An Introduction and Recent Advances in the Analytical Study of Early Synthetic Dyes and Organic Pigments in Cultural Heritage
Abstract
:1. Introduction
2. Categorisation and Chemistry of Early Synthetic Dyes
3. Synthetic Organic Pigments
- Organic pigments: these are the organic colourants that are insoluble in a medium as they are. Not all of these have a corresponding dye, e.g., Pigment Red 3, a β-naphthol dye (C.I. 12120) and Vat Blue 4/Pigment Blue 60/indanthrene blue (C.I. 69800).
- Toners: although this term is outdated, it was used to indicate a concentrated SOP. These are insoluble salts of water-soluble acid dyes with an appropriate counterion that acts as a precipitation agent, e.g., Ca, Ba, Mn, Sr inorganic salts, or the salts of water-soluble basic dyes complexed with acids, such as tannic acid. The C.I. records the different precipitation agents, e.g., Pigment Red 57:1 (C.I. 15850:1) is the calcium salt of PR57 and Pigment Red 57:2 (C.I.15850:2) is the barium salt of Pigment Red 57.
- Lakes: these are similar to toners but precipitated with a substrate of a light and neutral colour, e.g., alumina, blanc-fix, to form a metal complex, in analogy with historical lake-pigments prepared with natural dyes. Their tinctorial strength is lower than toners or organic pigments due to the lower organic dye content and the presence of a neutral-coloured substrate, hence, this class came into decline after WWI. Generally, dyes with only basic characteristics do not form lakes, whereas acid dyes do, e.g., Pigment Red 60 (C.I. 16105:1) is the barium lake of Mordant Red 9 (C.I. 16105)
- Extended pigments: these are pigments or toners (rarely lakes) diluted with a light and neutral-coloured extender (e.g., alumina, blanc-fix, calcium carbonate). The extender is not an integral part of the precipitated pigment but is incorporated in the pigment particles. Also, these are called ‘reduced’ pigments as the colour is reduced in intensity by the colour of the extender.
4. Analytical Techniques
4.1. Spectroscopic Techniques
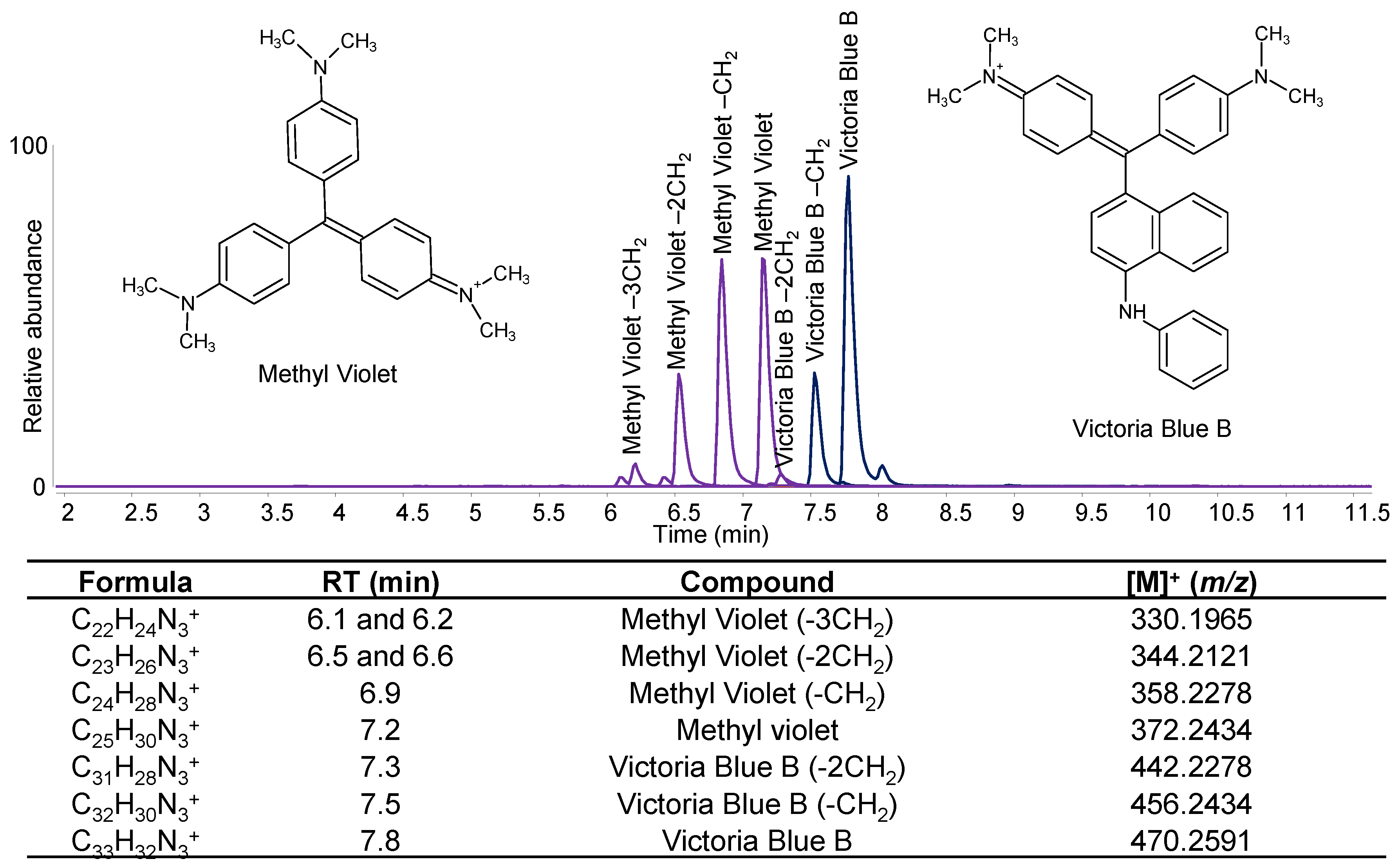
4.2. Mass Spectrometry Techniques (No or Minimal Sample Treatment)
4.3. Chromatographic Techniques
- Boiling the sample in a mixture of organic solvents and strong acids is the strongest and most aggressive method, which results in good molecular recovery but may be responsible for the cleavage of labile bonds and oxidation of moieties. HCl 0.5 M and MeOH (1:1) or HCl/MeOH/H2O (2:1:1) are the most used extraction solutions [152].
- Formic acid is a weak acid, which is used in solution HCOOH: MeOH (5:95 v/v) and allows one to perform a mild extraction [153].
- Ethylenediaminetetracetic acid (EDTA) is a complexation agent used because it is a strong chelator of aluminium and of other ions, thus releasing molecules from complexes without decomposing them. To improve the extraction yields, EDTA can be used in a mixture with an organic solvent such as dimethylformamide (DMF) [154].
- Ammonia-EDTA treatment of the sample can be followed by an ion pair dispersive liquid–liquid microextraction in chloroform using tetra-n-butylammonium bromide (TBAB) as ion pair reagent and methanol as disperser [156].
- Hydrofluoric acid (HF) is used for the mild extraction of unstable colourants belonging to a wide range of classes [157], but needs to be handled with special glassware and extra care.
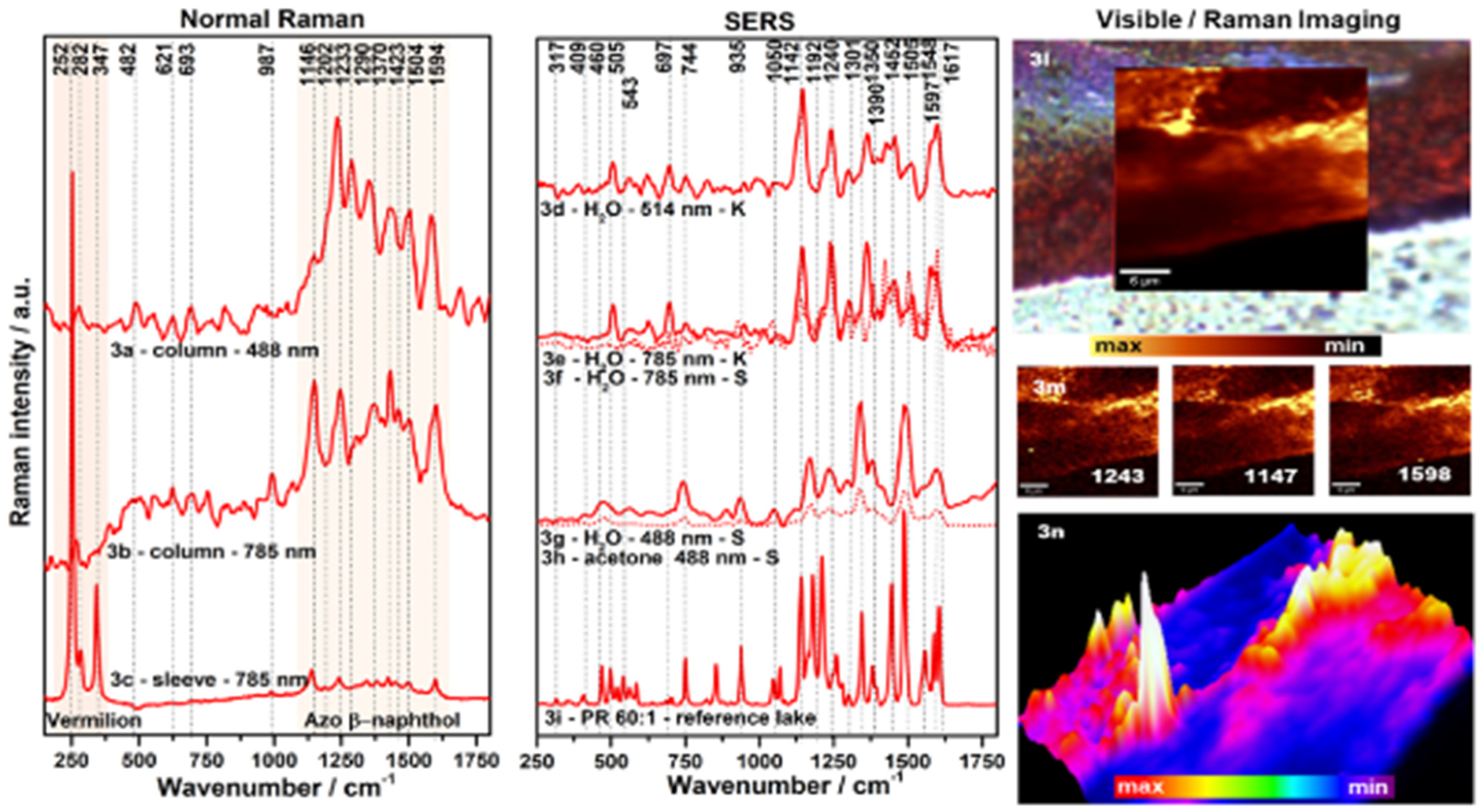
4.4. X-ray-Based Techniques
5. Degradation and Synthetic Pathways
5.1. Distinguishing Synthetic Pathways
5.2. Dealkylation, Desulphonation and Loss of Other Functional Groups
5.3. Other Photochemical Reactions
5.4. Chemical Fading
6. Case Studies
6.1. Burnished Indigo in Modern Textile-Making
6.2. Naphthol Green—A Forgotten Artists’ Pigment of the Early 20th Century
6.3. SOPs as Printing Inks
7. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Splitstoser, J.C.; Dillehay, T.D.; Wouters, J.; Claro, A. Early Pre-Hispanic Use of Indigo Blue in Peru. Sci. Adv. 2016, 2, e1501623. [Google Scholar] [CrossRef]
- Lehne, A. Tabellarische Uebersicht Uber Die Kunstlichen Organische Frabstoffe und Ihre Anwendung in Farbeirei und Zeugdruck; Springer: Berlin/Heidelberg, Germany, 1893. [Google Scholar]
- Jennison, F.H. The Manufacture of Lake Pigments from Artificial Colorus; Scott, Greenwood & Co.: London, UK, 1901. [Google Scholar]
- Bäumler, E. Die Rotfabriker, Familiengeschichte Eines Weltunternehmens; R. Piper GmbH & Co., KG: München, Germany, 1988. [Google Scholar]
- de Keijzer, M.; van Bommel, M.R.; Keijzer, R.H.; Knaller, R.; Oberhumer, E. Indigo Carmine: Understanding a Problematic Blue Dye. Stud. Conserv. 2012, 57, S87–S95. [Google Scholar] [CrossRef]
- van Bommel, M.R.; Vanden, I.; Wallert, A.M.; Boitelle, R.; Wouters, J.; Vanden Berghe, I.; Wallert, A.M. High-Performance Liquid Chromatography and Non-Destructive Three-Dimensional Fluorescence Analysis of Early Synthetic Dyes. J. Chromatogr. A 2007, 1157, 260–272. [Google Scholar]
- Woulfe, P. A Method of Dying Wool and Silk, of a Yellow Colour, with Indigo; and also with Several Other Blue and Red Colouring Substances and Receipt for Making the Yellow Dye. Philos. Trans. R. Soc. 1771, 61, 127–130. [Google Scholar]
- Sprengel, H. On a New Class of Explosives Which Are Non-Explosive during Their Manufacture, Storage, and Transport. J. Chem. Soc. 1873, 26, 796–808. [Google Scholar]
- Van Bommel, M.R.; Wallert, A.M.; Vanden Berghe, I.; Wouters, J.; Barnett, J.; Boitelle, R. Analysis of Synthetic Dyes in an Embroidery of Emile Bernard (c. 1892). In Proceedings of the ICOM-CC International Committee for Conservation, 14th Triennial Meeting, Hague, The Netherlands, 12–16 September 2005; James & James/Earthscan: London, UK, 2005. [Google Scholar]
- Cooksey, C.A.; Dronsfield, A. Pre-Perkin Synthetic Routes to Purple. Dye. Hist. Archaeol. 2003, 19, 118–124. [Google Scholar]
- Degano, I.; Tognotti, P.; Kunzelman, D.; Modugno, F. HPLC-DAD and HPLC-ESI-Q-ToF Characterisation of Early 20th Century Lake and Organic Pigments from Lefranc Archives. Herit. Sci. 2017, 5, 7. [Google Scholar] [CrossRef]
- Kauffman, G.B. Pittacal—The First Synthetic Dyestuff. J. Chem. Educ. 1977, 54, 753. [Google Scholar] [CrossRef]
- Nagendrappa, G. Sir William Henry Perkin: The Man and His ‘Mauve’. Resonance 2010, 15, 779–793. [Google Scholar] [CrossRef]
- Cooksey, C.; Dronsfield, A. Fuchsine or Magenta: The Second Most Famous Aniline Dye. A Short Memoir on the 150th Anniversary of the First Commercial Production of This Well Known Dye. Biotech. Histochem. 2009, 84, 179–183. [Google Scholar] [CrossRef]
- Van Den Belt, H. Why Monopoly Failed: The Rise and Fall of Société La Fuchsine. Br. J. Hist. Sci. 1992, 25, 45–63. [Google Scholar]
- de Keijzer, M.; van Bommel, M.R. Bright New Colours: The History and Analysis of Fluorescein, Eosin, Erythrosine, Rhodamine and Some of Their Derivatives. In The Diversity of Dyes in History and Archaeology; Kirby, J., Ed.; Archetype Publications: London, UK, 2017; pp. 326–338. [Google Scholar]
- Fieser, L.F. The Discovery of Synthetic Alizarin. J. Chem. Educ. 1930, 7, 2609. [Google Scholar] [CrossRef]
- Schmidt, H. Indigo—100 Jahre Industrielle Synthese. Chem. Unserer Zeit 1997, 31, 121–128. [Google Scholar] [CrossRef]
- Bird, C.L. Dyestuff Nomenclature*. J. Soc. Dye. Colour. 1945, 61, 321–328. [Google Scholar] [CrossRef]
- Tamburini, D. On the Reliability of Historic Books as Sources of Reference Samples of Early Synthetic Dyes—The Case of “The Coal Tar Colours of the Farbwerke Vorm. Meister, Lucius & Brüning, Höchst on the Main, Germany—A General Part” (1896). Dye. Pigment. 2024, 221, 111796. [Google Scholar] [CrossRef]
- Tamburini, D.; Shimada, C.M.; McCarthy, B. The Molecular Characterization of Early Synthetic Dyes in E. Knecht et al’s Textile Sample Book “A Manual of Dyeing” (1893) by High Performance Liquid Chromatography—Diode Array Detector—Mass Spectrometry (HPLC-DAD-MS). Dye. Pigment. 2021, 190, 109286. [Google Scholar] [CrossRef]
- Sessa, C.; Steuer, C.; Quintero Balbas, D.; Sciutto, G.; Prati, S.; Stege, H. Analytical Studies on Commercial Artists’ Colour Charts from Das Deutsche Farbenbuch (1925)—Identification of Synthetic and Natural Organic Colourants by Raman Microscopy, Surface-Enhanced Raman Spectroscopy and Metal Underlayer ATR-FTIR Spectroscopy. Herit. Sci. 2022, 10, 109. [Google Scholar] [CrossRef]
