Interaction of Vibrio to Biotic and Abiotic Surfaces: Relationship between Hydrophobicity, Cell Adherence, Biofilm Production, and Cytotoxic Activity
Abstract
:1. Introduction
2. Material and Methods
2.1. Molecular Identification of V. parahaemolyticus and V. alginolyticus
2.2. Hydrophobicity Analysis
2.3. Detection of Capsule Production
2.4. Adherence Assay
2.5. Determination of Cell Adherence and Invasion
2.6. Cytotoxicity Assay
2.7. Statistical Analysis
3. Results
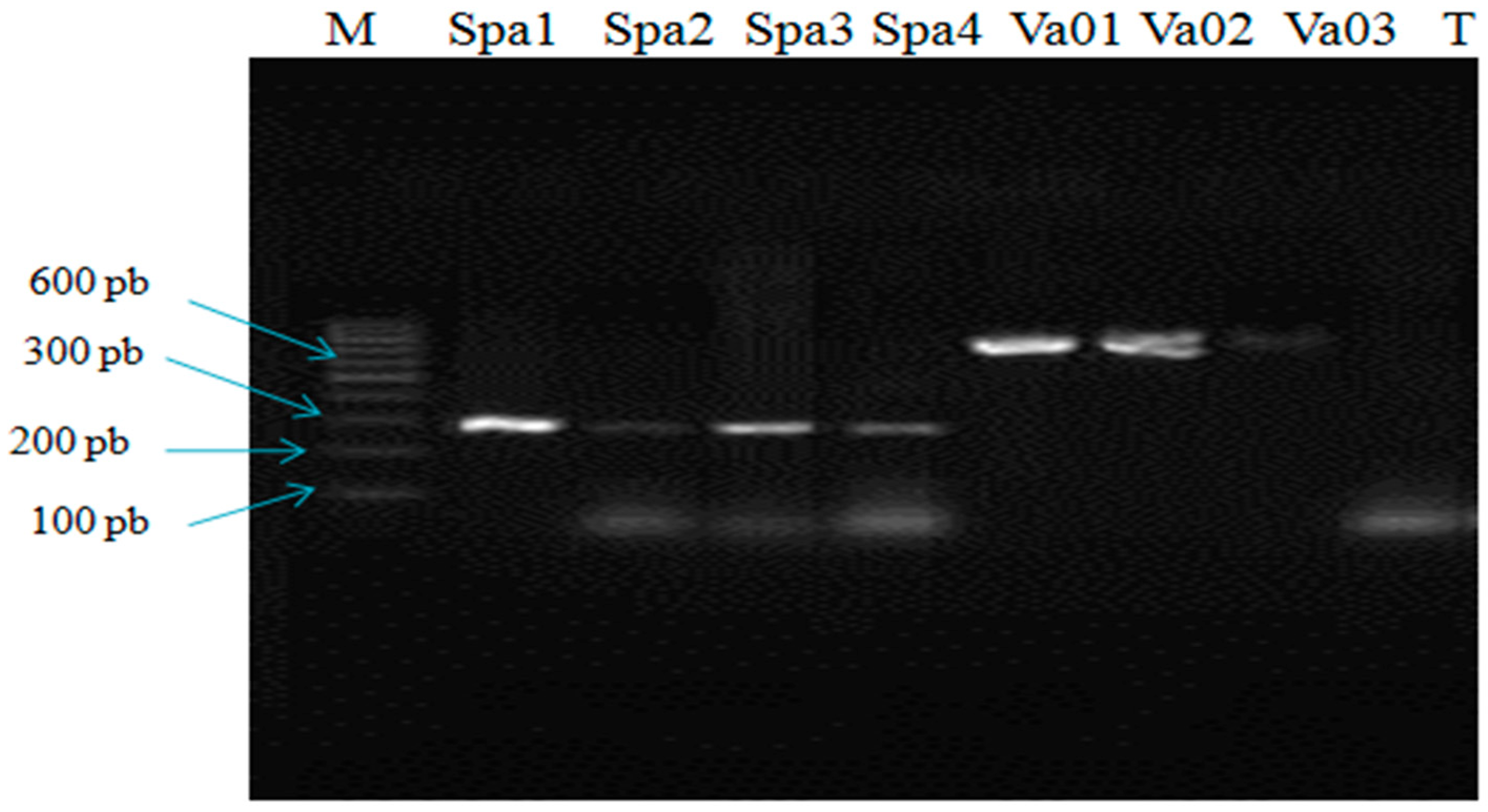
3.1. Molecular Identification of Isolates
3.2. Hydrophobicity
3.3. Capsule Production and Biofilm Formation on Polystyrene Plates
3.4. Adhesion and Invasion to Human Epithelial Cells
3.5. Cytotoxicity of the Bacterial Isolates to Hep-2 Cells
3.6. Relationship between Hydrophobicity, Biofilm, Adherence, Invasion and Cytotoxicity of the Vibrio Strains
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bondad-Reantaso, M.G.; Subasinghe, R.P.; Arthur, J.R.; Ogawa, K.; Chinabut, S.; Adlard, R.; Tan, Z.; Shariff, M. Disease and health management in Asian aquaculture. Vet. Parasitol. 2005, 132, 249–272. [Google Scholar] [CrossRef] [PubMed]
- Austin, B.; Austin, D.A. Bacterial Fish Pathogens, Disease of Farmed and Wild Fish; Springer Praxis: Godalming, UK, 2007. [Google Scholar]
- Ben Kahla-Nakbi, A.; Besbes, A.; Chaieb, K.; Rouabhia, M.; Bakhrouf, A. Survival of Vibrio alginolyticus in seawater and retention of virulence of its starved cells. Mar. Environ. Res. 2007, 64, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Torrecillas, S.; Makol, A.; Caballero, M.J.; Montero, D.; Dhanasiri, A.K.S.; Sweetman, J.; Izquierdo, M. Effects on mortality and stress response in European sea bass, Dicentrarchus labrax (L.), fed mannan oligosaccharides (MOS) after Vibrio anguillarum exposure. J. Fish Dis. 2012, 35, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Zavala-Norzagaray, A.A.; Aguirre, A.A.; Velazquez-Roman, J.; Flores-Villaseñor, H.; León-Sicairos, N.; Ley-Quiñonez, C.P.; Hernández-Díaz, L.D.J.; Canizalez-Roman, A. Isolation, characterization, and antibiotic resistance of Vibrio spp. in sea turtles from Northwestern Mexico. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Reilly, G.; Reilly, C.; Smith, E.; Baker-Austin, C. Vibrio alginolyticus-associated wound infection acquired in British waters, Guernsey, July 2011. Euro Surveill. 2011, 16, 10. [Google Scholar]
