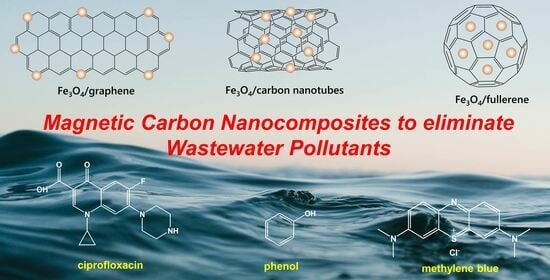
The Use of Magnetic Porous Carbon Nanocomposites for the Elimination of Organic Pollutants from Wastewater
Abstract
:1. Introduction
2. The Application of Magnetic Porous Carbon Nanocomposites
2.1. Magnetic Fullerene Nanocomposites
2.2. Magnetic Carbon-Dot Nanocomposites
2.3. Magnetic Carbon Nanotube Nanocomposites
2.4. Magnetic Graphite Nanocomposites
2.5. Magnetic Graphene Nanocomposites
2.6. Magnetic Graphene Oxide Nanocomposites
3. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Narain-Ford, D.M.; Bartholomeus, R.P.; Dekker, S.C.; van Wezel, A.P. Natural Purification Through Soils: Risks and Opportunities of Sewage Effluent Reuse in Sub-surface Irrigation. In Reviews of Environmental Contamination and Toxicology; Springer: Berlin/Heidelberg, Germany, 2020; Volume 250, pp. 85–117. [Google Scholar]
- Pham-Duc, P.; Nguyen-Viet, H.; Hattendorf, J.; Cam, P.D.; Zurbrügg, C.; Zinsstag, J.; Odermatt, P. Diarrhoeal diseases among adult population in an agricultural community Hanam province, Vietnam, with high wastewater and excreta re-use. BMC Public Health 2014, 14, 1–14. [Google Scholar] [CrossRef]
- Yang, J.; Jia, R.S.; Gao, Y.L.; Wang, W.F.; Cao, P.Q. The reliability evaluation of reclaimed water reused in power plant project. IOP Conf. Ser. Earth Environ. Sci. 2017, 100, 012189. [Google Scholar] [CrossRef]
- Clemmens, A.J.; Allen, R.G.; Burt, C.M. Technical concepts related to conservation of irrigation and rainwater in agricultural systems. Water Resour. Res. 2008, 44, W00E03. [Google Scholar] [CrossRef]
- Sato, T.; Qadir, M.; Yamamoto, S.; Endo, T.; Zahoor, A. Global, regional, and country level need for data on wastewater generation, treatment, and use. Agric. Water Manag. 2013, 130, 1–13. [Google Scholar] [CrossRef]
- Dickin, S.K.; Schuster-Wallace, C.J.; Qadir, M.; Pizzacalla, K. A review of health risks and pathways for exposure to wastewater Use in Agriculture. Environ. Health Perspect. 2016, 124, 900–909. [Google Scholar] [CrossRef]
- Duan, B.; Zhang, W.; Zheng, H.; Wu, C.; Zhang, Q.; Bu, Y. Comparison of health risk assessments of heavy metals and as in sewage sludge from wastewater treatment plants (WWTPs) for adults and children in the urban district of Taiyuan, China. Int. J. Environ. Res. Public Health 2017, 14, 1194. [Google Scholar] [CrossRef]
- Carr, R. Who guidelines for safe wastewater use—More than just numbers. Irrig. Drain. 2005, 54 (Suppl. S1), S103–S111. [Google Scholar] [CrossRef]
- Pedrero, F.; Kalavrouziotis, I.; Alarcón, J.J.; Koukoulakis, P.; Asano, T. Use of treated municipal wastewater in irrigated agriculture—Review of some practices in Spain and Greece. Agric. Water Manag. 2010, 97, 1233–1241. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Gkotsis, P.; Castellana, M.; Cartechini, F.; Zouboulis, A.I. Production of demineralized water for use in thermal power stations by advanced treatment of secondary wastewater effluent. J. Environ. Manag. 2017, 190, 132–139. [Google Scholar] [CrossRef]
- Chaoua, S.; Boussaa, S.; El Gharmali, A.; Boumezzough, A. Impact of irrigation with wastewater on accumulation of heavy metals in soil and crops in the region of Marrakech in Morocco. J. Saudi Soc. Agric. Sci. 2019, 18, 429–436. [Google Scholar] [CrossRef]
- Poustie, A.; Yang, Y.; Verburg, P.; Pagilla, K.; Hanigan, D. Reclaimed wastewater as a viable water source for agricultural irrigation: A review of food crop growth inhibition and promotion in the context of environmental change. Sci. Total Environ. 2020, 739, 139756. [Google Scholar] [CrossRef] [PubMed]
