The Vindolanda Vessel: pXRF and Microphotography of an Enamel-Painted Roman Gladiator Glass
Abstract
:1. Introduction
2. Roman Enamelled Glassware
3. Heritage Materials Science Technique
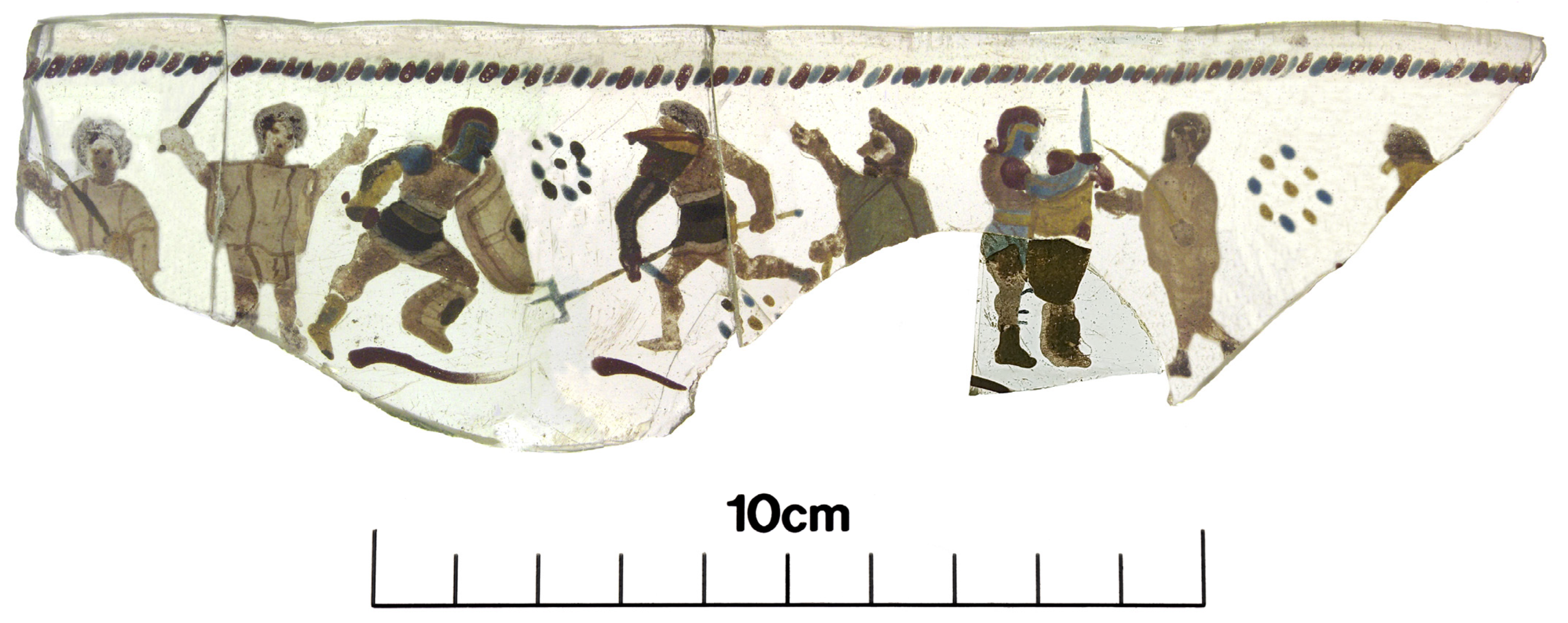
4. The Vindolanda Vessel
5. Methodology
5.1. Portable X-ray Flourescence (pXRF)
5.2. Microphotography
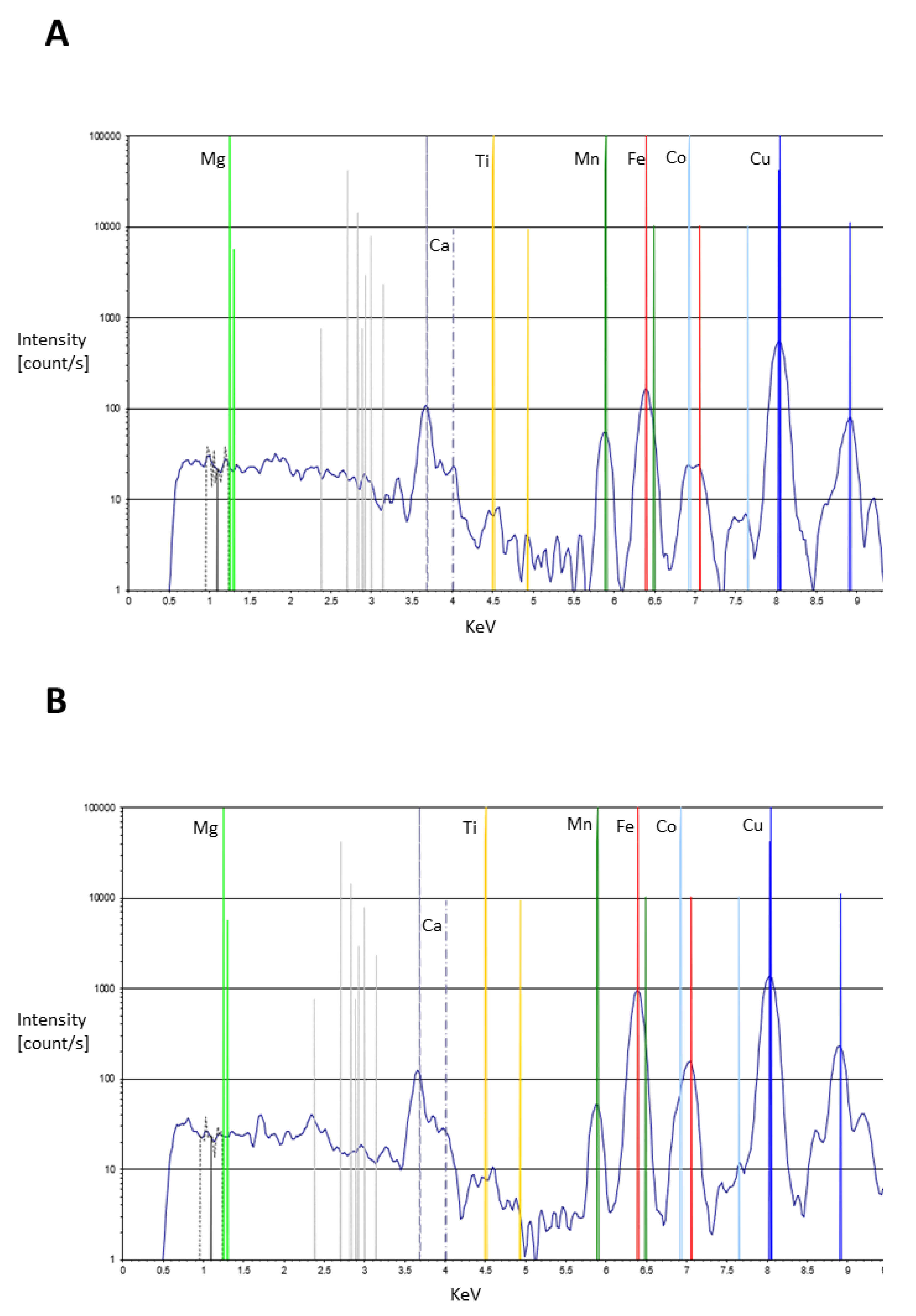
6. Results
| Sample | Colour | Mg | Al | P | S | K | Ca | Fe | Co | Cu | As | Sb | Hg | Pb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 26 | Colourless (ground) | <LOD | 10,157 | <LOD | 860 | 962 | 32,036 | 3528 | <LOD | 57 | 16 | 3653 | <LOD | 120 |
| n12 | red | <LOD | 3833 | 187 | <LOD | 442 | 22,201 | 22,562 | <LOD | 1270 | 153 | 5124 | <LOD | 753 |
| n20 | red | <LOD | 8558 | 831 | 5542 | 1729 | 16,613 | 31,627 | <LOD | 2694 | 1504 | 5867 | <LOD | 16,397 |
| n21 | red | <LOD | 10,194 | 1203 | 2031 | 1856 | 21,488 | 47,757 | <LOD | 5562 | 295 | 5320 | <LOD | 1966 |
| 2 | red | <LOD | 12,718 | 2363 | 10,592 | 1991 | 17,664 | 26,905 | <LOD | 413 | 1834 | 4595 | <LOD | 12,565 |
| 5 | red | <LOD | 11,242 | 1821 | 8498 | 1590 | 21,703 | 31,227 | <LOD | 6530 | 703 | 4507 | <LOD | 4459 |
| 17 | red | <LOD | 8650 | 2101 | 2465 | 2056 | 23,855 | 36,506 | <LOD | 1580 | 82 | 4473 | <LOD | 821 |
| 27 | red | <LOD | 10,504 | 2070 | 3459 | 927 | 19,062 | 59,304 | <LOD | 1109 | 146 | 3892 | 13 | 821 |
| n2 | brown | <LOD | 13,668 | 1207 | 291 | 1703 | 20,057 | 30,011 | <LOD | 206 | 42 | 4152 | 12 | 255 |
| n3 | brown (dark) | <LOD | 17,292 | 2086 | 284 | 2286 | 11,659 | 53,968 | <LOD | 375 | 80 | 4589 | <LOD | 488 |
| n7 | brown | <LOD | 4530 | <LOD | 345 | 428 | 21,635 | 20,817 | <LOD | 4777 | 99 | 5197 | 29 | 678 |
| n9 | brown | 23,447 | 22,425 | 1810 | 2037 | 1272 | 17,664 | 13,749 | <LOD | 193 | 32 | 3714 | <LOD | 213 |
| n17 | brown | <LOD | 19,724 | 1619 | 1214 | 2078 | 18,615 | 49,905 | <LOD | 2147 | 90 | 4610 | <LOD | 853 |
| n23 | brown | 13,352 | 28,441 | 5070 | 2926 | 6297 | 23,281 | 7605 | <LOD | 182 | 88 | 3447 | <LOD | 327 |
| n24 | brown | <LOD | 21,756 | 2834 | 6123 | 4201 | 13,764 | 9127 | <LOD | 240 | 2189 | 4263 | <LOD | 15,015 |
| n25 | brown | <LOD | 16,288 | 3099 | 11,575 | 4338 | 9827 | 8119 | <LOD | 363 | 4450 | 7778 | <LOD | 40,549 |
| 1 | brown | <LOD | 22,139 | 9014 | 7955 | 2401 | 16,571 | 50,660 | <LOD | 464 | 242 | 4246 | <LOD | 1159 |
| 3 | brown | <LOD | 11,729 | 2496 | 36,060 | 1364 | 11,551 | 14,553 | <LOD | 767 | 5715 | 7127 | <LOD | 46,865 |
| 4 | brown | <LOD | 12,236 | 3184 | 16,760 | 1332 | 17,364 | 12,927 | <LOD | 16,953 | 1348 | 5154 | <LOD | 11,495 |
| 8 | brown (mid) | 17,440 | 11,761 | 2124 | 40,961 | 580 | 8061 | 5542 | <LOD | 934 | 6293 | 7762 | <LOD | 54,009 |
| 9 | brown (dark) | <LOD | 8266 | 1941 | 18,832 | 1015 | 20,428 | 15,504 | <LOD | 21,480 | 1358 | 7630 | <LOD | 40,340 |