- Hagan, E.; Poulin, J. The Effect of Prior Exposure on the Lightfastness of Early Synthetic Dyes on Textiles. Herit. Sci. 2022, 10, 138. [Google Scholar] [CrossRef]
- Hagan, E.; Castro-Soto, I.; Breault, M.; Poulin, J. The Lightfastness of Early Synthetic Organic Dyes. Herit. Sci. 2022, 10, 50. [Google Scholar] [CrossRef]
- Caggiani, M.C.; Forleo, T.; Pojana, G.; Lagioia, G.; Mangone, A.; Giannossa, L.C. Characterization of Silk-Cotton and Wool-Cotton Blends Pattern Books by Fibre Optic Reflectance Spectroscopy. The Booming Market of First Synthetic Textile Dyes in Early 20th Century. Microchem. J. 2022, 175, 107178. [Google Scholar] [CrossRef]
- Pirok, B.W.J.; den Uijl, M.J.; Moro, G.; Berbers, S.V.J.; Croes, C.J.M.; van Bommel, M.R.; Schoenmakers, P.J. Characterization of Dye Extracts from Historical Cultural-Heritage Objects Using State-of-the-Art Comprehensive Two-Dimensional Liquid Chromatography and Mass Spectrometry with Active Modulation and Optimized Shifting Gradients. Anal. Chem. 2019, 91, 3062–3069. [Google Scholar] [CrossRef]
- Degano, I.; Sabatini, F.; Braccini, C.; Colombini, M.P. Triarylmethine Dyes: Characterization of Isomers Using Integrated Mass Spectrometry. Dye. Pigment. 2019, 160, 587–596. [Google Scholar] [CrossRef]
- Duxbury, D.F. The Photochemistry and Photophysics of Triphenylmethane Dyes in Solid and Liquid Media. Chem. Rev. 1993, 93, 381–433. [Google Scholar]
- Montagner, C.; Bacci, M.; Bracci, S.; Freeman, R.; Picollo, M. Library of UV–Vis–NIR Reflectance Spectra of Modern Organic Dyes from Historic Pattern-Card Coloured Papers. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 79, 1669–1680. [Google Scholar] [CrossRef]
- Claro, A.; Melo, M.J.; Seixas de Melo, J.S.; van den Berg, K.J.; Burnstock, A.; Montague, M.; Newman, R. Identification of Red Colorants in van Gogh Paintings and Ancient Andean Textiles by Microspectrofluorimetry. J. Cult. Herit. 2010, 11, 27–34. [Google Scholar] [CrossRef]
- Fremout, W.; Saverwyns, S. Identification of Synthetic Organic Pigments: The Role of a Comprehensive Digital Raman Spectral Library. J. Raman Spectrosc. 2012, 43, 1536–1544. [Google Scholar] [CrossRef]
- Tamburini, D.; Breitung, E.; Mori, C.; Kotajima, T.; Clarke, M.L.; McCarthy, B. Exploring the Transition from Natural to Synthetic Dyes in the Production of 19th-Century Central Asian Ikat Textiles. Herit. Sci. 2020, 8, 114. [Google Scholar] [CrossRef]
- Martins, A.; Prud’hom, A.C.; Duranton, M.; Haddad, A.; Daher, C.; Genachte-Le Bail, A.; Tang, T. Jazz Colors: Pigment Identification in the Gouaches Used by Henri Matisse. Heritage 2021, 4, 4205–4221. [Google Scholar] [CrossRef]
- Sessa, C.; Weiss, R.; Niessner, R.; Ivleva, N.P.; Stege, H. Towards a Surface Enhanced Raman Scattering (SERS) Spectra Database for Synthetic Organic Colourants in Cultural Heritage. The Effect of Using Different Metal Substrates on the Spectra. Microchem. J. 2018, 138, 209–225. [Google Scholar] [CrossRef]
- Degano, I.; La Nasa, J. Trends in High Performance Liquid Chromatography for Cultural Heritage. Top. Curr. Chem. 2016, 374, 20. [Google Scholar] [CrossRef]
- Groeneveld, I.; Pirok, B.W.J.; Molenaar, S.R.A.; Schoenmakers, P.J.; van Bommel, M.R. The Development of a Generic Analysis Method for Natural and Synthetic Dyes by Ultra-High-Pressure Liquid Chromatography with Photo-Diode-Array Detection and Triethylamine as an Ion-Pairing Agent. J. Chromatogr. A 2022, 1673, 463038. [Google Scholar] [CrossRef]
- Sabatini, F.; Degano, I. Investigating the Fragmentation Pathways of β-Naphthol Pigments Using Liquid Chromatography/Electrospray Ionization Quadrupole Time-of-Flight Mass Spectrometry. Rapid Commun. Mass Spectrom. 2020, 34, e8789. [Google Scholar] [CrossRef]
- Sandström, E.; Vettorazzo, C.; Mackay, C.L.; Troalen, L.G.; Hulme, A.N. Historical Textile Dye Analysis Using DESI-MS. Heritage 2023, 6, 4042–4053. [Google Scholar] [CrossRef]
- Alvarez-Martin, A.; Quanico, J.; Scovacricchi, T.; Avranovich Clerici, E.; Baggerman, G.; Janssens, K. Chemical Mapping of the Degradation of Geranium Lake in Paint Cross Sections by MALDI-MSI. Anal. Chem. 2023, 95, 18215–18223. [Google Scholar] [CrossRef]
- Centeno, S.A.; Hale, C.; Carò, F.; Cesaratto, A.; Shibayama, N.; Delaney, J.; Dooley, K.; van der Snickt, G.; Janssens, K.; Stein, S.A. Van Gogh’s Irises and Roses: The Contribution of Chemical Analyses and Imaging to the Assessment of Color Changes in the Red Lake Pigments. Herit. Sci. 2017, 5, 18. [Google Scholar] [CrossRef]
- Rowe, F.M. (Ed.) The Society of Dyers and Colourists Colour Index, 1st ed.; Society of Dyers and Colourists: Bradford, UK, 1924. [Google Scholar]
- Wich, E. The Colour Index. Color Res. Appl. 1977, 2, 77–80. [Google Scholar] [CrossRef]
- Society of Dyers and Colourists. American Association of Textile Chemists and Colorists. In Colour Index, 3rd ed.; Society of Dyers and Colourists: Bradford, UK, 1971. [Google Scholar]
- Knecht, E.; Rawson, C.; Loewenthal, R. A Manual of Dyeing: For the Use of Practical Dyers, Manufacturers, Students, and All Interested in the Art of Dyeing, 1st ed.; Charles Griffin and Company: London, UK, 1893. [Google Scholar]
- Fay, I.W. The Chemistry of the Coal-Tar Dyes, 2nd ed.; D. Van Nostrand Company: New York, NY, USA, 1919. [Google Scholar]
- Sekar, N. Direct Dyes. In Handbook of Textile and Industrial Dyeing; Elsevier: Amsterdam, The Netherlands, 2011; pp. 425–445. [Google Scholar]
- The Colour IndexTM Colour-Index.Com Published Online by Society of Dyers and Colourists and American Association of Textile Chemists and Colorists. Available online: https://colour-index.com/ (accessed on 7 February 2024).
- Kiernan, J. Classification and Naming of Dyes, Stains and Fluorochromes. Biotech. Histochem. 2001, 76, 261–278. [Google Scholar] [CrossRef]
- Griess, P. Preliminary Notice of the Reaction of Nitrous Acid with Picramic Acid and Aminonitrophenol. Ann. Chem. Pharm. 1858, 106, 123–125. [Google Scholar]
- Hunger, K.; Schmidt, M.U. Hydrazone Pigments (Formerly Called Azo Pigments). In Industrial Organic Pigments; Wiley: Hoboken, NJ, USA, 2018; pp. 193–424. [Google Scholar]
- Angelin, E.M.; Oliveira, M.C.; Nevin, A.; Picollo, M.; Melo, M.J. To Be or Not to Be an Azo Pigment: Chemistry for the Preservation of Historical β-Naphthol Reds in Cultural Heritage. Dye. Pigment. 2021, 190, 109244. [Google Scholar] [CrossRef]
- Vannucci, G.; Cañamares, M.V.; Prati, S.; Sanchez-Cortes, S. Study of the Azo-hydrazone Tautomerism of Acid Orange 20 by Spectroscopic Techniques: UV–Visible, Raman, and Surface-enhanced Raman Scattering. J. Raman Spectrosc. 2020, 51, 1295–1304. [Google Scholar] [CrossRef]
- Aiken, S.; Gabbutt, C.D.; Gillie, L.J.; Heywood, J.D.; Jacquemin, D.; Rice, C.R.; Heron, B.M. The Remarkable Hyperchromicity of Ketohydrazone Dyes and Pigment Lakes Derived from 4-Morpholino-2-naphthol. Eur. J. Org. Chem. 2013, 2013, 8097–8107. [Google Scholar] [CrossRef]
- Gessner, T.; Mayer, U. Triarylmethane and Diarylmethane Dyes. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Wanyonyi, W.C.; Onyari, J.M.; Shiundu, P.M.; Mulaa, F.J. Biodegradation and Detoxification of Malachite Green Dye Using Novel Enzymes from Bacillus Cereus Strain KM201428: Kinetic and Metabolite Analysis. Energy Procedia 2017, 119, 38–51. [Google Scholar] [CrossRef]
- Gürses, A.; Açıkyıldız, M.; Güneş, K.; Gürses, M.S. Classification of Dye and Pigments. In Dyes and Pigments; Springer: Cham, Switzerland, 2016; pp. 31–45. [Google Scholar]
- Wagner, H. Die Korperfarben; Wissenschaftliche Verlagsgesellschaft: Stuttgart, Germany, 1938. [Google Scholar]
- Zerr, G.; Rubencamp, R. Handbuch Der Farben-Fabrikation. Lehrbuch Der Fabrikation, Unterscuchung Unf Verwendung Aller in Der Praxis Vorkommenden Korperfarben, 1st ed.; Springer: Dresdeb, Germany, 1906. [Google Scholar]
- Fischer, M. The Permanent Palette; National Publishing Society: New York, NY, USA, 1930. [Google Scholar]
- Sabatini, F.; Eis, E.; Magini, F.; Degano, I.; Rickert, T. Bright Orange and Scarlet Red: Disclosing the Composition and Degradation Mechanisms of ‘Combined Lake’ Formulations. Dye. Hist. Archaeol. 2023, 37/40. Available online: https://www.researchgate.net/publication/369227280_Bright_Orange_and_Scarlet_Red_Disclosing_the_Composition_and_Degradation_Mechanisms_of_%27Combined_Lake%27_Formulations (accessed on 6 March 2024).