- Balkhair, K.S. Microbial contamination of vegetable crop and soil profile in arid regions under controlled application of domestic wastewater. Saudi J. Biol. Sci. 2016, 23, S83–S92. [Google Scholar] [CrossRef]
- Contreras, J.D.; Meza, R.; Siebe, C.; Rodríguez-Dozal, S.; López-Vidal, Y.A.; Castillo-Rojas, G.; Amieva, R.I.; Solano-Gálvez, S.G.; Mazari-Hiriart, M.; Silva-Magaña, M.A.; et al. Health risks from exposure to untreated wastewater used for irrigation in the Mezquital Valley, Mexico: A 25-year update. Water Res. 2017, 123, 834–850. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sawant, M.; Kamble, S.J.; Herlekar, M.; Starkl, M.; Aymerich, E.; Kazmi, A. Performance evaluation of a decentralized wastewater treatment system in India. Environ. Sci. Pollut. Res. 2019, 26, 21172–21188. [Google Scholar] [CrossRef]
- Ungureanu, N.; Vladut, V.; Dincă, M.; Zăbavă, B.-Ș.; Vlăduţ, V. Reuse of wastewater for irrigation. A sustainable practice in arid and semi-arid regions. In Proceedings of the 7th International Conference on Thermal Equipment, Renewable Energy and Rural Development (TE-RE-RD), Drobeta-Turnu Severin, Romania, 31 May–2 June 2018. [Google Scholar]
- Angelakis, A.N.; Snyder, S.A. Wastewater treatment and reuse: Past, present, and future. Water 2015, 7, 4887–4895. [Google Scholar] [CrossRef]
- Jeong, H.; Kim, H.; Jang, T. Irrigation water quality standards for indirect wastewater reuse in agriculture: A contribution toward sustainable wastewater reuse in South korea. Water 2016, 8, 169. [Google Scholar] [CrossRef]
- Kimura, K.; Honoki, D.; Sato, T. Effective physical cleaning and adequate membrane flux for direct membrane filtration (DMF) of municipal wastewater: Up-concentration of organic matter for efficient energy recovery. Sep. Purif. Technol. 2017, 181, 37–43. [Google Scholar] [CrossRef]
- Ding, W.; Cheng, S.; Yu, L.; Huang, H. Effective swine wastewater treatment by combining microbial fuel cells with flocculation. Chemosphere 2017, 182, 567–573. [Google Scholar] [CrossRef]
- Rott, E.; Minke, R.; Steinmetz, H. Removal of phosphorus from phosphonate-loaded industrial wastewaters via precipitation/flocculation. J. Water Process Eng. 2017, 17, 188–196. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chiang, C.C.; Thi Nguyen, M.L.; Lay, C.H. Enhancement of fermentative biohydrogen production from textile desizing wastewater via coagulation-pretreatment. Int. J. Hydrogen Energy 2017, 42, 12153–12158. [Google Scholar] [CrossRef]
- Cristóvão, R.O.; Gonçalves, C.; Botelho, C.M.; Martins, R.J.E.; Boaventura, R.A.R. Chemical oxidation of fish canning wastewater by Fenton’s reagent. J. Environ. Chem. Eng. 2014, 2, 2372–2376. [Google Scholar] [CrossRef]
- Li, R.; Yang, C.; Chen, H.; Zeng, G.; Yu, G.; Guo, J. Removal of triazophos pesticide from wastewater with Fenton reagent. J. Hazard. Mater. 2009, 167, 1028–1032. [Google Scholar] [CrossRef] [PubMed]
- Defaei, M.; Taheri-Kafrani, A.; Miroliaei, M.; Yaghmaei, P. Improvement of stability and reusability of α-amylase immobilized on naringin functionalized magnetic nanoparticles: A robust nanobiocatalyst. Int. J. Biol. Macromol. 2018, 113, 354–360. [Google Scholar] [CrossRef]