| 11 | brown (dark) | <LOD | 10,781 | 1877 | 6447 | 1197 | 27,506 | 6076 | <LOD | 4038 | 95 | 3999 | <LOD | 474 |
| 19 | brown | <LOD | 9139 | 2955 | 22,894 | 735 | 12,971 | 5131 | <LOD | 537 | 4193 | 6155 | <LOD | 30,275 |
| 23 | brown | <LOD | 12,999 | 4789 | 10,412 | 981 | 18,048 | 4291 | <LOD | 3313 | 2144 | 5154 | <LOD | 17,831 |
| n4 | fleshtone | <LOD | 3815 | <LOD | <LOD | 711 | 21,766 | 12,164 | <LOD | 400 | 129 | 4283 | <LOD | 379 |
| n11 | fleshtone | 34,323 | 17,071 | 1044 | 1613 | 1096 | 20,512 | 17,802 | <LOD | 231 | 47 | 4059 | <LOD | 301 |
| n16 | fleshtone | <LOD | <LOD | <LOD | <LOD | <LOD | 10,091 | 26,010 | <LOD | 4293 | 761 | 8689 | <LOD | 8448 |
| 7 | fleshtone | 13,209 | 17,949 | 7316 | 5647 | 1750 | 21,632 | 12,716 | <LOD | 574 | 93 | 3635 | <LOD | 677 |
| 14 | fleshtone | <LOD | 13,675 | 7030 | 3266 | 1901 | 23,663 | 21,062 | <LOD | 837 | 130 | 3842 | <LOD | 495 |
| 10 | cream | <LOD | 9269 | 1577 | 2542 | <LOD | 25,627 | 4332 | <LOD | 246 | 133 | 3631 | <LOD | 522 |
| 12 | cream-white | <LOD | 12,548 | 3835 | 5187 | <LOD | 23,964 | 12,861 | <LOD | 7581 | 135 | 4138 | <LOD | 1324 |
| n1 | blue | <LOD | 16,801 | 788 | 7869 | 1550 | 18,897 | 7488 | <LOD | 12,432 | 1717 | 5062 | <LOD | 10,790 |
| n6 | blue | <LOD | <LOD | <LOD | <LOD | <LOD | 25,136 | 13,150 | <LOD | 2354 | 57 | 4825 | <LOD | 352 |
| n10 | blue | <LOD | 6453 | 249 | 1962 | 924 | 31,400 | 4106 | 97 | 3105 | 150 | 4147 | <LOD | 1227 |
| n14 | blue | <LOD | <LOD | <LOD | <LOD | <LOD | 19,249 | 8734 | <LOD | 2166 | 328 | 6836 | <LOD | 1741 |
| n15 | blue | <LOD | <LOD | <LOD | <LOD | <LOD | 13,103 | 10,433 | <LOD | 123 | 127 | 6609 | <LOD | 629 |
| n22 | blue | 10,096 | 8657 | 569 | 1318 | 1415 | 31,592 | 4973 | 183 | 6818 | 154 | 4462 | <LOD | 849 |
| 6 | blue | <LOD | 8989 | 1363 | 5070 | 1479 | 30,326 | 7990 | 202 | 8616 | 260 | 4541 | <LOD | 2075 |
| 15 | blue | <LOD | 9186 | 2278 | 9022 | 1858 | 24,537 | 21,752 | 273 | 15,282 | 875 | 7040 | <LOD | 5197 |
| 20 | blue (dark) | <LOD | 10,241 | 4118 | 5095 | 2396 | 31,265 | 12,496 | 402 | 13,279 | 288 | 5762 | 13 | 1800 |
| 21 | blue (light) | <LOD | 7658 | 2215 | 3962 | 2206 | 32,639 | 8275 | 755 | 18,058 | 407 | 6784 | <LOD | 2743 |
| 24 | blue | <LOD | 13,197 | 1107 | 3404 | 1565 | 32,274 | 5705 | 257 | 10,179 | 188 | 4503 | <LOD | 1020 |
| n5 | yellow | <LOD | 9527 | 588 | 4074 | 424 | 19,045 | 6117 | <LOD | 4689 | 792 | 4443 | <LOD | 6009 |
| n8 | yellow | <LOD | 19,911 | 907 | 13,429 | 1831 | 18,391 | 5340 | <LOD | 283 | 2193 | 4092 | 27 | 15,369 |
| n13 | yellow | <LOD | <LOD | <LOD | <LOD | <LOD | 17,406 | 12,514 | <LOD | 2777 | <LOD | 6748 | <LOD | 573 |
| n18 | yellow | <LOD | 20,508 | 1497 | 9626 | 1715 | 19,830 | 8409 | 145 | 7033 | 1982 | 6013 | <LOD | 13,635 |
| n19 | yellow-cream | <LOD | 14,120 | 924 | 18,462 | 1992 | 10,459 | 6519 | <LOD | 509 | 4866 | 7795 | <LOD | 48,470 |
