Surface Properties and Biological Activities on Bacteria Cells by Biobased Surfactants for Antifouling Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Biobased Surfactants Derived from Vegetable Oil
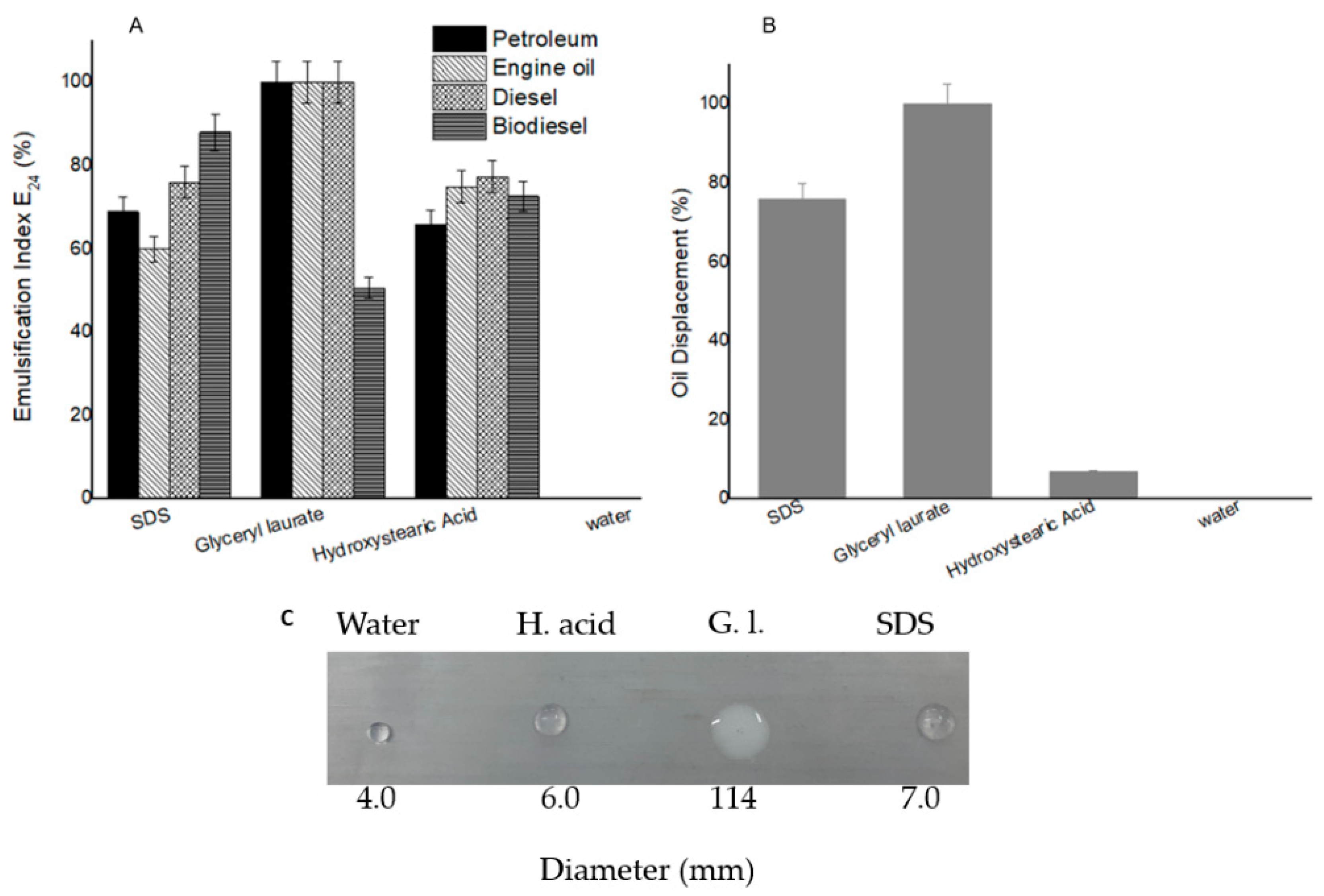
2.2. Emulsification Index
2.3. Oil Displacement and Drop Collapse Assay
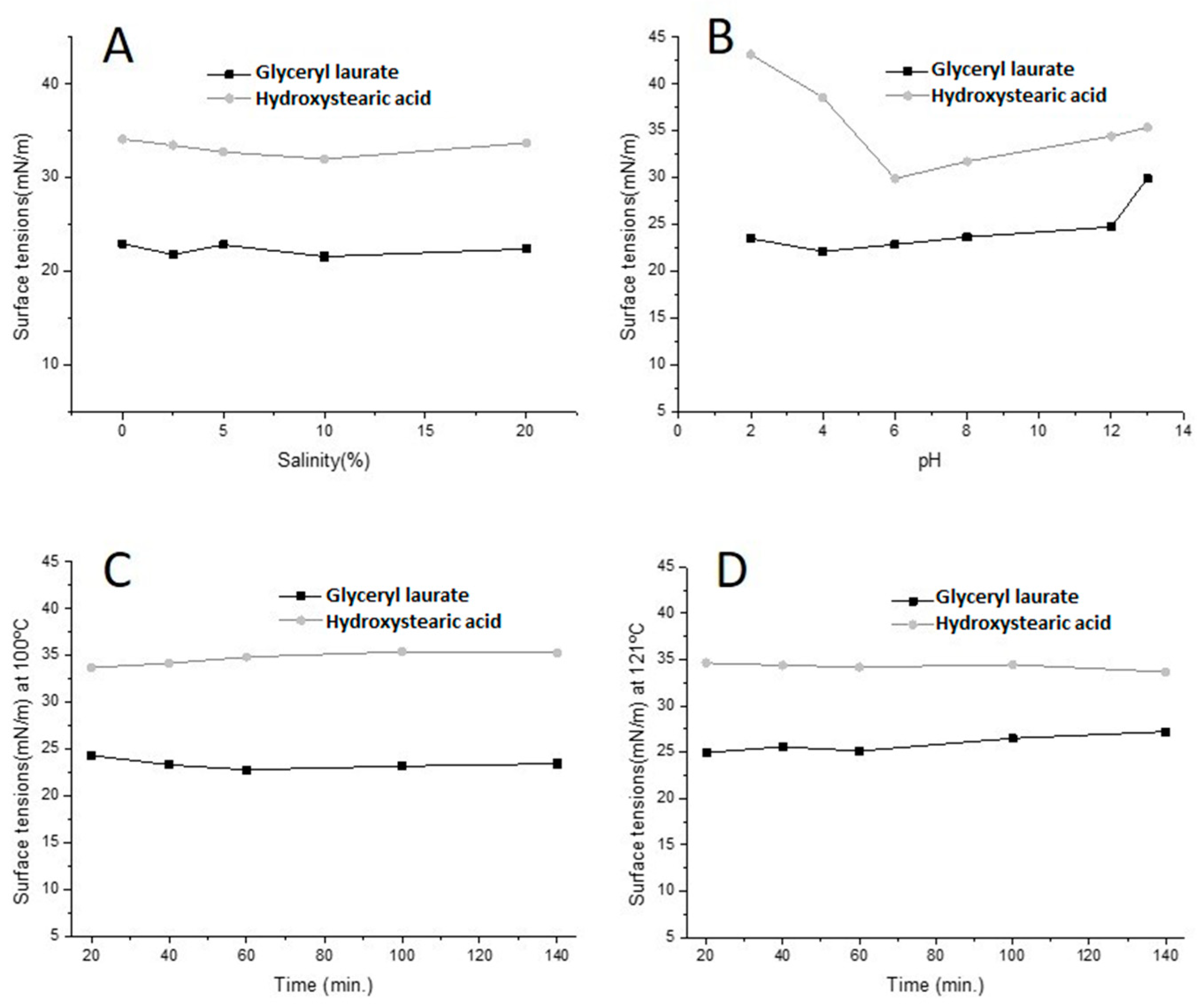
2.4. The pH, Salinity and Temperature Effect
2.5. Microorganisms: Growth Conditions
2.6. Antiadhesive Assay
2.7. Statistical Analysis
3. Results and Discussion
3.1. Surface Activities of Biobased Surfactants
3.2. Effects of pH, Temperature and Salinity on Surfactant against Tension
3.3. Antibacterial Bioactivity and Antiadhesive Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sherwood, J.; Clark, J.H.; Farmer, T.J.; Herrero-Davila, L.; Moity, L. Recirculation: A new concept to drive innovation in sustainable product design for bio-based products. Molecules 2017, 22, 48. [Google Scholar] [CrossRef]
- Escobar, N.; Laibach, N. Sustainability check for bio-based technologies: A review of process-based and life cycle approaches. Renew. Sustain. Energy Rev. 2021, 135, 110213. [Google Scholar] [CrossRef]
- Kandasamy, R.; Rajasekaran, M.; Venkatesan, S.K.; Uddin, M. New Trends in the Biomanufacturing of Green Surfactants: Biobased Surfactants and Biosurfactants. ACS Symp. Ser. 2019, 1329, 243–260. [Google Scholar] [CrossRef]
- Farias, C.B.B.; Almeida, F.C.G.; Silva, I.A.; Souza, T.C.; Meira, H.M.; Soares da Silva, R.d.C.F.; Luna, J.M.; Santos, V.A.; Converti, A.; Banat, I.M.; et al. Production of green surfactants: Market prospects. Electron. J. Biotechnol. 2021, 51, 28–39. [Google Scholar] [CrossRef]
- Nunes, A.; Marques, P.; Marto, J.; Ascenso, A.; Gonçalves, L.; Fitas, M.; Pinto, P.; Sotomayor, J.; Ribeiro, H.M. Sugar Surfactant-Based Shampoos. J. Surfactants Deterg. 2020, 23, 809–819. [Google Scholar] [CrossRef]
- Sivapathasekaran, C.; Sen, R. Origin, properties, production and purification of microbial surfactants as molecules with immense commercial potential. Tenside Surfactants Deterg. 2017, 54, 92–104. [Google Scholar] [CrossRef]
- Chowdhury, S.; Rakshit, A.; Acharjee, A.; Saha, B. Biodegradability and biocompatibility: Advancements in synthetic surfactants. J. Mol. Liq. 2021, 324, 115105. [Google Scholar] [CrossRef]
- Hayes, D.G.; Smith, G.A. Biobased Surfactants: Overview and Industrial State of the Art. In Biobased Surfactants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 3–38. ISBN 9780128127056. [Google Scholar]