- Berberidou, C.; Kitsiou, V.; Lambropoulou, D.A.; Antoniadis, A.; Ntonou, E.; Zalidis, G.C.; Poulios, I. Evaluation of an alternative method for wastewater treatment containing pesticides using solar photocatalytic oxidation and constructed wetlands. J. Environ. Manag. 2017, 195, 133–139. [Google Scholar] [CrossRef]
- Patel, H.; Vashi, R.T. Batch Adsorption Treatment of Textile Wastewater. In Characterization and Treatment of Textile Wastewater; Elsevier: Amsterdam, The Netherlands, 2015; pp. 111–125. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Fu, J.; Lazaridis, N.K.; Bikiaris, D.N.; Matis, K.A. New approaches on the removal of pharmaceuticals from wastewaters with adsorbent materials. J. Mol. Liq. 2015, 209, 87–93. [Google Scholar] [CrossRef]
- de Caprariis, B.; De Filippis, P.; Hernandez, A.D.; Petrucci, E.; Petrullo, A.; Scarsella, M.; Turchi, M. Pyrolysis wastewater treatment by adsorption on biochars produced by poplar biomass. J. Environ. Manag. 2017, 197, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Iglesias, O.; Collado, S.; Oulego, P.; Díaz, M. Graphene-family nanomaterials in wastewater treatment plants. Chem. Eng. J. 2017, 313, 121–135. [Google Scholar] [CrossRef]
- Delkash, M.; Ebrazi Bakhshayesh, B.; Kazemian, H. Using zeolitic adsorbents to cleanup special wastewater streams: A review. Microporous Mesoporous Mater. 2015, 214, 224–241. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Umejuru, E.C.; Mashifana, T.; Kandjou, V.; Amani-Beni, M.; Sadeghifar, H.; Fayazi, M.; Karimi-Maleh, H.; Sithole, N.T. Application of zeolite based nanocomposites for wastewater remediation: Evaluating newer and environmentally benign approaches. Environ. Res. 2023, 231, 116073. [Google Scholar] [CrossRef]
- Bialczyk, J.; Natkański, P.; Kuśtrowski, P.; Czaja-Prokop, U.; Bober, B.; Kaminski, A. Removal of cyanobacterial anatoxin-a from water by natural clay adsorbents. Appl. Clay Sci. 2017, 148, 17–24. [Google Scholar] [CrossRef]
- Khan, T.A.; Dahiya, S.; Ali, I. Use of kaolinite as adsorbent: Equilibrium, dynamics and thermodynamic studies on the adsorption of Rhodamine B from aqueous solution. Appl. Clay Sci. 2012, 69, 58–66. [Google Scholar] [CrossRef]
- Magriotis, Z.M.; Leal, P.V.B.; de Sales, P.F.; Papini, R.M.; Viana, P.R.M.; Arroyo, P.A. A comparative study for the removal of mining wastewater by kaolinite, activated carbon and beta zeolite. Appl. Clay Sci. 2014, 91–92, 55–62. [Google Scholar] [CrossRef]
- Struijk, M.; Rocha, F.; Detellier, C. Novel thio-kaolinite nanohybrid materials and their application as heavy metal adsorbents in wastewater. Appl. Clay Sci. 2017, 150, 192–201. [Google Scholar] [CrossRef]
- Gil, A.; Assis, F.C.C.; Albeniz, S.; Korili, S.A. Removal of dyes from wastewaters by adsorption on pillared clays. Chem. Eng. J. 2011, 168, 1032–1040. [Google Scholar] [CrossRef]
- Georgescu, A.M.; Nardou, F.; Zichil, V.; Nistor, I.D. Adsorption of lead(II) ions from aqueous solutions onto Cr-pillared clays. Appl. Clay Sci. 2018, 152, 44–50. [Google Scholar] [CrossRef]
- Rodrigues Mota, T.L.; Marques de Oliveira, A.P.; Nunes, E.H.M.; Houmard, M. Simple process for preparing mesoporous sol-gel silica adsorbents with high water adsorption capacities. Microporous Mesoporous Mater. 2017, 253, 177–182. [Google Scholar] [CrossRef]
- Banaei, A.; Samadi, S.; Karimi, S.; Vojoudi, H.; Pourbasheer, E.; Badiei, A. Synthesis of silica gel modified with 2,2′-(hexane-1,6-diylbis(oxy)) dibenzaldehyde as a new adsorbent for the removal of Reactive Yellow 84 and Reactive Blue 19 dyes from aqueous solutions: Equilibrium and thermodynamic studies. Powder Technol. 2017, 319, 60–70. [Google Scholar] [CrossRef]
