Evolution of the Design of CH4 Adsorbents
Abstract
:1. Introduction
2. CH4 Adsorption Measurements
3. Paradigm Shifts
3.1. Pore Size and Micropore Affinity
3.1.1. Pore Size Design of ACs, CNTs, ACFs, and Biocarbons
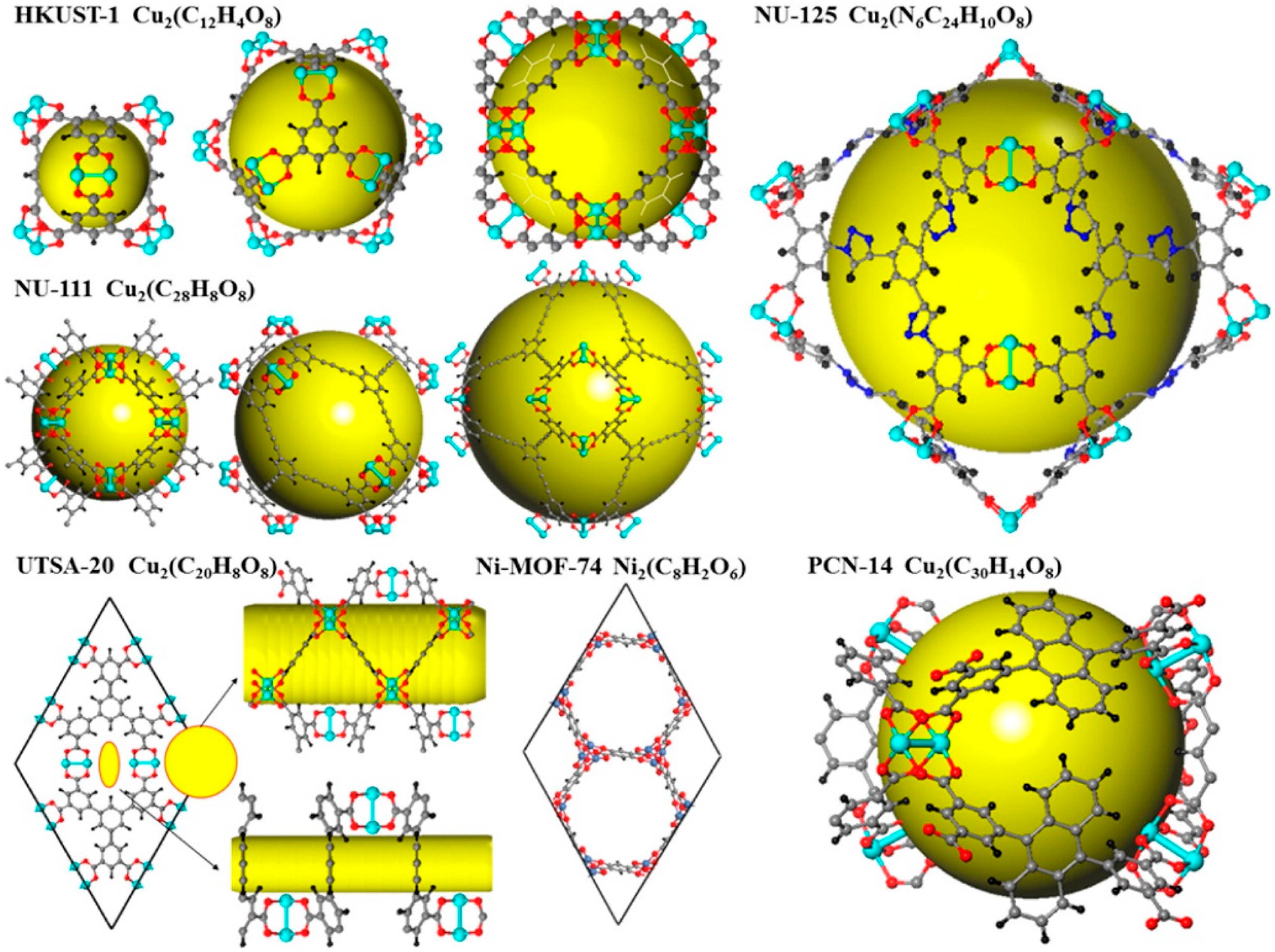
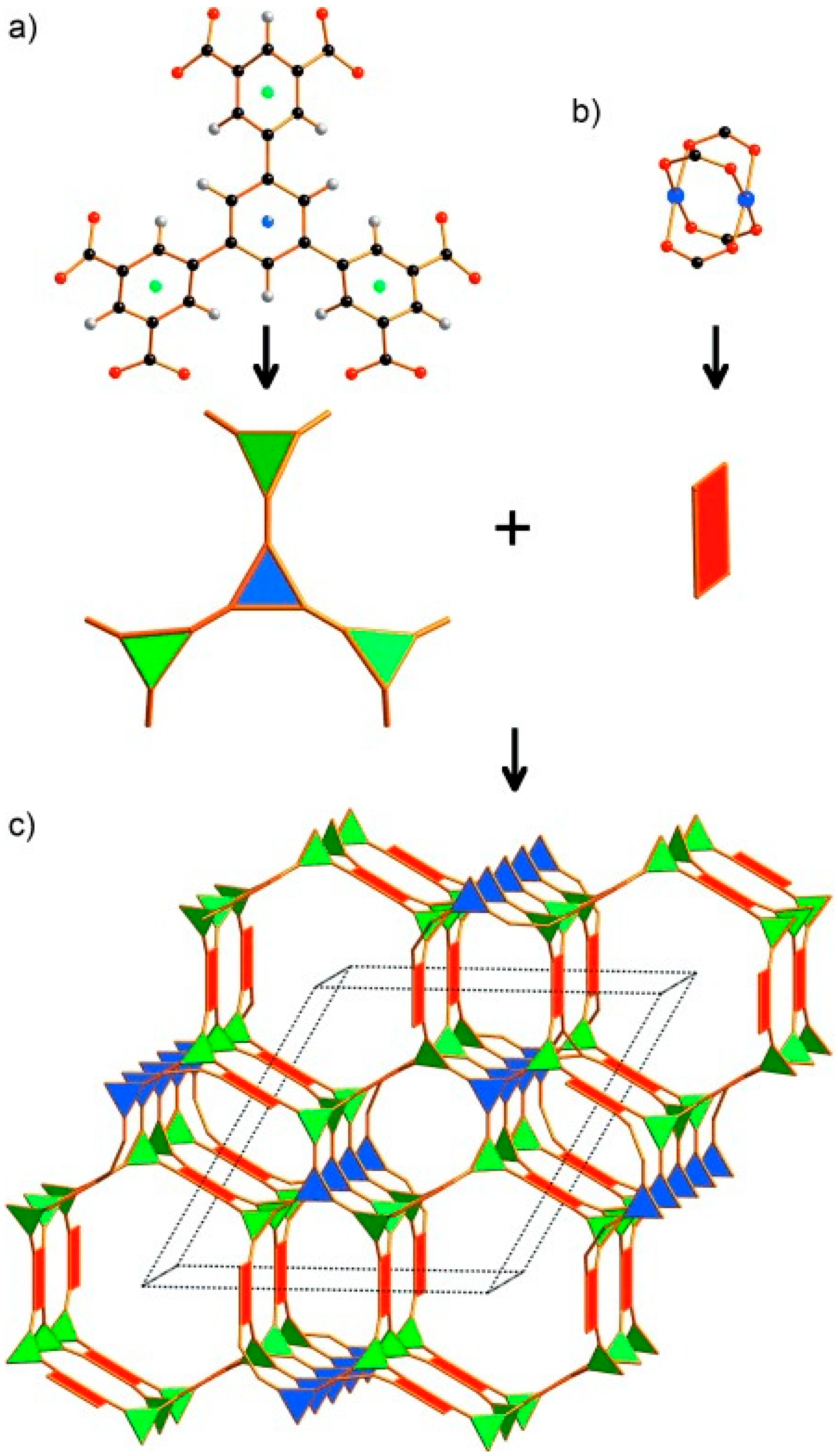
3.1.2. Pore Size Design of MOFs
3.2. Functionalization
3.2.1. Functionalization of Carbon-Based Materials
3.2.2. Functionalization of MOFs
3.3. Interplay between Surface Area, Volume, Density, and Qst
3.3.1. Interplay between Surface Area, Volume, Density, and Qst of Carbon Materials
3.3.2. Interplay between Surface Area, Volume, Density, and Qst of MOFs
3.4. Gas Adsorption Sites
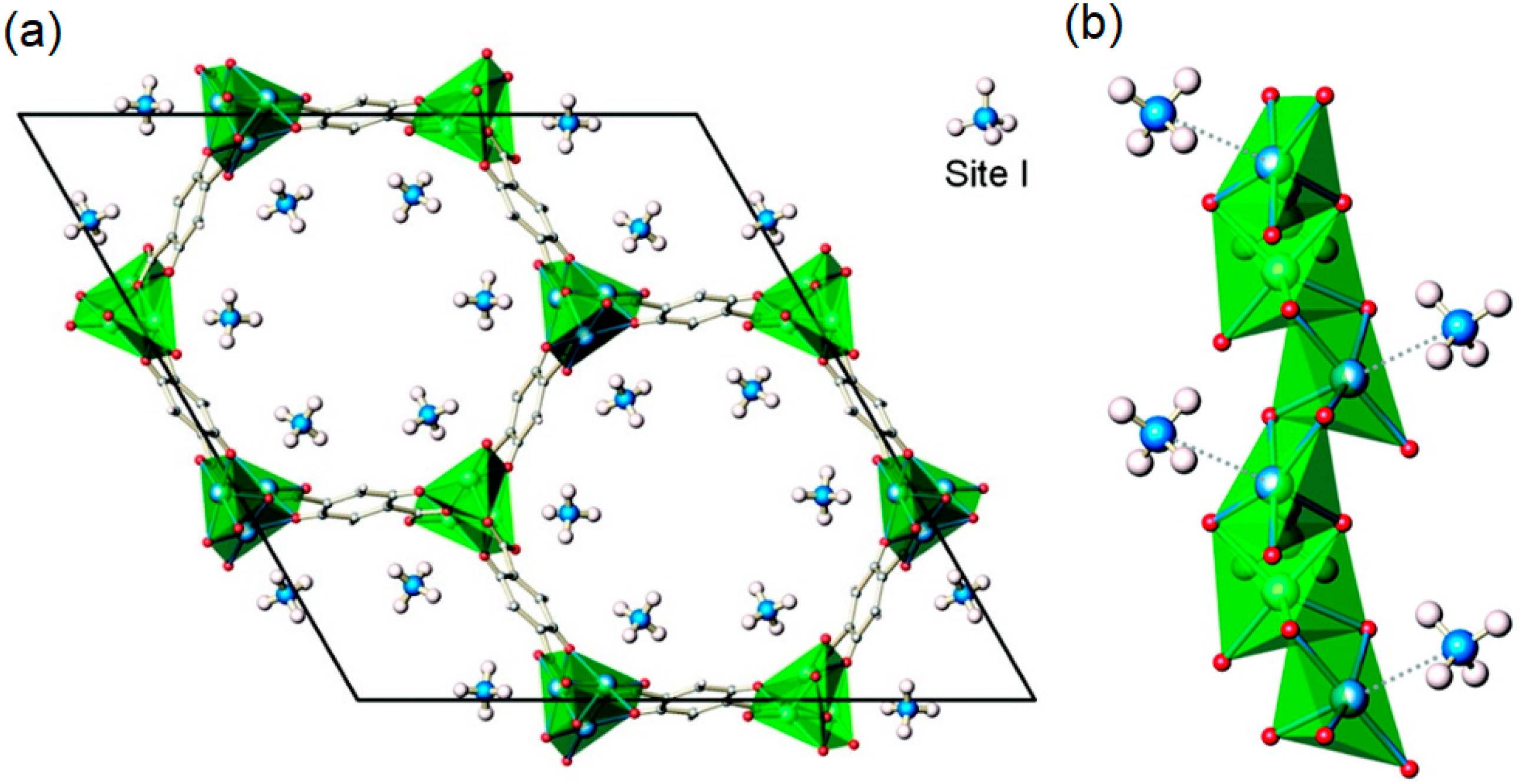
Gas Adsorption Sites of MOFs
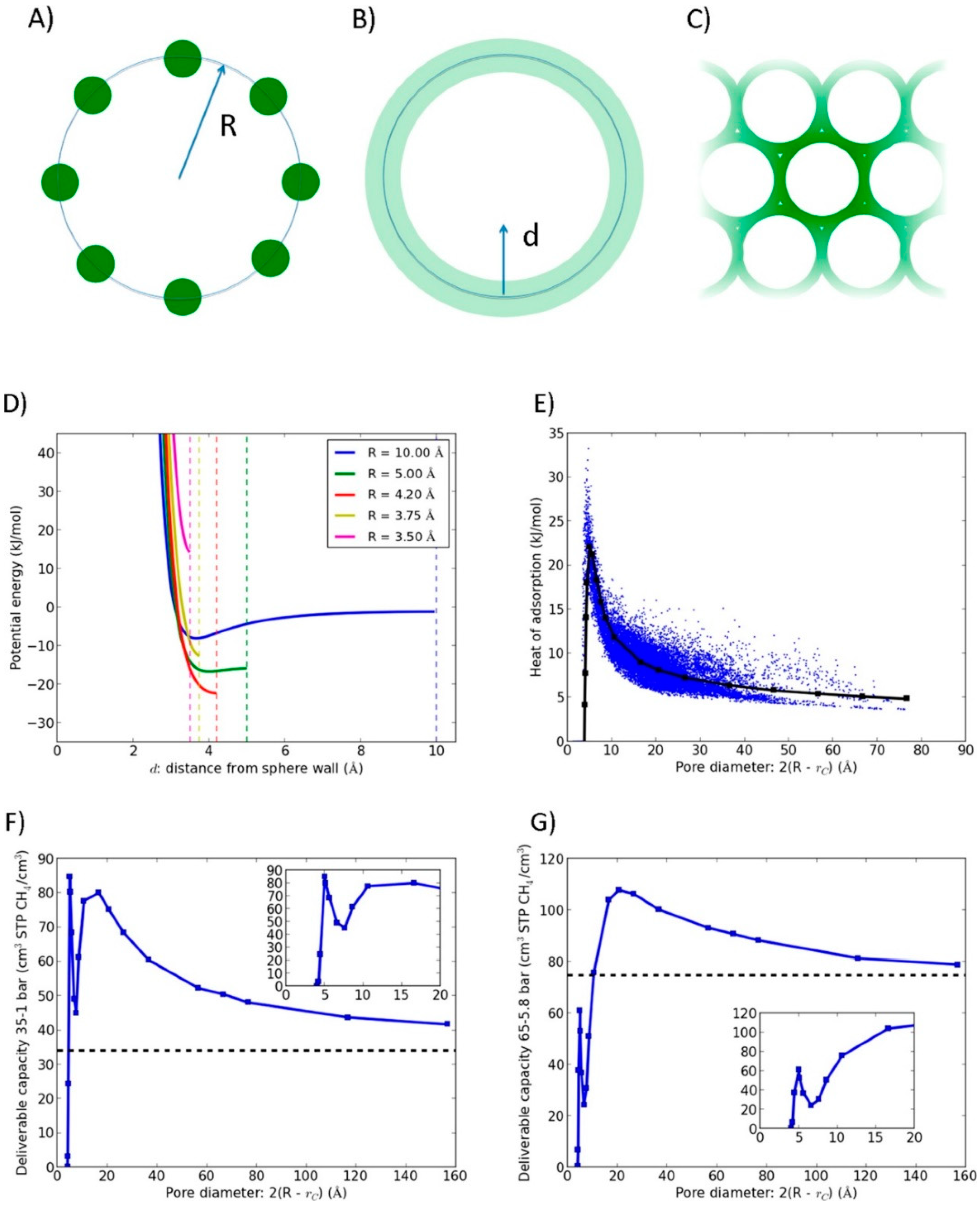
3.5. Topologically Guided Optimization of Packing Density
3.5.1. Optimization of the Packing Density of Carbon Materials
3.5.2. Optimization of the Packing Density of MOFs
3.6. High Throughput In Silico Screening
In Silico Screening of MOFs
3.7. Advanced Material Design
3.7.1. Flexibility
3.7.2. Mesoporosity and Ultraporosity
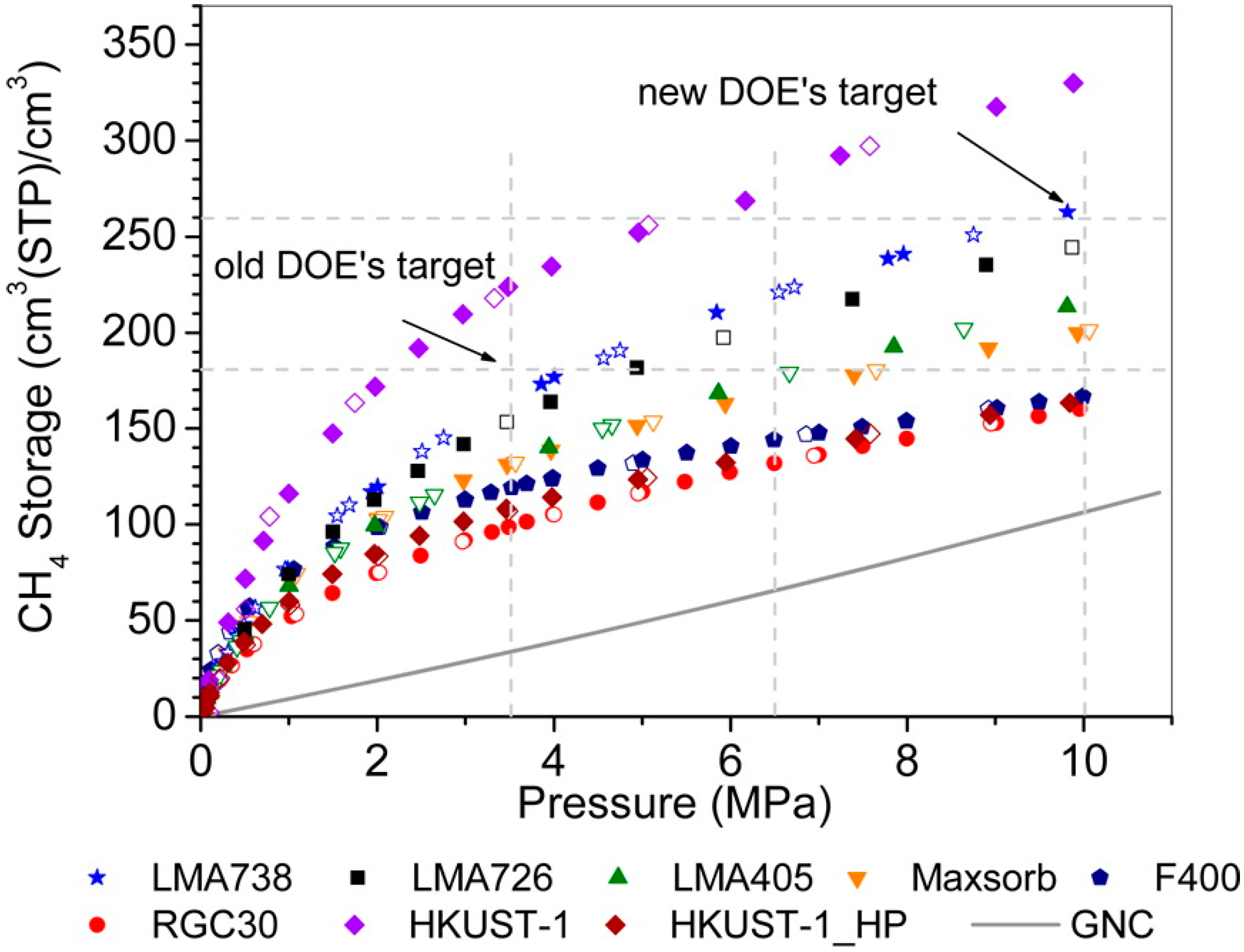
3.8. Process Conditions
Higher Pressure and Lower Temperature
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Shi, Z.L.; Zhang, Y.B. Renaissance of the Methane Adsorbents. Isr. J. Chem. 2018, 58, 985–994. [Google Scholar] [CrossRef]
- William, D.; Callister, J. Materials Science and Engineering 7th Ed.: An Introduction; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; ISBN 9783527631940. [Google Scholar]
- He, Y.; Zhou, W.; Qian, G.; Chen, B. Methane storage in metal–organic frameworks. Chem. Soc. Rev. 2014, 43, 5657–5678. [Google Scholar] [CrossRef] [PubMed]
- Makal, T.A.; Li, J.-R.R.; Lu, W.; Zhou, H.-C.C. Methane storage in advanced porous materials. Chem. Soc. Rev. 2012, 41, 7761–7779. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.E.; Wheatley, P.S. Gas storage in nanoporous materials. Angew. Chem. Int. Ed. 2008, 47, 4966–4981. [Google Scholar] [CrossRef]
- Robert, B. Smart materials: A review of capabilities and applications. Assem. Autom. 2014, 34, 16–22. [Google Scholar]
- Robert, B. Smart materials: A review of recent developments. Assem. Autom. 2012, 32, 3–7. [Google Scholar]
- New MOF Methane Storage Material Exceeds DOE Goals for Adsorbed Natural Gas Storage by 28%. Available online: https://www.greencarcongress.com/2008/01/new-mof-methane.html (accessed on 24 August 2020).
- Peplow, M. Metal-Organic Framework Compound Sets Methane Storage Record. Available online: https://cen.acs.org/articles/95/web/2017/12/Metal-organic-framework-compound-sets.html (accessed on 24 August 2020).
- ARPA-E MOVE Program Overview. Available online: https://arpa-e.energy.gov/sites/default/files/documents/files/MOVE_ProgramOverview.pdf (accessed on 23 August 2020).
- Varun Mehra ARPA-E Is Here to Stay. Available online: https://scienceprogress.org/wp-content/uploads/2013/01/ARPA-Ebrief.pdf (accessed on 23 August 2020).
- Jiang, J.; Furukawa, H.; Zhang, Y.B.; Yaghi, O.M. High Methane Storage Working Capacity in Metal-Organic Frameworks with Acrylate Links. J. Am. Chem. Soc. 2016, 138, 10244–10251. [Google Scholar] [CrossRef]
- Mason, J.A.; Veenstra, M.; Long, J.R. Evaluating metal-organic frameworks for natural gas storage. Chem. Sci. 2014, 5, 32–51. [Google Scholar] [CrossRef] [Green Version]
- Lozano-Castelló, D.; Alcañiz-Monge, J.; De La Casa-Lillo, M.A.; Cazorla-Amorós, D.; Linares-Solano, A. Advances in the study of methane storage in porous carbonaceous materials. Fuel 2002, 81, 1777–1803. [Google Scholar] [CrossRef]
- Antoniou, M.K.; Diamanti, E.K.; Enotiadis, A.; Policicchio, A.; Dimos, K.; Ciuchi, F.; Maccallini, E.; Gournis, D.; Agostino, R.G. Methane storage in zeolite-like carbon materials. Microporous Mesoporous Mater. 2014, 188, 16–22. [Google Scholar] [CrossRef]
- Tedesco, C.; Erra, L.; Brunelli, M.; Cipolletti, V.; Gaeta, C.; Fitch, A.N.; Atwood, J.L.; Neri, P. Methane Adsorption in a Supramolecular Organic Zeolite. Chem. A Eur. J. 2010, 16, 2371–2374. [Google Scholar] [CrossRef] [PubMed]
- Cavenati, S.; Grande, C.A.; Rodrigues, A.E. Adsorption Equilibrium of Methane, Carbon Dioxide, and Nitrogen on Zeolite 13X at High Pressures. J. Chem. Eng. Data 2004, 49, 1095–1101. [Google Scholar] [CrossRef]
- Mentasty, L.; Faccio, R.J.; Zgrablich, G. High-Pressure Methane Adsorption in 5A Zeolite and the Nature of Gas-Solid Interactions. Adsorpt. Sci. Technol. 1991, 8, 105–113. [Google Scholar] [CrossRef]
- Noro, S.; Kitagawa, S.; Kondo, M.; Seki, K. A New, Methane Adsorbent, Porous Coordination Polymer [{CuSiF6 (4, 4′-bipyridine)2}n]. Angew. Chem. Int. Ed. 2000, 39, 2081–2084. [Google Scholar] [CrossRef]
- Kizzie, A.C.; Dailly, A.; Perry, L.; Lail, M.A.; Lu, W.; Nelson, T.O.; Cai, M.; Zhou, H.-C. Enhanced Methane Sorption in Densified Forms of a Porous Polymer Network. Mater. Sci. Appl. 2014, 5, 387–394. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Chen, F.; Li, B.; Qian, G.; Zhou, W.; Chen, B. Porous metal–organic frameworks for fuel storage. Coord. Chem. Rev. 2018, 373, 167–198. [Google Scholar] [CrossRef]
- Li, B.; Wen, H.M.; Yu, Y.; Cui, Y.; Zhou, W.; Chen, B.; Qian, G. Nanospace within metal–organic frameworks for gas storage and separation. Mater. Today Nano 2018, 2, 21–49. [Google Scholar] [CrossRef]
- Farha, O.K.; Hupp, J.T. Rational design, synthesis, purification, and activation of metal-organic framework materials. Acc. Chem. Res. 2010, 43, 1166–1175. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Ding, X.; Jiang, D. Covalent organic frameworks. Chem. Soc. Rev. 2012, 41, 6010–6022. [Google Scholar] [CrossRef]