- Cerrone, F.; Radivojevic, J.; Nikodinovic-Runic, J.; Walsh, M.; Kenny, S.T.; Babu, R.; O’Connor, K.E. Novel sodium alkyl-1,3-disulfates, anionic biosurfactants produced from microbial polyesters. Colloids Surf. B Biointerfaces 2019, 182, 110333. [Google Scholar] [CrossRef] [PubMed]
- Adu, S.A.; Naughton, P.J.; Marchant, R.; Banat, I.M. Microbial biosurfactants in cosmetic and personal skincare pharmaceutical formulations. Pharmaceutics 2020, 12, 1099. [Google Scholar] [CrossRef]
- Pinto, M.I.S.; Guerra, J.M.C.; Meira, H.M.; Sarubbo, L.A.; de Luna, J.M. A Biosurfactant from Candida bombicola: Its Synthesis, Characterization, and its Application as a Food Emulsions. Foods 2022, 11, 561. [Google Scholar] [CrossRef]
- Markande, A.R.; Patel, D.; Varjani, S. A review on biosurfactants: Properties, applications and current developments. Bioresour. Technol. 2021, 330, 124963. [Google Scholar] [CrossRef] [PubMed]
- Moldes, A.; Vecino, X.; Rodríguez-López, L.; Rincón-Fontán, M.; Cruz, J.M. Biosurfactants: The use of biomolecules in cosmetics and detergents. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 163–185. [Google Scholar]
- Jahan, R.; Bodratti, A.M.; Tsianou, M.; Alexandridis, P. Biosurfactants, natural alternatives to synthetic surfactants: Physicochemical properties and applications. Adv. Colloid Interface Sci. 2020, 275, 102061. [Google Scholar] [CrossRef] [PubMed]
- Tmáková, L.; Sekretár, S.; Schmidt, Š. Plant-derived surfactants as an alternative to synthetic surfactants: Surface and antioxidant activities. Chem. Pap. 2015, 70, 188–196. [Google Scholar] [CrossRef]
- Ramanathan, R. Animal-derived surfactants: Where are we? The evidence from randomized, controlled clinical trials. J. Perinatol. 2009, 29, S38–S43. [Google Scholar] [CrossRef]
- Ziaee, F.; Ziaee, M.; Taseidifar, M. Synthesis and application of a green surfactant for the treatment of water containing PFAS/hazardous metal ions. J. Hazard. Mater. 2021, 407, 124800. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Toor, S.S.; Brandão, J.; Pedersen, T.H.; Rosendahl, L.A. Optimized conversion of waste cooking oil into ecofriendly bio-based polymeric surfactant- A solution for enhanced oil recovery and green fuel compatibility. J. Clean. Prod. 2021, 294, 126214. [Google Scholar] [CrossRef]