- Asfaram, A.; Ghaedi, M.; Ahmadi Azqhandi, M.H.; Goudarzi, A.; Hajati, S. Ultrasound-assisted binary adsorption of dyes onto Mn@ CuS/ZnS-NC-AC as a novel adsorbent: Application of chemometrics for optimization and modeling. J. Ind. Eng. Chem. 2017, 54, 377–388. [Google Scholar] [CrossRef]
- Mazaheri, H.; Ghaedi, M.; Asfaram, A.; Hajati, S. Performance of CuS nanoparticle loaded on activated carbon in the adsorption of methylene blue and bromophenol blue dyes in binary aqueous solutions: Using ultrasound power and optimization by central composite design. J. Mol. Liq. 2016, 219, 667–676. [Google Scholar] [CrossRef]
- Choleva, T.G.; Gatselou, V.A.; Tsogas, G.Z.; Giokas, D.L. Intrinsic peroxidase-like activity of rhodium nanoparticles, and their application to the colorimetric determination of hydrogen peroxide and glucose. Microchim. Acta 2018, 185, 22. [Google Scholar] [CrossRef]
- Novais, R.M.; Ascensão, G.; Tobaldi, D.M.; Seabra, M.P.; Labrincha, J.A. Biomass fly ash geopolymer monoliths for effective methylene blue removal from wastewaters. J. Clean. Prod. 2018, 171, 783–794. [Google Scholar] [CrossRef]
- Habibzadeh, M.; Chaibakhsh, N.; Naeemi, A.S. Optimized treatment of wastewater containing cytotoxic drugs by living and dead biomass of the freshwater microalga, Chlorella vulgaris. Ecol. Eng. 2018, 111, 85–93. [Google Scholar] [CrossRef]
- Vukelic, D.; Boskovic, N.; Agarski, B.; Radonic, J.; Budak, I.; Pap, S.; Sekulic, M.T. Eco-design of a low-cost adsorbent produced from waste cherry kernels. J. Clean. Prod. 2018, 174, 1620–1628. [Google Scholar] [CrossRef]
- Anjum, M.; Miandad, R.; Waqas, M.; Gehany, F.; Barakat, M.A. Remediation of wastewater using various nano-materials. Arab. J. Chem. 2019, 12, 4897–4919. [Google Scholar] [CrossRef]
- Tripathi, A.; Rawat Ranjan, M. Heavy Metal Removal from Wastewater Using Low Cost Adsorbents. J. Bioremediat. Biodegrad. 2015, 6, 315. [Google Scholar] [CrossRef]
- Abbaszadeh, S.; Wan Alwi, S.R.; Webb, C.; Ghasemi, N.; Muhamad, I.I. Treatment of lead-contaminated water using activated carbon adsorbent from locally available papaya peel biowaste. J. Clean. Prod. 2016, 118, 210–222. [Google Scholar] [CrossRef]
- Reck, I.M.; Paixão, R.M.; Bergamasco, R.; Vieira, M.F.; Vieira, A.M.S. Removal of tartrazine from aqueous solutions using adsorbents based on activated carbon and Moringa oleifera seeds. J. Clean. Prod. 2018, 171, 85–97. [Google Scholar] [CrossRef]
- Lladó, J.; Gil, R.R.; Lao-Luque, C.; Solé-Sardans, M.; Fuente, E.; Ruiz, B. Highly microporous activated carbons derived from biocollagenic wastes of the leather industry as adsorbents of aromatic organic pollutants in water. J. Environ. Chem. Eng. 2017, 5, 2090–2100. [Google Scholar] [CrossRef]
- Bendi, A.; Dharma Rao, G.B.; Sharma, N.; Singh, M.P. Results in Chemistry CoFe2O4/Cu(OH)2 Nanocomposite: Expeditious and magnetically recoverable heterogeneous catalyst for the four component Biginelli/transesterification reaction and their DFT studies. Results Chem. 2021, 3, 100202. [Google Scholar] [CrossRef]
- Bendi, A.; Dharma Rao, G.B.; Nancy; Nagakalyan, S. Synthesis and DFT studies of 1,2-disubstituted benzimidazoles using expeditious and magnetically recoverable CoFe2O4/Cu(OH)2 nanocomposite under solvent-free condition. J. Saudi Chem. Soc. 2021, 25, 101394. [Google Scholar] [CrossRef]