| 13 | yellowish | <LOD | 9124 | 1668 | 20,578 | <LOD | 14,987 | 6787 | <LOD | 8738 | 2831 | 5225 | 65 | 17,526 |
| 16 | yellow | <LOD | 7874 | 1863 | 23,349 | 1228 | 17,804 | 11,303 | 185 | 10,412 | 3227 | 9415 | <LOD | 24,662 |
| 18 | yellow | <LOD | 7630 | 2393 | 11,890 | 904 | 21,080 | 6800 | <LOD | 709 | 1479 | 4656 | <LOD | 8822 |
| 22 | yellow | <LOD | 7872 | 1966 | 5120 | 844 | 25,019 | 3436 | <LOD | 472 | 833 | 3778 | <LOD | 7061 |
| 25 | yellow | <LOD | 14,692 | 1438 | 11,442 | 1550 | 25,989 | 3810 | <LOD | 499 | 636 | 3687 | <LOD | 2814 |
7. Discussion
7.1. General Observations
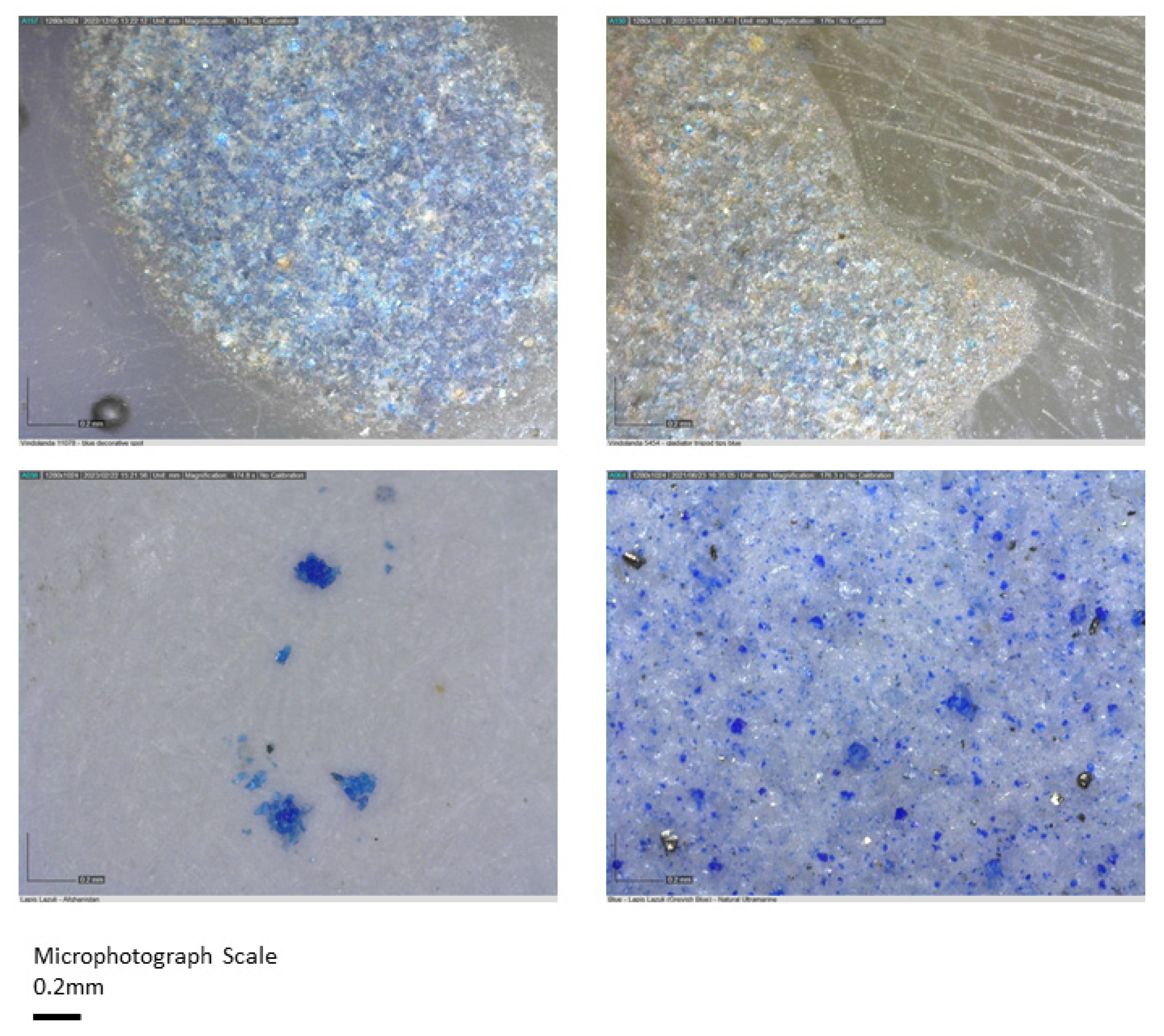
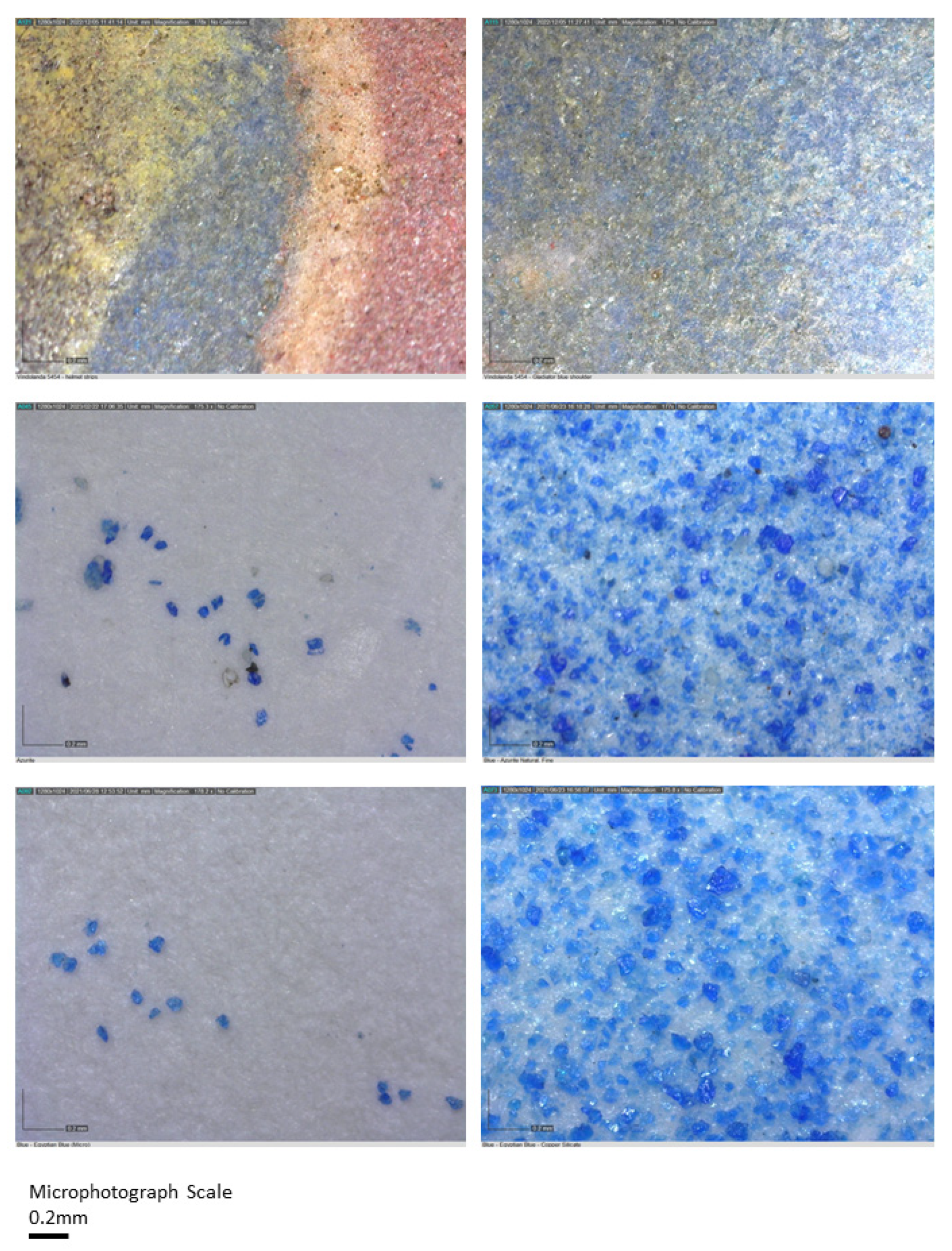
7.2. Blues
7.3. Reds and Browns
7.4. Yellows
7.5. Creams and Fleshtones
8. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Glassmakers: 3500 Years of Glass and Counting. Available online: http://www.theglassmakers.co.uk/ (accessed on 8 March 2023).
- Elder, P.T. Naturalis Historia; Rackham, H., Translator; Harvard University Press: Cambridge, UK, 1938. [Google Scholar] [CrossRef]
- Tatton-Brown, V.; Andrews, C. Before the Invention of Glassblowing. In Glass, 5,000 Years; Tait, H., Ed.; H.N. Abrams: New York, NY, USA, 1991; pp. 21–61. [Google Scholar]
- Cool, H.E.M. The Vessel Glass. In Silchester Insula IX: The Claudio-Neronian Occupation of the Iron Age Oppidum: The Early Roman Occupation at Silchester Insula IX, Britannia Monograph Series 33; Fulford, M., Clarke, A., Durham, E., Pankhurst, N., Eds.; Society for the Promotion of Roman Studies: London, UK, 2020; pp. 302–319. [Google Scholar]
- Harden, D.B.; Kemper, H.; Painter, K.; Whitehouse, D. (Eds.) Glass of the Caesars; Olivetti: Milan, Italy, 1987. [Google Scholar]
- Barag, D.P. Towards a Chronology of Syro-Palestinian Glass. In Annales du 8e Congrès International, d’Etude Historique du Verre: Londres-Liverpool, 18–25 September 1979; Association Internationale pur L’Histoire du Verre: Liège, Belgium, 1981; pp. 73–81. [Google Scholar]
- Cool, H.E.M. The Small Finds and Vessel Glass from Insula VI. 1 Pompeii: Excavations 1995–2006; Archaeopress: Oxford, UK, 2016. [Google Scholar]
- Hill, D.; (The Glassmakers, Andover, Hampshire, UK); Taylor, M.; (The Glassmakers, Andover, Hampshire, UK). Personal communication, 2023.
- Pitzer, A.P.S. Exploring Value through Roman Glass from Karanis, Egypt. Ph.D. Thesis, University of California, Los Angeles, CA, USA, 2015. Available online: https://escholarship.org/uc/item/9nt4r3vr (accessed on 12 January 2023).
- Cottam, S.E. Developments in Roman Glass Vessels in Italy, France, Britain and the Lower Rhineland c. AD40–AD110. Ph.D. Thesis, King’s College, London, UK, 2019. Unpublished. Available online: https://kclpure.kcl.ac.uk/portal/files/116534678/2019_Cottam_Sally_098227_ethesis.pdf (accessed on 12 January 2023).