- Ruiz, A.; Pinazo, A.; Pérez, L.; Manresa, A.; Marqués, A.M. Green Catanionic Gemini Surfactant-Lichenysin Mixture: Improved Surface, Antimicrobial, and Physiological Properties. ACS Appl. Mater. Interfaces 2017, 9, 22121–22131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fan, X.; Solaiman, D.K.Y.; Ashby, R.D.; Liu, Z.; Mukhopadhyay, S.; Yan, R. Inactivation of Escherichia coli O157:H7 in vitro and on the surface of spinach leaves by biobased antimicrobial surfactants. Food Control 2016, 60, 158–165. [Google Scholar] [CrossRef]
- Kumari, A.; Guliani, A.; Shukla, A.K.; Kumar, S.; Acharya, A. Green surfactant based synthesis of curcumin loaded poly lactic-co-glycolic acid nanoparticles with enhanced solubility, photo-stability and anti-biofilm activity. J. Drug Deliv. Sci. Technol. 2020, 59, 101884. [Google Scholar] [CrossRef]
- Labena, A.; Hegazy, M.A.; Sami, R.M.; Hozzein, W.N. Multiple applications of a novel cationic gemini surfactant: Anti-microbial, anti-biofilm, biocide, salinity corrosion inhibitor, and biofilm dispersion (Part II). Molecules 2020, 25, 1348. [Google Scholar] [CrossRef] [Green Version]
- Hess, D.J.; Henry-Stanley, M.J.; Wells, C.L. The Natural Surfactant Glycerol Monolaurate Significantly Reduces Development of Staphylococcus aureus and Enterococcus faecalis Biofilms. Surg. Infect. 2015, 16, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Czuba, U.; Quintana, R.; Lassaux, P.; Bombera, R.; Ceccone, G.; Bañuls-Ciscar, J.; Moreno-Couranjou, M.; Detrembleur, C.; Choquet, P. Anti-biofouling activity of Ranaspumin-2 bio-surfactant immobilized on catechol-functional PMMA thin layers prepared by atmospheric plasma deposition. Colloids Surf. B Biointerfaces 2019, 178, 120–128. [Google Scholar] [CrossRef]
- Song, B.; Wang, Y.-Z.; Wang, G.-Y.; Liu, G.-L.; Li, W.-Z.; Yan, F. The lipopeptide 6-2 produced by Bacillus amyloliquefaciens anti-CA has potent activity against the biofilm-forming organisms. Mar. Pollut. Bull. 2016, 108, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Alemán-Vega, M.; Sánchez-Lozano, I.; Hernández-Guerrero, C.J.; Hellio, C.; Quintana, E.T. Exploring antifouling activity of biosurfactants producing marine bacteria isolated from gulf of California. Int. J. Mol. Sci. 2020, 21, 6068. [Google Scholar] [CrossRef]
- Henderson, P. Fouling and Antifouling in Other Industries- Power Stations, Desalination Plants- Drinking Water Supplies and Sensors. In Biofouling; Dürr, S., Thomason, J.C., Eds.; Wiley-Blackwell: Oxford, UK, 2010; pp. 288–305. ISBN 9781444315462. [Google Scholar]
- de Carvalho, C.C.C.R. Marine Biofilms: A Successful Microbial Strategy with Economic Implications. Front. Mar. Sci. 2018, 5, 126. [Google Scholar] [CrossRef]