- Velazquez-Roman, J.; León-Sicairos, N.; de Jesus Hernández-Díaz, L.; Canizalez-Roman, A. Pandemic Vibrio parahaemolyticus O3: K6 on the American continent. Front. Cell Infect. Microbiol. 2013, 3, 110. [Google Scholar] [CrossRef] [PubMed]
- Secades, P.; Guijarro, J.A. Purification and characterization of an extracellular protease from the fish pathogen Yersinia ruckeri and effect of culture conditions on production. Appl. Environ. Microbiol. 1999, 65, 3969–3975. [Google Scholar] [PubMed]
- Di Pinto, A.; Ciccarese, G.; Tantillo, G.; Catalano, D.; Forte, V.T. A collagenase-targeted multiplex PCR assay for identification of Vibrio alginolyticus, Vibrio cholerae, and Vibrio parahaemolyticus. J. Food Prot. 2005, 68, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, S.-I.; Nitanda, Y.; Fujii, K.; Kawahara, K.; Li, T.; Maehara, Y.; Ramamurthy, T.; Takeda, Y.; Shinoda, S. Differential gene expression and extracellular secretion of the collagenolytic enzymes by the pathogen Vibrio parahaemolyticus. FEMS Microbiol. Lett. 2008, 283, 176–181. [Google Scholar] [CrossRef]
- Pizarro-Cerdá, J.; Cossart, P. Bacterial adhesion and entry into host cells. Cell 2006, 124, 715–727. [Google Scholar] [CrossRef]
- Silva-Dias, A.; Miranda, I.M.; Branco, J.; Monteiro-Soares, M.; Pina-Vaz, C.; Rodrigues, A.G. Adhesion, biofilm formation, cell surface hydrophobicity, and antifungal planktonic susceptibility: Relationship among Candida spp. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Gulig, P.A.; Bourdage, K.L.; Starks, A.M. Molecular pathogenesis of Vibrio vulnificus. J. Microbiol. 2005, 43, 118–131. [Google Scholar] [PubMed]
- Nakano, V.; Piazza, R.; Cianciarullo, A.; Bueris, V.; Santos, M.; Menezes, M.; Mendes-Ledesma, M.; Szulczewski, V.; Elias, W.; Pumbwe, L. Adherence and invasion of Bacteroidales isolated from the human intestinal tract. Clin. Microbiol. Infect. 2008, 14, 955–963. [Google Scholar] [CrossRef] [Green Version]
- Beena, V.; Shivananda, P. In vitro adhesiveness of Bacteroides fragilis group in relation to encapsulation. Indian J. Med. Res. 1997, 105, 258–261. [Google Scholar] [PubMed]
- Freeman, D.; Falkiner, F.; Keane, C. New method for detecting slime production by coagulase negative staphylococci. J. Clin. Pathol. 1989, 42, 872–874. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, R.; Frank, J. Biofilm formation and control in food processing facilities. Comprehensive reviews in food science and food safety. Compr. Rev. 2003, 2, 22–32. [Google Scholar]