- Choi, P.-S.; Jeong, J.-M.; Choi, Y.-K.; Kim, M.-S.; Shin, G.-J.; Park, S.-J. A review: Methane capture by nanoporous carbon materials for automobiles. Carbon Lett. 2016, 17, 18–28. [Google Scholar] [CrossRef] [Green Version]
- Chang, Z.; Zhang, D.-S.; Chen, Q.; Bu, X.-H. Microporous organic polymers for gas storage and separation applications. Phys. Chem. Chem. Phys. 2013, 15, 5430–5442. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, X.; Xue, H.; Pang, H.; Xu, Q. Metal–organic frameworks as a platform for clean energy applications. EnergyChem 2020, 1, 100006. [Google Scholar] [CrossRef]
- Zhang, H.; Nai, J.; Yu, L.; Lou, X.W.D. Metal-Organic-Framework-Based Materials as Platforms for Renewable Energy and Environmental Applications. Joule 2017, 1, 77–107. [Google Scholar] [CrossRef] [Green Version]
- Konstas, K.; Osl, T.; Yang, Y.; Batten, M.; Burke, N.; Hill, A.J.; Hill, M.R. Methane storage in metal organic frameworks. J. Mater. Chem. 2012, 22, 16698–16708. [Google Scholar] [CrossRef]
- Ji, Z.; Wang, H.; Canossa, S.; Wuttke, S.; Yaghi, O.M. Pore Chemistry of Metal–Organic Frameworks. Adv. Funct. Mater. 2020, 2000238. [Google Scholar] [CrossRef]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef]
- Furukawa, H.; Ko, N.; Go, Y.B.; Aratani, N.; Choi, S.B.; Choi, E.; Yazaydin, A.Ö.; Snurr, R.Q.; O’Keeffe, M.; Kim, J.; et al. Ultrahigh porosity in metal-organic frameworks. Science 2010, 329, 424–428. [Google Scholar] [CrossRef] [Green Version]
- Perry, J.J.; Perman, J.A.; Zaworotko, M.J. Design and synthesis of metal-organic frameworks using metal-organic polyhedra as supermolecular building blocks. Chem. Soc. Rev. 2009, 38, 1400–1417. [Google Scholar] [CrossRef]
- Wang, C.; Liu, D.; Lin, W. Metal-organic frameworks as a tunable platform for designing functional molecular materials. J. Am. Chem. Soc. 2013, 135, 13222–13234. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W. Methane storage in porous metal-organic frameworks: Current records and future perspectives. Chem. Rec. 2010, 10, 200–204. [Google Scholar] [CrossRef]
- Alahakoon, S.B.; Thompson, C.M.; Occhialini, G.; Smaldone, R.A. Design Principles for Covalent Organic Frameworks in Energy Storage Applications. ChemSusChem 2017, 10, 2116–2129. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Cao, D. Porous covalent–organic materials: Synthesis, clean energy application and design. J. Mater. Chem. A 2013, 1, 2691–2718. [Google Scholar] [CrossRef]
- Guan, X.; Chen, F.; Fang, Q.; Qiu, S. Design and applications of three dimensional covalent organic frameworks. Chem. Soc. Rev. 2020, 49, 1357–1384. [Google Scholar] [CrossRef]
- Zhan, X.; Chen, Z.; Zhang, Q. Recent progress in two-dimensional COFs for energy-related applications. J. Mater. Chem. A 2017, 5, 14463–14479. [Google Scholar] [CrossRef]
- Zhou, W.; Wu, H.; Hartman, M.R.; Yildirim, T. Hydrogen and methane adsorption in metal-organic frameworks: A high-pressure volumetric study. J. Phys. Chem. C 2007, 111, 16131–16137. [Google Scholar] [CrossRef]
- Keller, J.U.; Staudt, R. Gas Adsorption Equilibria: Experimental Methods and Adsorptive Isotherms; Springer US: New York, NY, USA, 2005; ISBN 0387235981. [Google Scholar]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders-Surface Area, Pore Size and Density; Springer: New York, NY, USA, 2004; ISBN 978-90-481-6633-6. [Google Scholar]
- Coudert, F.X.; Boutin, A.; Jeffroy, M.; Mellot-Draznieks, C.; Fuchs, A.H. Thermodynamic methods and models to study flexible metal-organic frameworks. ChemPhysChem 2011, 12, 247–258. [Google Scholar] [CrossRef] [Green Version]
- Myers, A.L.; Monson, P.A. Adsorption in porous materials at high pressure: Theory and experiment. Langmuir 2002, 18, 10261–10273. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K.S.W.; Llewellyn, P.; Maurin, G. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications; Elsevier: Oxford, UK, 2014; ISBN 978-0-08-097035-6. [Google Scholar]
- Myers, A.L. Thermodynamics of adsorption in porous materials. AIChE J. 2002, 48, 145–160. [Google Scholar] [CrossRef]
- Tykodi, R.J. Thermodynamics of adsorption. J. Chem. Phys. 1954, 22, 1647–1654. [Google Scholar] [CrossRef]
- Smith, J.M.; Van Ness, H.C.; Abbott, M.M. Introduction to Chemical Engineering Thermodynamics; McGraw-Hill Education: Boston, MA, USA, 2005; Volume 27, ISBN 0072402962. [Google Scholar]
- Zhang, M.; Zhou, W.; Pham, T.; Forrest, K.A.; Liu, W.; He, Y.; Wu, H.; Yildirim, T.; Chen, B.; Space, B.; et al. Fine Tuning of MOF-505 Analogues To Reduce Low-Pressure Methane Uptake and Enhance Methane Working Capacity. Angew. Chem. Int. Ed. 2017, 56, 11426–11430. [Google Scholar] [CrossRef]
- Liang, C.C.; Shi, Z.L.; He, C.T.; Tan, J.; Zhou, H.D.H.L.; Zhou, H.D.H.L.; Lee, Y.; Zhang, Y.B. Engineering of Pore Geometry for Ultrahigh Capacity Methane Storage in Mesoporous Metal-Organic Frameworks. J. Am. Chem. Soc. 2017, 139, 13300–13303. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.R.; Contescu, C.I.; Chisholm, M.F.; Cooper, V.R.; Guo, J.; He, L.; Ihm, Y.; Mamontov, E.; Melnichenko, Y.B.; Olsen, R.J.; et al. Modern approaches to studying gas adsorption in nanoporous carbons. J. Mater. Chem. A 2013, 1, 9341–9350. [Google Scholar] [CrossRef]