- Cassibry, K. Spectacular Translucence: The Games in Glass. Theor. Rom. Archaeol. J. 2018, 1, 5. [Google Scholar] [CrossRef]
- Price, A.J.; Cottam, S.E. Romano-British Glass Vessels: A Handbook, Practical Handbook in Archaeology 14; Council for British Archaeology: York, UK, 1998. [Google Scholar]
- Baxter, M.J.; Cool, H.E.M.; Heyworth, M.P.; Jackson, C.M. Further studies in the compositional variety of colourless Romano-British vessel glass. Archaeometry 2005, 37, 47–68. [Google Scholar] [CrossRef]
- Henderson, J. Ancient Glass; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Huisman, D.J.T.; Groot, D.E.; Pols, S.; Van Os, B.J.H.; Degryse, P. Compositional Variation in Roman Colourless Glass Objects from the Bocholtz Burial (The Netherlands). Archaeometry 2009, 51, 413–439. [Google Scholar] [CrossRef]
- Jackson, C.M. Making Colourless Glass in the Roman Period. Archaeometry 2005, 47, 763–780. [Google Scholar] [CrossRef]
- Degryse, P. Isotopes-Ratio Techniques in Glass Studies. In Modern Methods of Analysing Archaeological and Historical Glass, I; Janssens, K.A., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2013; pp. 235–245. [Google Scholar]
- Tantrakarn, K.; Kato, N.; Hokura, A.; Nakai, I.; Fujii, Y.; Gluščević, S. Archaeological analysis of Roman glass excavated from Zadar, Croatia by newly developed portable XRF spectrometer for glass. X Ray Spectrom. 2009, 38, 121–127. [Google Scholar] [CrossRef]
- Foy, D.; Picon, M.; Vichy, M.; Thirion-Merle, V. Characte’risation des verres de la fin de l’Antiquite’ en Mediterrane´e occidentale: L’e´mergence de nouveaux courants commerciaux. In Echanges et Commerce du verre dans le Monde Antique, Proceedings of the Acts of the AFAV Conference, Aix-en-Provence and Marseilles, France, 7–9 June 2001; Foy, D., Nenna, M.D., Eds.; Editions Mergoil: Dremil-Lafage, France, 2003; pp. 41–85. [Google Scholar]
- Price, J. Glass-working and glassworkers in cities and towns. In Roman Working Lives and Urban Living; MacMahon, A., Price, J., Eds.; Oxbow Books: Oxford, UK, 2005; pp. 167–190. [Google Scholar]
- Jackson, C.; Foster, H. Provenance Studies and Roman Glass. In Glass of the Roman World; Bayley, J., Freestone, I.C., Jackson, C.P., Eds.; Oxbow Books Ltd.: Oxford, UK, 2015; pp. 44–56. [Google Scholar]
- Freestone, I.C.; Bimson, M.; Buckton, D. Compositional categories of Byzantine glass tesserae. In Annales du 11e Congrès de l’Association Internationale pour l’Histoire du Verre, Bâle, 29 Août–3 Septembre 1988; AIHV: Amsterdam, The Netherlands, 1990; pp. 271–281. [Google Scholar]
- Turner, W.E.S.; Rooksby, H.P. A study of opalising agents in ancient opan glasses throughout three thousand four hundred years. Glastech. Ber. 1959, VII, 17–28. [Google Scholar]
- Henderson, J.; Warren, S.E. Analysis of prehistoric lead glass. In Proceedings of the 22nd Symposium on Archaeometry, Bradford, UK, 30 March–3 April 1982; Aspinall, A., Warren, S.E., Eds.; University of Bradford: Bradford, UK, 1983; pp. 83–94. [Google Scholar]
- Tite, M.; Pradell, T.; Shortland, A. Discovery, Production and use of tin-based opacifiers in glasses, enamels and glazes from the late Iron Age onwards: A reassessment. Archaeometry 2006, 50, 67–84. [Google Scholar] [CrossRef]
- Henderson, J. Scientific Analysis of ancient glass. In Scientific Analysis in Archaeology; Henderson, J., Ed.; Oxford University Committee for Archaeology Monograph No. 19: Oxford, UK, 1989; pp. 30–60. [Google Scholar]
- Turner, W.E.S. Studies in ancient glasses and glassmaking processes, part III: The chronology of the glass-making constituents. J. Glass Technol. 1956, 40, 39–52. [Google Scholar]
- Paynter, S. Analyses of Colourless Roman Glass from Binchester, County Durham. J. Archaeol. Sci. 2006, 33, 1037–1057. [Google Scholar] [CrossRef]
- Foster, H.E.; Jackson, C.M. The Composition of Late Romano-British Colourless Vessel Glass: Glass Production and Consumption. J. Archaeol. Sci. 2010, 37, 3068–3080. [Google Scholar] [CrossRef]
- Freestone, I.C.; Stapleton, C.P. Composition, Technology and Production of Coloured Glasses from Roman Mosaic Vessels. In Glass of the Roman World; Bayley, J., Freestone, I.C., Jackson, C.P., Eds.; Oxbow Books Ltd.: Oxford, UK, 2015; pp. 61–76. [Google Scholar]
- Jackson, C.M.; Paynter, S. A Great Big Melting Pot: Exploring patterns of glass supply, consumption and recycling in Roman Coppergate, York. Archaeometry 2016, 58, 68–95. [Google Scholar] [CrossRef] [Green Version]
- Dalton, O.M. Byzantine Art and Archaeology; Dover Publications: New York, NY, USA, 1961. [Google Scholar]
- Pierides, A. Jewellery in the Cyprus Museum; Department of Antiquities: Nicosia, Cyprus, 1971. [Google Scholar]
- Cooper, E. Ten Thousand Years of Pottery, 4th ed.; University of Pennsylvania Press: Philadelphia, PY, USA, 2000. [Google Scholar]
- Van der Linden, V.; Meedsom, E.; Devos, A.; Dooren, R.V.; Nieuwdorp, H.; Janssen El Balance, S.; Vekemans, B.; Vincze, L.; Janssen, K. PXRF, μ-XRF, Vacuum μ-XRF, and EPMA Analysis of Email Champlevé Objects Present in Belgian Museums. Microsc. Microanal. 2011, 17, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Gudenrath, W. Enameled Glass Vessels, 1425 B.C.E.-1800: The Decorating Process. J. Glass Stud. 2006, 48, 23–70. [Google Scholar]
- Taylor, M.; (Owner, The Glassmakers, Andover, Hampshire, UK). Personal communication, 2023.
- Norling-Christensen, H. Romerske glaskar i Danmark. Nationmuseets Arb. 1953, 81–90. [Google Scholar]
- Ekholm, G. Westeuropäische Gläser in Skandinavien während der späten Kaiser—Und der frühen Merowingerzeit. Acta Archaeol. 1958, 29, 21–50. [Google Scholar]
- Greiff, S.; Hartman, S. Scientific studies on fragments of enamelled glass from a ‘circus cup’. In Aarbøger for Nordisk Oldkyndighed og Historie; J.H. Lynge & Son: Copenhagen, Denmark, 2009; pp. 121–132. [Google Scholar]
- Słowińska, D.; Kejtrowska, K.; Hansen, U.L. A Roman painted glass beaker from Przeworsk culture cemetery at Zaborów, Western Mazowsze. Wiadomości Archeol. 2008, 60, 125–160. [Google Scholar]