- Farghali, A.A.; Bahgat, M.; Enaiet Allah, A.; Khedr, M.H. Adsorption of Pb(II) ions from aqueous solutions using copper oxide nanostructures. Beni-Suef Univ. J. Basic Appl. Sci. 2013, 2, 61–71. [Google Scholar] [CrossRef]
- Dissanayake, M.A.K.L.; Divarathna, H.K.D.W.M.N.; Dissanayake, C.B.; Senadeera, G.K.R.; Ekanayake, P.M.P.C.; Thotawattage, C.A. An innovative TiO2 nanoparticle/nanofibre/nanoparticle, three layer composite photoanode for efficiency enhancement in dye-sensitized solar cells. J. Photochem. Photobiol. A Chem. 2016, 322–323, 110–118. [Google Scholar] [CrossRef]
- Muhamad, S.U.; Idris, N.H.; Yusoff, H.M.; Din, M.F.M.; Majid, S.R. In-situ encapsulation of nickel nanoparticles in polypyrrole nanofibres with enhanced performance for supercapacitor. Electrochim. Acta 2017, 249, 9–15. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C.; Dharma Rao, G.B.; Singh, L.; Anjaneyulu, B.; Afshari, M. CuFe2O4 Magnetic Nanoparticles as Heterogeneous Catalysts for Synthesis of Dihydropyrimidinones as Inhibitors of SARS-CoV-2 Surface Proteins—Insights from Molecular Docking Studies. Processes 2023, 11, 2294. [Google Scholar] [CrossRef]
- Asfaram, A.; Ghaedi, M.; Goudarzi, A.; Rajabi, M. Response surface methodology approach for optimization of simultaneous dye and metal ion ultrasound-assisted adsorption onto Mn doped Fe3O4-NPs loaded on AC: Kinetic and isothermal studies. Dalton Trans. 2015, 44, 14707–14723. [Google Scholar] [CrossRef]
- Ganesan, V.; Louis, C.; Damodaran, S.P. Graphene oxide-wrapped magnetite nanoclusters: A recyclable functional hybrid for fast and highly efficient removal of organic dyes from wastewater. J. Environ. Chem. Eng. 2018, 6, 2176–2190. [Google Scholar] [CrossRef]
- Asfaram, A.; Ghaedi, M.; Hajati, S.; Goudarzi, A. Ternary dye adsorption onto MnO2 nanoparticle-loaded activated carbon: Derivative spectrophotometry and modeling. RSC Adv. 2015, 5, 72300–72320. [Google Scholar] [CrossRef]
- Anjaneyulu, B.; Chinmay; Chauhan, V.; Sonia, A.C.C.; Mozhgan, A. Recent advances on zinc ferrite and its derivatives as the forerunner of the nanomaterials in catalytic applications. J. Inorg. Organomet. Polym. 2023, 1–21. [Google Scholar] [CrossRef]
- Siqueira, J.R.; Oliveira, O.N. 9–Carbon-Based Nanomaterials. In Nanostructures; Elsevier: Oxford, UK, 2017. [Google Scholar] [CrossRef]
- Ren, X.; Zeng, G.; Tang, L.; Wang, J.; Wan, J.; Feng, H.; Song, B.; Huang, C.; Tang, X. Effect of exogenous carbonaceous materials on the bioavailability of organic pollutants and their ecological risks. Soil Biol. Biochem. 2018, 116, 70–81. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, X.; Yang, Y.; Yuan, Q. Aptamer-functionalized carbon nanomaterials electrochemical sensors for detecting cancer relevant biomolecules. Carbon 2018, 129, 380–395. [Google Scholar] [CrossRef]
- Malhotra, B.D.; Ali, M.A. Functionalized Carbon Nanomaterials for Biosensors. In Nanomaterials for Biosensors; Elsevier: Amsterdam, The Netherlands, 2018; Chapter 2; pp. 75–103. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Kim, Y.; Jeon, H.; Cho, S.; Lee, W.; Lee, J.U. Graphene/carbon nanotube hybrid as a multi-functional interfacial reinforcement for carbon fiber-reinforced composites. Compos. Part B Eng. 2017, 122, 23–30. [Google Scholar] [CrossRef]
- Imani Yengejeh, S.; Kazemi, S.A.; Öchsner, A. Carbon nanotubes as reinforcement in composites: A review of the analytical, numerical and experimental approaches. Comput. Mater. Sci. 2017, 136, 85–101. [Google Scholar] [CrossRef]