- Lin, J.M.; He, C.T.; Liu, Y.; Liao, P.Q.; Zhou, D.D.; Zhang, J.P.; Chen, X.M. A Metal-Organic Framework with a Pore Size/Shape Suitable for Strong Binding and Close Packing of Methane. Angew. Chem. Int. Ed. 2016, 55, 4674–4678. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Castelló, D.; Cazorla-Amorós, D.; Linares-Solano, A.; Quinn, D.F. Influence of pore size distribution on methane storage at relatively low pressure: Preparation of activated carbon with optimum pore size. Carbon N. Y. 2002, 40, 989–1002. [Google Scholar] [CrossRef]
- Alcañiz-Monge, J.; De La Casa-Lillo, M.A.; Cazorla-Amorós, D.; Linares-Solano, A. Methane storage in activated carbon fibres. Carbon N. Y. 1997, 35, 291–297. [Google Scholar] [CrossRef]
- Chui, S.S.Y.; Lo, S.M.F.; Charmant, J.P.H.; Orpen, A.G.; Williams, I.D. A chemically functionalizable nanoporous material [Cu3(TMA)2 (H2O)3](n). Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef]
- Hamza, U.D.; Nasri, N.S.; Mohammed, J.; Majid, Z.A. Natural gas adsorption on biomass derived activated carbons: A mini review. MATEC Web Conf. 2016, 60, 1–5. [Google Scholar] [CrossRef]
- Sircar, S.; Golden, T.C.; Rao, M.B. Activated carbon for gas separation and storage. Carbon N. Y. 1996, 34, 1–12. [Google Scholar] [CrossRef]
- Matranga, K.R.; Myers, A.L.; Glandt, E.D. Storage of Natural-Gas By Adsorption on Activated Carbon. Chem. Eng. Sci. 1992, 47, 1569–1579. [Google Scholar] [CrossRef]
- Himeno, S.; Komatsu, T.; Fujita, S. High-Pressure Adsorption Equilibria of Methane and Carbon Dioxide on Several Activated Carbons. J. Chem. Eng. Data 2005, 50, 369–376. [Google Scholar] [CrossRef]
- Kondo, M.; Yoshitomi, T.; Seki, K.; Matsuzaka, H.; Kitagawa, S. Three-Dimensional Framework with Channeling Cavities for Small Molecules: {[M2(4,4′-bpy)3(NO3)4]·xH2O}n(M = Co, Ni, Zn). Angew. Chem. (Int. Ed. Engl.) 1997, 36, 1725–1727. [Google Scholar] [CrossRef]
- Chen, Z.; Li, P.; Anderson, R.; Wang, X.; Zhang, X.; Robison, L.; Redfern, L.R.; Moribe, S.; Islamoglu, T.; Gómez-Gualdrón, D.A.; et al. Balancing volumetric and gravimetric uptake in highly porous materials for clean energy. Science 2020, 368, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Düren, T.; Sarkisov, L.; Yaghi, O.M.; Snurr, R.Q. Design of New Materials for Methane Storage. Langmuir 2004, 20, 2683–2689. [Google Scholar] [CrossRef] [PubMed]
- Gulati, A.; Kakkar, R. DFT studies on storage and adsorption capacities of gases on MOFs. Phys. Sci. Rev. 2018. [Google Scholar]
- Guo, Z.; Wu, H.; Srinivas, G.; Zhou, Y.; Xiang, S.; Chen, Z.; Yang, Y.; Zhou, W.; O’Keeffe, M.; Chen, B. A metal-organic framework with optimized open metal sites and pore spaces for high methane storage at room temperature. Angew. Chem. Int. Ed. 2011, 50, 3178–3181. [Google Scholar] [CrossRef]
- Martin, R.L.; Simon, C.M.; Smit, B.; Haranczyk, M. In silico Design of Porous Polymer Networks: High-Throughput Screening for Methane Storage Materials. J. Am. Chem. Soc. 2014, 136, 5006–5022. [Google Scholar] [CrossRef]
- Wen, H.M.; Li, B.; Li, L.; Lin, R.B.; Zhou, W.; Qian, G.; Chen, B. A Metal–Organic Framework with Optimized Porosity and Functional Sites for High Gravimetric and Volumetric Methane Storage Working Capacities. Adv. Mater. 2018, 30, 1704792. [Google Scholar] [CrossRef]
- Moellmer, J.; Moeller, A.; Dreisbach, F.; Glaeser, R.; Staudt, R. High pressure adsorption of hydrogen, nitrogen, carbon dioxide and methane on the metal-organic framework HKUST-1. Microporous Mesoporous Mater. 2011, 138, 140–148. [Google Scholar] [CrossRef]
- Yan, Y.; Kolokolov, D.I.; Da Silva, I.; Stepanov, A.G.; Blake, A.J.; Dailly, A.; Manuel, P.; Tang, C.C.; Yang, S.; Schröder, M. Porous Metal-Organic Polyhedral Frameworks with Optimal Molecular Dynamics and Pore Geometry for Methane Storage. J. Am. Chem. Soc. 2017, 139, 13349–13360. [Google Scholar] [CrossRef]
- Alezi, D.; Belmabkhout, Y.; Suyetin, M.; Bhatt, P.M.; Weseliński, L.J.; Solovyeva, V.; Adil, K.; Spanopoulos, I.; Trikalitis, P.N.; Emwas, A.H.; et al. MOF Crystal Chemistry Paving the Way to Gas Storage Needs: Aluminum-Based soc -MOF for CH4, O2, and CO2 Storage. J. Am. Chem. Soc. 2015, 137, 13308–13318. [Google Scholar] [CrossRef]
- Peng, Y.; Krungleviciute, V.; Eryazici, I.; Hupp, J.T.; Farha, O.K.; Yildirim, T. Methane storage in metal-organic frameworks: Current records, surprise findings, and challenges. J. Am. Chem. Soc. 2013, 135, 11887–11894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, H.; Doonan, C.J.; Furukawa, H.; Ferreira, R.B.; Towne, J.; Knobler, C.B.; Wang, B.; Yaghi, O.M. Multiple functional groups of varying ratios in metal-organic frameworks. Science 2010, 327, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.A.; Oktawiec, J.; Taylor, M.K.; Hudson, M.R.; Rodriguez, J.; Bachman, J.E.; Gonzalez, M.I.; Cervellino, A.; Guagliardi, A.; Brown, C.M.; et al. Methane storage in flexible metal-organic frameworks with intrinsic thermal management. Nature 2015, 527, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tang, S.; Wang, C.; Lv, X.; Jiang, M.; Wu, H.; Zhao, X. High gas storage capacities and stepwise adsorption in a UiO type metal-organic framework incorporating Lewis basic bipyridyl sites. Chem. Commun. 2014, 50, 2304–2307. [Google Scholar] [CrossRef]
- Wilmer, C.E.; Farha, O.K.; Yildirim, T.; Eryazici, I.; Krungleviciute, V.; Sarjeant, A.A.; Snurr, R.Q.; Hupp, J.T. Gram-scale, high-yield synthesis of a robust metal–organic framework for storing methane and other gases. Energy Environ. Sci. 2013, 6, 1158–1163. [Google Scholar] [CrossRef]
- Wen, H.-M.M.; Li, B.; Yuan, D.; Wang, H.; Yildirim, T.; Zhou, W.; Chen, B. A porous metal-organic framework with an elongated anthracene derivative exhibiting a high working capacity for the storage of methane. J. Mater. Chem. A 2014, 2, 11516–11522. [Google Scholar] [CrossRef]