- Cantet, F.; Hervio-Heath, D.; Caro, A.; Le Mennec, C.; Monteil, C.; Quemere, C.; Jolivet-Gougeon, A.; Colwell, R.R.; Monfort, P. Quantification of Vibrio parahaemolyticus, Vibrio vulnificus and Vibrio cholerae in French Mediterranean coastal lagoons. Res. Microbiol. 2013, 164, 867–874. [Google Scholar] [CrossRef] [Green Version]
- Snoussi, M.; Noumi, E.; Cheriaa, J.; Usai, D.; Sechi, L.A.; Zanetti, S.; Bakhrouf, A. Adhesive properties of environmental Vibrio alginolyticus strains to biotic and abiotic surfaces. New Microbiol. 2008, 31, 489–500. [Google Scholar]
- Fabbro, C.; Celussi, M.; Russell, H.; Del Negro, P. Phenotypic and genetic diversity of coexisting Listonella anguillarum, Vibrio harveyi and Vibrio chagassi recovered from skin haemorrhages of diseased sand smelt, Atherina boyeri, in the Gulf of Trieste (NE Adriatic Sea). Lett. Appl. Microbiol. 2012, 54, 153–159. [Google Scholar] [CrossRef]
- Hara-Kudo, Y.; Nishina, T.; Nakagawa, H.; Konuma, H.; Hasegawa, J.; Kumagai, S. Improved method for detection of Vibrio parahaemolyticus in seafood. Appl. Environ. Microbiol. 2001, 67, 5819–5823. [Google Scholar] [CrossRef]
- Chapman, P.; Ellin, M.; Ashton, R.; Shafique, W. Comparison of culture, PCR and immunoassays for detecting Escherichia coli O157 following enrichment culture and immunomagnetic separation performed on naturally contaminated raw meat products. Int. J. Food Microbiol. 2001, 68, 11–20. [Google Scholar] [CrossRef]
- Zaranza, A.V.; Morais, F.C.; do Carmo, M.S.; de Mendonça Marques, A.; Andrade-Monteiro, C.; Ferro, T.F.; Monteiro-Neto, V.; Figueiredo, P.d.M.S. Antimicrobial susceptibility, biofilm production and adhesion to HEp-2 cells of Pseudomonas aeruginosa strains isolated from clinical samples. J. Biomater. Nanobiotechnol. 2013, 4, 98–106. [Google Scholar] [CrossRef]
- Chaieb, K.; Zmantar, T.; Souiden, Y.; Mahdouani, K.; Bakhrouf, A. XTT assay for evaluating the effect of alcohols, hydrogen peroxide and benzalkonium chloride on biofilm formation of Staphylococcus epidermidis. Microb. Pathog. 2011, 50, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jensen, H.; Grimmer, S.; Naterstad, K.; Axelsson, L. In vitro testing of commercial and potential probiotic lactic acid bacteria. Int. J. Food Microbiol. 2012, 153, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Foubister, V.; Pucciarelli, M.G.; Finlay, B.B. Methods to study bacterial invasion. J. Microbiol. Methods 1993, 18, 227–240. [Google Scholar] [CrossRef]