- Fehr, H.; Welker, E. Reiche römische Brandbestattung mit bemaltem Glasbecher aus Bassenheim Kreis Mayen-Koblenz. Archäologisches Korresp. 1986, 16, 193–198. [Google Scholar]
- Allen, D.; (Independent researcher, Devon, UK). Personal communication, 2023.
- Coarelli, F. The Painted Cups of Begram and the Ambrosian Iliad. East West 1962, 13, 317–335. [Google Scholar]
- Youso, K. Afghanistan: Hidden Treasures from the National Museum, Kabul; Asian Art Museum: San Francisco, CA, USA, 2008. [Google Scholar]
- Max, Y. The Enameled Cups from Masada. J. Glass Stud. 2021, 63, 11–32. [Google Scholar]
- Bayley, J. Roman Enamels and Enamelling. In Glass of the Roman World; Bayley, J., Freestone, I.C., Jackson, C.P., Eds.; Oxbow Books Ltd.: Oxford, UK, 2015; pp. 178–189. [Google Scholar]
- Biek, L.; Butcher, S.A.; Carruthers, T.G.; Rooksby, H.P.; Warren, S.E.; Crummett, J.G.; Hedges, R.E.M.; Kaczmarczyk, A. Enamels and glass pastes on Roman-period ‘bronzes’ found at Nornour, Isles of Scilly. In Proceedings of the 16th International Symposium on Archaeometry and Archaeological Prospection; Slater, E.A., Tate, J.O., Eds.; National Museum of Antiquities of Scotland: Edinburgh, Scotland, 1980; pp. 51–79. [Google Scholar]
- Colomban, P. The Destructive/Non-Destructive Identification of Enameled Pottery, Glass Artifacts and Associated Pigments—A Brief Overview. Arts 2013, 2, 77–110. [Google Scholar] [CrossRef] [Green Version]
- Caggiani, M.C.; Colomban, P.; Valotteau, C.; Mangone, A.; Cambon, P. Mobile Raman spectroscopy analysis of ancient enamelled glass masterpieces. Anal. Methods 2013, 5, 4345. [Google Scholar] [CrossRef]
- Greiff, S.; Schuster, J. Technological study of enamelling on Roman glass: The nature of opacifying, decolourizing and fining agents used with the glass beakers of Lübsow (Lubieszewo, Poland). J. Cult. Herit. 2008, 9, 27–32. [Google Scholar] [CrossRef]
- Dussubieux, L.; Gratuze, B. Non-Destructive Characterization of Glass Beads: An application to the study of glass trade between India and Southeast Asia. In Proceedings of the 9th International Conference of the European Association of Southeast Asian Archaeologists, Sigtuna, Stockholm, Sweden, 27 May–2 June 2002; pp. 135–148. [Google Scholar]
- Glascock, M.D. Application of Neutron Activation Analysis to Archaeological Studies of Natural and Man-Made Glasses. In Modern Methods of Analysing Archaeological and Historical Glass, I; Janssens, K.A., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2013; pp. 185–197. [Google Scholar]
- Shackley, M. X-ray Flourescence (XRF) Analysis in Archaeology. In X-ray Flourescence Spectrometry (XRF) in Geoarchaeology; Shackley, M., Ed.; Springer: New York, NY, USA, 2011; pp. 7–44. [Google Scholar]
- Baert, K.; Meulebroeck, W.; Wouters, H.; Ceglia, A.; Nys, K.; Thienpont, H.; Terryn, H. Raman spectroscopy as a rapid screening method for ancient plain window glass. J. Raman Spectrosc. 2011, 42, 1055–1061. [Google Scholar] [CrossRef]
- Weber, G.; Strivay, D.; Martinot, L.; Gamir, H. Use of PIXE-PIGE Under Variable Incident Angle for Ancient Glass Corrosion Measurements. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2002, 189, 350–357. [Google Scholar] [CrossRef]
- Šmit, Ž. Ion-Beam Analysis Methods. In Modern Methods of Analysing Archaeological and Historical Glass, I; Janssens, K.A., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2013; pp. 155–184. [Google Scholar]
- Fiorentino, S.; Chinni, T.; Vandini, M. Materials Inspiring Methodology: Reflecting the Potential of Transdisciplinary Approaches to the Study of Archaeological Glass. Appl. Sci. 2021, 11, 8049. [Google Scholar] [CrossRef]
- Janssens, K. Electron Microscopy. In Modern Methods of Analysing Archaeological and Historical Glass, I; Janssens, K.A., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2013; pp. 129–154. [Google Scholar]
- Campbell, L.; Smith, M. Multi-technique analysis of pigments on sandstone sculptures: Renaissance re-painting of a Roman relief. Herit. Sci. 2022, 10, 156. [Google Scholar] [CrossRef]
- Dyer, J.; Sotiropoulou, S. A technical step forward in the integration of visible-induced luminescence imaging methods for the study of ancient polychromy. Herit. Sci. 2017, 5, 24. [Google Scholar] [CrossRef] [Green Version]
- Bracci, S.; Vettori, S.; Cantisani, E.; Degnano, I.; Galli, M. The ancient use of colouring on the marble statues of Hierapolis of Phrygia (Turkey): An integrated multi-analytical approach. Archaeol. Anthropol. Sci. 2019, 11, 1611–1619. [Google Scholar] [CrossRef]
- Christie, H.R. Pushing Boundaries: Spectral Imaging of Archaeological Small Finds. Ph.D. Thesis, University of Glasgow, Glasgow, Scotland, 2019. Unpublished. [Google Scholar]
- Oujja, M.; Sanz, M.; Agua, F.; Conde, J.F.; Garcia-Heras, M.; Dávila, A.; Oñate, P.; Sanguino, J.; Vázquez de Aldana, J.R.; Moreno, P.; et al. Multianalytical characterization of Late Roman glasses including nanosecond and femtosecond laser induced breakdown spectroscopy. J. Anal. At. Spectrom. 2015, 30, 1590. [Google Scholar] [CrossRef] [Green Version]
- Donais, M.K.; Van Pevenage, J.; Sparks, A.; Redente, M.; George, D.B.; Moens, L.; Vincze, L.; Vandenabeele, P. Characterizion of Roman glass tesserae from Coriglia excavation site (Italy) via energy-dispersive X-ray fluorescence spectrometry and Raman spectroscopy. Appl. Phys. A 2016, 122, 1050. [Google Scholar] [CrossRef] [Green Version]
- Colomban, P.; Kirmizi, B.; Gougeon, C.; Gironda, M.; Cardinal, C. Pigments and glassy matrix of the 17th–18th Century enamelled French watches: A non-invasive on-site Raman and pXRF study. J. Cult. Herit. 2020, 44, 1–14. [Google Scholar] [CrossRef]
- Collins, R.; Birley, B.; Croom, A.; Laskey, J.; McIntosh, F.; Padley, T.; Parking, A.; Price, E. Living on the Edge of Empire: The Objects and People of Hadrian’s Wall; Pen and Sword Books Limited: Barnsley, UK, 2020. [Google Scholar]