- Gándara, F.; Furukawa, H.; Lee, S.; Yaghi, O.M. High methane storage capacity in aluminum metal-organic frameworks. J. Am. Chem. Soc. 2014, 136, 5271–5274. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, C.; Wang, Q.; Fu, W.; Huang, K.; Zhou, W. A metal-organic framework functionalized with piperazine exhibiting enhanced CH4 storage. J. Mater. Chem. A 2017, 5, 349–354. [Google Scholar] [CrossRef]
- Spanopoulos, I.; Tsangarakis, C.; Klontzas, E.; Tylianakis, E.; Froudakis, G.; Adil, K.; Belmabkhout, Y.; Eddaoudi, M.; Trikalitis, P.N. Reticular Synthesis of HKUST-like tbo-MOFs with Enhanced CH4 Storage. J. Am. Chem. Soc. 2016, 138, 1568–1574. [Google Scholar] [CrossRef] [Green Version]
- Bolinois, L.; Kundu, T.; Wang, X.; Wang, Y.; Hu, Z.; Koh, K.; Zhao, D. Breathing-induced new phase transition in an MIL-53(Al)–NH 2 metal–organic framework under high methane pressures. Chem. Commun. 2017, 53, 8118–8121. [Google Scholar] [CrossRef]
- Mendoza-Cortés, J.L.; Han, S.S.; Furukawa, H.; Yaghi, O.M.; Goddard, W.A. Adsorption Mechanism and Uptake of Methane in Covalent Organic Frameworks: Theory and Experiment. J. Phys. Chem. A 2010, 114, 10824–10833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furukawa, H.; Yaghi, O.M. Storage of Hydrogen, Methane, and Carbon Dioxide in Highly Porous Covalent Organic Frameworks for Clean Energy Applications. J. Am. Chem. Soc. 2009, 131, 8875–8883. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.D.; Tan, B.; Trewin, A.; Su, F.; Rosseinsky, M.J.; Bradshaw, D.; Sun, Y.; Zhou, L.; Cooper, A.I. Microporous organic polymers for methane storage. Adv. Mater. 2008, 20, 1916–1920. [Google Scholar] [CrossRef]
- Yuan, D.; Lu, W.; Zhao, D.; Zhou, H.-C. Highly Stable Porous Polymer Networks with Exceptionally High Gas-Uptake Capacities. Adv. Mater. 2011, 23, 3723–3725. [Google Scholar] [CrossRef]
- Menon, V.C.; Komarneni, S. Porous adsorbents for vehicular natural gas storage: A review. J. Porous Mater. 1998, 5, 43–58. [Google Scholar] [CrossRef]
- Policicchio, A.; MacCallini, E.; Agostino, R.G.; Ciuchi, F.; Aloise, A.; Giordano, G. Higher methane storage at low pressure and room temperature in new easily scalable large-scale production activated carbon for static and vehicular applications. Fuel 2013, 104, 813–821. [Google Scholar] [CrossRef]
- Golebiowska, M.; Roth, M.; Firlej, L.; Kuchta, B.; Wexler, C. The reversibility of the adsorption of methane–methyl mercaptan mixtures in nanoporous carbon. Carbon N. Y. 2012, 50, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Burchell, T.; Rogers, M. Low Pressure Storage of Natural Gas for Vehicular Applications. SAE Trans. 2000, 109, 2242–2246. [Google Scholar]
- Guan, C.; Loo, L.S.; Wang, K.; Yang, C. Methane storage in carbon pellets prepared via a binderless method. Energy Convers. Manag. 2011, 52, 1258–1262. [Google Scholar] [CrossRef]
- Casco, M.E.; Martínez-Escandell, M.; Gadea-Ramos, E.; Kaneko, K.; Silvestre-Albero, J.; Rodríguez-Reinoso, F. High-pressure methane storage in porous materials: Are carbon materials in the pole position? Chem. Mater. 2015, 27, 959–964. [Google Scholar] [CrossRef] [Green Version]
- Rahman, K.A.; Loh, W.S.; Yanagi, H.; Chakraborty, A.; Saha, B.B.; Chun, W.G.; Ng, K.C. Experimental Adsorption Isotherm of Methane onto Activated Carbon at Sub- and Supercritical Temperatures. J. Chem. Eng. Data 2010, 55, 4961–4967. [Google Scholar] [CrossRef]
- Park, J.E.; Lee, G.B.; Hwang, S.Y.; Kim, J.H.; Hong, B.U.; Kim, H.; Kim, S. The effects of methane storage capacity using upgraded activated carbon by KOH. Appl. Sci. 2018, 8, 1596. [Google Scholar] [CrossRef] [Green Version]
- Cook, T.L.; Komodromos, C.; Quinn, D.F.; Ragan, S. Adsorbent Storage for Natural Gas Vehicles. In Carbon Materials for Advanced Technologies; Elsevier: Oak Ridge, TN, USA, 1999. [Google Scholar]
- Kumar, K.V.; Preuss, K.; Titirici, M.M.; Rodríguez-Reinoso, F. Nanoporous Materials for the Onboard Storage of Natural Gas. Chem. Rev. 2017, 117, 1796–1825. [Google Scholar] [CrossRef] [PubMed]
- Delavar, M.; Ghoreyshi, A.A.; Jahanshahi, M.; Nabian, N. Comparative experimental study of methane adsorption on multi-walled carbon nanotubes and granular activated carbons. J. Exp. Nanosci. 2014, 9, 310–328. [Google Scholar] [CrossRef]
- Bekyarova, E.; Murata, K.; Yudasaka, M.; Kasuya, D.; Iijima, S.; Tanaka, H.; Kahoh, H.; Kaneko, K. Single-wall nanostructured carbon for methane storage. J. Phys. Chem. B 2003, 107, 4681–4684. [Google Scholar] [CrossRef]
- Pfeifer, P.; Aston, L.; Banks, M.; Barker, S.; Burress, J.; Carter, S.; Coleman, J.; Crockett, S.; Faulhaber, C.; Flavin, J.; et al. Complex pore spaces create record-breaking methane storage system for natural-gas vehicles. Chaos Interdiscip. J. Nonlinear Sci. 2007, 17, 41108. [Google Scholar] [CrossRef] [Green Version]
- Tsivadze, A.Y.; Aksyutin, O.E.; Ishkov, A.G.; Men’shchikov, I.E.; Fomkin, A.A.; Shkolin, A.V.; Khozina, E.V.; Grachev, V.A. Porous carbon-based adsorption systems for natural gas (methane) storage. Russ. Chem. Rev. 2018, 87, 950–983. [Google Scholar] [CrossRef]
- Tu, T.N.; Nguyen, H.T.D.; Tran, N.T. Tailoring the pore size and shape of the one-dimensional channels in iron-based MOFs for enhancing the methane storage capacity. Inorg. Chem. Front. 2019, 6, 2441–2447. [Google Scholar] [CrossRef]
