Citric Acid Regulated Fabrication of Macroporous TiO2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
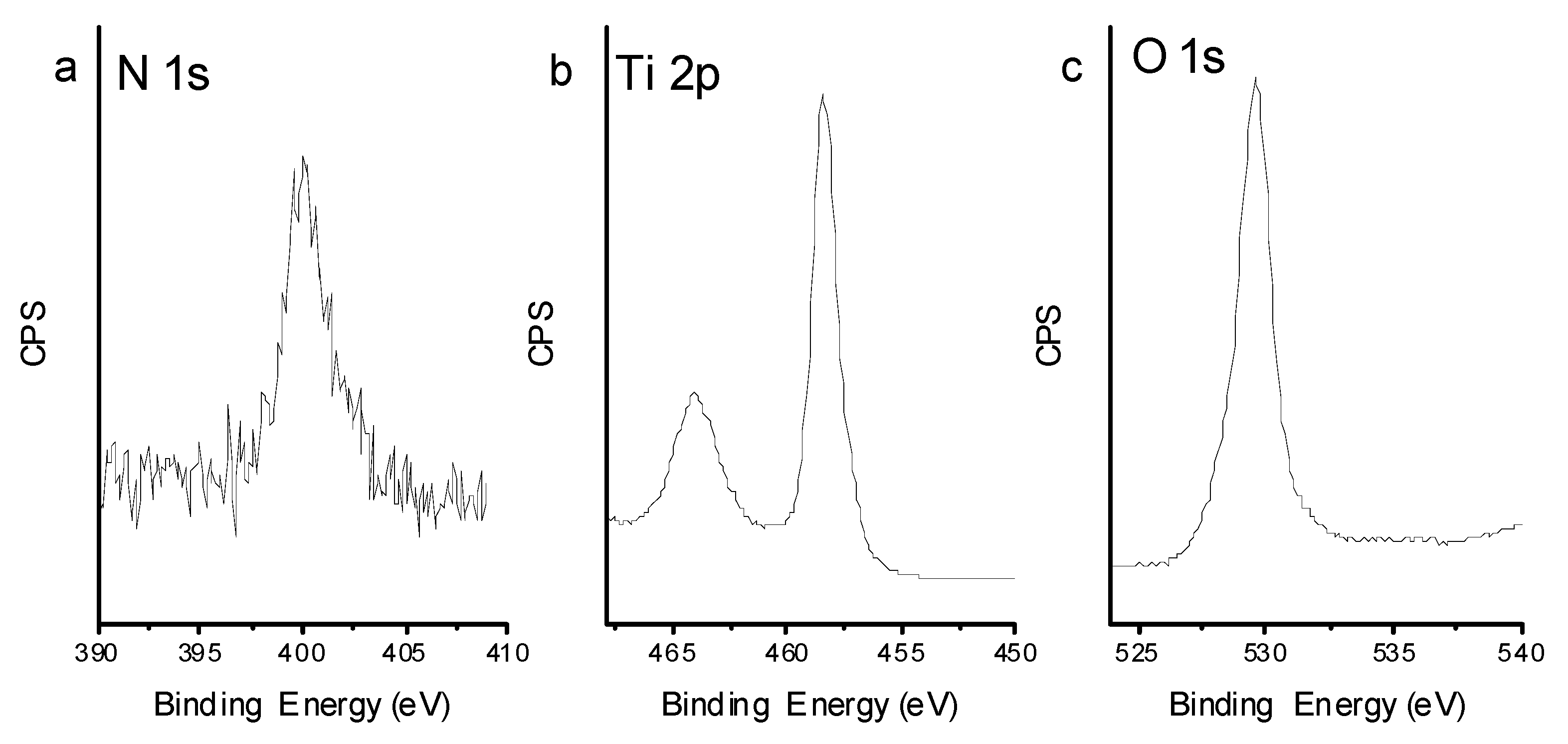
2.3. Characterization
2.4. Photocatalytic Performance
3. Results and Discussion
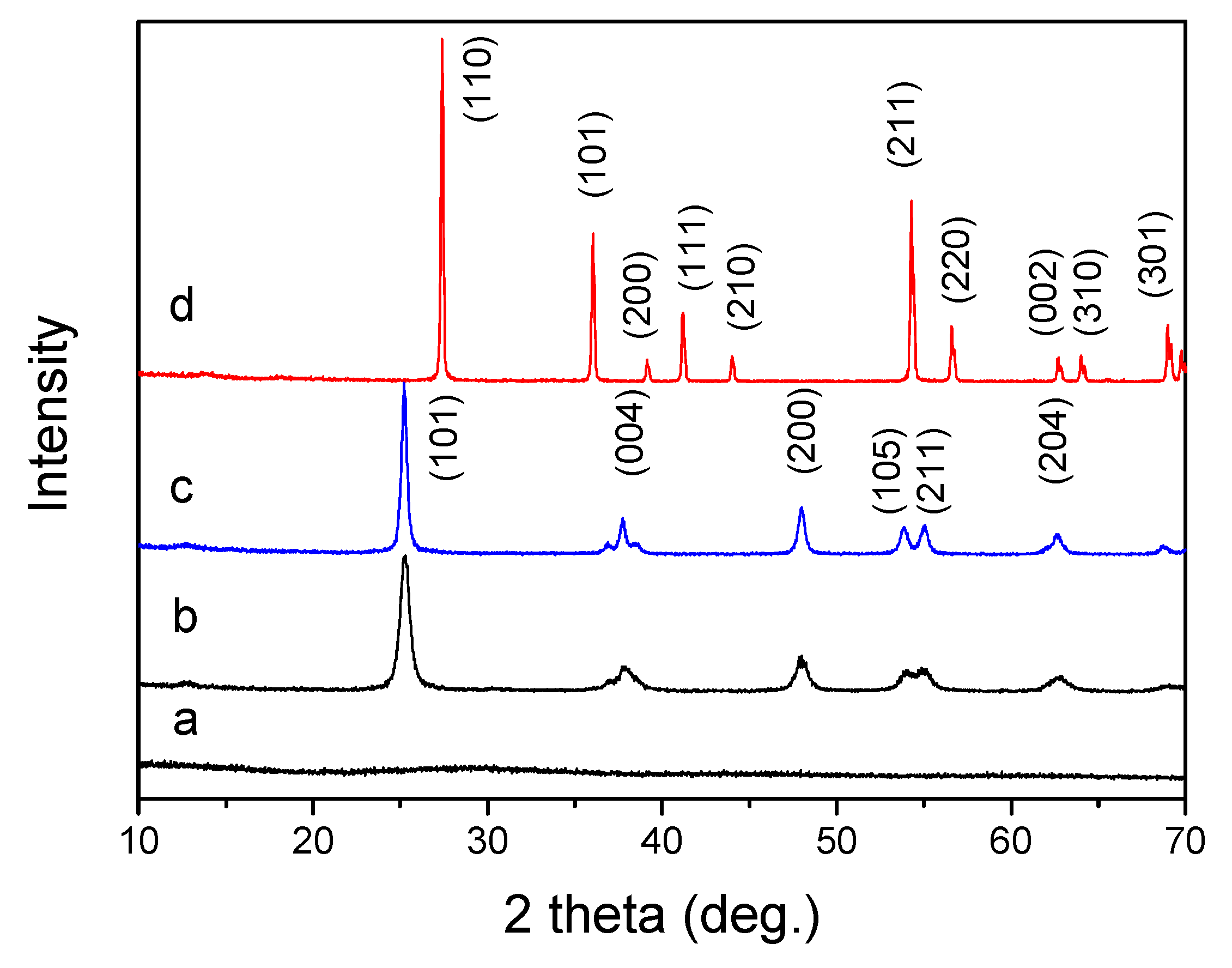
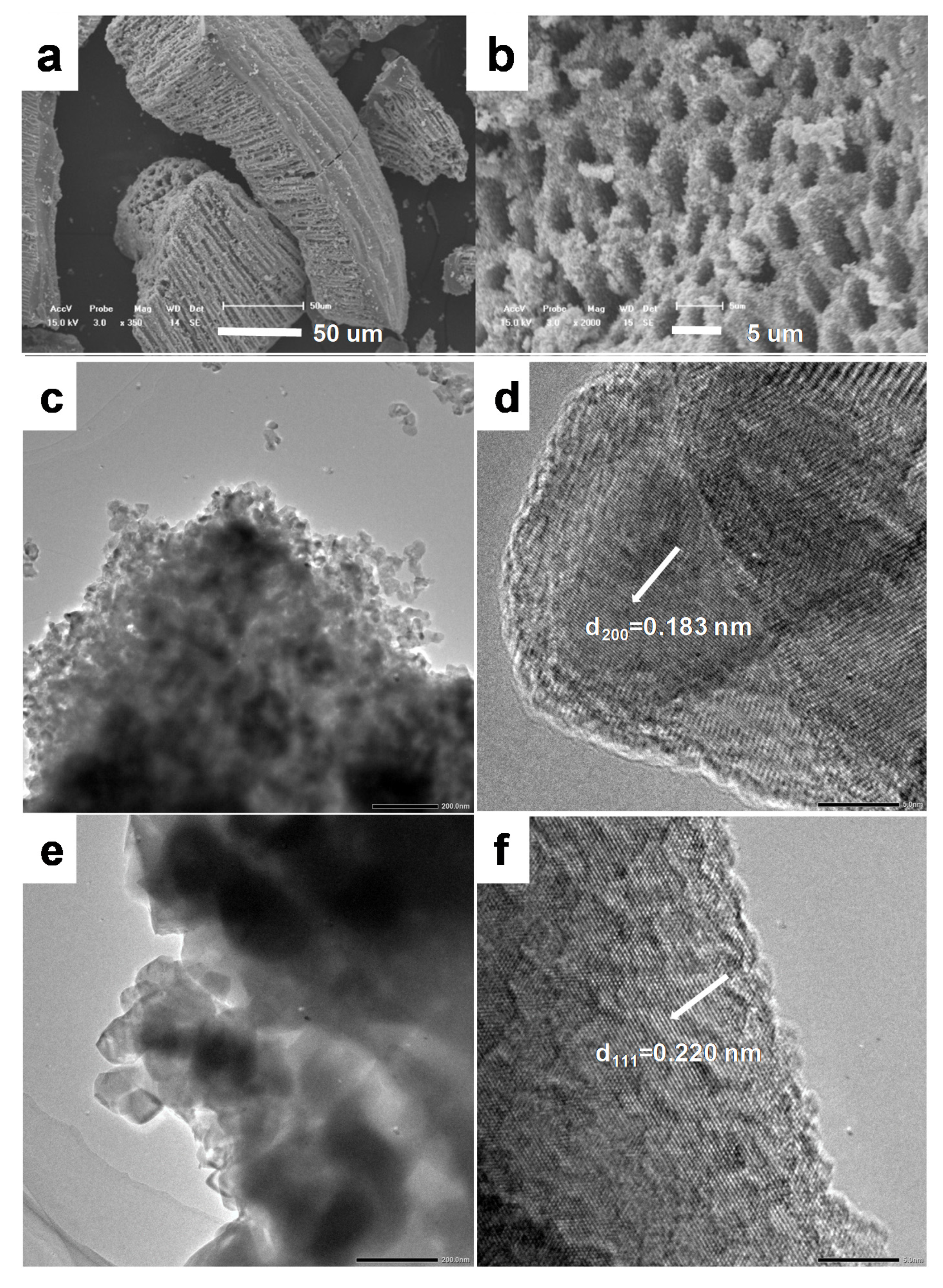
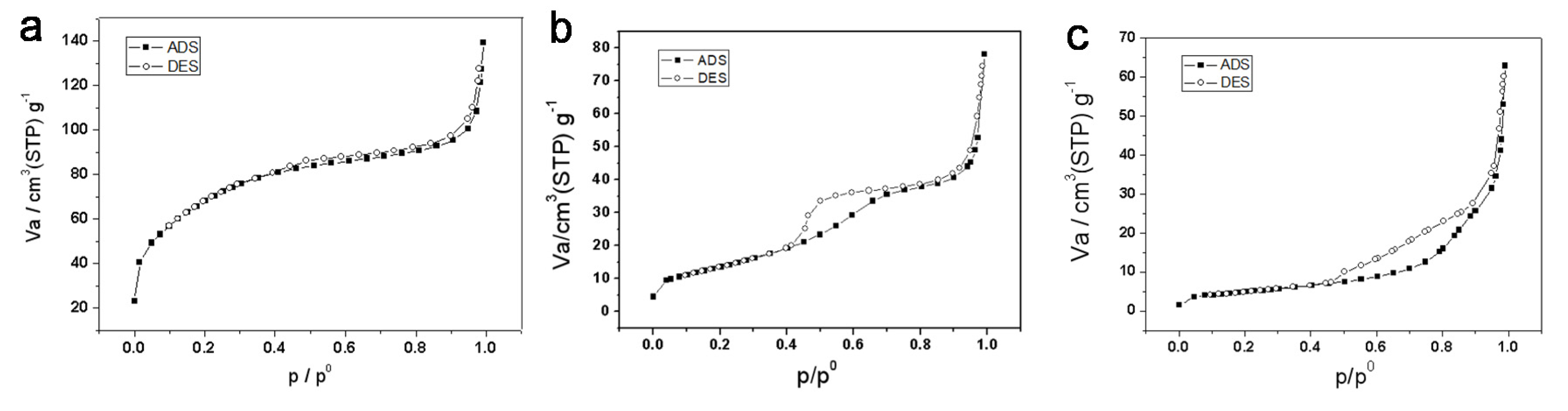
3.1. Macroporous TiO2
3.2. Effect of Citric Acid on the Formation of Macroporous Structure
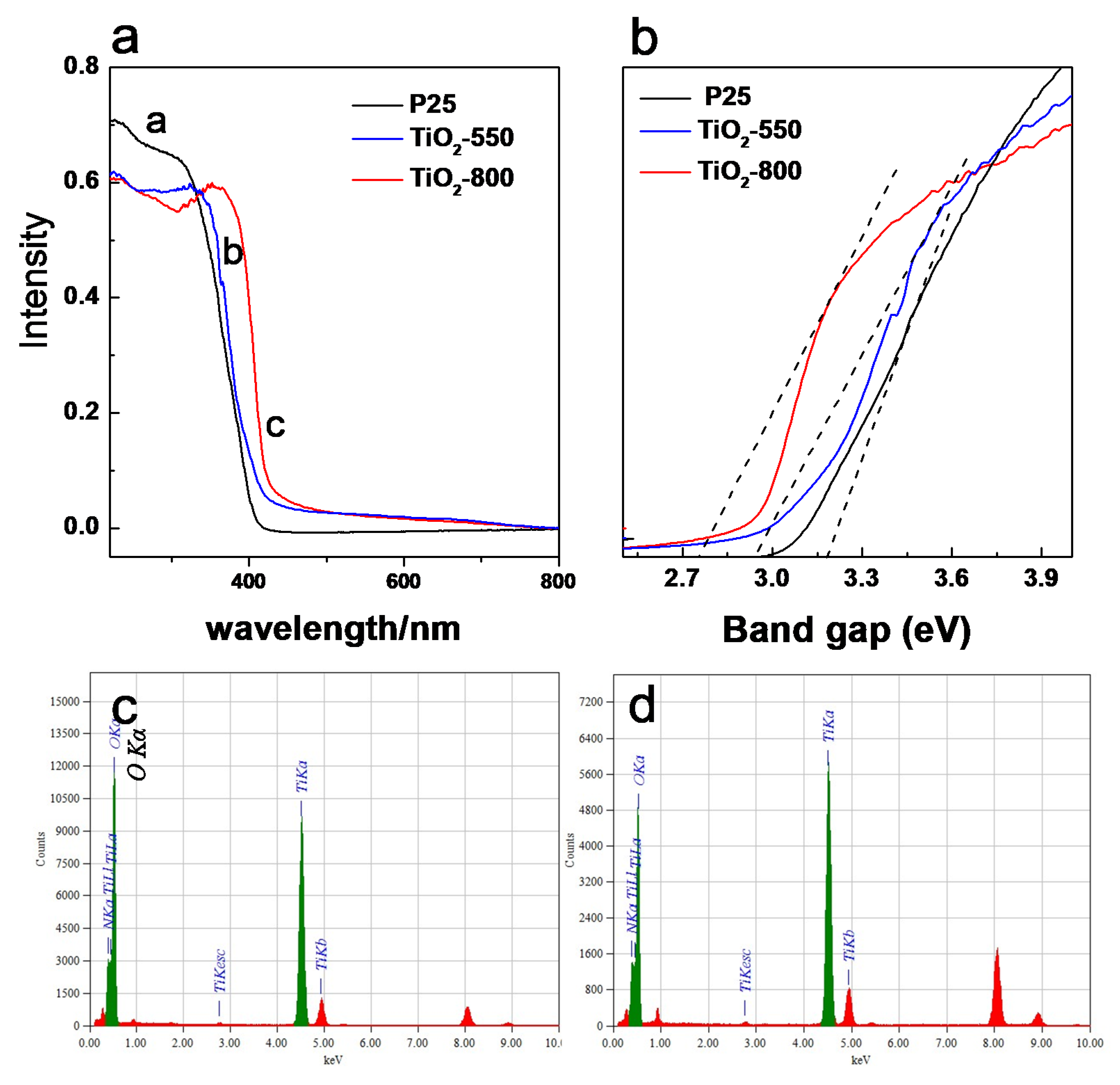
3.3. Photocatlytic Activity of Macroporous TiO2 with Different Crystalline Strcuture, (a) P25, (b) TiO2-550, (c) TiO2-800
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, X.B.; Burda, C. The Electronic Origin of the Visible-Light Absorption Properties of C-, N- and S-Doped TiO2 Nanomaterials. J. Am. Chem. Soc. 2008, 130, 5018–5019. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.B.; Liu, L.; Huang, F.Q. Black Titanium Dioxide (TiO2) Nanomaterials. Chem. Soc. Rev. 2015, 44, 1861–1885. [Google Scholar] [CrossRef] [PubMed]