- Little, B.J.; Lee, J.S.; Ray, R.I. The influence of marine biofilms on corrosion: A concise review. Electrochim. Acta 2008, 54, 2–7. [Google Scholar] [CrossRef]
- Bhoj, Y.; Tharmavaram, M.; Rawtani, D. A comprehensive approach to antifouling strategies in desalination, marine environment, and wastewater treatment. Chem. Phys. Impact 2021, 2, 100008. [Google Scholar] [CrossRef]
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling technology—past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat. 2004, 50, 75–104. [Google Scholar] [CrossRef]
- Fryer, P.J.; Robbins, P.T.; Asteriadou, I.K. Current Knowledge in Hygienic Design: Can We Minimise Fouling and Speed Cleaning? Food Eng. Ser. 2013, 1, 209–227. [Google Scholar] [CrossRef]
- Simões, M.; Simões, L.C.; Vieira, M.J. A review of current and emergent biofilm control strategies. LWT-Food Sci. Technol. 2010, 43, 573–583. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.G.C.; Sarubbo, L. Synthetic and biological surfactants used to mitigate biofouling on industrial facilities surfaces. Biointerface Res. Appl. Chem. 2022, 12, 2560–2585. [Google Scholar] [CrossRef]
- Percival, S.L.; Mayer, D.; Kirsner, R.S.; Schultz, G.; Weir, D.; Roy, S.; Alavi, A.; Romanelli, M. Surfactants: Role in biofilm management and cellular behaviour. Int. Wound J. 2019, 16, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Satpute, S.K.; Banpurkar, A.G.; Banat, I.M.; Sangshetti, J.N.; Patil, R.H.; Gade, W.N. Multiple Roles of Biosurfactants in Biofilms. Curr. Pharm. Des. 2016, 22, 1429–1448. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhao, F.; Wang, J.; Zhong, N. Biofilm formation and control strategies of foodborne pathogens: Food safety perspectives. RSC Adv. 2017, 7, 36670–36683. [Google Scholar] [CrossRef]
- Ghilini, F.; Pissinis, D.E.; Miñán, A.; Schilardi, P.L.; Diaz, C. How functionalized surfaces can inhibit bacterial adhesion and viability. ACS Biomater. Sci. Eng. 2019, 5, 4920–4936. [Google Scholar] [CrossRef] [PubMed]
- Bucci, A.R.; Marcelino, L.; Mendes, R.K.; Etchegaray, A. The antimicrobial and antiadhesion activities of micellar solutions of surfactin, CTAB and CPCl with terpinen-4-ol: Applications to control oral pathogens. World J. Microbiol. Biotechnol. 2018, 34, 86. [Google Scholar] [CrossRef]
- Ceresa, C.; Fracchia, L.; Williams, M.; Banat, I.M.; Díaz De Rienzo, M.A. The effect of sophorolipids against microbial biofilms on medical-grade silicone. J. Biotechnol. 2020, 309, 34–43. [Google Scholar] [CrossRef]