- Saliba, A.M.; Filloux, A.; Ball, G.; Silva, A.S.; Assis, M.-C.; Plotkowski, M.-C. Type III secretion-mediated killing of endothelial cells by Pseudomonas aeruginosa. Microb. Pathog. 2002, 33, 153–166. [Google Scholar] [CrossRef]
- Murakami, J.; Kishi, K.; Hirai, K.; Hiramatsu, K.; Yamasaki, T.; Nasu, M. Macrolides and clindamycin suppress the release of Shiga-like toxins from Escherichia coli O157: H7 in vitro. Int. J. Antimicrob. Agents 2000, 15, 103–109. [Google Scholar] [CrossRef]
- El-Housseiny, G.S.; Aboulwafa, M.M.; Hassouna, N.A. Adherence, invasion and cytotoxicity of some bacterial pathogens. J. Am. Sci. 2010, 6, 260–268. [Google Scholar]
- Da Silva, M.E.Z.; Camargo Filho, I.; Endo, E.H.; Nakamura, C.V.; Ueda-Nakamura, T.; Dias Filho, B.P. Characterisation of potential virulence markers in Pseudomonas aeruginosa isolated from drinking water. Antonie van Leeuwenhoek 2008, 93, 323–334. [Google Scholar] [CrossRef]
- Hsern Malcolm, T.T.; Cheah, Y.K.; Radzi, C.W.J.W.M.; Kasim, F.A.; Kantilal, H.K.; Huat John, T.Y.; Martinez-Urtaza, J.; Nakaguchi, Y.; Nishibuchi, M.; Son, R. Detection and quantification of pathogenic Vibrio parahaemolyticus in shellfish by using multiplex PCR and loop-mediated isothermal amplification assay. Food Control 2015, 47, 664–671. [Google Scholar] [CrossRef]
- Lafisca, A.; Pereira, C.S.; Giaccone, V.; Rodrigues, D.d.P. Enzymatic characterization of Vibrio alginolyticus strains isolated from bivalves harvested at Venice Lagoon (Italy) and Guanabara Bay (Brazil). Rev. Inst. Med. Trop. São Paulo 2008, 50, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, C.; Oliver, J.D. A comparison of thiosulphate-citrate-bile salts-sucrose (TCBS) agar and thiosulphate-chloride-iodide (TCI) agar for the isolation of Vibrio species from estuarine environments. Lett. Appl. Microbiol. 2003, 36, 150–151. [Google Scholar] [CrossRef] [Green Version]
- Strand, M.; Hedström, M.; Seth, H.; McEvoy, E.G.; Jacobsson, E.; Göransson, U.; Andersson, H.S.; Sundberg, P. The Bacterial (Vibrio alginolyticus) Production of Tetrodotoxin in the Ribbon Worm Lineus longissimus —Just a False Positive? Mar. Drugs 2016, 14, 63. [Google Scholar] [CrossRef] [PubMed]
- Linke, D.; Goldman, A. Bacterial Adhesion: Chemistry, Biology and Physics; Springer Science & Business Media: Berlin, Germany, 2011; Volume 715. [Google Scholar]
- Kauppi, A. Chemical Attenuation of Bacterial Virulence: Small Molecule Inhibitors of Type III Secretion. 2006. Available online: http://www.diva-portal.org/smash/record.jsf?pid=diva2%3A145112&dswid=2468 (accessed on 1 June 2017).