- Wood, B.C.; Bhide, S.Y.; Dutta, D.; Kandagal, V.S.; Pathak, A.D.; Punnathanam, S.N.; Ayappa, K.G.; Narasimhan, S. Methane and carbon dioxide adsorption on edge-functionalized graphene: A comparative DFT study. J. Chem. Phys. 2012, 137, 054702. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.F.; Cronin, L. Postsynthetic covalent modification of metal-organic framework (MOF) materials. Angew. Chem. Int. Ed. 2008, 47, 4635–4637. [Google Scholar] [CrossRef]
- Fischer, R.A.; Wöll, C. Functionalized coordination space in metal-organic frameworks. Angew. Chem. Int. Ed. 2008, 47, 8164–8168. [Google Scholar] [CrossRef] [PubMed]
- Bunck, D.N.; Dichtel, W.R. Mixed linker strategies for organic framework functionalization. Chem. A Eur. J. 2013, 19, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Go, Y.B.; Ko, N.; Park, Y.K.; Uribe-Romo, F.J.; Kim, J.; O’Keeffe, M.; Yaghi, O.M. Isoreticular expansion of metal-organic frameworks with triangular and square building units and the lowest calculated density for porous crystals. Inorg. Chem. 2011, 50, 9147–9152. [Google Scholar] [CrossRef] [PubMed]
- Eubank, J.F.; Wojtas, L.; Hight, M.R.; Bousquet, T.; Kravtsov, V.C.; Eddaoudi, M. The next chapter in MOF pillaring strategies: Trigonal heterofunctional ligands to access targeted high-connected three dimensional nets, isoreticular platforms. J. Am. Chem. Soc. 2011, 133, 17532–17535. [Google Scholar] [CrossRef]
- Yuan, D.; Zhao, D.; Sun, D.; Zhou, H.C. An isoreticular series of metal-organic frameworks with dendritic hexacarboxylate ligands and exceptionally high gas-uptake capacity. Angew. Chem. Int. Ed. 2010, 49, 5357–5361. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, R.D.R.D.; Krungleviciute, V.; Clingerman, D.J.D.J.; Mondloch, J.E.J.E.; Peng, Y.; Wilmer, C.E.C.E.; Sarjeant, A.A.A.; Snurr, R.Q.R.Q.; Hupp, J.T.J.T.; Yildirim, T.; et al. Carborane-based metal-organic framework with high methane and hydrogen storage capacities. Chem. Mater. 2013, 25, 3539–3543. [Google Scholar] [CrossRef] [Green Version]
- Xue, D.X.; Wang, Q.; Bai, J. Amide-functionalized metal–organic frameworks: Syntheses, structures and improved gas storage and separation properties. Coord. Chem. Rev. 2019, 387, 2–16. [Google Scholar] [CrossRef]
- Wu, H.; Yildirim, T.; Zhou, W. Exceptional mechanical stability of highly porous zirconium metal-organic framework UiO-66 and its important implications. J. Phys. Chem. Lett. 2013, 4, 925–930. [Google Scholar] [CrossRef]
- Rao, X.; Cai, J.; Yu, J.; He, Y.; Wu, C.; Zhou, W.; Yildirim, T.; Chen, B.; Qian, G. A microporous metal–organic framework with both open metal and Lewis basic pyridyl sites for high C2H2 and CH4 storage at room temperature. Chem. Commun. 2013, 49, 6719–6721. [Google Scholar] [CrossRef]
- Kayal, S.; Sun, B.; Chakraborty, A. Study of metal-organic framework MIL-101(Cr) for natural gas (methane) storage and compare with other MOFs (metal-organic frameworks). Energy 2015, 91, 772–781. [Google Scholar] [CrossRef]
- Li, B.; Wen, H.-M.M.; Wang, H.; Wu, H.; Yildirim, T.; Zhou, W.; Chen, B. Porous metal-organic frameworks with Lewis basic nitrogen sites for high-capacity methane storage. Energy Environ. Sci. 2015, 8, 2504–2511. [Google Scholar] [CrossRef]
- He, Y.; Zhou, W.; Yildirim, T.; Chen, B. A series of metal-organic frameworks with high methane uptake and an empirical equation for predicting methane storage capacity. Energy Environ. Sci. 2013, 6, 2735–2744. [Google Scholar] [CrossRef]
- Lin, X.; Telepeni, I.; Blake, A.J.; Dailly, A.; Brown, C.M.; Simmons, J.M.; Zoppi, M.; Walker, G.S.; Thomas, K.M.; Mays, T.J.; et al. High capacity hydrogen adsorption in Cu(II) tetracarboxylate framework materials: The role of pore size, ligand functionalization, and exposed metal sites. J. Am. Chem. Soc. 2009, 131, 2159–2171. [Google Scholar] [CrossRef] [PubMed]
- Burgess, C.G.V.; Everett, D.H.; Nuttall, S. Adsorption hysteresis in porous materials. Pure Appl. Chem. 1989, 61, 1845–1852. [Google Scholar] [CrossRef] [Green Version]
- Donohue, M.D.; Aranovich, G.L. Adsorption hysteresis in porous solids. J. Colloid Interface Sci. 1998, 205, 121–130. [Google Scholar] [CrossRef]
- Ye, Y.; Lin, R.B.; Cui, H.; Alsalme, A.; Zhou, W.; Yildirim, T.; Zhang, Z.; Xiang, S.; Chen, B. A microporous metal-organic framework with naphthalene diimide groups for high methane storage. Dalton Trans. 2020, 49, 3658–3661. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, C.; Shi, Z.; Huang, K.; Fu, W.; Zhou, W. Inserting Amide into NOTT-101 to Sharply Enhance Volumetric and Gravimetric Methane Storage Working Capacity. Inorg. Chem. 2019, 58, 13782–13787. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, C.; Jiang, J.; Duan, X.; Zhang, L.; Jiang, K.; Qian, G. A fluorinated Zr-based MOF of high porosity for high CH4 storage. J. Solid State Chem. 2019, 277, 139–142. [Google Scholar] [CrossRef]
- Chang, G.; Wen, H.; Li, B.; Zhou, W.; Wang, H.; Alfooty, K.; Bao, Z.; Chen, B. A Fluorinated Metal-Organic Framework for High Methane Storage at Room Temperature. Cryst. Growth Des. 2016, 16, 3395–3399. [Google Scholar] [CrossRef]