- Rebello, S.; Asok, A.K.; Mundayoor, S.; Jisha, M.S. Surfactants: Toxicity, remediation and green surfactants. Environ. Chem. Lett. 2014, 12, 275–287. [Google Scholar] [CrossRef]
- Kabara, J.J.; Swieczkowski, D.M.; Conley, A.J.; Truant, J.P. Fatty Acids and Derivatives as Antimicrobial Agents. Antimicrob. Agents Chemother. 1972, 2, 23. [Google Scholar] [CrossRef]
- Nitbani, F.O.; Tjitda, P.J.P.; Nurohmah, B.A.; Wogo, H.E. Preparation of Fatty Acid and Monoglyceride from Vegetable Oil. J. Oleo Sci. 2020, 69, 277–295. [Google Scholar] [CrossRef] [Green Version]
- Abraham, M.E.; Benenati, R.F. Kinetics and mechanism of the epoxidation of unsaturated fatty acids. AIChE J. 1972, 18, 807–811. [Google Scholar] [CrossRef]
- Wai, P.T.; Jiang, P.; Shen, Y.; Zhang, P.; Gu, Q.; Leng, Y. Catalytic developments in the epoxidation of vegetable oils and the analysis methods of epoxidized products. RSC Adv. 2019, 9, 38119–38136. [Google Scholar] [CrossRef] [PubMed]
- Yunfei, H.; Yazhuo, S.; Honglai, L.; Dominique, L.; Anniina, S. Surfactant Adsorption onto Interfaces: Measuring the Surface Excess in Time. Langmuir 2012, 28, 3146–3151. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.G.; Goldenberg, B.G. Surface-active agents from two Bacillus species. Appl. Environ. Microbiol. 1987, 53, 224–229. [Google Scholar] [CrossRef]
- Morikawa, M.; Hirata, Y.; Imanaka, T. A study on the structure-function relationship of lipopeptide biosurfactants. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2000, 1488, 211–218. [Google Scholar] [CrossRef]
- Gudiña, E.J.; Fernandes, E.C.; Teixeira, J.A.; Rodrigues, L.R. Antimicrobial and anti-adhesive activities of cell-bound biosurfactant from Lactobacillus agilis CCUG31450. RSC Adv. 2015, 5, 90960–90968. [Google Scholar] [CrossRef]
- Karmakar, G.; Ghosh, P.; Sharma, B.K. Chemically modifying vegetable oils to prepare green lubricants. Lubricants 2017, 5, 44. [Google Scholar] [CrossRef]
- Rodríguez-López, L.; Rincón-Fontán, M.; Vecino, X.; Cruz, J.M.; Moldes, A.B. Biological Surfactants vs. Polysorbates: Comparison of Their Emulsifier and Surfactant Properties. Tenside Surfactants Deterg. 2018, 55, 273–280. [Google Scholar] [CrossRef]
- Tugrul, T.; Cansunar, E. Detecting surfactant-producing microorganisms by the drop-collapse test. World J. Microbiol. Biotechnol. 2005, 21, 851–853. [Google Scholar] [CrossRef]
- Walter, V.; Syldatk, C.; Hausmann, R. Screening concepts for the isolation of biosurfactant producing microorganisms. Adv. Exp. Med. Biol. 2010, 672, 1–13. [Google Scholar] [CrossRef]