- Peters, G.; Pulverer, G. Pathogenesis and management of Staphylococcus epidermidis ‘plastic’ foreign body infections. J. Antimicrob. Chemother. 1984, 14, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Sinde, E.; Carballo, J. Attachment of Salmonella spp. and Listeria monocytogenes to stainless steel, rubber and polytetrafluorethylene: The influence of free energy and the effect of commercial sanitizers. Food Microbiol. 2000, 17, 439–447. [Google Scholar] [CrossRef]
- Fletcher, M.; Loeb, G. Influence of substratum characteristics on the attachment of a marine pseudomonad to solid surfaces. Appl. Environ. Microbiol. 1979, 37, 67–72. [Google Scholar] [PubMed]
- Bendinger, B.; Rijnaarts, H.H.; Altendorf, K.; Zehnder, A.J. Physicochemical cell surface and adhesive properties of coryneform bacteria related to the presence and chain length of mycolic acids. Appl. Environ. Microbiol. 1993, 59, 3973–3977. [Google Scholar]
- Niderman-Meyer, O.; Zeidman, T.; Shimoni, E.; Kashi, Y. Mechanisms Involved in Governing Adherence of Vibrio cholerae to Granular Starch. Appl. Environ. Microbiol. 2010, 76, 1034–1043. [Google Scholar] [CrossRef]
- Van Loosdrecht, M.C.; Norde, W.; Zehnder, A. Physical chemical description of bacterial adhesion. J. Biomater. Appl. 1990, 5, 91–106. [Google Scholar] [CrossRef]
- Jain, A.; Agarwal, A. Biofilm production, a marker of pathogenic potential of colonizing and commensal staphylococci. J. Microbiol. Methods 2009, 76, 88–92. [Google Scholar] [CrossRef]
- Carbone, M.; Maugeri, T.L.; Giannone, M.; Gugliandolo, C.; Midiri, A.; Fera, M.T. Adherence of environmental Arcobacter butzleri and Vibrio spp. isolates to epithelial cells in vitro. Food Microbiol. 2003, 20, 611–616. [Google Scholar] [CrossRef]
- Kabha, K.; Schmegner, J.; Keisari, Y.; Parolis, H.; Schlepper-Schaeffer, J.; Ofek, I. SP-A enhances phagocytosis of Klebsiella by interaction with capsular polysaccharides and alveolar macrophages. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1997, 272, L344–L352. [Google Scholar] [CrossRef]
- Davis, C.; Avots-Avotins, A.; Fader, R. Evidence for a bladder cell glycolipid receptor for Escherichia coli and the effect of neuraminic acid and colominic acid on adherence. Infect. Immunity 1981, 34, 944–948. [Google Scholar]
- Chan, R.; Acres, S.; Costerton, J. Use of specific antibody to demonstrate glycocalyx, K99 pili, and the spatial relationships of K99+ enterotoxigenic Escherichia coli in the ileum of colostrum-fed calves. Infect. Immunity 1982, 37, 1170–1180. [Google Scholar]
- Jacques, M.; Bélanger, M.; Roy, G.; Foiry, B. Adherence of Actinobacillus pleuropneumoniae to porcine tracheal epithelial cells and frozen lung sections. Vet. Microbiol. 1991, 27, 133–143. [Google Scholar] [CrossRef]
- Jacques, M.; Kobisch, M.; Belanger, M.; Dugal, F. Virulence of capsulated and noncapsulated isolates of Pasteurella multocida and their adherence to porcine respiratory tract cells and mucus. Vet. Microbiol. 1993, 61, 4785–4792. [Google Scholar]
- Vesterlund, S.; Paltta, J.; Karp, M.; Ouwehand, A.C. Measurement of bacterial adhesion—In vitro evaluation of different methods. J. Microbiol. Methods 2005, 60, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, P.A.; Pereira, A.C.M.; Ferreira, A.F.; de Mattos Alves, M.A.; Rosa, A.C.P.; Freitas-Almeida, A.C. Adhesion, invasion, intracellular survival and cytotoxic activity of strains of Aeromonas spp. in HEp-2, Caco-2 and T-84 cell lines. Antonie van Leeuwenhoek 2015, 107, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Soto-Rodriguez, S.A.; Gomez-Gil, B.; Lozano, R.; del Rio-Rodríguez, R.; Diéguez, A.L.; Romalde, J.L. Virulence of Vibrio harveyi responsible for the “Bright-red” Syndrome in the Pacific white shrimp Litopenaeus vannamei. J. Invertebr. Pathol. 2012, 109, 307–317. [Google Scholar] [CrossRef]