- Garibay, S.J.; Wang, Z.; Tanabe, K.K.; Cohen, S.M. Postsynthetic modification: A versatile approach toward multifunctional metal-organic frameworks. Inorg. Chem. 2009, 48, 7341–7349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, S.; Lu, W.; Chen, Y.P.; Zhang, Q.; Liu, T.F.; Feng, D.; Wang, X.; Qin, J.; Zhou, H.C. Sequential linker installation: Precise placement of functional groups in multivariate metal-organic frameworks. J. Am. Chem. Soc. 2015, 137, 3177–3180. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.X.; Wei, Z.W.; Jiang, J.J.; Zheng, S.P.; Wang, H.P.; Qiu, Q.F.; Cao, C.C.; Fenske, D.; Su, C.Y. Dynamic Spacer Installation for Multirole Metal-Organic Frameworks: A New Direction toward Multifunctional MOFs Achieving Ultrahigh Methane Storage Working Capacity. J. Am. Chem. Soc. 2017, 139, 6034–6037. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.A.A.; Masoomi, M.Y.; Islamoglu, T.; Morsali, A.; Xu, Y.; Hupp, J.T.; Farha, O.K.; Wang, J.; Junk, P.C. Improvement of Methane-Framework Interaction by Controlling Pore Size and Functionality of Pillared MOFs. Inorg. Chem. 2017, 56, 2581–2588. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhao, J.; Yan, T. Methane Uptakes in Covalent Organic Frameworks with Double Halogen Substitution. J. Phys. Chem. C 2015, 119, 2010–2014. [Google Scholar] [CrossRef]
- Zhao, J.; Yan, T. Effects of substituent groups on methane adsorption in covalent organic frameworks. RSC Adv. 2014, 4, 15542–15551. [Google Scholar] [CrossRef]
- Sharma, A.; Babarao, R.; Medhekar, N.V.; Malani, A. Methane Adsorption and Separation in Slipped and Functionalized Covalent Organic Frameworks. Ind. Eng. Chem. Res. 2018, 57, 4767–4778. [Google Scholar] [CrossRef]
- Cao, D.; Zhang, X.; Chen, J.; Wang, W.; Yun, J. Optimization of single-walled carbon nanotube arrays for methane storage at room temperature. J. Phys. Chem. B 2003, 107, 13286–13292. [Google Scholar] [CrossRef]
- Arean, C.O.; Bonelli, B.; Delgado, M.R.; Garrone, E. Hydrogen storage via physisorption: The combined role of adsorption enthalpy and entropy. Turk. J. Chem. 2009, 33, 599–606. [Google Scholar]
- Ding, L.; Yazaydin, A.O. Hydrogen and methane storage in ultrahigh surface area Metal–Organic Frameworks. Microporous Mesoporous Mater. 2013, 182, 185–190. [Google Scholar] [CrossRef]
- Rowsell, J.L.C.; Yaghi, O.M. Effects of functionalization, catenation, and variation of the metal oxide and organic linking units on the low-pressure hydrogen adsorption properties of metal-organic frameworks. J. Am. Chem. Soc. 2006, 128, 1304–1315. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Bae, Y.S. Can metal-organic frameworks attain new DOE targets for on-board methane storage by increasing methane heat of adsorption? J. Phys. Chem. C 2014, 118, 19833–19841. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Myers, A.L. Optimum conditions for adsorptive storage. Langmuir 2006, 22, 1688–1700. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.S.; Snurr, R.Q. Optimal isosteric heat of adsorption for hydrogen storage and delivery using metal-organic frameworks. Microporous Mesoporous Mater. 2010, 132, 300–303. [Google Scholar] [CrossRef]
- Gedrich, K.; Senkovska, I.; Klein, N.; Stoeck, U.; Henschel, A.; Lohe, M.R.; Baburin, I.A.; Mueller, U.; Kaskel, S. A highly porous metal-organic framework with open nickel sites. Angew. Chem. Int. Ed. 2010, 49, 8489–8492. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, W.; Yildirim, T. High-capacity methane storage in metal-organic frameworks M2(dhtp): The important role of open metal sites. J. Am. Chem. Soc. 2009, 131, 4995–5000. [Google Scholar] [CrossRef]
- Gómez-Gualdrón, D.A.; Simon, C.M.; Lassman, W.; Chen, D.; Martin, R.L.; Haranczyk, M.; Farha, O.K.; Smit, B.; Snurr, R.Q. Impact of the strength and spatial distribution of adsorption sites on methane deliverable capacity in nanoporous materials. Chem. Eng. Sci. 2017, 159, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Tsivion, E.; Mason, J.A.; Gonzalez, M.I.; Long, J.R.; Head-Gordon, M. A computational study of CH4 storage in porous framework materials with metalated linkers: Connecting the atomistic character of CH4 binding sites to usable capacity. Chem. Sci. 2016, 7, 4503–4518. [Google Scholar] [CrossRef] [Green Version]
- Tsivion, E.; Head-Gordon, M. Methane Storage: Molecular Mechanisms Underlying Room-Temperature Adsorption in Zn 4 O(BDC) 3 (MOF-5). J. Phys. Chem. C 2017, 121, 12091–12100. [Google Scholar] [CrossRef] [Green Version]
- Dietzel, P.D.C.; Morita, Y.; Blom, R.; Fjellvåg, H. An in situ high-temperature single-crystal investigation of a dehydrated metal-organic framework compound and field-induced magnetization of one-dimensional metal-oxygen chains. Angew. Chem. Int. Ed. 2005, 44, 6354–6358. [Google Scholar] [CrossRef]
- Rosi, N.L.; Kim, J.; Eddaoudi, M.; Chen, B.; O’Keeffe, M.; Yaghi, O.M. Rod packings and metal-organic frameworks constructed from rod-shaped secondary building units. J. Am. Chem. Soc. 2005, 127, 1504–1518. [Google Scholar] [CrossRef] [PubMed]
- Wilmer, C.E.; Leaf, M.; Lee, C.Y.; Farha, O.K.; Hauser, B.G.; Hupp, J.T.; Snurr, R.Q. Large-scale screening of hypothetical metal-organic frameworks. Nat. Chem. 2012, 4, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Yu, L.; Ren, Q.; Lu, X.; Deng, S. Adsorption of CO2 and CH4 on a magnesium-based metal organic framework. J. Colloid Interface Sci. 2011, 353, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Thornton, A.W.; Nairn, K.M.; Hill, J.M.; Hill, A.J.; Hill, M.R. Metal-organic frameworks impregnated with magnesium-decorated fullerenes for methane and hydrogen storage. J. Am. Chem. Soc. 2009, 131, 10662–10669. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.S.; Rana, M.K.; Hwang, J.; Siegel, D.J. Thermodynamic screening of metal-substituted MOFs for carbon capture. Phys. Chem. Chem. Phys. 2013, 15, 4573–4581. [Google Scholar] [CrossRef] [Green Version]