- Chen, Y.F.; Tan, Y.J.; Li, J.; Hao, Y.B.; Shi, Y.D.; Wang, M. Graphene oxide-assisted dispersion of multi-walled carbon nanotubes in biodegradable Poly(ε-caprolactone) for mechanical and electrically conductive enhancement. Polym. Test. 2018, 65, 387–397. [Google Scholar] [CrossRef]
- Deb, A.; Vimala, R. Camptothecin loaded graphene oxide nanoparticle functionalized with polyethylene glycol and folic acid for anticancer drug delivery. J. Drug Deliv. Sci. Technol. 2018, 43, 333–342. [Google Scholar] [CrossRef]
- Ahmed, W.; Elhissi, A.; Dhanak, V.; Subramani, K. Carbon nanotubes: Applications in cancer therapy and drug delivery research. In Emerging Nanotechnologies in Dentistry; Elsevier: Amsterdam, The Netherlands, 2017; pp. 371–389. [Google Scholar] [CrossRef]
- Ioniță, M.; Crică, L.E.; Voicu, S.I.; Dinescu, S.; Miculescu, F.; Costache, M.; Iovu, H. Synergistic effect of carbon nanotubes and graphene for high performance cellulose acetate membranes in biomedical applications. Carbohydr. Polym. 2018, 183, 50–61. [Google Scholar] [CrossRef]
- Li, Z.-F.; Xin, L.; Yang, F.; Liu, Y.; Liu, Y.; Zhang, H.; Stanciu, L.; Xie, J. Hierarchical polybenzimidazole-grafted graphene hybrids as supports for Pt nanoparticle catalysts with excellent PEMFC performance. Nano Energy 2015, 16, 281–292. [Google Scholar] [CrossRef]
- Jeng, K.T.; Hsu, N.Y.; Chien, C.C. Synthesis and evaluation of carbon nanotube-supported RuSe catalyst for direct methanol fuel cell cathode. Int. J. Hydrogen Energy 2011, 36, 3997–4006. [Google Scholar] [CrossRef]
- Teow, Y.H.; Mohammad, A.W. New generation nanomaterials for water desalination: A review. Desalination 2019, 451, 2–17. [Google Scholar] [CrossRef]
- Thines, R.K.; Mubarak, N.M.; Nizamuddin, S.; Sahu, J.N.; Abdullah, E.C.; Ganesan, P. Application potential of carbon nanomaterials in water and wastewater treatment: A review. J. Taiwan Inst. Chem. Eng. 2017, 72, 116–133. [Google Scholar] [CrossRef]
- Ray, S.C.; Jana, N.R. Application of Carbon-Based Nanomaterials for Removal of Biologically Toxic Materials. In Carbon Nanomaterials for Biological and Medical Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 43–86. [Google Scholar] [CrossRef]
- Kong, L.P.; Gan, X.J.; bin Ahmad, A.L.; Hamed, B.H.; Evarts, E.R.; Ooi, B.S.; Lim, J. Design and synthesis of magnetic nanoparticles augmented microcapsule with catalytic and magnetic bifunctionalities for dye removal. Chem. Eng. J. 2012, 197, 350–358. [Google Scholar] [CrossRef]
- Lotfi Zadeh Zhad, H.R.; Aboufazeli, F.; Sadeghi, O.; Amani, V.; Najafi, E.; Tavassoli, N. Tris(2-Aminoethyl)amine-functionalized Femagnetic nanoparticles as a selective sorbent for separation of silver and gold ions in different pHs. J. Chem. 2013, 2013, 482793. [Google Scholar] [CrossRef]
- Carreño, N.L.V.; Escote, M.T.; Valentini, A.; McCafferty, L.; Stolojan, V.; Beliatis, M.; Mills, C.A.; Rhodes, R.; Smith, C.T.G.; Silva, S.R.P. Adsorbent 2D and 3D carbon matrices with protected magnetic iron nanoparticles. Nanoscale 2015, 7, 17441–17449. [Google Scholar] [CrossRef]
- Chen, C.L.; Wang, X.K.; Nagatsu, M. Europium adsorption on multiwall carbon nanotube/iron oxide magnetic composite in the presence of polyacrylic acid. Environ. Sci. Technol. 2009, 43, 2362–2367. [Google Scholar] [CrossRef]
- Lu, A.H.; Salabas, E.L.; Schüth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef]