- Thompson, T.L.; Yates, J.T. Surface Science Studies of the Photoactivation of TiO2 New Photochemical Processes. Chem. Rev. 2006, 106, 4428–4453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, Q.; Feng, Z.C.; Li, M.J.; Li, C. Importance of the Relationship Between Surface Phases and Photocatalytic Activity of TiO2. Angew. Chem. Int. Ed. 2008, 47, 1766–1769. [Google Scholar] [CrossRef]
- Gutierrez, J.; Tercjak, A.; Mondragon, I. Conductive Behavior of High TiO2 Nanoparticle Content of Inorganic/Organic Nanostructured Composites. J. Am. Chem. Soc. 2010, 132, 873–878. [Google Scholar] [CrossRef]
- Kwon, D.H.; Kim, K.M.; Jang, J.H.; Jeon, J.M.; Lee, M.H.; Kim, G.H.; Li, X.S.; Park, G.S.; Lee, B.; Han, S.; et al. Atomic Structure of Conducting Nanofilaments in TiO2 Resistive Switching Memory. Nat. Nanotechnol. 2010, 5, 148–153. [Google Scholar] [CrossRef]
- Bavykin, D.V.; Friedrich, J.M.; Walsh, F.C. Protonated Titanates and TiO2 Nanostructured Materials: Synthesis, Properties, and Applications. Adv. Mater. 2006, 18, 2807–2824. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D. TiO2 Photocatalysis and Related Surface Phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Khan, M.M.; Ansari, S.A.; Pradhan, D.; Ansari, M.O.; Han, D.H.; Lee, J.; Cho, M.H. Band Gap Engineered TiO2 Nanoparticles for Visible Light Induced Photoelectrochemical and Photocatalytic Studies. J. Mater. Chem. A 2014, 2, 637–644. [Google Scholar] [CrossRef]
- Li, W.; Wu, Z.X.; Wang, J.X.; Elzatahry, A.A.; Zhao, D.Y. A Perspective on Mesoporous TiO2 Materials. Chem. Mater. 2014, 26, 287–298. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zhao, Z.H.; Kumar, A.; Boughton, R.I.; Liu, H. Recent Progress in Design, Synthesis, and Applications of One-dimensional TiO2 Nanostructured Surface Heterostructures: A review. Chem. Soc. Rev. 2014, 43, 6920–6937. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.Y.; Ren, T.Z.; Azioune, A.; Pireaux, J.J.; Su, B.L. Self-Assembly of Hierarchically Mesoporous−Macroporous Phosphated Nanocrystalline Aluminum (Oxyhydr)oxide Materials. Chem. Mater. 2006, 18, 1753–1767. [Google Scholar] [CrossRef]
- Shi, M.M.; Bao, D.; Wulan, B.R.; Li, Y.H.; Zhang, Y.F.; Yan, J.M.; Jiang, Q. Au Sub-Nanoclusters on TiO2 toward Highly Efficient and Selective Electrocatalyst for N2 Conversion to NH3 at Ambient Conditions. Adv. Mater. 