- León, K.; Mery, D.; Pedreschi, F.; León, J. Color Measurement in L∗a∗b∗ Units from RGB Digital Images. Food Res. Int. 2006, 39, 1084–1091. [Google Scholar] [CrossRef]
- Alvarez-Martin, A.; Trashin, S.; Cuykx, M.; Covaci, A.; De Wael, K.; Janssens, K. Photodegradation Mechanisms and Kinetics of Eosin-Y in Oxic and Anoxic Conditions. Dye. Pigment. 2017, 145, 376–384. [Google Scholar] [CrossRef]
- Izzo, F.C.; Vitale, V.; Fabbro, C.; Van Keulen, H. Multi-Analytical Investigation on Felt-Tip Pen Inks: Formulation and Preliminary Photo-Degradation Study. Microchem. J. 2016, 124, 919–928. [Google Scholar] [CrossRef]
- Ghelardi, E.; Degano, I.; Colombini, M.P.; Mazurek, J.; Schilling, M.; Khanjian, H.; Learner, T. A Multi-Analytical Study on the Photochemical Degradation of Synthetic Organic Pigments. Dye. Pigment. 2015, 123, 396–403. [Google Scholar] [CrossRef]
- Sabatini, F.; Eis, E.; Degano, I.; Thoury, M.; Bonaduce, I.; Lluveras-Tenorio, A. The Issue of Eosin Fading: A Combined Spectroscopic and Mass Spectrometric Approach Applied to Historical Lakes. Dye. Pigment. 2020, 180, 108436. [Google Scholar] [CrossRef]
- Germinario, G.; Garrappa, S.; D’Ambrosio, V.; van der Werf, I.D.; Sabbatini, L. Chemical Composition of Felt-Tip Pen Inks. Anal. Bioanal. Chem. 2018, 410, 1079–1094. [Google Scholar] [CrossRef]
- Micheluz, A.; Angelin, E.M.; Lopes, J.A.; Melo, M.J.; Pamplona, M. Discoloration of Historical Plastic Objects: New Insight into the Degradation of β-Naphthol Pigment Lakes. Polymers 2021, 13, 2278. [Google Scholar] [CrossRef]
- Picollo, M.; Bacci, M.; Casini, A.; Lotti, F.; Porcinai, S.; Radicati, B.; Stefani, L. Fiber Optics Reflectance Spectroscopy: A Non-Destructive Technique for the Analysis of Works of Art. In Optical Sensors and Microsystems: New Concepts, Materials, Technologies; Martellucci, S., Chester, A.N., Mignani, A.G., Eds.; Springer: Boston, MA, USA, 2000; pp. 259–265. ISBN 978-0-306-47099-8. [Google Scholar]
- IFAC. Fiber Optics Reflectance Spectra (FORS) of Pictorial Materials in the 270–1700 Nm Range; Institute for Applied Physics “Nello Carrara” of the National Research Council (IFAC-CNR): Florence, Italy, 2020. [Google Scholar]
- Carlesi, S.; Bartolozzi, G.; Cucci, C.; Marchiafava, V.; Picollo, M. The Artists’ Materials of Fernando Melani: A Precursor of the Poor Art Artistic Movement in Italy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 104, 527–537. [Google Scholar] [CrossRef]
- Zaffino, C.; Passaretti, A.; Poldi, G.; Fratelli, M.; Tibiletti, A.; Bestetti, R.; Saccani, I.; Guglielmi, V.; Bruni, S. A Multi-Technique Approach to the Chemical Characterization of Colored Inks in Contemporary Art: The Materials of Lucio Fontana. J. Cult. Herit. 2017, 23, 87–97. [Google Scholar] [CrossRef]
- Bacci, M.; Picollo, M.; Trumpy, G.; Tsukada, M.; Kunzelman, D. Non-Invasive Identification of White Pigments on 20Th-Century Oil Paintings by Using Fiber Optic Reflectance Spectroscopy. J. Am. Inst. Conserv. 2007, 46, 27–37. [Google Scholar] [CrossRef]
- Bacci, M.; Magrini, D.; Picollo, M.; Vervat, M. A Study of the Blue Colors Used by Telemaco Signorini (1835–1901). J. Cult. Herit. 2009, 10, 275–280. [Google Scholar] [CrossRef]
- Haddad, A.; Nakie-Miller, T.; Jenks, J.B.; Kowach, G. Andy Warhol and His Amazing Technicolor Shoes: Characterizing the Synthetic Dyes Found in Dr. Ph. Martin’s Synchromatic Transparent Watercolors and Used in À La Recherche Du Shoe Perdu. Colorants 2022, 2, 1–21. [Google Scholar] [CrossRef]
- Cesaratto, A.; Luo, Y.-B.; Smith, H.D.; Leona, M. A Timeline for the Introduction of Synthetic Dyestuffs in Japan during the Late Edo and Meiji Periods. Herit. Sci. 2018, 6, 22. [Google Scholar] [CrossRef]
- Chavanne, C.; Verney, A.; Paquier-Berthelot, C.; Bostal, M.; Buléon, P.; Walter, P. Bayeux Tapestry: First Use of Early Synthetic Dyes for the Restoration of a Masterpiece. Dye. Pigment. 2023, 208, 110798. [Google Scholar] [CrossRef]
- Ciccola, A.; Serafini, I.; D’Agostino, G.; Giambra, B.; Bosi, A.; Ripanti, F.; Nucara, A.; Postorino, P.; Curini, R.; Bruno, M. Dyes of a Shadow Theatre: Investigating Tholu Bommalu Indian Puppets through a Highly Sensitive Multi-Spectroscopic Approach. Heritage 2021, 4, 1807–1820. [Google Scholar] [CrossRef]
- Tamburini, D.; Dyer, J.; Cartwright, C.; Green, A. Changes in the Production Materials of Burmese Textiles in the Nineteenth Century—Dyes, Mordants and Fibres of Karen Garments from the British Museum’s Collection. Herit. Sci. 2023, 11, 150. [Google Scholar] [CrossRef]
- Tamburini, D.; Kim-Marandet, M.; Kim, S.A. Dye Identification in Mounting Textiles of Traditional Korean Paintings from the Late Joseon Dynasty. Heritage 2023, 6, 44–66. [Google Scholar] [CrossRef]
- Carlesi, S.; Bartolozzi, G.; Cucci, C.; Marchiafava, V.; Picollo, M.; La Nasa, J.; Di Girolamo, F.; Dilillo, M.; Modugno, F.; Degano, I.; et al. Discovering “the Italian Flag” by Fernando Melani (1907–1985). Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 168, 52–59. [Google Scholar] [CrossRef]
- Cucci, C.; Bartolozzi, G.; De Vita, M.; Marchiafava, V.; Picollo, M.; Casadio, F. The Colors of Keith Haring: A Spectroscopic Study on the Materials of the Mural Painting Tuttomondo and on Reference Contemporary Outdoor Paints. Appl. Spectrosc. 2016, 70, 186–196. [Google Scholar] [CrossRef]
- Vermeulen, M.; Smith, K.; Eremin, K.; Rayner, G.; Walton, M. Application of Uniform Manifold Approximation and Projection (UMAP) in Spectral Imaging of Artworks. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 252, 119547. [Google Scholar] [CrossRef]
- Pérez-Arantegui, J.; Rupérez, D.; Almazán, D.; Díez-de-Pinos, N. Colours and Pigments in Late Ukiyo-e Art Works: A Preliminary Non-Invasive Study of Japanese Woodblock Prints to Interpret Hyperspectral Images Using in-Situ Point-by-Point Diffuse Reflectance Spectroscopy. Microchem. J. 2018, 139, 94–109. [Google Scholar] [CrossRef]
- Biron, C.; Mounier, A.; Le Bourdon, G.; Servant, L.; Chapoulie, R.; Daniel, F. Revealing the Colours of Ukiyo-e Prints by Short Wave Infrared Range Hyperspectral Imaging (SWIR). Microchem. J. 2020, 155, 104782. [Google Scholar] [CrossRef]
- De La Codre, H.; Daniel, F.; Chapoulie, R.; Servant, L.; Mounier, A. Investigating the Materials Used in Eighteenth-Century Tapestries from the Three French Royal Manufactories: Inputs of Hyperspectral Approaches. Eur. Phys. J. Plus 2021, 136, 1193. [Google Scholar] [CrossRef]
- Vermeulen, M.; Tamburini, D.; McGeachy, A.C.; Meyers, R.D.; Walton, M.S. Multiscale Characterization of Shellfish Purple and Other Organic Colorants in 20th-Century Traditional Enredos from Oaxaca, Mexico. Dye. Pigment. 2022, 206, 110663. [Google Scholar] [CrossRef]
- Vlachou-Mogire, C.; Danskin, J.; Gilchrist, J.R.; Hallett, K. Mapping Materials and Dyes on Historic Tapestries Using Hyperspectral Imaging. Heritage 2023, 6, 3159–3182. [Google Scholar] [CrossRef]
- Claro, A.; Melo, M.J.; Schäfer, S.; de Melo, J.S.S.; Pina, F.; van den Berg, K.J.; Burnstock, A. The Use of Microspectrofluorimetry for the Characterization of Lake Pigments. Talanta 2008, 74, 922–929. [Google Scholar] [CrossRef]
- Nevin, A.; Comelli, D.; Valentini, G.; Anglos, D.; Burnstock, A.; Cather, S.; Cubeddu, R. Time-Resolved Fluorescence Spectroscopy and Imaging of Proteinaceous Binders Used in Paintings. Anal. Bioanal. Chem. 2007, 338, 1897–1905. [Google Scholar]
- Nevin, A.; Cesaratto, A.; Bellei, S.; D’Andrea, C.; Toniolo, L.; Valentini, G.; Comelli, D. Time-Resolved Photoluminescence Spectroscopy and Imaging: New Approaches to the Analysis of Cultural Heritage and Its Degradation. Sensors 2014, 14, 6338–6355. [Google Scholar] [CrossRef]
- Doherty, B.; Vagnini, M.; Dufourmantelle, K.; Sgamellotti, A.; Brunetti, B.; Miliani, C. A Vibrational Spectroscopic and Principal Component Analysis of Triarylmethane Dyes by Comparative Laboratory and Portable Instrumentation. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 121, 292–305. [Google Scholar] [CrossRef]
- Haddad, A.; Rogge, C.E. Is There an International Klein Pink? Colorants 2023, 2, 194–208. [Google Scholar] [CrossRef]
- Moretti, P.; Germinario, G.; Doherty, B.; van der Werf, I.D.; Sabbatini, L.; Mirabile, A.; Sgamellotti, A.; Miliani, C. Disclosing the Composition of Historical Commercial Felt-Tip Pens Used in Art by Integrated Vibrational Spectroscopy and Pyrolysis-Gas Chromatography/Mass Spectrometry. J. Cult. Herit. 2019, 35, 242–253. [Google Scholar] [CrossRef]
- Neugebauer, W.; Sessa, C.; Steuer, C.; Allscher, T.; Stege, H. Naphthol Green—A Forgotten Artists’ Pigment of the Early 20th Century. History, Chemistry and Analytical Identification. J. Cult. Herit. 2018, 36, 153–165. [Google Scholar] [CrossRef]
- Anselmi, C.; Capitani, D.; Tintaru, A.; Doherty, B.; Sgamellotti, A.; Miliani, C. Beyond the Color: A Structural Insight to Eosin-Based Lakes. Dye. Pigment. 2017, 140, 297–311. [Google Scholar] [CrossRef]
- Alvarez-Martin, A.; Janssens, K. Protecting and Stimulating Effect on the Degradation of Eosin Lakes. Part 1: Lead White and Cobalt Blue. Microchem. J. 2018, 141, 51–63. [Google Scholar] [CrossRef]
- Stenger, J.; Kwan, E.E.; Eremin, K.; Speakman, S.; Kirby, D.; Stewart, H.; Huang, S.G.; Kennedy, A.R.; Newman, R.; Khandekar, N. Lithol Red Salts: Characterization and Deterioration. e-Preservation Sci. 2010, 7, 147–157. [Google Scholar]
- Lomax, S.Q.; Lomax, J.F. The Synthesis Characterization of Historical Novel Azo Pigments: Implications for Conservation Science. Herit. Sci. 2019, 7, 101. [Google Scholar] [CrossRef]
- Beltran, V.; Marchetti, A.; De Meyer, S.; Nuyts, G.; De Wael, K. Geranium Lake Pigments: The Role of the Synthesis on the Structure and Composition. Dye. Pigment. 2021, 189, 109260. [Google Scholar] [CrossRef]
- Wertz, J.H.; Tang, P.L.; Quye, A.; France, D.J. Characterisation of Oil and Aluminium Complex on Replica and Historical 19th c. Turkey Red Textiles by Non-Destructive Diffuse Reflectance FTIR Spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 204, 267–275. [Google Scholar] [CrossRef]
- Prati, S.; Milosevic, M.; Sciutto, G.; Bonacini, I.; Kazarian, S.G.; Mazzeo, R. Analyses of Trace Amounts of Dyes with a New Enhanced Sensitivity FTIR Spectroscopic Technique: MU-ATR (Metal Underlayer ATR Spectroscopy). Anal. Chim. Acta 2016, 941, 67–79. [Google Scholar] [CrossRef]
- Quintero Balbas, D.; Prati, S.; Sciutto, G.; Catelli, E.; Mazzeo, R. Thin-Layer Chromatography/Metal Underlayer-ATR FTIR Methodology for the Study of Synthetic Dyes Extracted from Degraded Wool Fibres. New J. Chem. 2019, 43, 9411–9419. [Google Scholar] [CrossRef]
- Beltran, V.; Salvadó, N.; Butí, S.; Cinque, G. Micro Infrared Spectroscopy Discrimination Capability of Compounds in Complex Matrices of Thin Layers in Real Sample Coatings from Artworks. Microchem. J. 2015, 118, 115–123. [Google Scholar] [CrossRef]
- Geiman, I.; Leona, M.; Lombardi, J.R. Application of Raman Spectroscopy and Surface-Enhanced Raman Scattering to the Analysis of Synthetic Dyes Found in Ballpoint Pen Inks. J. Forensic Sci. 2009, 54, 947–952. [Google Scholar] [CrossRef]
- Vandenabeele, P.; Moens, L.; Edwards, H.G.M.; Dams, R. Raman Spectroscopic Database of Azo Pigments and Application to Modern Art Studies. J. Raman Spectrosc. 2000, 31, 509–517. [Google Scholar]
- Lomax, S.Q.; Lomax, J.F.; De Luca-Westrate, A. The Use of Raman Microscopy and Laser Desorption Ionization Mass Spectrometry in the Examination of Synthetic Organic Pigments in Modern Works of Art. J. Raman Spectrosc. 2014, 45, 448–455. [Google Scholar] [CrossRef]
- Scherrer, N.C.; Stefan, Z.; Francoise, D.; Annette, F.; Renate, K. Synthetic Organic Pigments of the 20th and 21st Century Relevant to Artist’s Paints: Raman Spectra Reference Collection. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 73, 505–524. [Google Scholar] [CrossRef]
- Colombini, A.; Kaifas, D. Characterization of Some Orange and Yellow Organic and Fluorescent Pigments by Raman Spectroscopy. e-Preservation Sci. 2010, 7, 14–21. [Google Scholar]
- Ropret, P.; Centeno, S.A.; Bukovec, P. Raman Identification of Yellow Synthetic Organic Pigments in Modern and Contemporary Paintings: Reference Spectra and Case Studies. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2008, 69, 486–497. [Google Scholar] [CrossRef]
- Pause, R.; van der Werf, I.D.; van den Berg, K.J. Identification of Pre-1950 Synthetic Organic Pigments in Artists’ Paints. A Non-Invasive Approach Using Handheld Raman Spectroscopy. Heritage 2021, 4, 1348–1365. [Google Scholar] [CrossRef]
- Royal Institute for Cultural Heritage (KIK/IRPA) Modern and Contemporary Art in the Laboratory. Available online: https://modern.kikirpa.be (accessed on 6 March 2024).