- Birley, B.; (Vindolanda Trust, Hexham, UK). Personal communication, 2023.
- Isings, C. Roman Glass from Dated Finds; J.B. Wolters: Groningen, The Netherlands, 1957. [Google Scholar]
- ©Carole Raddato (CC BY-SA). Available online: https://www.flickr.com/search/?user_id=41523983%40N08&sort=date-taken-desc&text=glass%20gladiator&view_all=1 (accessed on 8 March 2023).
- Hertzberg, G.F. A History of All Nations from the Earliest Times; Being a Universal Historical Library, Volume 5: Imperial Rome; Wright, J.H., Translator; Lea Brothers and Company: New York, NY, USA, 1905. [Google Scholar]
- Moioli, P.; Seccaroni, C. Analysis of Art Objects Using Portable X-ray Flourescence Spectrometer. X-ray Spectrom. 2000, 29, 48–52. [Google Scholar] [CrossRef]
- Fermo, P.; Andreoli, M.; Bonizzoni, L.; Fantauzzi, M.; Guibertoni, G.; Ludwig, N.; Rossi, A. Characterisation of Roman and Byzantine glasses from the surroundings of Thugga (Tunisia): Raw materials and colours. Microchem. J. 2016, 129, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Blair, E.H. pXRF Analysis of Heritage Glass. In Advances in Portable X-ray Flourescence Spectrometry: Instrumentation, Application and Interpretation; Drake, B.L., MacDonald, B.I., Eds.; Royal Society of Chemistry: London, UK, 2022; pp. 400–423. [Google Scholar]
- Goldman, Y.; Linn, R.; Shamir, O.; Weinstein-Evron, M. Micro-RTI as a novel technology for the investigation and documentation of archaeological textiles. J. Archaeol. Sci. Rep. 2018, 19, 1–10. [Google Scholar] [CrossRef]
- McConaughy, M.A.; Anderson, G.E.; Harding, D.G. A microscopic examination of materials adhering to two early woodland copper objects from West Virginia and Pennsylvania. Archaeol. East. N. Am. 2014, 42, 15–24. [Google Scholar]
- Jordan, J.M.; Peuramaki-Brown, M.M.; Chiac, S.; Saqui, A.; Tzib, F. It’s what’s inside that counts: Developing a paste group typology in Belize. J. Archaeol. Sci. Rep. 2021, 37, 103019. [Google Scholar] [CrossRef]
- Bustos-Pérez, G.; Díaz, S.; Baena, J. An experimental approach to degrees of rounding among lithic artefifacts. J. Archaeol. Method Theory 2019, 26, 1243–1275. [Google Scholar] [CrossRef]
- Kononenko, N.; Torrence, R.; Barton, H.; Hennell, A. Cross-cultural interaction on Wuvulu Island, Papua New Guinea: The perspective from use-wear and residue analysis of turtle bone artifacts. J. Archaeol. Sci. 2010, 37, 2911–2919. [Google Scholar] [CrossRef]
- Antinozzi, S.; Ronchi, D.; Fiorillo, F.; Barba, S. 3Dino: Configuration for a Micro-Photogrammetric Survey. Digit. Herit. 2021, 2, 211–222. [Google Scholar]
- Tantrakarn, K.; Kato, N.; Nakai, I. The Application of a Portable X-ray Flourescence Spectrometer to the On-Site Analysis of Glass Vessel Fragments from Southern Thailand. Archaeometry 2012, 55, 508–527. [Google Scholar] [CrossRef]
- Freestone, I.C.; Bimson, M. The Possible Early Use of Chromium as a Glass Colorant. J. Glass Stud. 2003, 45, 183–185. [Google Scholar]
- Colomban, P.; Tournié, A.; Caggiani, M.C.; Paris, C. Pigments and enamelling/gilding technology of Mamluk mosque lamps and bottle. J. Raman Spectrosc. 2012, 43, 1975–1984. [Google Scholar] [CrossRef]
- Colomban, P. Polymerization degree and Raman identification of ancient glasses used for jewelry, ceramic enamels and mosaics. J. Non Cryst. Solids 2003, 323, 180–187. [Google Scholar] [CrossRef]
- Galli, A.; Bonizzoni, L. True versus forged in the cultural heritage materials: The role of pXRF analysis. X-ray Spectrom. 2014, 43, 22–28. [Google Scholar] [CrossRef]
- Feller, R.L. ‘Artists’ Pigments: A Handbook of Their History and Characteristics; Cambridge University Press: Cambridge, UK, 1986. [Google Scholar]
- Bedford, C.; Robinson, D.W.; Gandy, D. Emigdiano Blues: The California indigenous pigment palette and an in situ analysis of an exotic colour. Open Archaeol. 2018, 4, 152–172. [Google Scholar] [CrossRef]
- Ağtürk, T.S. The Painted Tetrarchic Reliefs of Nicomedia: Uncovering the Colourful Life of Diocletian’s Forgotten Capital; Brepols: Turnhout, Belgium, 2021. [Google Scholar]
- Constantinescu, B.; Cristea-Stan, D.; Szökefalvi-Nagy, Z.; Kovács, I.; Harsány, I.; Kasztovskzky, Z. PIXE and PGAA—Complementary methods for studies on ancient glass artefacts (from Bysantine, late medieval to modern Murano glass). Nucl. Instrum. Methods Phys. Res. 2018, B 417, 105–109. [Google Scholar] [CrossRef]
- Amadori, M.L.; Constantini, I.; Madariaga Mota, J.M.; Valentini, L.; Ferrucci, F.; Mengacci, V.; Camaiti, M. Calcium antimonate: A new discovery in colour palette of Paestum wall paintings. Microchem. J. 2021, 168, 106401. [Google Scholar] [CrossRef]
- Rütti, B. Les verres Peints du Haut Empire Romain: Centres de Production et de Diffusion; Musée Romain: Lausanne, Switzerland, 2001. [Google Scholar]
- Whitehouse, D. A Fragment of Roman Glass Decorated with Enamel. J. Glass Stud. 2008, 50, 306–309. [Google Scholar]
- Hassan, I. Transmission electron microscopy and differential thermal studies of lazurite polymorphs. Am Miner. 2000, 85, 1383–1389. [Google Scholar] [CrossRef]









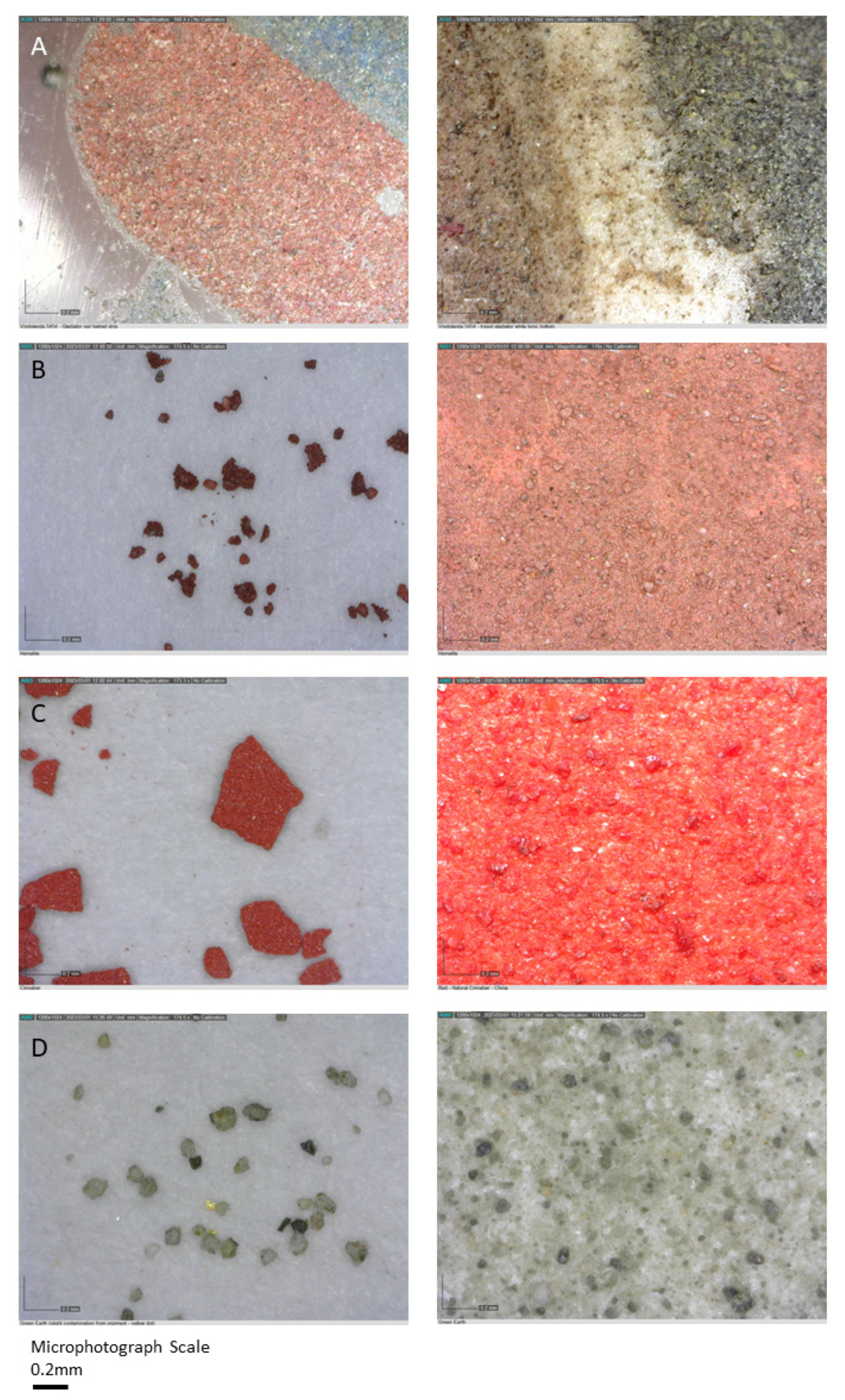
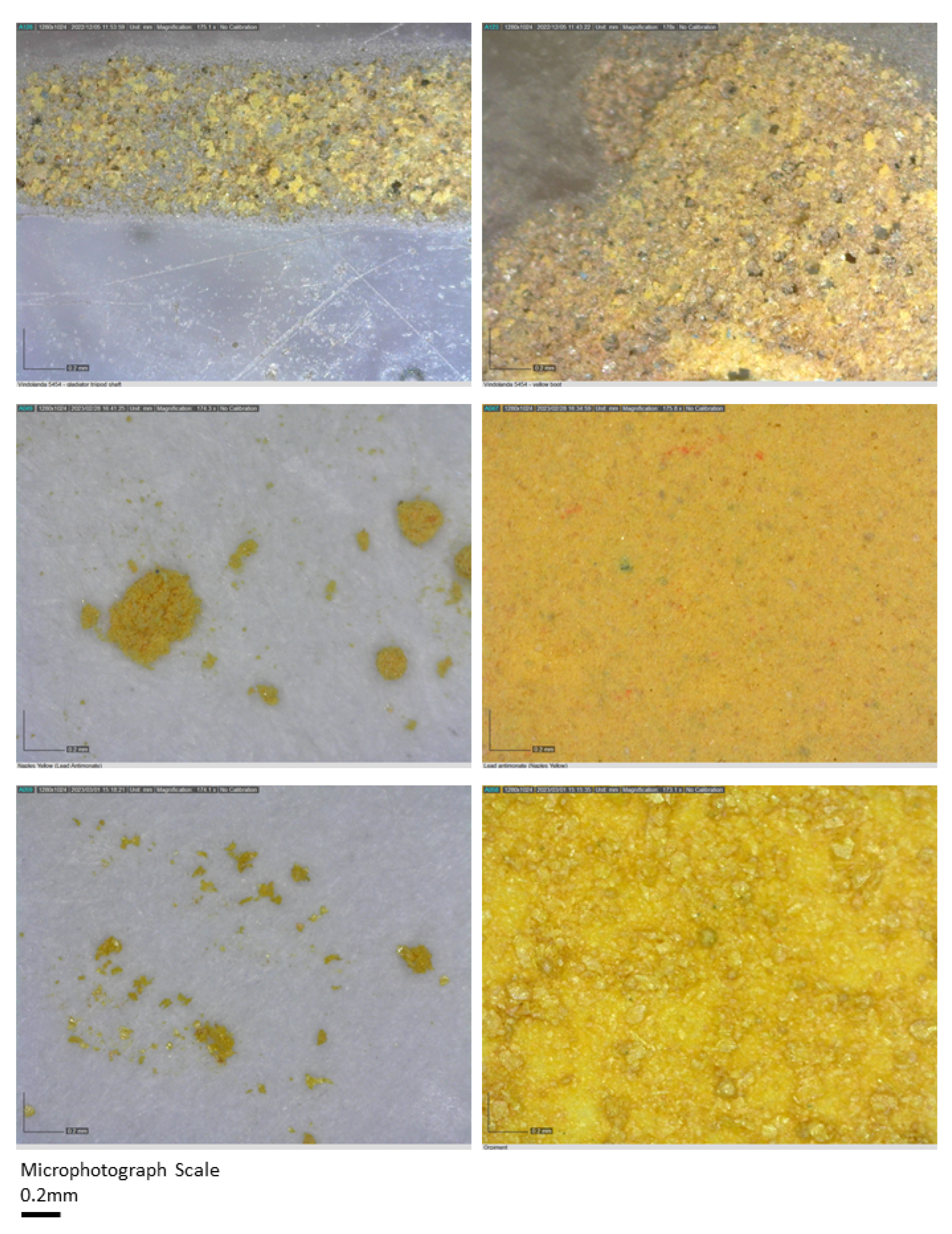
| Enamel Colour | Opaque | Translucent (Metals in Solution) |
|---|---|---|
| Black | Commonly very dark translucent olive-green glass or possibly combinations of cobalt oxide (CoO), iron oxide (Fe2O3), copper oxide (Cu2O) and/or manganese dioxide (MnO2) | Iron (Fe) |
| Blue | Cobalt oxide (CoO) | Copper oxide (Cu2O) OR Lapis lazuli (Na7Al6Si6O24S3) |
| Blue (Dark) | Commonly translucent glass with added calcium antimonate (CaSb2O6) | Cobalt (Co) |
| Blue-green | Commonly translucent glass with added calcium antimonate (CaSb2O6) | Copper (Cu) or iron (Fe) |
| Brown | Iron oxide—hematite (Fe2O3) | Manganese dioxide (MnO2) |
| Brown (golden) | - | Manganese (Mn) |
| Orange | Cuprous oxide (Cu2O) | - |