2017, 29, 1606550. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Sofranko, A.C.; Dionysiou, D.D. Nanocrystalline TiO2 Photocatalytic Membranes with a Hierarchical Mesoporous Multilayer Structure: Synthesis, Characterization, and Multifunction. Adv. Funct. Mater. 2006, 16, 1067–1074. [Google Scholar] [CrossRef]
- Kandiel, T.A.; Dillert, R.; Feldhoff, A.; Bahnemann, D.W. Direct Synthesis of Photocatalytically Active Rutile TiO2 Nanorods Partly Decorated with Anatase Nanoparticles. J. Phys. Chem. C 2010, 114, 4909–4915. [Google Scholar] [CrossRef]
- Kim, S.H.; Cho, Y.S.; Jeon, S.J.; Eun, T.H.; Yi, G.R.; Yang, S.M. Microspheres with Tunable Refractive Index by Controlled Assembly of Nanoparticles. Adv. Mater. 2008, 20, 3268–3273. [Google Scholar] [CrossRef]
- Li, H.X.; Bian, Z.F.; Zhu, J.; Zhang, D.Q.; Li, G.S.; Huo, Y.N.; Li, H.; Lu, Y.F. Mesoporous Titania Spheres with Tunable Chamber Stucture and Enhanced Photocatalytic Activity. J. Am. Chem. Soc. 2007, 129, 8406–8407. [Google Scholar] [CrossRef]