- Rousaki, A.; Vandenabeele, P. In Situ Raman Spectroscopy for Cultural Heritage Studies. J. Raman Spectrosc. 2021, 52, 2178–2189. [Google Scholar] [CrossRef]
- Ciccola, A.; Tozzi, L.; Romani, M.; Serafini, I.; Ripanti, F.; Curini, R.; Vitucci, F.; Cestelli Guidi, M.; Postorino, P. Lucio Fontana and the Light: Spectroscopic Analysis of the Artist’s Collection at the National Gallery of Modern and Contemporary Art. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 236, 118319. [Google Scholar] [CrossRef]
- Schulte, F.; Brzezinka, K.-W.; Lutzenberger, K.; Stege, H.; Panne, U. Raman Spectroscopy of Synthetic Organic Pigments Used in 20th Century Works of Art. J. Raman Spectrosc. 2008, 39, 1455–1463. [Google Scholar] [CrossRef]
- Lomax, S.Q.; Lomax, J.F.; Graham, T.K.; Moore, T.J.T.; Knapp, C.G. Historical Azo Pigments: Synthesis and Characterization. J. Cult. Herit. 2018, 35, 218–224. [Google Scholar] [CrossRef]
- Sodo, A.; Bicchieri, M.; Guiso, M.; Ricci, M.A.; Ricci, G. Raman Investigations on Marker Pen Inks. J. Raman Spectrosc. 2012, 43, 1781–1787. [Google Scholar] [CrossRef]
- Rosi, F.; Grazia, C.; Fontana, R.; Gabrieli, F.; Pensabene Buemi, L.; Pampaloni, E.; Romani, A.; Stringari, C.; Miliani, C. Disclosing Jackson Pollock’s Palette in Alchemy (1947) by Non-Invasive Spectroscopies. Herit. Sci. 2016, 4, 18. [Google Scholar] [CrossRef]
- Haddad, A.; Neufeld, L.; Martins, A. Realizing Sensations: Analyzing Paul Cezanne’s Watercolors and Assessing Their Light Sensitivity with Microfade Testing. Herit. Sci. 2023, 11, 39. [Google Scholar] [CrossRef]
- Angelin, E.M.; de Sá, S.; Picollo, M.; Nevin, A.; Callapez, M.E.; Melo, M.J. The Identification of Synthetic Organic Red Pigments in Historical Plastics: Developing an in Situ Analytical Protocol Based on Raman Microscopy. J. Raman Spectrosc. 2021, 52, 145–158. [Google Scholar] [CrossRef]
- Haddad, A.; Randall, M.; Zycherman, L.; Martins, A. Reviving Alexander Calder’s Man-Eater with Pennants: A Technical Examination of the Original Paint Palette. Heritage 2021, 4, 1920–1937. [Google Scholar] [CrossRef]
- Amato, F.; Micciche’, C.; Cannas, M.; Gelardi, F.M.; Pignataro, B.; Li Vigni, M.; Agnello, S. Ag Nanoparticles Agargel Nanocomposites for SERS Detection of Cultural Heritage Interest Pigments. Eur. Phys. J. Plus 2018, 133, 74. [Google Scholar] [CrossRef]
- Longoni, M.; Gavazzi, M.; Monti, D.; Bruni, S. Early Synthetic Textile Dyes of the Late 19th Century from the “Primo Levi” Chemistry Museum (Rome): A Multi-Technique Analytical Investigation. J. Cult. Herit. 2023, 59, 131–139. [Google Scholar] [CrossRef]
- Leona, M.; Decuzzi, P.; Kubic, T.A.; Gates, G.; Lombardi, J.R. Non Destructive Identification of Natural and Synthetic Organic Colorants in Works of Art by Surface Enhanced Raman Scattering. Anal. Chem. 2011, 83, 3990–3993. [Google Scholar] [CrossRef]
- Reggio, D.; Mirabile, A.; Lazzari, M. Sensing Soluble Molecules through SERS Substrates in One-Step Procedure: Unrevealing the Meiji Woodblock Printing Materials. Talanta 2023, 254, 124177. [Google Scholar] [CrossRef]
- Anghelone, M.; Stoytschew, V.; Jembrih-Simbürger, D.; Schreiner, M. Spectroscopic Methods for the Identification and Photostability Study of Red Synthetic Organic Pigments in Alkyd and Acrylic Paints. Microchem. J. 2018, 139, 155–163. [Google Scholar] [CrossRef]
- Coccato, A.; Caggiani, M.C. An Overview of Principal Components Analysis Approaches in Raman Studies of Cultural Heritage Materials. J. Raman Spectrosc. 2023, 55, 125–147. [Google Scholar] [CrossRef]
- Manfredi, M.; Barberis, E.; Aceto, M.; Marengo, E. Non-Invasive Characterization of Colorants by Portable Diffuse Reflectance Infrared Fourier Transform (DRIFT) Spectroscopy and Chemometrics. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 181, 171–179. [Google Scholar] [CrossRef]
- Poulin, J. A New Methodology for the Characterisation of Natural Dyes on Museum Objects Using Gas Chromatography–Mass Spectrometry. Stud. Conserv. 2018, 63, 36–61. [Google Scholar] [CrossRef]
- Degani, L.; Riedo, C.; Gulmini, M.; Chiantore, O. From Plant Extracts to Historical Textiles: Characterization of Dyestuffs by GC–MS. Chromatographia 2014, 77, 1683–1696. [Google Scholar] [CrossRef]
- Lomax, S.; Schilling, M.; Learner, T. The Identification of Synthetic Organic Pigments by FTIR and DTMS. In Modern Paints Uncovered; Getty Publications: Los Angeles, CA, USA, 2007. [Google Scholar]
- Boon, J.J.; Learner, T. Analytical Mass Spectrometry of Artists’ Acrylic Emulsion Paints by Direct Temperature Resolved Mass Spectrometry and Laser Desorption Ionisation Mass Spectrometry. J. Anal. Appl. Pyrolysis 2002, 64, 327–344. [Google Scholar] [CrossRef]
- Menke, C.A.; Rivenc, R.; Learner, T. The Use of Direct Temperature-Resolved Mass Spectrometry (DTMS) in the Detection of Organic Pigments Found in Acrylic Paints Used by Sam Francis. Int. J. Mass Spectrom. 2009, 284, 2–11. [Google Scholar] [CrossRef]
- Rehorek, A.; Plum, A. Characterization of Sulfonated Azo Dyes and Aromatic Amines by Pyrolysis Gas Chromatography/Mass Spectrometry. Anal. Bioanal. Chem. 2007, 388, 1653–1662. [Google Scholar] [CrossRef]
- Russell, J.; Singer, B.W.; Perry, J.J.; Bacon, A. The Identification of Synthetic Organic Pigments in Modern Paints and Modern Paintings Using Pyrolysis-Gas Chromatography--Mass Spectrometry. Anal. Bioanal. Chem. 2011, 400, 1473. [Google Scholar] [CrossRef]
- Germinario, G.; Rigante, E.C.L.; van der Werf, I.D.; Sabbatini, L. Pyrolysis Gas Chromatography–Mass Spectrometry of Triarylmethane Dyes. J. Anal. Appl. Pyrolysis 2017, 127, 229–239. [Google Scholar] [CrossRef]
- La Nasa, J.; Biale, G.; Sabatini, F.; Degano, I.; Colombini, M.P.M.P.; Modugno, F. Synthetic Materials in Art: A New Comprehensive Approach for the Characterization of Multi-Material Artworks by Analytical Pyrolysis. Herit. Sci. 2019, 7, 8. [Google Scholar] [CrossRef]
- Fardi, T.; Pintus, V.; Kampasakali, E.; Pavlidou, E.; Schreiner, M.; Kyriacou, G. Analytical Characterization of Artist’s Paint Systems Based on Emulsion Polymers and Synthetic Organic Pigments. J. Anal. Appl. Pyrolysis 2018, 135, 231–241. [Google Scholar] [CrossRef]
- Soltzberg, L.J.; Hagar, A.; Kridaratikorn, S.; Mattson, A.; Newman, R. MALDI-TOF Mass Spectrometric Identification of Dyes and Pigments. J. Am. Soc. Mass Spectrom. 2007, 18, 2001–2006. [Google Scholar] [CrossRef]
- Kirby, D.P.; Khandekar, N.; Sutherland, K.; Price, B.A. Applications of Laser Desorption Mass Spectrometry for the Study of Synthetic Organic Pigments in Works of Art. Int. J. Mass Spectrom. 2009, 284, 115–122. [Google Scholar] [CrossRef]
- Schreiver, I.; Eschner, L.-M.; Luch, A. Matrix-Assisted Laser Desorption/Ionization Tandem Mass Spectrometry for Identification of Organic Tattoo Pigments in Inks and Tissue Samples. Analyst 2018, 143, 3941–3950. [Google Scholar]
- Dunn, J.; Siegel, J.; Allison, J. Photodegradation and Laser Desorption Mass Spectrometry for the Characterization of Dyes Used in Red Pen Inks. Sci. J. Forensic 2003, 48, 652–657. [Google Scholar]
- Weyermann, C.; Kirsch, D.; Costa-Vera, C.; Spengler, B. Photofading of Ballpoint Dyes Studied on Paper by LDI and MALDI MS. J. Am. Soc. Mass Spectrom. 2006, 17, 297–306. [Google Scholar] [CrossRef]