| Green | Commonly translucent glass with added lead antimonate (Pb2Sb2O7) Alternatively, decayed reds or oranges Alternatively, lead antimonate (Pb2Sb2O7) with added lapis lazuli (Na7Al6Si6O24S3) | Iron (Fe), or copper (Cu)—in lead (Pb)-rich glass |
| Green (pale) | Iron oxide (Fe2O3) | Copper oxide (Cu2O) with iron oxide (Fe2O3) |
| Pink | Manganese dioxide (MnO2) | |
| Purple | - | Manganese (Mn) |
| Red | Iron oxide -hematite (Fe2O3); Copper (Cu) or cuprous oxide (Cu2O) | - |
| Yellow | Lead antimonate (Pb2Sb2O7) | - |
| White | Calcium antimonate (CaSb2O6) | - |
| Sample | Feature | Colour/s |
|---|---|---|
| Artefact No. 5454 | ||
| 1 | Retiarius helmet | Brown/red |
| 2 | Retiarius facemask | Red |
| 3 | Retiarius facemask | Brown |
| 4 | Retiarius manica | Brown |
| 5 | Retiarius pugio pommel | Red |
| 6 | Retiarius pugio blade (possible leg interference) | Blue |
| 7 | Retiarius thigh | Beige (skintone) |
| 8 | Retiarius subligaculum centre | Light Brown |
| 9 | Retiarius balteus/cigulum | Dark Brown |
| 10 | Secutor scutum | Cream |
| 11 | Secutor scutum boss | Dark brown |
| 12 | Secutor subligaculum bottom trimming | Cream |
| 13 | Secutor balteus/cigulum | Yellowish |
| 14 | Secutor chest | Beige (skintone) |
| 15 | Secutor galea facemask | Blue |
| 16 | Secutor galea facemask | Yellow |
| 17 | Secutor galea | Red |
| 18 | Secutor manica front | Yellow |
| 19 | Secutor manica elbow | Brown |
| 20 | Secutor manica shoulder guard (exterior) | Dark Blue |
| 21 | Secutor manica shoulder guard (central diagonal stripe) | Light Blue |
| 22 | Secutor boot base | Yellow |
| 23 | Secutor boot centre | Brown |
| 24 | Retiarius fascina central prong | Blue |
| 25 | Retiarius fascina shaft | Yellow |
| 27 | Decorated band under Retiarius | Red |
| 26 | Ground—plain, unpainted glass | Colourless |
| Artefact Nos. 11078 and 711 | ||
| n1 | Judge/editor? tunic | Blue |
| n2 | Judge/editor? hair | Brown |
| n3 | Judge/editor? eye | Dark Brown |
| n4 | Judge/editor? forearm | Beige (skintone) |
| n5 | Judge/editor? wand | Yellow |
| n6 | Decorative dot | Blue |
| n7 | Decorative dot | Brown |
| n8 | Decorative dot | Yellow |
| n9 | Retiarius knee guard | Brown |
| n10 | Retiarius’ fascina terminal | Blue |
| n11 | Retiarius forearm | Beige (skintone) |
| n12 | Secutor helmet | Brown |
| n13 | Secutor facemask (possible interference from blue area) | Yellow |
| n14 | Secutor facemask | Blue |
| n15 | Secutor manica | Blue |
| n16 | Secutor torso | Beige (skintone) |
| n17 | Secutor manica shoulder guard | Brown |
| n18 | Secutor balteus/cigulum | Yellow |
| n19 | Secutor scutum front | Yellow-cream |
| n20 | Secutor scutum—inner top | Red |
| n21 | Secutor gladius pommel | Red |
| n22 | Secutor gladius blade | Blue |
| n23 | Secutor knee | Brown |
| n24 | Secutor boot | Brown |
| n25 | Secutor scutum | Brown |
| Element | Note |
|---|---|
| Mg | below LOD on colourless glass, but very high on some brown samples, especially on the Retiarius knee guard and subligaculum centre non-combatant Secutor’s knee and gladius blade (artefact 11078), and on the fleshtones of the Retiarius’ forearm and thigh |
| Si | high level present in the colourless glass is overwhelmed by pigments on ALL painted areas |
| P | below LOD in colourless glass, but elevated in almost all samples, particularly high in the brown Retiarius galea and fleshtones of the thigh of the Retiarius and chest of the Secutor on artefact 5454 |
| S | slightly elevated in the colourless glass, but high readings in most samples, especially the gladiatorial accountrements, e.g., Retiarius’ galerus and subligaculum where it is exceptionally high and his balteus/cigulum and manica; and the Secutor’s manica elbow padding which is visibly a similar colour to the Retiarius’s galerus and subligaculum, his scutum (in both the degraded area and yellowish area); and yellow areas of the 11078 Secutor’s scutum, balteus/cigulum and galea facemask |
| K | relatively low in the colourless glass and elevated on many samples, especially in brown and red features and some blue spots, but particularly high in the areas where pigment is degraded on artefact 711 (could be salt crustation from post-depositional position) |
| Ca | as with Si, the high level present in the colourless glass is overwhelmed by pigments on almost all painted features, except those coloured with blue |
| Ti | trace levels in some features, e.g., brown judge/editor?’s eye, and degraded pigment on the Secutor’s boot (artefact 711) and Retiarius’ manica, as well as the fleshtone of the Secutor’s torso and two shades of blue on the Secutor’s manica shoulder guard; and the yellows of the Secutor on 11078 facemask (but the spot could have interference from the blue area here), and his balteus/cigulum and scutum |
| V | very low level on colourless glass and only trace levels on a few areas dispersed across samples with no consistent pattern to colours, except for fleshtones, where most samples have slightly elevated levels |
| Mn | present at low levels on the colourless glass and even lower on painted features |
| Cr | below LOD in colourless glass and trace levels from some samples, e.g., red on the Secutor’s galea (5454) and browns in the Retiarius’s accoutrements, including knee guard, galerus, manica and scutum boss; also on several blue samples, especially weapon blades, shoulder guards, decorative circle and judge/editor’s tunic |
| Fe | only trace levels on the colourless glass but elevated on ALL samples except two yellow features, especially in all red and almost all brown samples and highest in areas where layered pigment is most likely (features painted on top of others), e.g., judge/editor? eye and hair, Secutor’s manica shoulder guard, galea facemask and shoulder guard, Retiarius’ galerus, manica and balteus/cigulum. Also high in fleshtone areas, especially the chests of both Secutores |