- Getzschmann, J.; Senkovska, I.; Wallacher, D.; Tovar, M.; Fairen-Jimenez, D.; Düren, T.; Van Baten, J.M.; Krishna, R.; Kaskel, S. Methane storage mechanism in the metal-organic framework Cu 3(btc)2: An in situ neutron diffraction study. Microporous Mesoporous Mater. 2010, 136, 50–58. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, X.; Huang, H.; Zhang, Z.; Yildirim, T.; Zhou, W.; Xiang, S.; Chen, B. A microporous aluminum-based metal-organic framework for high methane, hydrogen, and carbon dioxide storage. Nano Res. 2020. [Google Scholar] [CrossRef]
- Ma, S.; Sun, D.; Simmons, J.M.; Collier, C.D.; Yuan, D.; Zhou, H.-C.C. Metal-organic framework from an anthracene derivative containing nanoscopic cages exhibiting high methane uptake. J. Am. Chem. Soc. 2008, 130, 1012–1016. [Google Scholar] [CrossRef]
- Dietzel, P.D.C.; Besikiotis, V.; Blom, R. Application of metal-organic frameworks with coordinatively unsaturated metal sites in storage and separation of methane and carbon dioxide. J. Mater. Chem. 2009, 19, 7362–7370. [Google Scholar] [CrossRef]
- Wu, H.; Simmons, J.M.; Liu, Y.; Brown, C.M.; Wang, X.S.; Shengqian, M.; Peterson, V.K.; Southon, P.D.; Kepert, C.J.; Zhou, H.C.; et al. Metal-organic frameworks with exceptionally high methane uptake: Where and how is methane stored? Chem. - A Eur. J. 2010, 16, 5205–5214. [Google Scholar] [CrossRef]
- Sculley, J.; Yuan, D.; Zhou, H.-C. The current status of hydrogen storage in metal–organic frameworks—Updated. Energy Environ. Sci. 2011, 4, 2721–2735. [Google Scholar] [CrossRef]
- Chen, L.; Morrison, C.A.; Düren, T. Improving predictions of gas adsorption in metal-organic frameworks with coordinatively unsaturated metal sites: Model potentials, ab initio parameterization, and gcmc simulations. J. Phys. Chem. C 2012, 116, 18899–18909. [Google Scholar] [CrossRef]
- Rana, M.K.; Koh, H.S.; Zuberi, H.; Siegel, D.J. Methane storage in metal-substituted metal-organic frameworks: Thermodynamics, usable capacity, and the impact of enhanced binding sites. J. Phys. Chem. C 2014, 118, 2929–2942. [Google Scholar] [CrossRef]
- Collins, S.P.; Perim, E.; Daff, T.D.; Skaf, M.S.; Galvão, D.S.; Woo, T.K. Idealized Carbon-Based Materials Exhibiting Record Deliverable Capacities for Vehicular Methane Storage. J. Phys. Chem. C 2019, 123, 1050–1058. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Gualdron, D.A.; Gutov, O.V.; Krungleviciute, V.; Borah, B.; Mondloch, J.E.; Hupp, J.T.; Yildirim, T.; Farha, O.K.; Snurr, R.Q. Computational design of metal-organic frameworks based on stable zirconium building units for storage and delivery of methane. Chem. Mater. 2014, 26, 5632–5639. [Google Scholar] [CrossRef] [Green Version]
- Getman, R.B.; Bae, Y.-S.; Wilmer, C.E.; Snurr, R.Q. Review and Analysis of Molecular Simulations of Methane, Hydrogen, and Acetylene Storage in Metal–Organic Frameworks. Chem. Rev. 2012, 112, 703–723. [Google Scholar] [CrossRef]
- Simon, C.M.; Kim, J.; Gomez-Gualdron, D.A.; Camp, J.S.; Chung, Y.G.; Martin, R.L.; Mercado, R.; Deem, M.W.; Gunter, D.; Haranczyk, M.; et al. The materials genome in action: Identifying the performance limits for methane storage. Energy Environ. Sci. 2015, 8, 1190–1199. [Google Scholar] [CrossRef]
- Mendoza-Cortes, J.L.; Pascal, T.A.; Goddard, W.A. Design of Covalent Organic Frameworks for Methane Storage. J. Phys. Chem. A 2011, 115, 13852–13857. [Google Scholar] [CrossRef] [Green Version]
- Mercado, R.; Fu, R.-S.; Yakutovich, A.V.; Talirz, L.; Haranczyk, M.; Smit, B. In Silico Design of 2D and 3D Covalent Organic Frameworks for Methane Storage Applications. Chem. Mater. 2018, 30, 5069–5086. [Google Scholar] [CrossRef]
- Loiseau, T.; Serre, C.; Huguenard, C.; Fink, G.; Taulelle, F.; Henry, M.; Bataille, T.; Férey, G. A Rationale for the Large Breathing of the Porous Aluminum Terephthalate (MIL-53) Upon Hydration. Chem. A Eur. J. 2004, 10, 1373–1382. [Google Scholar] [CrossRef]
- Boutin, A.; Coudert, F.-X.; Springuel-Huet, M.-A.; Neimark, A.V.; Férey, G.; Fuchs, A.H. The behavior of flexible MIL-53(Al) upon CH 4 and CO 2 adsorption. J. Phys. Chem. C 2010, 114, 22237–22244. [Google Scholar] [CrossRef] [Green Version]
- Kundu, T.; Shah, B.B.; Bolinois, L.; Zhao, D. Functionalization-Induced Breathing Control in Metal-Organic Frameworks for Methane Storage with High Deliverable Capacity. Chem. Mater. 2019, 31, 2842–2847. [Google Scholar] [CrossRef]
- Rallapalli, P.; Patil, D.; Prasanth, K.P.; Somani, R.S.; Jasra, R.V.; Bajaj, H.C. An alternative activation method for the enhancement of methane storage capacity of nanoporous aluminium terephthalate, MIL-53(Al). J. Porous Mater. 2010, 17, 523–528. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kang, J.H.; Kim, S.I.; Bae, Y.S. Extraordinarily large and stable methane delivery of MIL-53(Al) under LNG-ANG conditions. Chem. Eng. J. 2019, 365, 242–248. [Google Scholar] [CrossRef]
- Saha, D.; Fieback, T.M.; Tom, B. Characteristics of Methane Adsorption in Micro–Mesoporous Carbons at Low and Ultra-High Pressure. Energy Technol. 2016, 4, 1392–1400. [Google Scholar] [CrossRef]
- Verma, G.; Kumar, S.; Vardhan, H.; Ren, J.; Niu, Z.; Pham, T.; Wojtas, L.; Butikofer, S.; Echeverria Garcia, J.C.; Chen, Y.-S.; et al. A robust soc-MOF platform exhibiting high gravimetric uptake and volumetric deliverable capacity for on-board methane storage. Nano Res. 2020. [Google Scholar] [CrossRef]
- Mueller, U.; Schubert, M.; Teich, F.; Puetter, H.; Schierle-Arndt, K.; Pastré, J. Metal–organic frameworks—Prospective industrial applications. J. Mater. Chem. 2006, 16, 626–636. [Google Scholar] [CrossRef]