- Weyermann, C.; Kirsch, D.; Vera, C.C.; Spengler, B. Evaluation of the Photodegradation of Crystal Violet upon Light Exposure by Mass Spectrometric and Spectroscopic Methods. J. Forensic Sci. 2009, 54, 339–345. [Google Scholar] [CrossRef]
- Weyermann, C.; Spengler, B. The Potential of Artificial Aging for Modelling of Natural Aging Processes of Ballpoint Ink. Forensic Sci. Int. 2008, 180, 23–31. [Google Scholar] [CrossRef]
- Taverna, D.; Di Donna, L.; Mazzotti, F.; Policicchio, B.; Sindona, G. High-Throughput Determination of Sudan Azo-Dyes within Powdered Chili Pepper by Paper Spray Mass Spectrometry. J. Mass Spectrom. 2013, 48, 544–547. [Google Scholar] [CrossRef]
- Guo, T.; Zhang, Z.; Yannell, K.E.; Dongb, Y.; Cooks, R.G. Paper Spray Ionization Mass Spectrometry for Rapid Quantification of Illegal Beverage Dyes. Anal. Methods 2017, 9, 6273–6279. [Google Scholar]
- Alvarez-Martin, A.; Cleland, T.P.; Kavich, G.M.; Janssens, K.; Newsome, G.A. Rapid Evaluation of the Debromination Mechanism of Eosin in Oil Paint by Direct Analysis in Real Time and Direct Infusion-Electrospray Ionization Mass Spectrometry. Anal. Chem. 2019, 91, 10856–10863. [Google Scholar] [CrossRef]
- Sandström, E.; Vettorazzo, C.; Mackay, C.L.; Troalen, L.G.; Hulme, A.N. Development and Application of Desorption Electrospray Ionization Mass Spectrometry for Historical Dye Analysis. Anal. Chem. 2023, 95, 4846–4854. [Google Scholar] [CrossRef]
- Astefanei, A.; van Bommel, M.R.; Corthals, G.L. Surface Acoustic Wave Nebulisation Mass Spectrometry for the Fast and Highly Sensitive Characterisation of Synthetic Dyes in Textile Samples. J. Am. Soc. Mass Spectrom. 2017, 28, 2108–2116. [Google Scholar] [CrossRef]
- Wei, L.; Ma, Y.; Guo, Z.; Ding, J.; Jin, G.; Gu, A.; Lei, Y. Application of Advanced Analytical Techniques in Organic Cultural Heritage: A Case Study of Ancient Architecture Relics in the Palace Museum (Beijing). Coatings 2022, 12, 636. [Google Scholar] [CrossRef]
- Krmpotić, M.; Jembrih-Simbürger, D.; Siketić, Z.; Marković, N.; Anghelone, M.; Tadić, T.; Plavčić, D.; Malloy, M.; Radović, I.B. Identification of Synthetic Organic Pigments (SOPs) Used in Modern Artist’s Paints with Secondary Ion Mass Spectrometry with MeV Ions. Anal. Chem. 2020, 92, 9287–9294. [Google Scholar] [CrossRef]
- Degano, I.; Ribechini, E.; Modugno, F.; Colombini, M.P. Analytical Methods for the Characterization of Organic Dyes in Artworks and in Historical Textiles. Appl. Spectrosc. Rev. 2009, 44, 363–410. [Google Scholar] [CrossRef]
- Zhang, X.; Laursen, R.A. Development of Mild Extraction Methods for the Analysis of Natural Dyes in Textiles of Historical Interest Using LC-Diode Array Detector-MS. Anal. Chem. 2005, 77, 2022–2025. [Google Scholar] [CrossRef]
- Surowiec, I. Application of High-Performance Separation Techniques in Archaeometry. Microchim. Acta 2008, 162, 289–302. [Google Scholar] [CrossRef]
- Manhita, A.; Ferreira, T.; Candeias, A.; Barrocas Dias, C. Extracting Natural Dyes from Wool---an Evaluation of Extraction Methods. Anal. Bioanal. Chem. 2011, 400, 1501. [Google Scholar] [CrossRef]
- Serafini, I.; McClure, K.R.; Ciccola, A.; Vincenti, F.; Bosi, A.; Peruzzi, G.; Montesano, C.; Sergi, M.; Favero, G.; Curini, R. Inside the History of Italian Coloring Industries: An Investigation of ACNA Dyes through a Novel Analytical Protocol for Synthetic Dye Extraction and Characterization. Molecules 2023, 28, 5331. [Google Scholar] [CrossRef]
- Sanyova, J.; Reisse, J. Development of a Mild Method for the Extraction of Anthraquinones from Their Aluminum Complexes in Madder Lakes Prior to HPLC Analysis. J. Cult. Herit. 2006, 7, 229–235. [Google Scholar] [CrossRef]
- Christie, R.M.; Standring, P.N. Colour and Constitution Relationships in Organic Pigments. Part 2—Disazoacetoacetanilides11Part 1-Monoazoacetoacetanilides: Dyes and Pigments, 9(1). Dye. Pigment. 1989, 11, 109–121. [Google Scholar] [CrossRef]
- Christie, R.M.; Standring, P.N.; Griffiths, J. Colour and Constitution Relationships in Organic Pigments. Part 1—Monoazoacetoacetanilides. Dye. Pigment. 1988, 9, 37–56. [Google Scholar] [CrossRef]
- Souto, C.S.C.N. Analysis of Early Synthetic Dyes with HPLC-DAD-MS. Master’s Thesis, Universidade de Lisboa, Lisboa, Portugal, 2010. [Google Scholar]
- Talsky, G.; Ristić-Šolajić, M. High-Resolution/Higher-Order Derivative Spectrophotometry for Identification and Estimation of Synthetic Organic Pigments in Artists’ Paints. Anal. Chim. Acta 1987, 196, 123–134. [Google Scholar] [CrossRef]
- Deomartins, A.; Canalli, V.M.; Azevedo, C.M.N.; Pires, M. Degradation of Pararosaniline (C.I. Basic Red 9 Monohydrochloride) Dye by Ozonation and Sonolysis. Dye. Pigment. 2006, 68, 227–234. [Google Scholar] [CrossRef]
- Jain, R.; Sikarwar, S. Photodegradation of Hazardous Dye Naphthol Yellow S Over Titanium Dioxide. J. Dispers. Sci. Technol. 2011, 32, 1345–1352. [Google Scholar] [CrossRef]
- Zaied, M.; Chutet, E.; Peulon, S.; Bellakhal, N.; Desmazières, B.; Dachraoui, M.; Chaussé, A. Spontaneous Oxidative Degradation of Indigo Carmine by Thin Films of Birnessite Electrodeposited onto SnO2. Appl. Catal. B 2011, 107, 42–51. [Google Scholar] [CrossRef]
- Sherma, J. Advances in the Thin-Layer Chromatographic Forensic Analysis of Inks. J. Liq. Chromatogr. Relat. Technol. 2016, 39, 549–557. [Google Scholar] [CrossRef]
- Causin, V.; Casamassima, R.; Marega, C.; Maida, P.; Schiavone, S.; Marigo, A.; Villari, A. The Discrimination Potential of Ultraviolet-Visible Spectrophotometry, Thin Layer Chromatography, and Fourier Transform Infrared Spectroscopy for the Forensic Analysis of Black and Blue Ballpoint Inks. J. Forensic Sci. 2008, 53, 1468–1473. [Google Scholar] [CrossRef]
- Cañamares, M.V.; Reagan, D.A.; Lombardi, J.R.; Leona, M. TLC-SERS of Mauve, the First Synthetic Dye. J. Raman Spectrosc. 2014, 45, 1147–1152. [Google Scholar] [CrossRef]
- Ferretti, A.; Degano, I.; Legnaioli, S.; Campanella, B.; Sainati, A.; Colombini, M.P. Shedding Light on the Composition and Degradation Mechanism of Dyes in Historical Ink’s Collection (19th–20th Century). Dye. Pigment. 2023, 220, 111672. [Google Scholar] [CrossRef]
- Campanella, B.; Botti, J.; Cavaleri, T.; Cicogna, F.; Legnaioli, S.; Pagnotta, S.; Poggialini, F.; Poli, T.; Scalarone, D.; Palleschi, V. The Shining Brightness of Daylight Fluorescent Pigments: Raman and SERS Study of a Modern Class of Painting Materials. Microchem. J. 2020, 152, 104292. [Google Scholar] [CrossRef]
- Troalen, L.G.; Phillips, A.S.; Peggie, D.A.; Barran, P.E.; Hulme, A.N. Historical Textile Dyeing with Genista tinctoria L.: A Comprehensive Study by UPLC-MS/MS Analysis. Anal. Methods 2014, 6, 8915–8923. [Google Scholar] [CrossRef]
- Douglas, J.G.; Kavich, G.; Mori, C.; Wallace, D.; Barden, R. Materials Characterization of the Ruby Slippers from the 1939 Classic Film, The Wizard of Oz. Herit. Sci. 2018, 6, 49. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Y.; Zhao, P.; Peng, Z.; Wang, S. Identification of Early Synthetic Dyes in Historical Chinese Textiles of the Late Nineteenth Century by High-Performance Liquid Chromatography Coupled with Diode Array Detection and Mass Spectrometry. Color. Technol. 2016, 132, 177–185. [Google Scholar] [CrossRef]