- Song, X.F.; Gao, L. Fabrication of Hollow Hybrid Microspheres Coated with Silica/Titania via Sol−Gel Process and Enhanced Photocatalytic Activities. J. Phys. Chem. C 2007, 111, 8180–8187. [Google Scholar] [CrossRef]
- Wang, M.Y.; Wang, C.L.; Xie, K.P.; Sun, L.; Lin, C.J. Fabrication of a Sponge like Nanostructured TiO2 Film and Its Photocatalytic Activity. Acta Phys. Chim. Sin. 2009, 25, 2475–2480. [Google Scholar]
- Xu, P.C.; Liu, Y.; Wei, J.H.; Xiong, R.; Pan, C.X.; Shi, J. Solvothermal Preparation of Ag/TiO2 Nanoparticles and Their Photocatalytic Activity. Acta Phys. Chim. Sin 2010, 26, 2261–2266. [Google Scholar]
- Yu, J.G.; Su, Y.R.; Cheng, B. Template-Free Fabrication and Enhanced Photocatalytic Activity of Hierarchical Macro-/Mesoporous Titania. Adv. Funct. Mater. 2007, 17, 1984–1990. [Google Scholar] [CrossRef]
- Wu, Q.Z.; Shen, Y.; Liao, J.F.; Li, Y.G. Synthesis and Characterization of Three-dimensionally Ordered Macroporous Rare Earth Oxides. Mater. Lett. 2004, 58, 2688–2691. [Google Scholar] [CrossRef]