- Yu, C.; Geng, J.; Zhuang, Y.; Zhao, J.; Chu, L.; Luo, X. Preparation of the chitosan grafted poly (quaternary ammonium)/Fe3O4 nanoparticles and its adsorption performance for food yellow 3. Carbohydr. Polym. 2016, 152, 327–336. [Google Scholar] [CrossRef]
- Sayana, K.V.; Prajwal, K.; Deeksha, K.J.; Vishalakshi, B.; Vishwanath, T. Magnetized CNTs incorporated MBA cross-linked guar gum nano-composite for methylene blue dye removal. J. Appl. Polym. Sci. 2024, 141, e54868. [Google Scholar] [CrossRef]
- Ege, K.; Arzum, Ç.; Alattin, Ç.; Elif, A. Enhanced photocatalytic dye degradation using surface-modified tungstophosphoric acid-iron magnetic nanocatalyst. Opt. Mater. 2024, 148, 114816. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, X.; Zhang, S.; Sun, N.; Zhou, H.; Wang, G.; Zhang, Y.; Zhang, H.; Zhao, H. Porous carbon nanosheets functionalized with Fe3O4 nanoparticles for capacitive removal of heavy metal ions from water. Environ. Sci. Water Res. Technol. 2020, 6, 331–340. [Google Scholar] [CrossRef]
- Yang, W.; Chen, H.; Han, X.; Ding, S.; Shan, Y.; Liu, Y. Preparation of magnetic Co-Fe modified porous carbon from agricultural wastes by microwave and steam activation for mercury removal. J. Hazard. Mater. 2020, 5, 120981. [Google Scholar] [CrossRef] [PubMed]
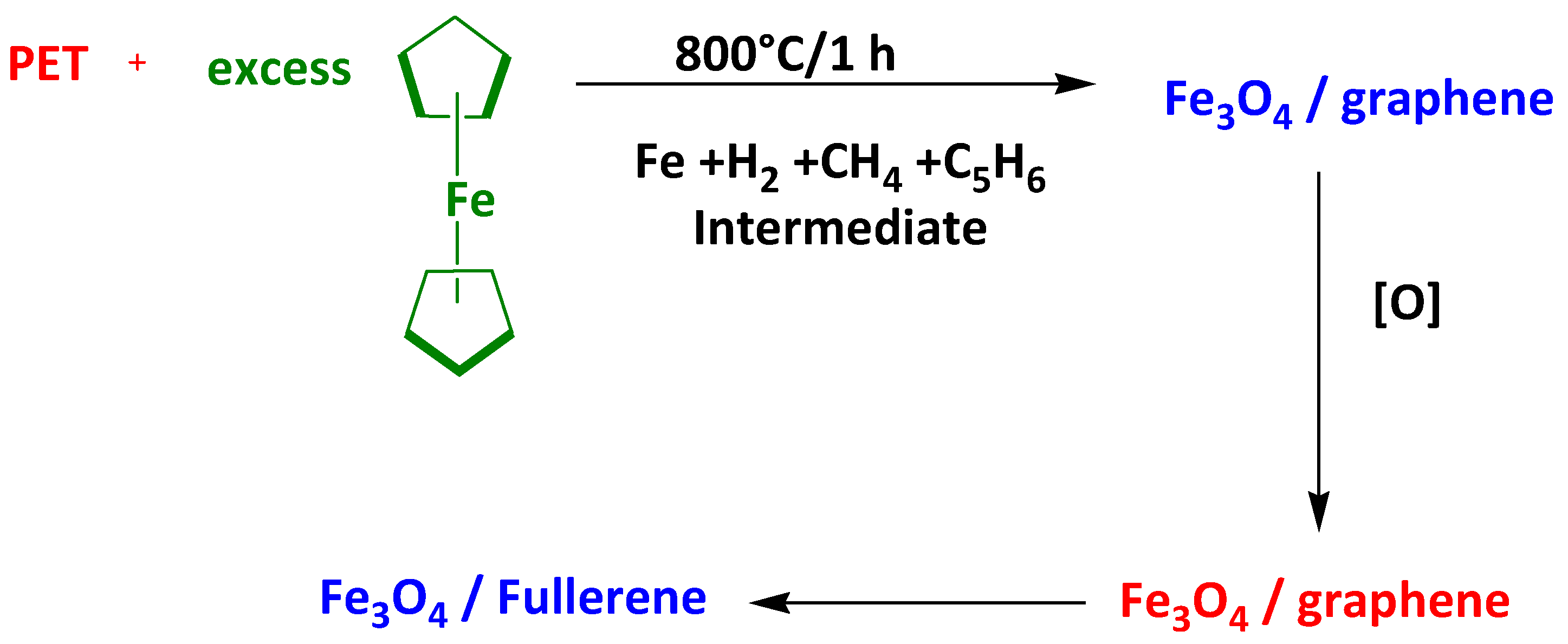
- Elessawy, N.A.; El-Sayed, E.M.; Ali, S.; Elkady, M.F.; Elnouby, M.; Hamad, H.A. One-pot green synthesis of magnetic fullerene nanocomposite for adsorption characteristics. J. Water Process Eng. 2020, 34, 101047. [Google Scholar] [CrossRef]
- Elessawy, N.A.; Elnouby, M.; Gouda, M.H.; Hamad, H.A.; Taha, N.A.; Gouda, M.; Eldin, M.S.M. Ciprofloxacin removal using magnetic fullerene nanocomposite obtained from sustainable PET bottle wastes: Adsorption process optimization, kinetics, isotherm, regeneration and recycling studies. Chemosphere 2020, 239, 124728. [Google Scholar] [CrossRef]
- Deng, Y.; Ok, Y.S.; Mohan, D.; Pittman, C.U.; Dou, X. Carbamazepine removal from water by carbon dot-modified magnetic carbon nanotubes. Environ. Res. 2019, 169, 434–444. [Google Scholar] [CrossRef]
- Sun, A.C. Synthesis of magnetic carbon nanodots for recyclable photocatalytic degradation of organic compounds in visible light. Adv. Powder Technol. 2018, 29, 719–725. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, S.; Zhang, H.; Fan, X.; Gao, C.; Yu, H.; Quan, X. Carbon nanotubes-incorporated MIL-88B-Fe as highly efficient Fenton-like catalyst for degradation of organic pollutants. Front. Environ. Sci. Eng. 2019, 13, 18. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, H.; Wu, W.; Pang, W.; Yan, G. Efficient removal of Alizarin Red S from aqueous solution by polyethyleneimine functionalized magnetic carbon nanotubes. Bioresour. Technol. 2019, 293, 122100. [Google Scholar] [CrossRef]
- Salam, M.A.; El-Shishtawy, R.M.; Obaid, A.Y. Synthesis of magnetic multi-walled carbon nanotubes/magnetite/chitin magnetic nanocomposite for the removal of Rose Bengal from real and model solution. J. Ind. Eng. Chem. 2014, 20, 3559–3567. [Google Scholar] [CrossRef]
- Cheng, J.; Chang, P.R.; Zheng, P.; Ma, X. Characterization of magnetic carbon nanotube-cyclodextrin composite and its adsorption of dye. Ind. Eng. Chem. Res. 2014, 53, 1415–1421. [Google Scholar] [CrossRef]
- Zhu, H.; Fu, Y.; Jiang, R.; Yao, J.; Liu, L.; Chen, Y.; Xiao, L.; Zeng, G. Preparation, characterization and adsorption properties of chitosan modified magnetic graphitized multi-walled carbon nanotubes for highly effective removal of a carcinogenic dye from aqueous solution. Appl. Surf. Sci. 2013, 285, 865–873. [Google Scholar] [CrossRef]