- Confortin, D.; Neevel, H.; Brustolon, M.; Franco, L.; Kettelarij, A.J.A.J.; Williams, R.M.R.M.; van Bommel, M.R. Crystal Violet: Study of the Photo-Fading of an Early Synthetic Dye in Aqueous Solution and on Paper with HPLC-PDA, LC-MS and FORS. J. Phys. Conf. Ser. 2010, 231, 012011. [Google Scholar] [CrossRef]
- Chen, V.J.; Smith, G.D.; Holden, A.; Paydar, N.; Kiefer, K. Chemical Analysis of Dyes on an Uzbek Ceremonial Coat: Objective Evidence for Artifact Dating and the Chemistry of Early Synthetic Dyes. Dye. Pigment. 2016, 131, 320–332. [Google Scholar] [CrossRef]
- de Melo, J.S.; Takato, S.; Sousa, M.; Melo, M.J.; Parola, A.J. Revisiting Perkin’s Dye(s): The Spectroscopy and Photophysics of Two New Mauveine Compounds (B2 and C). Chem. Commun. 2007, 25, 2624–2626. [Google Scholar]
- Kucharska, M.; Grabka, J. A Review of Chromatographic Methods for Determination of Synthetic Food Dyes. Talanta 2010, 80, 1045–1051. [Google Scholar] [CrossRef]
- Cai, M.; Jin, M.; Weavers, L.K. Analysis of Sonolytic Degradation Products of Azo Dye Orange G Using Liquid Chromatography–Diode Array Detection-Mass Spectrometry. Ultrason. Sonochemistry 2011, 18, 1068–1076. [Google Scholar] [CrossRef]
- Petroviciu, I.; Teodorescu, I.C.; Vasilca, S.; Albu, F. Transition from Natural to Early Synthetic Dyes in the Romanian Traditional Shirts Decoration. Heritage 2023, 6, 505–523. [Google Scholar] [CrossRef]
- Geldof, M.; de Keijzer, M.; van Bommel, M.; Pilz, K.; Salvant, J.; van Keulen, H.; Megens, L. Van Gogh’s Geranium Lake. In Van Gogh’s Studio Practice; Mercatorfonds: Brussels, Belgium, 2013. [Google Scholar]
- Plater, M.J.; Raab, A. Mauveine and the Mauve Shade Six Pence Stamp. J. Chem. Res. 2016, 40, 648–651. [Google Scholar]
- Sousa, M.M.; Melo, M.J.; Parola, A.J.; Morris, P.J.T.; Rzepa, H.S.; de Melo, J.S.S. A Study in Mauve: Unveiling Perkin’s Dye in Historic Samples. Chem.–A Eur. J. 2008, 14, 8507–8513. [Google Scholar] [CrossRef]
- Lech, K.; Wilicka, E.; Witowska-Jarosz, J.; Jarosz, M. Early Synthetic Dyes—A Challenge for Tandem Mass Spectrometry. J. Mass Spectrom. 2012, 48, 141–147. [Google Scholar] [CrossRef]
- Pirok, B.W.J.; Knip, J.; van Bommel, M.R.; Schoenmakers, P.J. Characterization of Synthetic Dyes by Comprehensive Two-Dimensional Liquid Chromatography Combining Ion-Exchange Chromatography and Fast Ion-Pair Reversed-Phase Chromatography. J. Chromatogr. A 2016, 1436, 141–146. [Google Scholar] [CrossRef]
- Tamburini, D.; Dyer, J.; Cartwright, C. First Evidence and Characterisation of Rare Chrome-Based Colourants Used on 19th-Century Textiles from Myanmar. Dye. Pigment. 2023, 218, 111472. [Google Scholar] [CrossRef]
- Sundberg, B.N.; Pause, R.; van der Werf, I.D.; Astefanei, A.; van den Berg, K.J.; van Bommel, M.R. Analytical Approaches for the Characterization of Early Synthetic Organic Pigments for Artists’ Paints. Microchem. J. 2021, 170, 106708. [Google Scholar] [CrossRef]
- Alvarez-Martin, A.; Newsome, G.A.; Janssens, K. High-Resolution Mass Spectrometry and Nontraditional Mass Defect Analysis of Brominated Historical Pigments. Anal. Chem. 2021, 93, 14851–14858. [Google Scholar] [CrossRef]
- Ferreira, B.R.V.; Correa, D.N.; Eberlin, M.N.; Vendramini, P.H. Fragmentation Reactions of Rhodamine B and 6G as Revealed by High Accuracy Orbitrap Tandem Mass Spectrometry. J. Braz. Chem. Soc. 2017, 28, 136–142. [Google Scholar]
- Vermeulen, M.; Miranda, A.S.O.; Tamburini, D.; Delgado, S.E.R.; Walton, M. A Multi-Analytical Study of the Palette of Impressionist and Post-Impressionist Puerto Rican Artists. Herit. Sci. 2022, 10, 44. [Google Scholar] [CrossRef]
- Sabatini, F.; Manariti, A.; di Girolamo, F.; Bonaduce, I.; Tozzi, L.; Rava, A.; Colombini, M.P.; Lluveras-Tenorio, A. Painting on Polyurethane Foam: “Composizione-Superficie Lunare” by Giulio Turcato. Microchem. J. 2020, 156, 104872. [Google Scholar] [CrossRef]
- Sabatini, F.; Degano, I.; Colombini, M.P. Development of a Method Based on High-Performance Liquid Chromatography Coupled with Diode Array, Fluorescence, and Mass Spectrometric Detectors for the Analysis of Eosin at Trace Levels. Sep. Sci. Plus 2020, 3, 207–215. [Google Scholar] [CrossRef]
- La Nasa, J.; Nodari, L.; Nardella, F.; Sabatini, F.; Degano, I.; Modugno, F.; Legnaioli, S.; Campanella, B.; Tufano, M.K.K.; Zuena, M.; et al. Chemistry of Modern Paint Media: The Strained and Collapsed Painting by Alexis Harding. Microchem. J. 2020, 155, 104659. [Google Scholar] [CrossRef]
- Reiß, L.; Machill, S.; Lübken, T.; Herm, C. HPLC–HR-ESI–MS/MS Identification of Fluorescent Dyes and Optical Brighteners and Their Degradation Products in Daylight Fluorescent Paints. Herit. Sci. 2023, 11, 146. [Google Scholar] [CrossRef]
- La Nasa, J.; Campanella, B.; Sabatini, F.; Rava, A.; Shank, W.; Lucero-Gomez, P.; De Luca, D.; Legnaioli, S.; Palleschi, V.; Colombini, M.P.; et al. 60 Years of Street Art: A Comparative Study of the Artists’ Materials through Spectroscopic and Mass Spectrometric Approaches. J. Cult. Herit. 2021, 48, 129–140. [Google Scholar] [CrossRef]
- Sabatini, F.; La Nasa, J.; Degano, I.; Campanella, B.; Legnaioli, S.; Saccani, I.; Modugno, F. Fluorescent Paints in Contemporary Murals: A Case Study. Heritage 2023, 6, 5689–5699. [Google Scholar] [CrossRef]
- Serrano, A.; van Bommel, M.R.; Hallett, J. Evaluation between Ultrahigh Pressure Liquid Chromatography and High-Performance Liquid Chromatography Analytical Methods for Characterizing Natural Dyestuffs. J. Chromatogr. A 2013, 1318, 102–111. [Google Scholar] [CrossRef]
- Wertz, J.H.; Quye, A.; France, D.; Tang, P.L.; Richmond, L. Authenticating Turkey Red Textiles through Material Investigations by FTIR and UHPLC. In Proceedings of the ICOM-CC 18th Triennial Meeting, Copenhagen, Denmark, 4–8 September 2017. [Google Scholar]
- den Uijl, M.J.; van der Wijst, Y.J.H.L.; Groeneveld, I.; Schoenmakers, P.J.; Pirok, B.W.J.; van Bommel, M.R. Combining Photodegradation in a Liquid-Core-Waveguide Cell with Multiple-Heart-Cut Two-Dimensional Liquid Chromatography. Anal. Chem. 2022, 94, 11055–11061. [Google Scholar] [CrossRef]
- Groeneveld, I.; Bagdonaite, I.; Beekwilder, E.; Ariese, F.; Somsen, G.W.; van Bommel, M.R. Liquid Core Waveguide Cell with In Situ Absorbance Spectroscopy and Coupled to Liquid Chromatography for Studying Light-Induced Degradation. Anal. Chem. 2022, 94, 7647–7654. [Google Scholar] [CrossRef]
- Groeneveld, I.; Schoemaker, S.E.; Somsen, G.W.; Ariese, F.; Van Bommel, M.R. Characterization of a Liquid-Core Waveguide Cell for Studying the Chemistry of Light-Induced Degradation. Analyst 2021, 146, 3197–3207. [Google Scholar]
- den Uijl, M.J.; Lokker, A.; van Dooren, B.; Schoenmakers, P.J.; Pirok, B.W.J.; van Bommel, M.R. Comparing Different Light-Degradation Approaches for the Degradation of Crystal Violet and Eosin Y. Dye. Pigment. 2022, 197, 109882. [Google Scholar] [CrossRef]
- Campanella, B.; Degano, I.; Grifoni, E.; Legnaioli, S.; Lorenzetti, G.; Pagnotta, S.; Poggialini, F.; Palleschi, V. Identification of Inorganic Dyeing Mordant in Textiles by Surface-Enhanced Laser-Induced Breakdown Spectroscopy. Microchem. J. 2018, 139, 230–235. [Google Scholar] [CrossRef]
- Campanella, B.; Grifoni, E.; Hidalgo, M.; Legnaioli, S.; Lorenzetti, G.; Pagnotta, S.; Poggialini, F.; Ripoll-Seguer, L.; Palleschi, V. Multi-Technique Characterization of Madder Lakes: A Comparison between Non- and Micro-Destructive Methods. J. Cult. Herit. 2018, 33, 208–212. [Google Scholar] [CrossRef]