- Song, L.H.; Li, L.; Gao, X.; Zhao, J.X.; Lu, T.; Liu, Z. A Facile Synthesis of a Uniform Constitution of Three-Dimensionally Ordered Macroporous TiO2–carbon Nanocomposites with Hierarchical Pores for Lithium Ion Batteries. J. Mater. Chem. A 2015, 3, 6862–6872. [Google Scholar] [CrossRef]
- Fan, Y.; Bao, X.J.; Lin, X.Y.; Shi, G.; Liu, H.Y. Acidity Adjustment of HZSM-5 Zeolites by Dealumination and Realumination with Steaming and Citric Acid Treatments. J. Phys. Chem. B 2006, 110, 15411–15416. [Google Scholar] [CrossRef]
- Shen, X.Q.; Zhou, J.X.; Jing, M.X.; Shen, Y.J. Nanosized Nickel Oxides Derived from the Citrate Gel Process and Performances for Electrochemical Capacitors. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2007, 22, 179–182. [Google Scholar] [CrossRef]
- Liu, G.; Jia, M.J.; Zhou, Z.; Zhang, W.X.; Wu, T.H.; Jiang, D.Z. Synthesis of Amorphous Mesoporous Aluminophosphate Materials with High Thermal Stability Using a Citric Acid Route. Chem. Commun. 2004, 1660. [Google Scholar] [CrossRef]
- Delekar, S.; Yadav, H.; Achary, S.N.; Meena, S.S.; Pawar, S. Structural Refinement and Photocatalytic Activity of Fe-doped Anatase TiO2 Nanoparticles. Appl. Surf. Sci. 2012, 263, 536–545. [Google Scholar] [CrossRef]