- Vecino Bello, X.; Devesa-Rey, R.; Cruz, J.M.; Moldes, A.B. Study of the Synergistic Effects of Salinity, pH, and Temperature on the Surface-Active Properties of Biosurfactants Produced by Lactobacillus pentosus. J. Agric. Food Chem. 2012, 60, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, A.; Ricker, E.B.; Nuxoll, E. Thermal mitigation of Pseudomonas aeruginosa biofilms. Biofouling 2015, 31, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Neu, T.R. Significance of bacterial surface-active compounds in interaction of bacteria with interfaces. Microbiol. Rev. 1996, 60, 151–166. [Google Scholar] [CrossRef]
- Khalid, H.F.; Tehseen, B.; Sarwar, Y.; Hussain, S.Z.; Khan, W.S.; Raza, Z.A.; Bajwa, S.Z.; Kanaras, A.G.; Hussain, I.; Rehman, A. Biosurfactant coated silver and iron oxide nanoparticles with enhanced anti-biofilm and anti-adhesive properties. J. Hazard. Mater. 2019, 364, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Padmapriya, B.; Suganthi, S. Antimicrobial and anti adhesive activity of purified biosurfactants produced by candida species. Middle East J. Sci. Res. 2013, 14, 1359–1369. [Google Scholar] [CrossRef]
- Allan, V.J.M.; Callow, M.E.; Macaskie, L.E.; Paterson-Beedle, M. Biofilm Highlights; Flemming, H.-C., Wingender, J., Szewzyk, U., Eds.; Springer Series on Biofilms; Springer: Berlin/Heidelberg, Germany, 2011; Volume 5, ISBN 978-3-642-19939-4. [Google Scholar]
- Costerton, J.W. Introduction to biofilm. Int. J. Antimicrob. Agents 1999, 11, 217–221. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial Life on Surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Lopez, D.; Vlamakis, H.; Kolter, R. Biofilms. Cold Spring Harb. Perspect. Biol. 2010, 2, a000398. [Google Scholar] [CrossRef]
- Poli, A.; Anzelmo, G.; Nicolaus, B. Bacterial Exopolysaccharides from Extreme Marine Habitats: Production, Characterization and Biological Activities. Mar. Drugs 2010, 8, 1779–1802. [Google Scholar] [CrossRef]
- Azzam, E.M.S.; Zaki, M.F. Surface and antibacterial activity of synthesized nonionic surfactant assembled on metal nanoparticles. Egypt. J. Pet. 2016, 25, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Paraszkiewicz, K.; Moryl, M.; Płaza, G.; Bhagat, D.; Satpute, S.K.; Bernat, P. Surfactants of microbial origin as antibiofilm agents. Int. J. Environ. Health Res. 2021, 31, 401–420. [Google Scholar] [CrossRef] [PubMed]
- Mueller, E.A.; Schlievert, P.M. Non-Aqueous Glycerol Monolaurate Gel Exhibits Antibacterial and Anti-Biofilm Activity against Gram-Positive and Gram-Negative Pathogens. PLoS ONE 2015, 10, e0120280. [Google Scholar] [CrossRef]