- Rubio-Martinez, M.; Avci-Camur, C.; Thornton, A.W.; Imaz, I.; Maspoch, D.; Hill, M.R. New synthetic routes towards MOF production at scale. Chem. Soc. Rev. 2017, 46, 3453–3480. [Google Scholar] [CrossRef] [Green Version]
- DeSantis, D.; Mason, J.A.; James, B.D.; Houchins, C.; Long, J.R.; Veenstra, M. Techno-economic Analysis of Metal–Organic Frameworks for Hydrogen and Natural Gas Storage. Energy Fuels 2017, 31, 2024–2032. [Google Scholar] [CrossRef]
- Rubio-Martinez, M.; Batten, M.P.; Polyzos, A.; Carey, K.C.; Mardel, J.I.; Lim, K.S.; Hill, M.R. Versatile, high quality and scalable continuous flow production of metal-organic frameworks. Sci. Rep. 2014, 4, 5443. [Google Scholar] [CrossRef]
- Dunne, P.W.; Walton, R.I. Towards scalable and controlled synthesis of metal–organic framework materials using continuous flow reactors. React. Chem. Eng. 2016, 1, 352–360. [Google Scholar] [CrossRef] [Green Version]
- Gaab, M.; Trukhan, N.; Maurer, S.; Gummaraju, R.; Müller, U. The progression of Al-based metal-organic frameworks—From academic research to industrial production and applications. Microporous Mesoporous Mater. 2012, 157, 131–136. [Google Scholar] [CrossRef]
- Silva, P.; Vilela, S.M.F.; Tomé, J.P.C.; Almeida Paz, F.A. Multifunctional metal-organic frameworks: From academia to industrial applications. Chem. Soc. Rev. 2015, 44, 6774–6803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoud, E.; Ali, L.; El Sayah, A.; Alkhatib, A.S.; Abdulsalam, H.; Juma, M.; Al-Muhtaseb, H.A. Implementing Metal-Organic Frameworks for Natural Gas Storage. Crystals 2019, 9, 406. [Google Scholar] [CrossRef] [Green Version]




| MOF | VP (cm3 g−1) a | BET (m2 g−1) | Uptake b (cm3 cm−3) | Delivery c (cm3 cm−3) | T (K) | P (bar) | Qst (kJ mol−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| UTSA-110a | 1.263 | 3241 | 241 | 190 | 273.15 | 65 | 14.5 | [66] |
| HKUST-1 | 0.78 | 1850 | 267 | 183 | 298 | 65 | 17.0 | [67] |
| MFM-112a | 1.62 | 3800 | 236 | 200 | 298 | 80 | 16.2 | [68] |
| NU-1501-Al | - | 7310 | 262 | 238 | 270 | 100 | - | [61] |
| Al-soc-MOF-1 | 2.3 | 5585 | - | 264 | 258 | 80 | - | [69] |
| PCN-14 | 0.85 | 2170 | 230 | 154 | 298 | 65 | 17.6 | [70] |
| NJU-Bai 43 | 1.22 | 3090 | 254 | 198 | 298 | 65 | 14.45 | [49] |
| PCN-61 | 1.36 | 3000 | 219 | 174 | 298 | 65 | - | [71] |
| ST-2 | 2.67 | 5660 | - | 303 | 283 | 200 | - | [50] |
| ST-2 | 2.67 | 5660 | - | 289 | 298 | 200 | 9.2 | [50] |
| Co(bpd) | - | 2911 | - | 197 | 298 | 65 | 8.3 | [72] |
| NU-135 | 1.02 | 2530 | 230 | 170 | 298 | 65 | 16.6 | [73] |
| NU-125 | 1.29 | 3120 | 228 | 180 | 298 | 58 | 15.5 | [74] |
| ZJU-25 | 1.183 | 2124 | 229 | 181 | 300 | 63 | 15.1 | [75] |
| MOF-519 | 0.938 | 2400 | 355 | 306 | 298 | 250 | 14.6 | [76] |
| MOF-5 | 1.4 | - | 214 | 182 | 298 | 65 | 12.3 | [13] |
| NJU-Bai 19 | 1.063 | 2803 | 246 | 185 | 298 | 65 | 14.8 | [77] |
| Cu-tbo-MOF 5 | 0.595 | 3971 | 216 | 175 | 298 | 80 | 20.4 | [78] |
| MIL-53(Al)–NH2 | 0.50 | 947 | 123 | 119 | 298 | 65 | - | [79] |
| MOF-905 | 1.34 | 3490 | 207 | 175 | 298 | 65 | 11.7 | [12] |
| Compound | VP (cm3 g−1) a | BET (m2 g−1) | Uptake b (cm3 cm−3) | Uptake (g g−1) | T (K) | P (bar) | Qst (kJ mol−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| COF | ||||||||
| COF-1 | 0.38 | 1230 | - | 0.109 | 298 | 100 | 24.9 | [80] |
| COF-5 | 1.17 | 1520 | - | 0.169 | 298 | 100 | 12.5 | [80] |
| COF-6 | 0.32 | 750 | - | 0.062 | 298 | 35 | 19 | [81] |
| COF-8 | 0.69 | 1350 | - | 0.08 | 298 | 35 | 12 | [81] |
| COF-10 | 1.44 | 1760 | - | 0.074 | 298 | 35 | 8.5 | [81] |
| COF-102 | 1.81 | 4940 | 255 | 0.284 | 298 | 100 | 9.6 | [80] |
| COF-103 | 2.05 | 5230 | 260 | 0.31 | 298 | 100 | 9.8 | [80] |
| COF-105 | 4.94 | 6450 | - | 0.405 | 298 | 100 | 8.5 | [80] |
| COF-108 | 5.40 | 6280 | - | 0.415 | 298 | 100 | 8.5 | [80] |
| HCPs | ||||||||
| HCP-1 | 0.54 | 1904 | - | 0.077 | 298 | 20 | - | [82] |
| HCP-2 | 0.36 | 1307 | - | 0.067 | 298 | 20 | - | [82] |
| HCP-3 | 0.32 | 963 | - | 0.049 | 298 | 20 | - | [82] |
| HCP-4 | 0.55 | 1366 | - | 0.065 | 298 | 20 | 20.8 | [82] |
| PPNs | ||||||||
| PPN-1 | 1249 | 0.45 | - | 0.076 | 295 | 35 | 18.1 | [83] |
| PPN-2 | 1764 | 1.26 | - | 0.098 | 295 | 35 | 16.4 | [83] |
| PPN-3 | 2840 | 1.7 | - | 0.122 | 295 | 35 | 15.2 | [83] |
| PPN-4 | 6461 | 3.04 | - | 0.389 | 295 | 55 | - | [83] |
| Carbon | VP (cm3 g−1) a | BET (m2 g−1) | Uptake b (cm3 cm−3) | Uptake (g g−1) | T (K) | P (bar) | Ref. |
|---|---|---|---|---|---|---|---|
| Kansai Maxsorb | 0.93 | 2671 | 127 | 0.26421 | 298 | 35 | [84] |
| NUCHAR-RGC | 1.1 | 1600 | 120.3 | 0.15117 | 299 | 35 | [85] |
| Darco® AC | 0.131 | 651.69 | 145.5 | 0.11302 | 298 | 35 | [86] |
| SMS-30 | 0.98 | 2860 | 120 | 0.21964 | 298 | 35 | [86] |
| SMS-22 | 0.84 | 2451 | 150 | 0.16996 | 298 | 35 | [86] |
| SMS-19 | 0.88 | 2552 | 149 | 0.16363 | 298 | 35 | [87] |
| HSAC-21 | 0.83 | 1466 | 147.2 | 0.16166 | 296 | 35 | [85] |
| HSAC-23 | 0.58 | 1784 | 146.4 | 0.16078 | 300 | 35 | [85] |
| C1050P | 0.4 | 932 | 127 | 0.09251 | 298 | 35 | [88] |
| LMA738 | - | - | 276 | - | 298 | 200 | [89] |
| MAXSORB III | 0.179 | 3140 | 60 | 0.40758 | 300 | 35 | [90] |
| Activated Carbon | - | 3052 | - | 0.32 | 298 | 35 | [91] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, E. Evolution of the Design of CH4 Adsorbents. Surfaces 2020, 3, 433-466. https://doi.org/10.3390/surfaces3030032
Mahmoud E. Evolution of the Design of CH4 Adsorbents. Surfaces. 2020; 3(3):433-466. https://doi.org/10.3390/surfaces3030032
Chicago/Turabian StyleMahmoud, Eyas. 2020. "Evolution of the Design of CH4 Adsorbents" Surfaces 3, no. 3: 433-466. https://doi.org/10.3390/surfaces3030032