- Fieberg, J.E.; Knutås, P.; Hostettler, K.; Smith, G.D. “Paintings Fade Like Flowers”: Pigment Analysis and Digital Reconstruction of a Faded Pink Lake Pigment in Vincent van Gogh’s Undergrowth with Two Figures. Appl. Spectrosc. 2017, 71, 794–808. [Google Scholar]
- Hendriks, E.; Jansen, L.; Salvant, J.; Ravaud, E.; Eveno, M.; Menu, M.; Fiedler, I.; Geldof, M.; Megens, L.; van Bommel, M.R.; et al. A Comparative Study of Vincent van Gogh’s Bedroom Series. In Studying Old Master Paintings: Technology and Practice-The National Gallery Technical Bulletin; Spring, M., Ed.; Archetype Publications: London, UK, 2011; pp. 237–243. [Google Scholar]
- Lomax, S.Q. The Application of X-Ray Powder Diffraction for the Analysis of Synthetic Organic Pigments. Part 2: Artists’ Paints. J. Coat. Technol. Res. 2010, 7, 325–330. [Google Scholar]
- Whitaker, A. Crystal Structures of Azo Pigments Derived from Acetoacetanilide. J. Soc. Dye. Colour. 1988, 104, 294–300. [Google Scholar] [CrossRef]
- Whitaker, A. X-ray Powder Diffraction of Synthetic Organic Colorants. In Analytical Chemistry of Synthetic Colorants; Peters, A., Freeman, H., Eds.; Blackie: London, UK, 1995. [Google Scholar]
- Sabatini, F.; Giugliano, R.; Degano, I. Photo-Oxidation Processes of Rhodamine B: A Chromatographic and Mass Spectrometric Approach. Microchem. J. 2018, 140, 114–122. [Google Scholar] [CrossRef]
- Pirok, B.W.J.; Moro, G.; Meekel, N.; Berbers, S.V.J.; Schoenmakers, P.J.; van Bommel, M.R. Mapping Degradation Pathways of Natural and Synthetic Dyes with LC-MS: Influence of Solvent on Degradation Mechanisms. J. Cult. Herit. 2019, 38, 29–36. [Google Scholar] [CrossRef]
- Chieli, A.; Romani, A.; Degano, I.; Sabatini, F.; Tognotti, P.; Miliani, C. New Insights into the Fading Mechanism of Geranium Lake in Painting Matrix. Dye. Pigment. 2020, 181, 108600. [Google Scholar]
- Sabatini, F.; Degano, I.; van Bommel, M. Investigating the In-Solution Photodegradation Pathway of Diamond Green G by Chromatography and Mass Spectrometry. Color. Technol. 2021, 137, 456–467. [Google Scholar] [CrossRef]
- Chen, V.J.; Minto, R.E.; Manicke, N.; Smith, G.D. Structural Elucidation of Two Congo Red Derivatives on Dyed Historical Objects Indicative of Formaldehyde Exposure and the Potential for Chemical Fading. Dye. Pigment. 2022, 201, 110173. [Google Scholar] [CrossRef]
- Favaro, G.; Confortin, D.; Pastore, P.; Brustolon, M. Application of LC-MS and LC-MS-MS to the Analysis of Photo-Decomposed Crystal Violet in the Investigation of Cultural Heritage Materials Aging. J. Mass Spectrom. 2012, 47, 1660–1670. [Google Scholar] [CrossRef]
- Chen, V.J.; Smith, G.D.; Whitaker, M.R.; von Rabenau, B. Identification of Red Dyes in Selected Textiles from Chin and Karen Ethnic Groups of Myanmar by LC-DAD-ESI-MS. In Dyes in History and Archaeology 33/34; Kirby, J., Ed.; Archetype Publications: London, UK, 2021; pp. 92–101. [Google Scholar]
- Smith, G.D.; Esson, J.M.; Chen, V.J.; Hanson, R.M. Forensic Dye Analysis in Cultural Heritage: Unraveling the Authenticity of the Earliest Persian Knotted-Pile Silk Carpet. Forensic Sci. Int. 2021, 3, 100130. [Google Scholar] [CrossRef]
- Aceto, M.; Agostino, A.; Fenoglio, G.; Idone, A.; Gulmini, M.; Picollo, M.; Ricciardi, P.; Delaney, J.K. Characterisation of Colourants on Illuminated Manuscripts by Portable Fibre Optic UV-Visible-NIR Reflectance Spectrophotometry. Anal. Methods 2014, 6, 1488. [Google Scholar] [CrossRef]
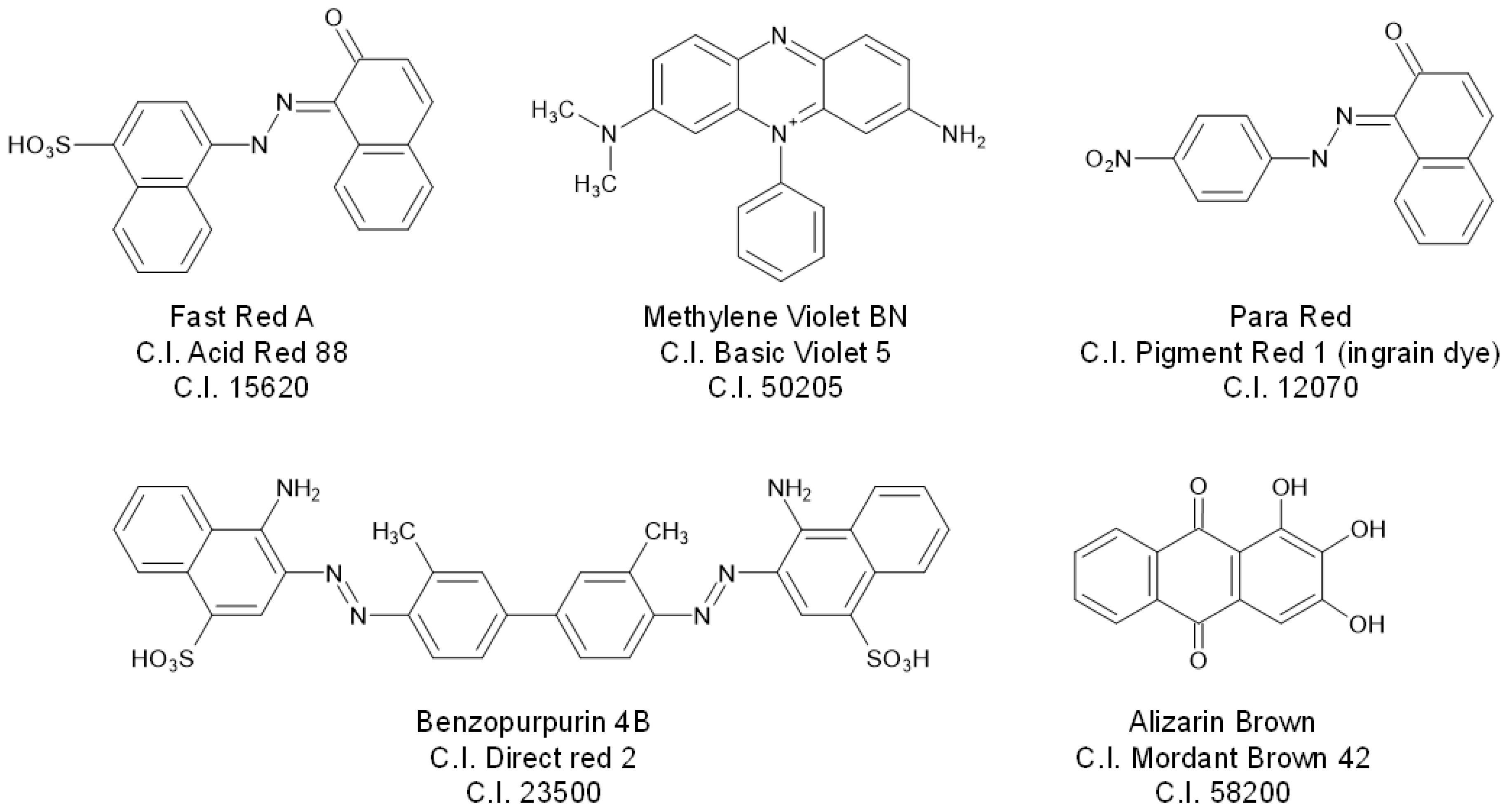
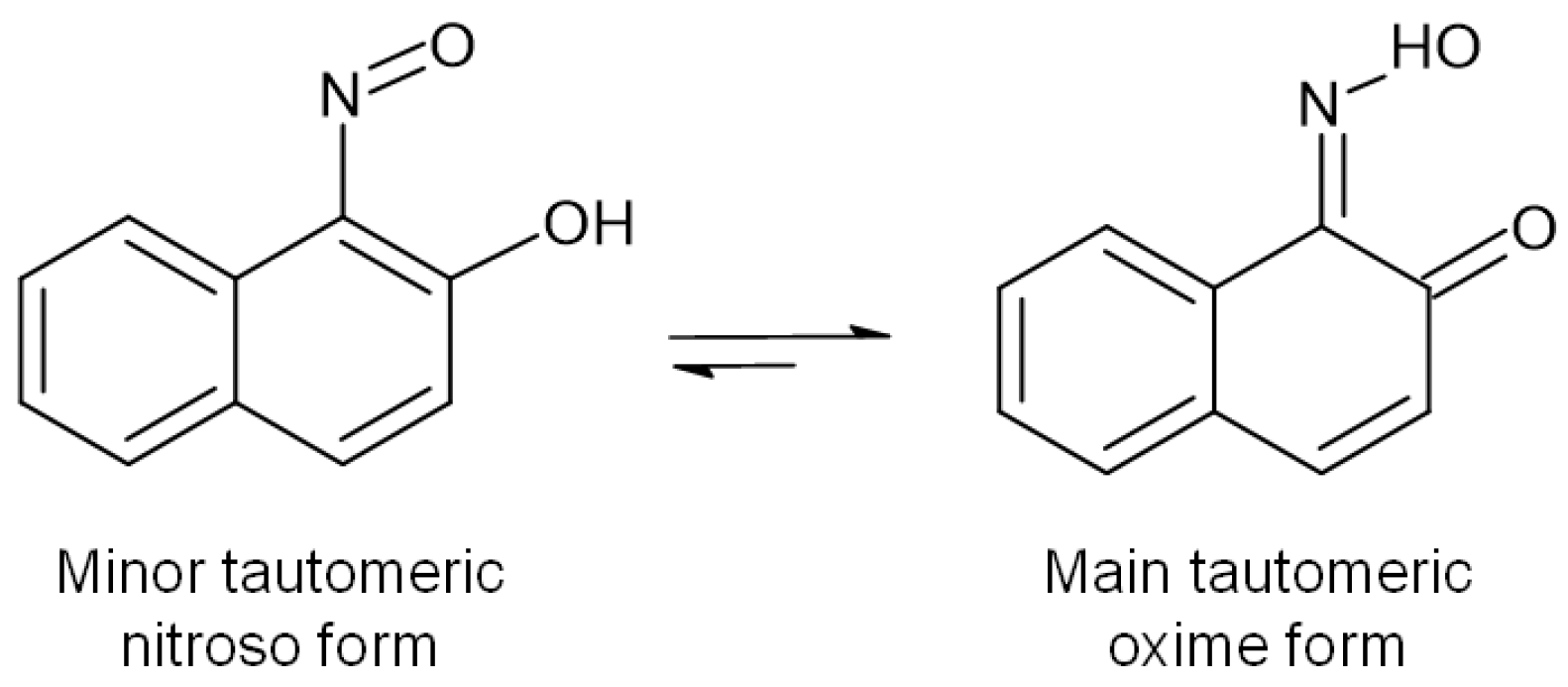


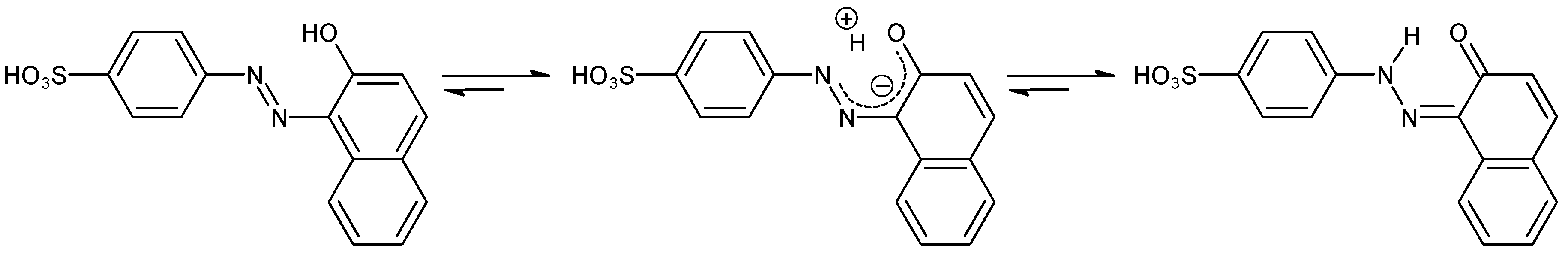
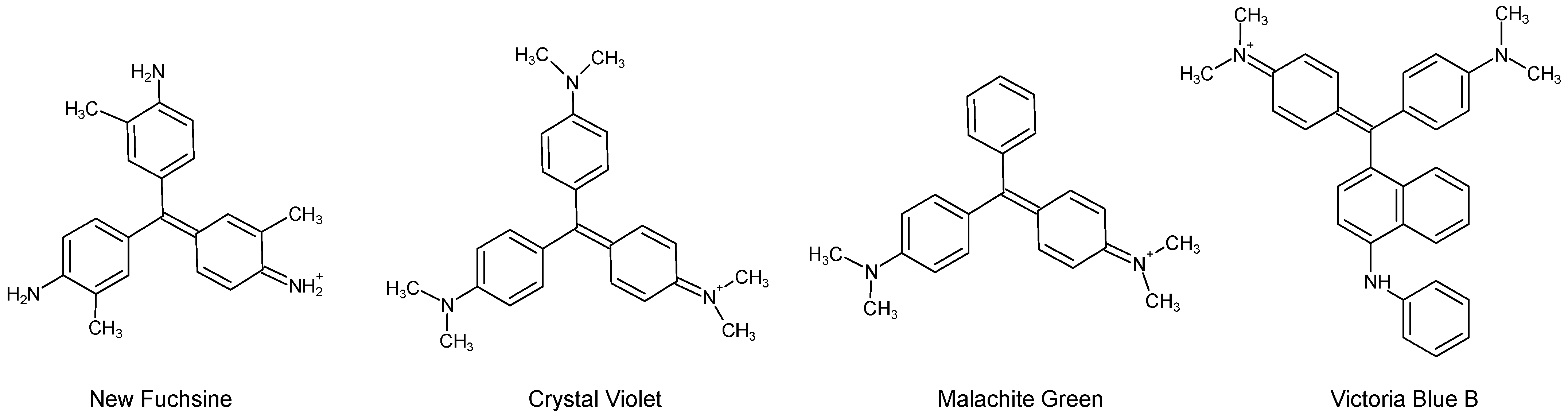
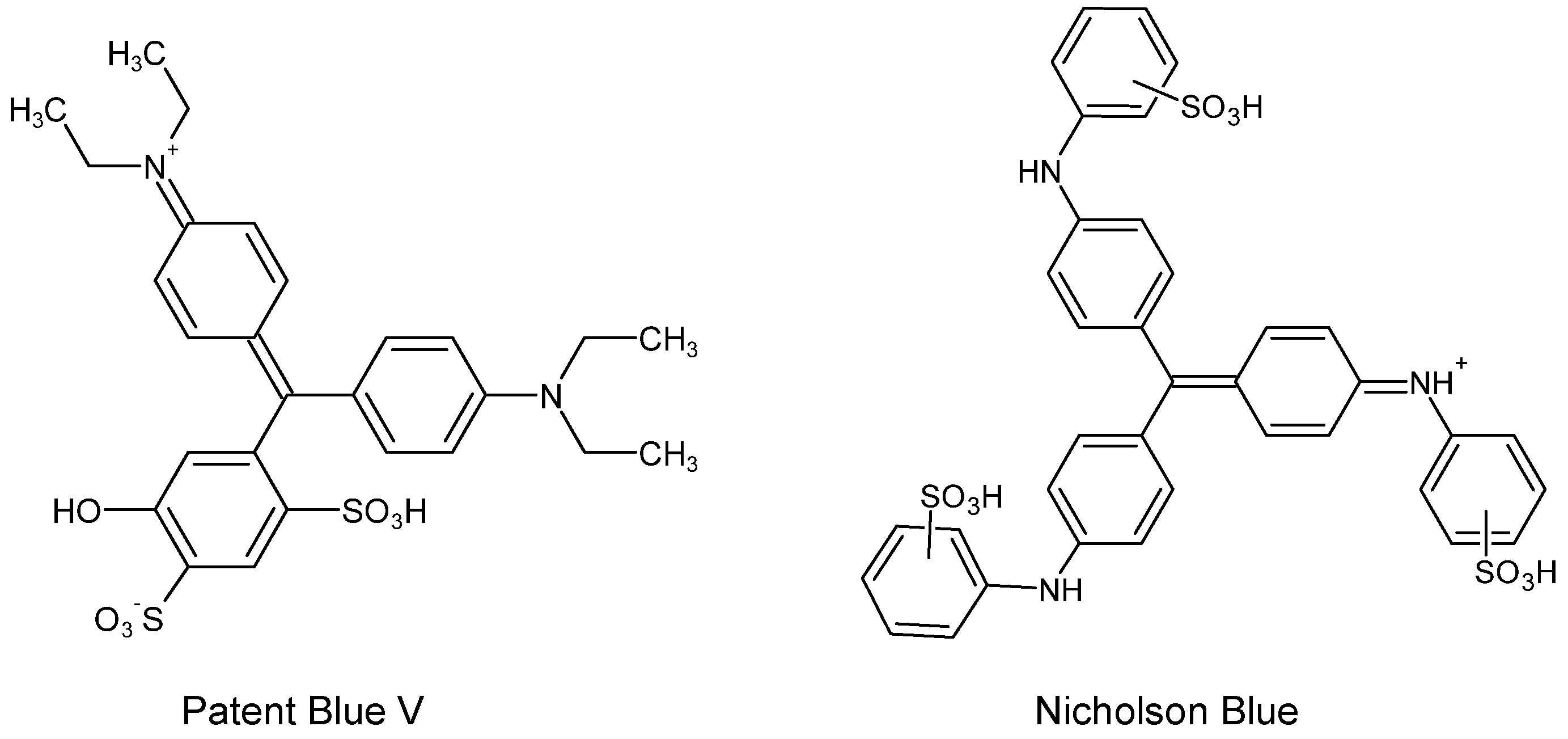

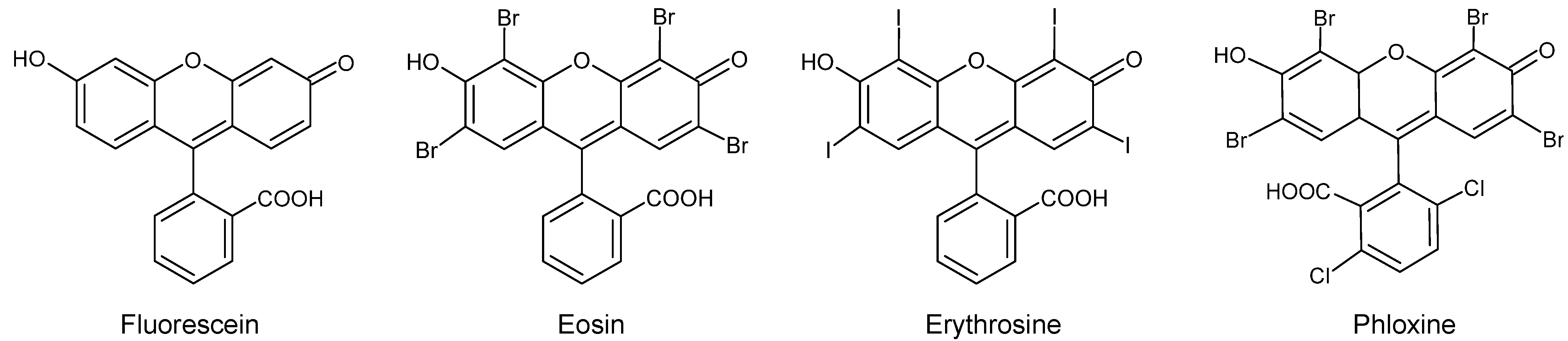
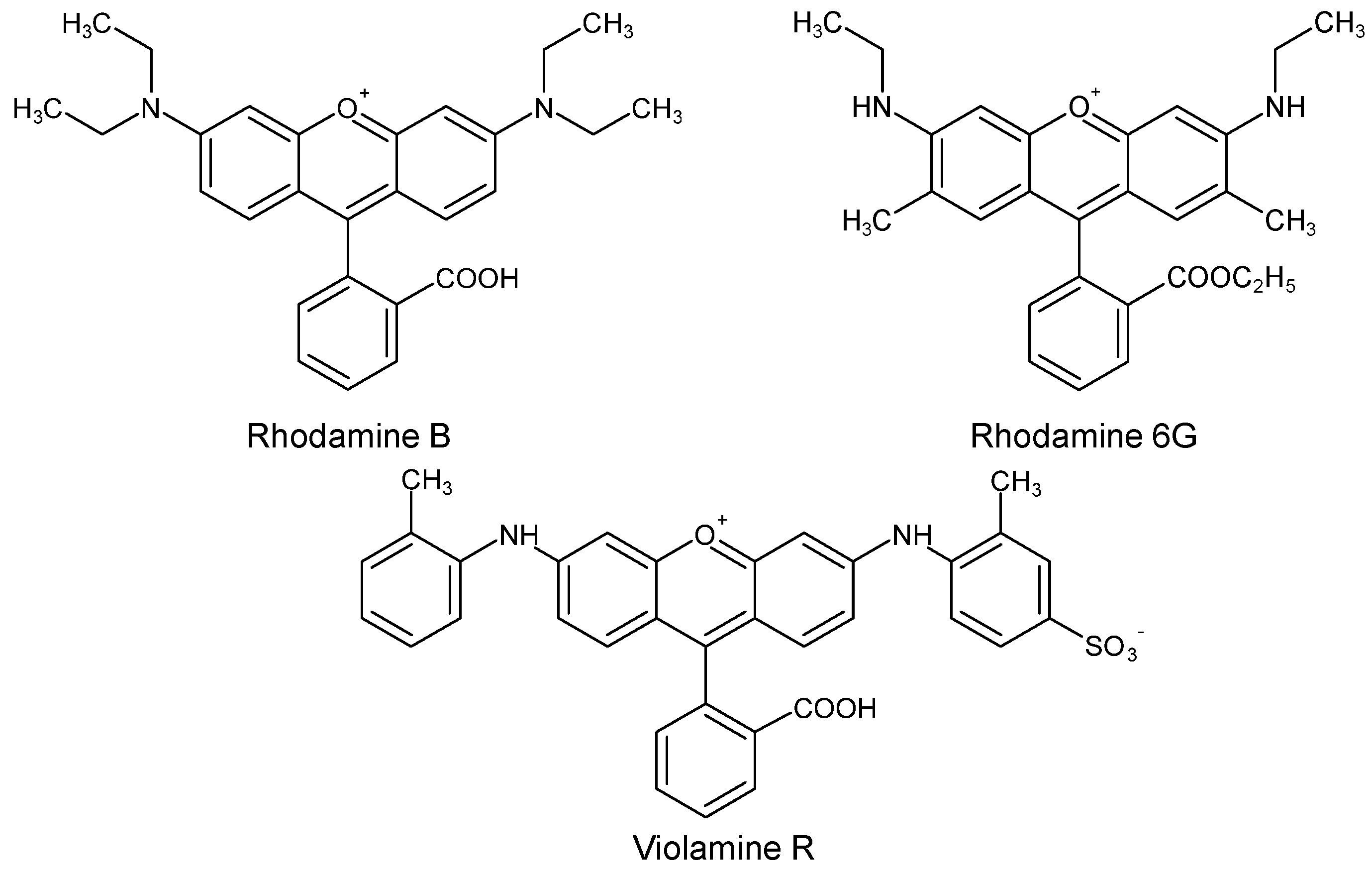

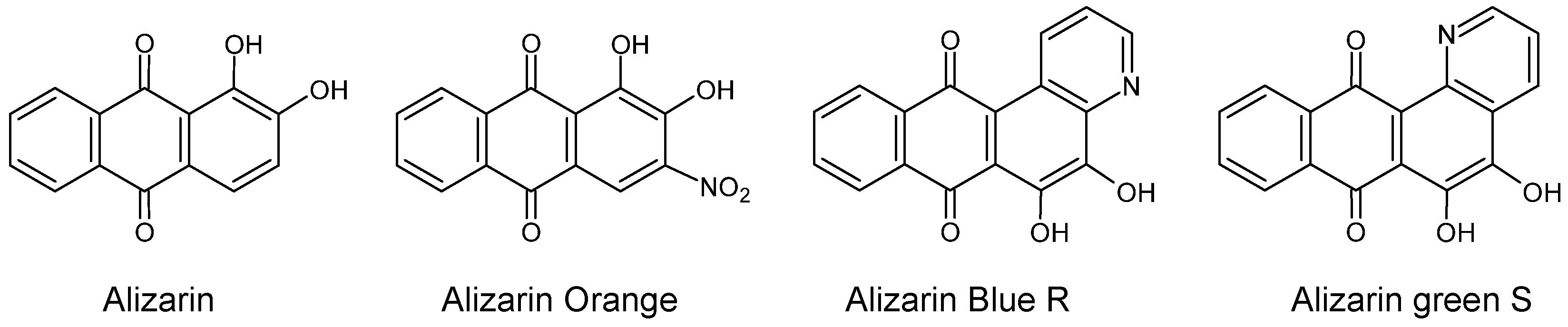
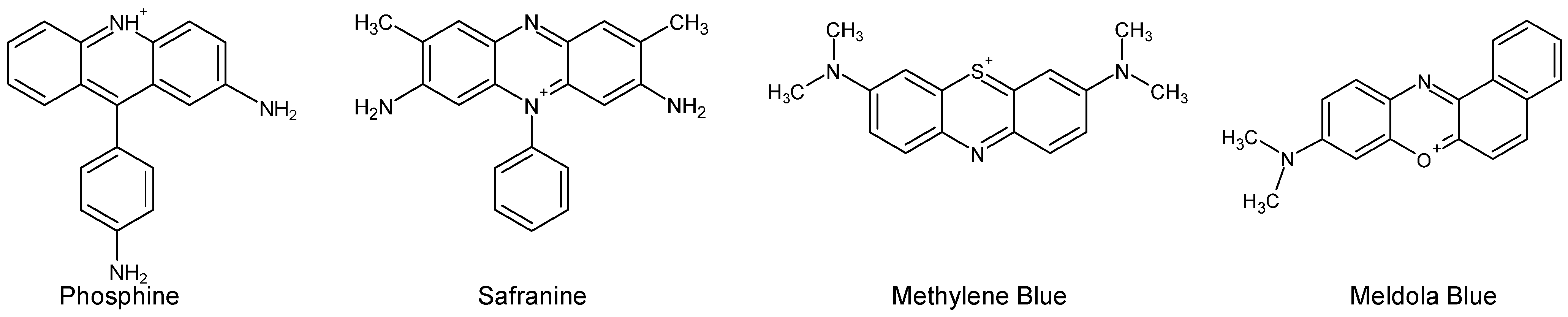
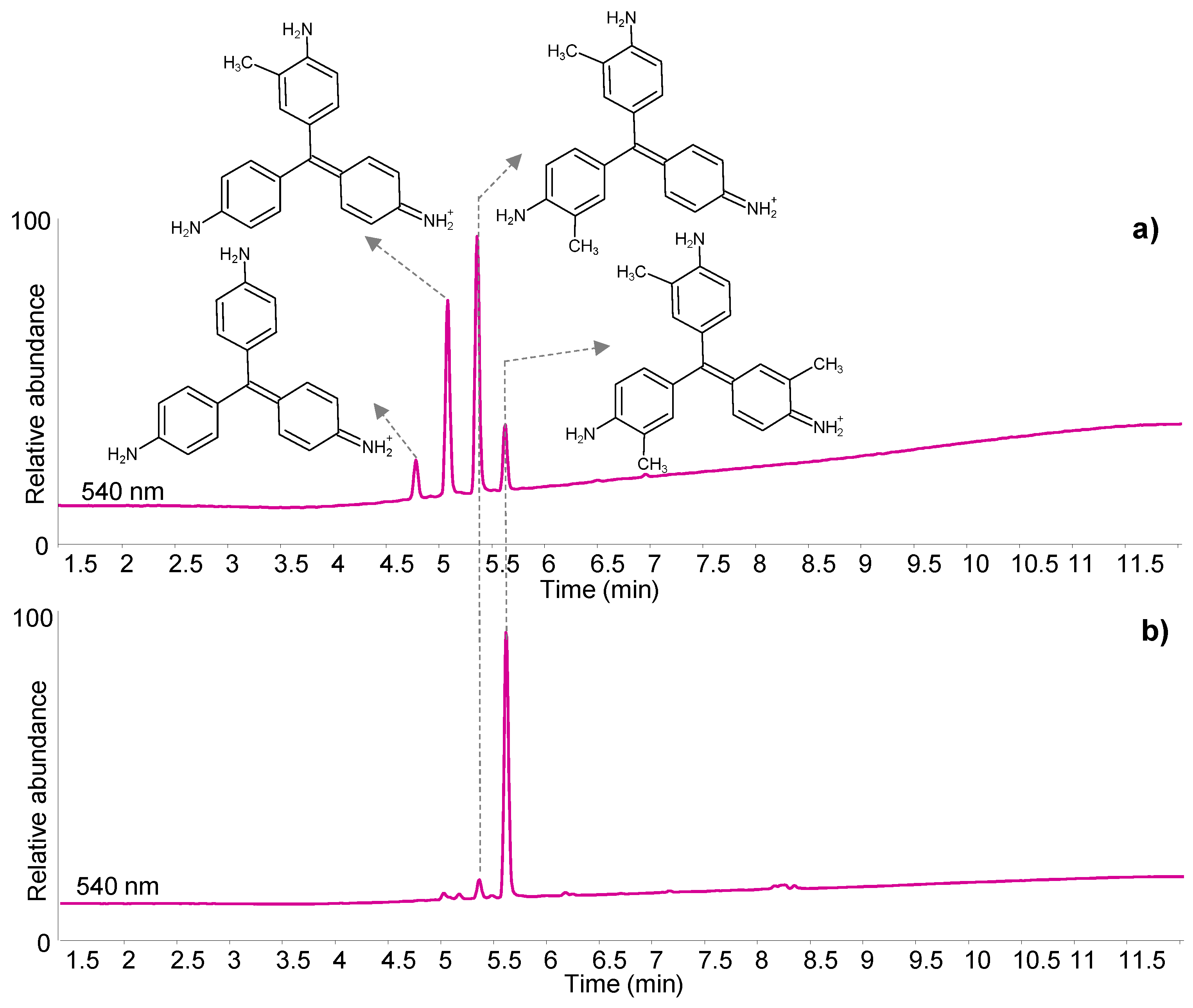
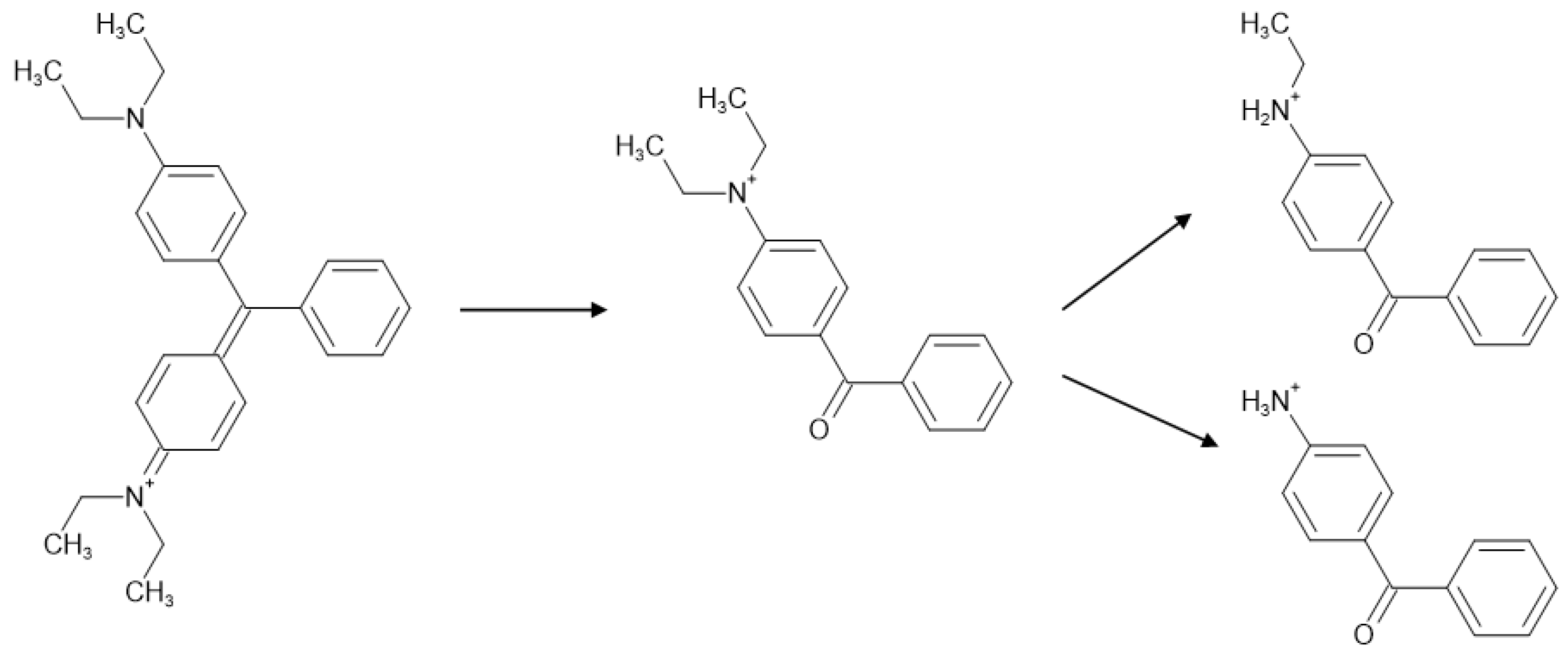
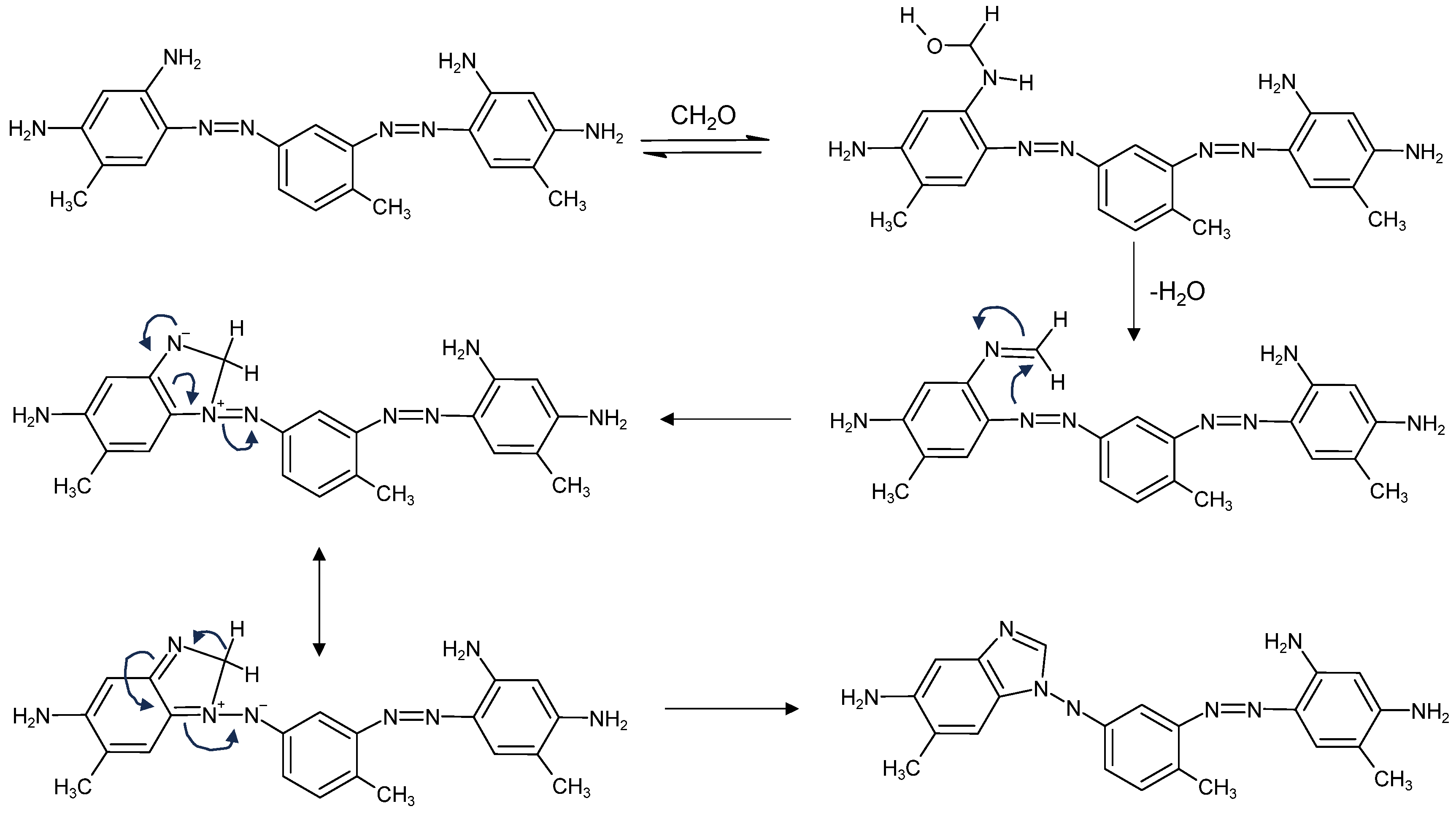




| C.I. Numbers | C.I. Numbers | ||
|---|---|---|---|
| Nitroso | 10000–10299 | Quinoline | 47000–47999 |
| Nitro | 10300–10999 | Thiazole | 49000–49399 |
| Monoazo | 11000–19999 | Azine | 50000–50999 |
| Disazo | 20000–29999 | Oxazine | 51000–51999 |
| Trisazo | 30000–34999 | Thiazine | 52000–52999 |
| Polyazo | 35000–36999 | Anthraquinone | 58000–72999 |
| Diarylmethane | 41000–41999 | Indigoid | 73000–73999 |
| Triarylmethane | 42000–44999 | Phthalocyanine | 74000–74999 |
| Xanthene | 45000–45999 | Natural | 75000–75999 |
| Acridine | 46000–46999 | Inorganic | 77000–77999 |
| Dye | Type | Precipitation Agent/Substrate |
|---|---|---|
| Acid dyes * | Toners | Barium chloride, lead nitrate, lead acetate, zinc sulphate, aluminium sulphate, aluminium acetate, manganese sulphate, tin chloride, antimony chloride, calcium nitrate, calcium acetate |
| Basic dyes * | Toners | Tannic acid, phosphoric acid, arsenic acid, antimonious acid, stannic acid, resinic acid, palmitic acid, stearic acid (and other similar fatty acids) |
| Intended use/dye dependent * | Lakes and extenders | Barium sulphate (natural or blanc-fix), kaolin (China clay), calcium sulphate (Paris white, gypsum, satin white), potassium aluminium sulphate, alumina, diatomaceous earth (Kiezelguhr), red lead, zinc oxide, lead sulphate, aluminium hydrate, calcium carbonate (chalk), barium phosphate, lead carbonate (white lead), calcium phosphate, carbon (lamp and vegetable black), green earth |
| Category | Technique | Expected Information | Advantages (+)/Disadvantages (−) | |
|---|---|---|---|---|
Non-invasive Analysis Preliminary indications of the presence of ESDs and SOPs and their distribution + decision on sampling areas | Visual examination | Optical microscopy | Technical examination of production processes, paint application, ink strokes, fibre classification | |
| Electronic spectroscopy | Colourimetry | Colour change measurements and colour matching | + Utilisable in situ; enables colour to be measured and systematically defined − No information useful for identification | |
| Fibre optic reflectance/Fluorescence spectroscopy | Preliminary indications of the colourants present and their spatial distribution, colour matching | + Utilisable in situ; enables large numbers of spectra to be quickly recorded and visualised in real time; inexpensive; safe; database of spectra available − Relatively poor spatial (probes with aperture diameters > 1 mm) and wavelength resolution (ca. 2–5 nm); limited “fingerprinting” ability; results strongly affected by substrate, pH and other materials present | ||
| Imaging | Broadband multispectral imaging (MSI)/Hyperspectral imaging (HSI) | False-colour images are created, showing the distribution of materials on the surface | + Utilisable in situ; capability to scan wide surfaces; some dye identification possible − Few applications due to limited fingerprinting ability; complex data elaboration; sensitivity to external illumination conditions | |
| X-ray-based techniques | X-ray fluorescence spectroscopy (XRF) | Investigation of the inorganic components (elemental analysis and distribution) | + Utilisable in situ; sensitive and selective − Poor sensitivity to light elements | |