- Moitra, S.; Seethalakshmi, I.; Jeyanthi Rebecca, L. Toxicity analysis of vibrio species from fish samples. J. Chem. Pharm. Res. 2013, 5, 128–130. [Google Scholar]
- Jones, M.K.; Oliver, J.D. Vibrio vulnificus: Disease and Pathogenesis. Infect. Immun. 2009, 77, 1723–1733. [Google Scholar] [CrossRef] [Green Version]
- Ghobashy, M.O.I.; Abu shady, H.M.; Rashad, S.S.; Shahlol, A.M.A. Klebsiella pneumoniae invasion, adherence and cytotoxicity are independent events mechanisms. Egypt. J. Exp. Biol. (Bot.) 2012, 8, 251–259. [Google Scholar]
- Lamari, F.; Chakroun, I.; Rtimi, S. Assessment of the correlation among antibiotic resistance, adherence to abiotic and biotic surfaces, invasion and cytotoxicity of Pseudomonas aeruginosa isolated from diseased gilthead sea bream. Colloids Surf. B Biointerfaces 2017, 158, 229–236. [Google Scholar] [CrossRef]




| Strains | Capsule Production on CRA | * Adhesion to N-Octane (Hydrophobicity) | ** Adhesion to Polystyrene (Biofilm Formation) | |||
|---|---|---|---|---|---|---|
| Phenotypes on CRA | Capsule Production | % Change in Absorbance ± (S.E.) | * Adhesion Index | ** Score Adhesion OD570 ± (S.E) | Adhesion Index | |
| V. parahaemolyticus: (Spa1) | Black | Capsule producer | 60.8 ± 2.8 | Strong | 0.57 ± 0.25 | Moderately to weakly positive |
| V. parahaemolyticus: (Spa2) | Black | Capsule producer | 88.2 ± 0.0 | Strong | 1.64 ± 0.20 | Highly positive |
| V.parahaemolyticus: (Spa3) | Black | Capsule producer | 87.3 ± 1.4 | Strong | 1.36 ± 0.27 | Highly positive |
| V. parahaemolyticus: (Spa4) | Pinkish red | Capsule non producer | 38.2 ± 1.4 | Moderate | 0.27 ± 0.03 | Moderately to weakly positive |
| V. alginolyticus: (Va01) | Black | Capsule producer | 74.5 ± 0.0 | Strong | 1.21 ± 0.27 | Highly positive |
| V. alginolyticus: (Va02) | Pinkish red | Capsule non producer | 32.4 ± 1.4 | Moderate | 0.36 ± 0.20 | Moderately to weakly positive |
| V. alginolyticus: (Va03) | Red | Capsule non producer | 39.2 ± 2.8 | Moderate | 0.45 ± 0.02 | Moderately to weakly positive |
| Bacterial Isolates | Cytotoxicity Level of Bacterial Isolates | |||
|---|---|---|---|---|
| No Cytotoxicity | Low ** | Moderate ** | High ** | |
| V. parahaemolyticus | - | Sp4 | Spa1 | - |
| - | - | Spa2 | - | |
| - | - | Spa3 | - | |
| V. alginolyticus | - | Va02 | Va01 | - |
| - | Va03 | - | - | |
| Bacterial Isolates | Virulence Properties | ||||
|---|---|---|---|---|---|
| Hydrophobicity Using N-Octane vs. Adhesion to Polystyrene Plates | Adhesion to Polystyrene Plates vs. Adhesion to Hep-2 Cells | Adhesion vs. Invasion (to Hep-2 Cells) | Adhesion vs. Cytotoxicity (to Hep-2 Cells) | Invasion vs. Cytotoxicity (to Hep-2 Cells) | |
| Vibrio species | +0.89 * | +0.92 * | +0.70 * | +0.80 * | +0.86 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamari, F.; Khouadja, S.; Rtimi, S. Interaction of Vibrio to Biotic and Abiotic Surfaces: Relationship between Hydrophobicity, Cell Adherence, Biofilm Production, and Cytotoxic Activity. Surfaces 2018, 1, 187-201. https://doi.org/10.3390/surfaces1010014
Lamari F, Khouadja S, Rtimi S. Interaction of Vibrio to Biotic and Abiotic Surfaces: Relationship between Hydrophobicity, Cell Adherence, Biofilm Production, and Cytotoxic Activity. Surfaces. 2018; 1(1):187-201. https://doi.org/10.3390/surfaces1010014
Chicago/Turabian StyleLamari, Faouzi, Sadok Khouadja, and Sami Rtimi. 2018. "Interaction of Vibrio to Biotic and Abiotic Surfaces: Relationship between Hydrophobicity, Cell Adherence, Biofilm Production, and Cytotoxic Activity" Surfaces 1, no. 1: 187-201. https://doi.org/10.3390/surfaces1010014