- Meylheuc, T.; Methivier, C.; Renault, M.; Herry, J.-M.; Pradier, C.-M.; Bellon-Fontaine, M.N. Adsorption on stainless steel surfaces of biosurfactants produced by gram-negative and gram-positive bacteria: Consequence on the bioadhesive behavior of Listeria monocytogenes. Colloids Surf. B Biointerfaces 2006, 52, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.G.C.; Medeiros, A.O.; Almeida, D.G.; Meire, H.M.; Almeida, F.C.; Soares da Silva, R.C.F.; Sarubbo, L.A. Antifouling protection of surfaces immersed in marine environment by natural surfactants as bioactive contained in coating based on natural resin. Chem. Eng. Trans. 2019, 74, 1507–1512. [Google Scholar] [CrossRef]
- Faÿ, F.; Carteau, D.; Linossier, I.; Delbury, M.; Vallée-Réhel, K. Joint-action of antifouling substances in copper-free paints. Colloids Surf. B Biointerfaces 2013, 102, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-J.; Yu, H.-Y.; Wang, S.-Y.; Xu, Z.-K. Improvement of antifouling characteristics in a bioreactor of polypropylene microporous membrane by the adsorption of Tween 20. J. Environ. Sci. 2007, 19, 1461–1465. [Google Scholar] [CrossRef]
- Cowie, P.R.; Smith, M.J.; Hannah, F.; Cowling, M.J.; Hodgkeiss, T. The prevention of microfouling and macrofouling on hydrogels impregnated with either Arquad 2C-75® or benzalkonium chloride. Biofouling 2006, 22, 195–207. [Google Scholar] [CrossRef]
- López-Galindo, C.; Casanueva, J.F.; Nebot, E. Efficacy of different antifouling treatments for seawater cooling systems. Biofouling 2010, 26, 923–930. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, M.d.G.C.; da Silva, M.E.P.; de Medeiros, A.O.; Meira, H.M.; Sarubbo, L.A. Surface Properties and Biological Activities on Bacteria Cells by Biobased Surfactants for Antifouling Applications. Surfaces 2022, 5, 383-394. https://doi.org/10.3390/surfaces5030028
da Silva MdGC, da Silva MEP, de Medeiros AO, Meira HM, Sarubbo LA. Surface Properties and Biological Activities on Bacteria Cells by Biobased Surfactants for Antifouling Applications. Surfaces. 2022; 5(3):383-394. https://doi.org/10.3390/surfaces5030028
Chicago/Turabian Styleda Silva, Maria da Gloria C., Maria Eduarda P. da Silva, Anderson O. de Medeiros, Hugo M. Meira, and Leonie A. Sarubbo. 2022. "Surface Properties and Biological Activities on Bacteria Cells by Biobased Surfactants for Antifouling Applications" Surfaces 5, no. 3: 383-394. https://doi.org/10.3390/surfaces5030028






