in Polymer Fibers. Macromol. Mater. Eng. 2017, 302, 1600544. [Google Scholar] [CrossRef]
- Int’l, A. Standard Specifications and Operating Instructions for Glass Capillary Kinematic Viscometers; ASTM: Salt Lake City, UT, USA, 2009. [Google Scholar]
- Bhushan, B. Modern Tribology Handbook. In Modern Tribology Handbook; Bhushan, B., Ed.; CRC Press: Boca Raton, FL, USA, 2000; Volume 2, pp. 1413–1515. [Google Scholar]
- Shi, X.; Ostrovidov, S.; Zhao, Y.; Liang, X.; Kasuya, M.; Kurihara, K.; Nakajima, K.; Bae, H.; Wu, H.; Khademhosseini, A. Microfluidic Spinning of Cell-Responsive Grooved Microfibers. Adv. Funct. Mater. 2015, 25, 2250–2259. [Google Scholar] [CrossRef]


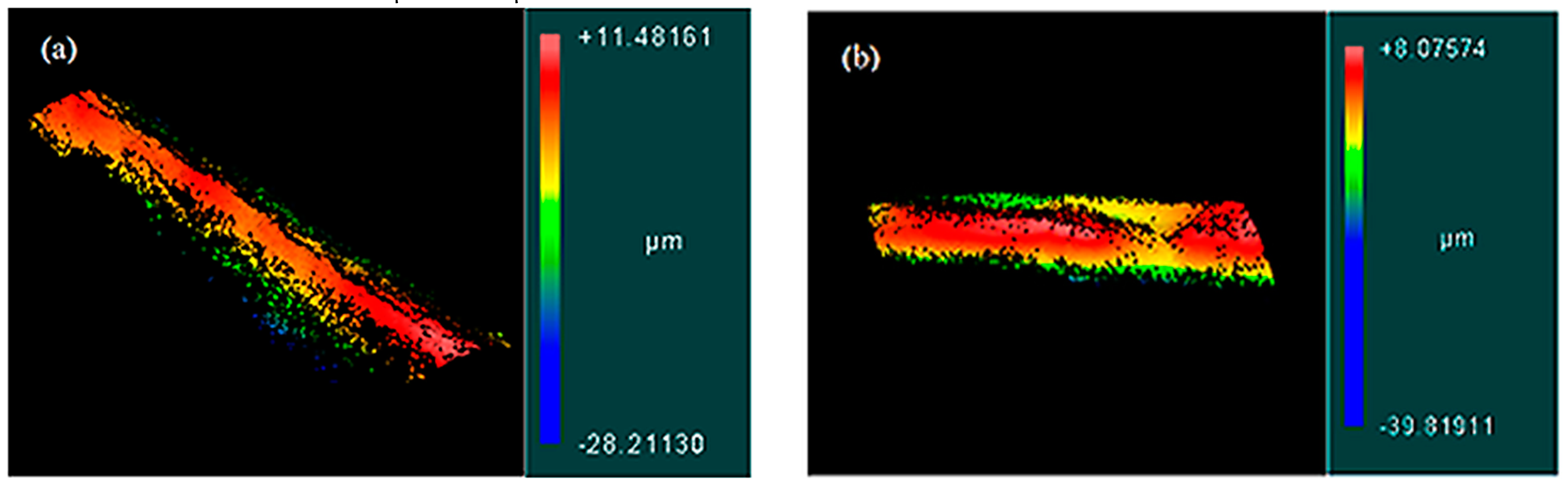
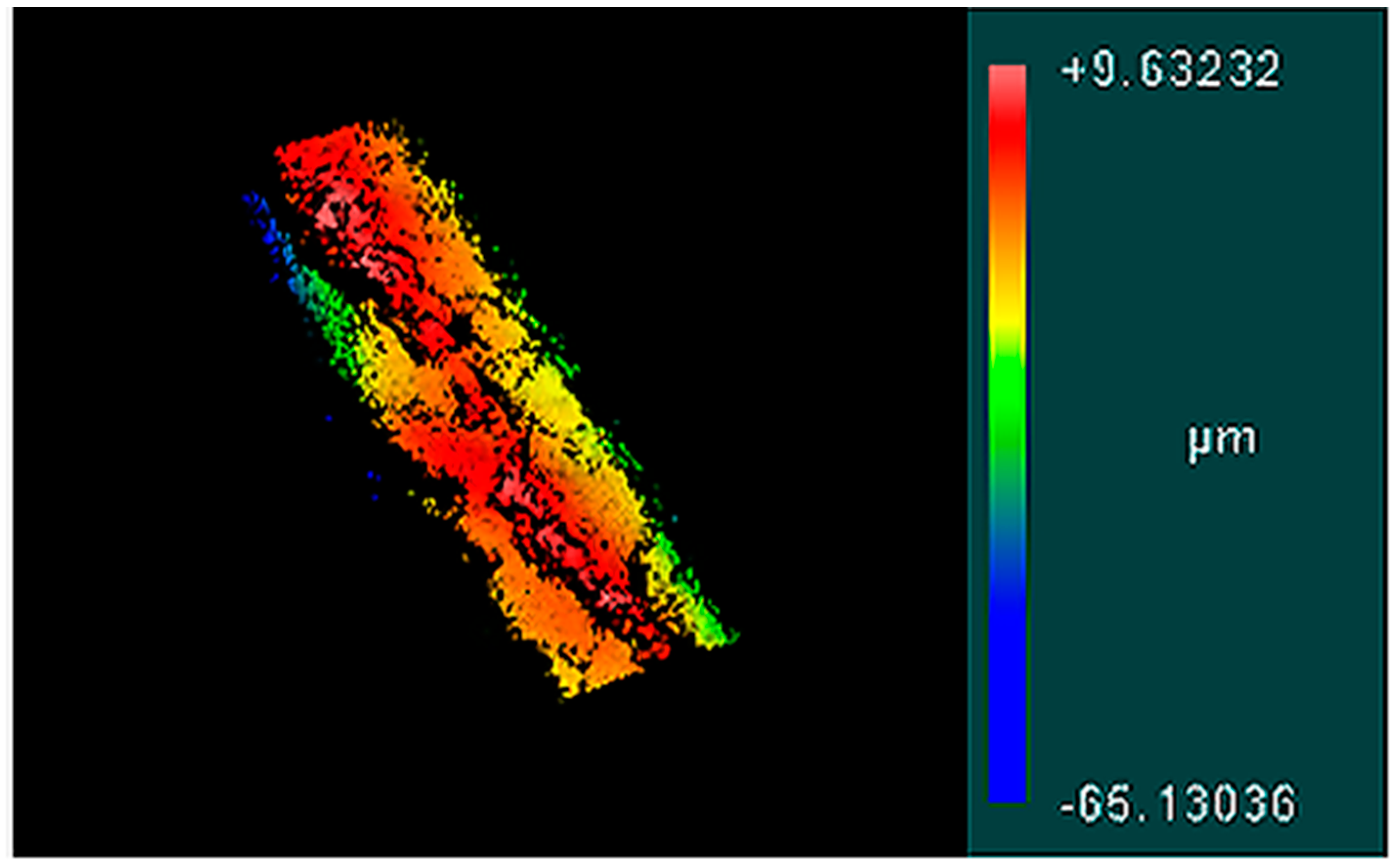
| FRRs (µL/min:µL/min) | Filtration | Ra (µm) |
|---|---|---|
| 50:10 | N/A | 1.294 ± 0.324 |
| 50:10 | 0.45 µm pore size | 2.35 ± 0.33 |
| 75:5 | N/A | 3.10 ± 0.58 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McNamara, M.C.; Pretzer, R.J.; Montazami, R.; Hashemi, N.N. Shear at Fluid-Fluid Interfaces Affects the Surface Topologies of Alginate Microfibers. Clean Technol. 2019, 1, 265-272. https://doi.org/10.3390/cleantechnol1010018
McNamara MC, Pretzer RJ, Montazami R, Hashemi NN. Shear at Fluid-Fluid Interfaces Affects the Surface Topologies of Alginate Microfibers. Clean Technologies. 2019; 1(1):265-272. https://doi.org/10.3390/cleantechnol1010018
Chicago/Turabian StyleMcNamara, Marilyn C., Ryan J. Pretzer, Reza Montazami, and Nicole N. Hashemi. 2019. "Shear at Fluid-Fluid Interfaces Affects the Surface Topologies of Alginate Microfibers" Clean Technologies 1, no. 1: 265-272. https://doi.org/10.3390/cleantechnol1010018