| Co | below LOD on colourless glass and elevated exclusively on blue areas as well as two slightly elevated yellow samples, both of which either overly or sit immediately beside blue features. Highest levels from the light and dark blue of the combatant Secutor’s shoulder guard |
| Ni | below LOD on colourless glass with trace levels on a few blue samples, e.g., Secutor’s galea facemask and two shades of blue in the Secutor’s shoulder guard as well as the Retiarius’ pugio blade. One elevated reading on a yellow sample likely derives from the blue on the combatant Secutor’s galea facemask since the yellow has been visibly painted over a blue base here |
| Cu | very low trace level on colourless glass and elevated levels on almost all painted features, mostly at unremarkable levels, but high levels evident from all but one blue sample (Secutor’s manica on 11078, which could suggest the analysis spot had interference from several coloured features here as most of the elements detected are anomalous with other blue samples), especially elevated at the judge/editor? tunic, and combatant Secutor’s shoulder guard and galea facemask as well as the Retiarius’ fascina prong. The highest levels of Cu on brown samples are from the Retiarius’ manica and balteus/cigulum which may suggest mixing with blue on these features. One yellow sample has high Cu, at the Secutor’s galea facemask, which likely derives from the blue feature here since the yellow has been visible painted over a blue base here |
| Zn | low trace levels on colourless glass and elevated in some spots, including the Retiarius’ manica and balteus/cigulum aligning with the high Cu here as well as the blue spots aligning with high copper on the judge/editor? tunic, the Secutor’ galea facemask and shoulder guard and also on the yellow spots of the Secutor galea facemask and balteus/cigulum. Again, it is visible evident these yellow features were painted over underlying colours in these areas |
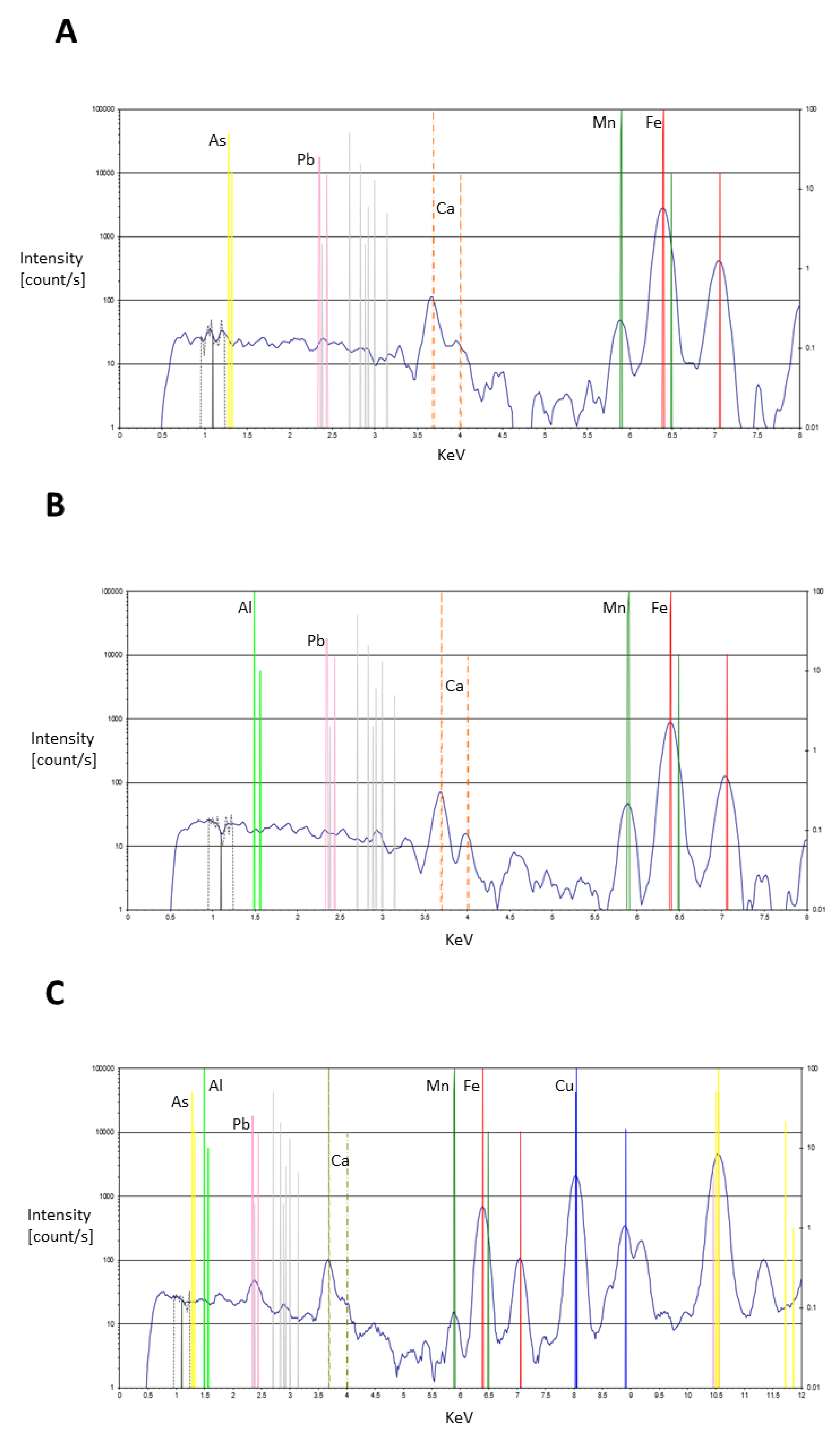
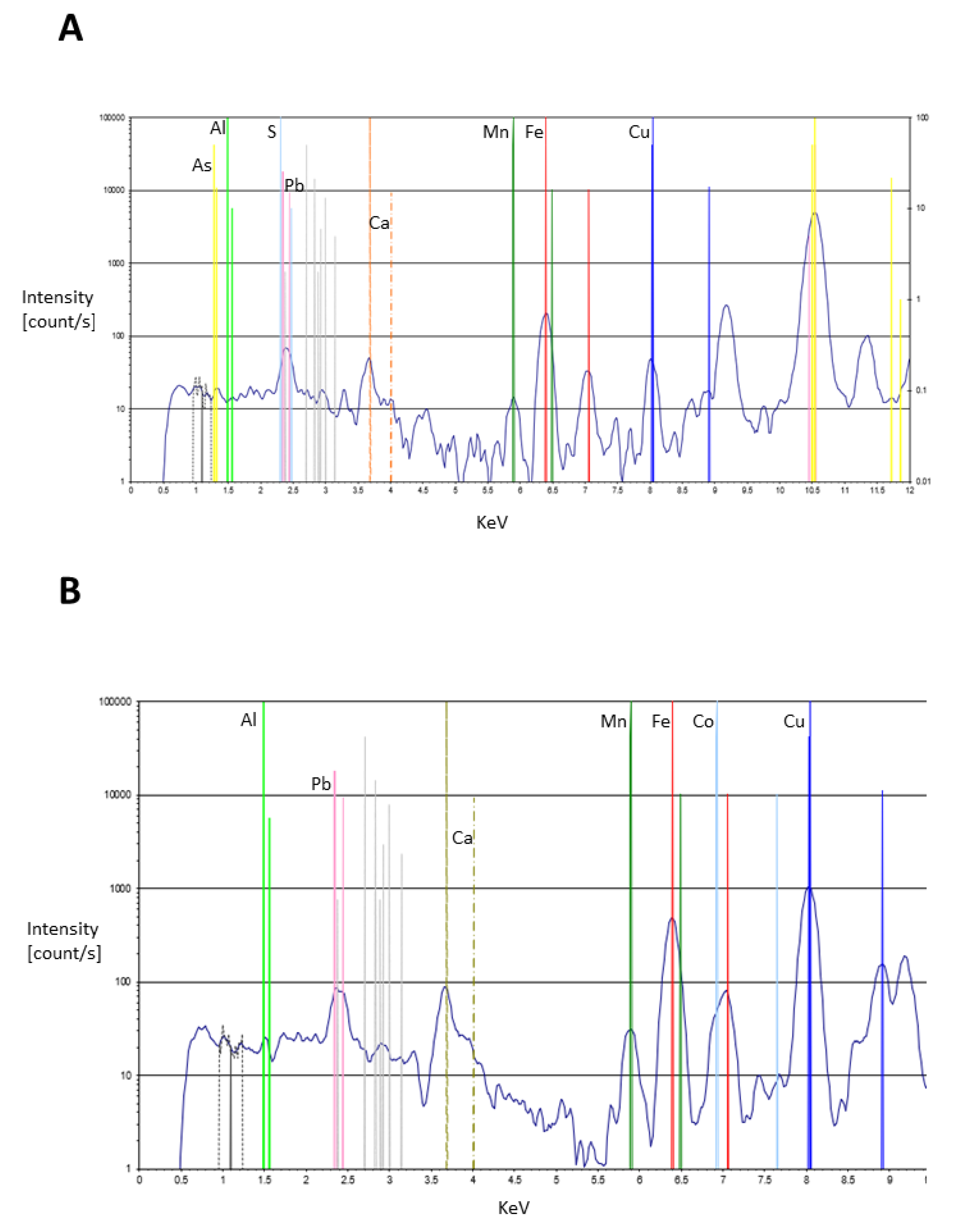
| As | very low trace levels on colourless glass and elevated levels on many samples. For example, elevated on yellow features, especially the non-combatant Secutor’s scutum front, galea facemask/balteus/cigulum as well as the decorative circle and manica front, and highest readings in some brown samples, e.g., Retiarius’ subligaculum and galerus as well as the Secutor’s boot and knee (degraded pigment on 711) and combatant Secutor’s manica elbow pad and boot central feature. These correspond with the highest levels of pb, suggesting a mix or layer of arsenic and lead-based pigment or antimony, lots of black dots suggest antimony on microphotographs. |
| Sb | present in the colourless glass with slight elevations on red samples, accoutrements of the combatants, especially the degraded scutum on 711, the Retiarius galerus, subligaculum, balteus/cigulum and the Secutor combatant’s manica elbow pad. One fleshtone at the Secutor’s torso (11078); blue areas, with highest levels on both Secutors’ facemasks and shoulder guard; several yellow samples have high levels, especially the Secutores’ galea facemasks (aligning with the blues here), balteus/cigulum and scutum front |
| Hg | below LOD on colourless glass and low elevation on a small number of samples, including brown decorative circle, judge/editor? Hair, blue Secutor shoulder guard and yellow decorative circle and secutor balteus/cigulum. May indicate mixing of cinnabar at these features |
| Pb | very low traces on the colourless glass. Elevated on a large number of features, especially high on brown Retiarius’ subligaculum, galerus, balteus/cigulum and the Secutor’s manica below the elbow on 5454 as well as the Secutor scutum with degraded pigment on 711. Also high on the yellow of the Secutor’s scutum on 11078, Secutor’s (5454) galea facemask and elevated on the yellow decorative circle, Secutor’s (11078) balteus/cigulum and in one blue sample of the judge/editor?’s tunic as well as brown of the Secutor’s boot degraded pigment (711) and Retiarius’ manica and the red inner top of the Secutor’s scutum (11078) and Retiarius’ galerus |
| Coloured Features | Elements Detected by pXRF | Pigment/s | Compound/s |
|---|---|---|---|
| Blue (1) | Al, Ca, Fe, K (trace) | Lapis lazuli | Na7Al6Si6O24S3 |
| Blue (2) | Ca, Fe, Cu, Co | Azurite | Cu3(CO3)2(OH)2 |
| Blue (3) | Ca, Fe, Cu, Si (trace) | Egyptian Blue | CaCuSi4O10 |
| Brown | Mg, Al, Fe, Cu, As, Sb, Pb, Hg (trace) | Iron oxide (hematite); Green Earth? Azurite?; Lead antimonate; Realgar; Cinnabar (trace) | Fe2O3 K[(Al,FeIII), (FeII,Mg)](AlSi3,Si4)O10(OH)2 Cu3(CO3)2(OH)2 Pb2Sb2O7 AS2S3 HgS |
| Cream | Fe, Cu, Pb | Iron oxide (hematite); Lead antimonate; Azurite? | Fe2O3 Pb2Sb2O7 Cu3(CO3)2(OH)2 |
| Red | Fe, Cu, As, Sb, Pb, Hg (trace) | Iron oxide (hematite); Minium?; Azurite?; Lead antimonate; Realgar; Cinnabar (trace) | Fe2O3 Pb3O4 Cu3(CO3)2(OH)2 Pb2Sb2O7 AS2S3 HgS |
| Fleshtone | Mg, Al, Ca, Fe, Ti, Cu, As, Sb, Pb | Iron oxide (hematite); Lead antimonate; Green earth; Orpiment; Azurite? | Fe2O3 Pb2Sb2O7 K[(Al,FeIII), (FeII,Mg)](AlSi3,Si4)O10(OH)2 AS2S3 Cu3(CO3)2(OH)2 |
| Yellow | Al, Ti, Fe, Cu, As, Sb, Pb, Hg (trace) | Iron oxide (hematite or geothite) Lead antimonate; Orpiment; Green earth; Azurite? | Fe2O3, or α-FeOOH Pb2Sb2O7 AS2S3 K[(Al,FeIII), (FeII,Mg)](AlSi3,Si4)O10(OH)2 Cu3(CO3)2(OH)2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campbell, L. The Vindolanda Vessel: pXRF and Microphotography of an Enamel-Painted Roman Gladiator Glass. Heritage 2023, 6, 3638-3672. https://doi.org/10.3390/heritage6040194
Campbell L. The Vindolanda Vessel: pXRF and Microphotography of an Enamel-Painted Roman Gladiator Glass. Heritage. 2023; 6(4):3638-3672. https://doi.org/10.3390/heritage6040194
Chicago/Turabian StyleCampbell, Louisa. 2023. "The Vindolanda Vessel: pXRF and Microphotography of an Enamel-Painted Roman Gladiator Glass" Heritage 6, no. 4: 3638-3672. https://doi.org/10.3390/heritage6040194