- Yadav, H.M.; Otari, S.V.; Koli, V.B.; Mali, S.S.; Hong, C.K.; Pawar, S.H.; Delekar, S.D. Preparation and Characterization of Copper-doped Anatase TiO2 Nanoparticles with Visible Light Photocatalytic Antibacterial Activity. J. Photochem. Photobiol. A Chem. 2014, 280, 32–38. [Google Scholar] [CrossRef]
- Gao, C.M.; Wei, T.; Zhang, Y.Y.; Song, X.H.; Huan, Y.; Liu, H.; Zhao, M.W.; Yu, J.H.; Chen, X.D. A Photoresponsive Rutile TiO2 Heterojunction with Enhanced Electron-Hole Separation for High-Performance Hydrogen Evolution. Adv. Mater. 2019, 31, 1806596. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.; Shao, N.; Zhou, X.; Chen, T. Citric Acid Regulated Fabrication of Macroporous TiO2. Surfaces 2020, 3, 50-60. https://doi.org/10.3390/surfaces3010006
Chen R, Shao N, Zhou X, Chen T. Citric Acid Regulated Fabrication of Macroporous TiO2. Surfaces. 2020; 3(1):50-60. https://doi.org/10.3390/surfaces3010006
Chicago/Turabian StyleChen, Rui, Ningning Shao, Xiaoquan Zhou, and Tiehong Chen. 2020. "Citric Acid Regulated Fabrication of Macroporous TiO2" Surfaces 3, no. 1: 50-60. https://doi.org/10.3390/surfaces3010006