| Vibrational spectroscopies | Portable FTIR spectroscopy | Information on ESDs, SOPs and other materials present in the sample | + Utilisable in situ; provides information on other materials present in the sample and on the recipe used for SOPs − Spectra affected by the matrix; reference spectra are needed for interpretation; poor spatial resolution; surface scattering effect | |
| Portable Raman spectroscopy | Information on ESDs and SOPs in several different matrices and on their spatial distribution | + Utilisable in situ; versatile irradiation source; high sensitivity; databases available − Background fluorescence signal produced by the organic matrix or support | ||
Invasive/non-destructive analyses Detailed information related to the samples | Microscopy | Optical microscopy (possibly on cross-sections) | Indication of dye technology, removal of dirt/contaminants on the fibre surface, assessment of the stratigraphy for paint cross-sections | + Relatively easy to use − Only preliminary information; if the sample is treated, it cannot be used for further analysis |
| Benchtop vibrational spectroscopies | FTIR/Raman spectroscopy | Information on ESDs, SOPs and other materials present in the sample | See above for portable FTIR + higher spectral and spatial resolution compared to the portable equipment − possible signal overlap; minor components often non-detectable | |
| Surface-enhanced Raman spectroscopy (SERS) | Information on ESDs and SOPs in several different matrices | See above for portable Raman + Signal enhanced by several orders of magnitude; reduced problem of background fluorescence − The chromophore-containing molecule can be unavailable for the desired SERS effect due to the matrix; uneven signal enhancement; minor compounds not always detectable | ||
| X-ray-based techniques | Scanning electron microscopy Energy dispersive X-ray spectroscopy (SEM-EDX) | Identification of fibres and mordant analysis/identification of precipitating agents in SOPs or additives | + Combined visual and elemental analysis; high magnification and spatial resolution; mapping capabilities − Sample cannot be used for further analysis; morphological information difficult to be interpreted | |
| Laser-induced breakdown spectroscopy (LIBS) | Investigation of the inorganic components (elemental analysis) | + Almost non-destructive (the laser beam might leave a small mark on the fibre); quick; relatively inexpensive − No information about molecular structure of the inorganic components | ||
| X-ray powder diffraction (XRD) | Identification of the crystalline structure of SOPs | + Suitable for studying the interaction between the dye and its substrate − Only applicable to crystalline phases | ||
| X-ray fluorescence spectroscopy (XRF) | Investigation of the inorganic components (elemental analysis) | + Sensitive and selective; − Detection of light atoms is difficult; no information about molecular structure of the inorganic components | ||
Invasive/micro-destructive analyses Highly detailed molecular information | Spectroscopies entailing modification of the sample | Surface-enhanced Raman spectroscopy (SERS) applied to a sample extract | Detailed information on ESDs and SOPs in several different matrices | + Very high sensitivity − Uneven signal enhancement; spectra dominated by the main component of a mixture |
| Fourier Transform infrared spectroscopy (FTIR) in transmission mode (KBr pellet, diamond cell, etc.) | Detailed information on some ESDs and SOPs as well as substrates and other materials | + High sensitivity; particle localisation with microscope − Overlapping signals; low specificity | ||
| Mass spectrometry techniques (no or minimal sample treatment) | Direct temperature resolved-mass spectrometry (DT-MS) | Molecular information on ESDs, SOPs and other organic material in the sample (e.g., binding medium, additives, etc.) | + Quick acquisition; no sample treatment − Matrix ionisation can produce fragments preventing SOPs or ESDs detection/identification | |
| Pyrolysis gas chromatography coupled to mass spectrometry (Py-GC/MS) * | Rapid identification of some synthetic colourants; information on binding media and any other organic material | + No sample treatment; useful for insoluble pigments; libraries available for SOPs − Pigments of the same chemical class cannot often be distinguished; interferences due to textile matrices | ||
| Laser desorption ionisation coupled to mass spectrometry (LDI-MS) and matrix-assisted laser desorption ionisation (MALDI-MS) | Information at molecular level of ESDs and SOPs; in imaging mode information on the spatial distribution of the analytes is provided | + Minimal or no sample treatment − No separation of the analytes; scarce insight on minor/degradation products; complex spectral interpretation | ||
| Ambient ionisation mass spectrometry (AMS) | Information at molecular level of ESDs and SOPs; in imaging mode information on the spatial distribution of the analytes is provided | + Minimal pre-treatment; possibility to be used in situ; DESI can be implemented in imaging mode (DESI-MSI) − No separation of the analytes; scarce insight on minor/degradation products; complex spectral interpretation | ||
| Secondary ions mass spectrometry (SIMS) | Information at molecular level of SOPs and binding media; in imaging mode information on the spatial distribution | + Identification of both the pigment and the binder in a short analysis time. − No separation of the analytes; complex spectral interpretation | ||
| Chromatographic techniques | Thin-layer chromatography (TLC) | Pre-screening for main dye components; separation for further spectroscopic analyses | + Possibility to be directly combined with FTIR and SERS; fraction recollection − Separation not highly precise | |
| High-pressure liquid chromatography—diode array detector—tandem mass spectrometry (HPLC-DAD-MS/MS) | Identification of colourants and dyestuff sources at a molecular level; identification of synthetic by-products and unknown degradation products | + Ultimate sensitivity and selectivity; highly efficient chromatographic separation; high-resolution mass information − Expensive equipment; time-consuming sample treatment; possible modifications of the analytes induced by sample treatment; difficult data interpretation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamburini, D.; Sabatini, F.; Berbers, S.; van Bommel, M.R.; Degano, I. An Introduction and Recent Advances in the Analytical Study of Early Synthetic Dyes and Organic Pigments in Cultural Heritage. Heritage 2024, 7, 1969-2010. https://doi.org/10.3390/heritage7040094
Tamburini D, Sabatini F, Berbers S, van Bommel MR, Degano I. An Introduction and Recent Advances in the Analytical Study of Early Synthetic Dyes and Organic Pigments in Cultural Heritage. Heritage. 2024; 7(4):1969-2010. https://doi.org/10.3390/heritage7040094
Chicago/Turabian StyleTamburini, Diego, Francesca Sabatini, Sanne Berbers, Maarten R. van Bommel, and Ilaria Degano. 2024. "An Introduction and Recent Advances in the Analytical Study of Early Synthetic Dyes and Organic Pigments in Cultural Heritage" Heritage 7, no. 4: 1969-2010. https://doi.org/10.3390/heritage7040094