- Gao, H.; Zhao, S.; Cheng, X.; Wang, X.; Zheng, L. Removal of anionic azo dyes from aqueous solution using magnetic polymer multi-wall carbon nanotube nanocomposite as adsorbent. Chem. Eng. J. 2013, 223, 84–90. [Google Scholar] [CrossRef]
- Madrakian, T.; Afkhami, A.; Ahmadi, M.; Bagheri, H. Removal of some cationic dyes from aqueous solutions using magnetic-modified multi-walled carbon nanotubes. J. Hazard. Mater. 2011, 196, 109–114. [Google Scholar] [CrossRef]
- Yan, L.; Chang, P.R.; Zheng, P.; Ma, X. Characterization of magnetic guar gum-grafted carbon nanotubes and the adsorption of the dyes. Carbohydr. Polym. 2012, 87, 1919–1924. [Google Scholar] [CrossRef]
- Qu, S.; Huang, F.; Yu, S.; Chen, G.; Kong, J. Magnetic removal of dyes from aqueous solution using multi-walled carbon nanotubes filled with Fe2O3 particles. J. Hazard. Mater. 2008, 160, 643–647. [Google Scholar] [CrossRef]
- Ranjbar, E.; Ghiassi, R.; Baghdadi, M.; Ruhl, A.S. Bisphenol A removal in treated wastewater matrix at neutral pH using magnetic graphite intercalation compounds as persulfate activators. Water Environ. Res. 2023, 95, e10835. [Google Scholar] [CrossRef]
- Ruan, C.P.; Ai, K.L.; Lu, L.H. An Acid-resistant Magnetic Co/C Nanocomposite for Adsorption and Separation of Organic Contaminants from Water. Chin. J. Anal. Chem. 2016, 44, 224–231. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Q.; Hou, J.; Yan, J.; Zhang, F.; Zhao, J.; Ding, H.; Li, Y.; Ding, L. One-step solvothermal synthesis of magnetic Fe3O4-graphite composite for Fenton-like degradation of levofloxacin. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2016, 51, 52–62. [Google Scholar] [CrossRef]
- Bharath, G.; Alhseinat, E.; Ponpandian, N.; Khan, M.A.; Siddiqui, M.R.; Ahmed, F.; Alsharaeh, E.H. Development of adsorption and electrosorption techniques for removal of organic and inorganic pollutants from wastewater using novel magnetite/porous graphene-based nanocomposites. Sep. Purif. Technol. 2017, 188, 206–218. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, X.; Xie, J.; Wu, R.; Liu, X.; Li, H.; Chen, F.; Yang, H.; Ming, Z.; Yang, S.-T. Magnetic graphene sponge for the removal of methylene blue. Appl. Surf. Sci. 2015, 351, 765–771. [Google Scholar] [CrossRef]
- Zhao, G.; Mo, Z.; Zhang, P.; Wang, B.; Zhu, X.; Guo, R. Synthesis of graphene/Fe3O4/NiO magnetic nanocomposites and its application in photocatalytic degradation the organic pollutants in wastewater. J. Porous Mater. 2015, 22, 1245–1253. [Google Scholar] [CrossRef]
- Yang, X.; Li, J.; Wen, T.; Ren, X.; Huang, Y.; Wang, X. Adsorption of naphthalene and its derivatives on magnetic graphene composites and the mechanism investigation. Colloids Surf. A Physicochem. Eng. Asp. 2013, 422, 118–125. [Google Scholar] [CrossRef]
- Yao, Y.; Miao, S.; Liu, S.; Ma, L.P.; Sun, H.; Wang, S. Synthesis, characterization, and adsorption properties of magnetic Fe3O 4@graphene nanocomposite. Chem. Eng. J. 2012, 184, 326–332. [Google Scholar] [CrossRef]
- Li, N.; Zheng, M.; Chang, X.; Ji, G.; Lu, H.; Xue, L.; Pan, L.; Cao, J. Preparation of magnetic CoFe2O4-functionalized graphene sheets via a facile hydrothermal method and their adsorption properties. J. Solid State Chem. 2011, 184, 953–958. [Google Scholar] [CrossRef]
- Wang, C.; Feng, C.; Gao, Y.; Ma, X.; Wu, Q.; Wang, Z. Preparation of a graphene-based magnetic nanocomposite for the removal of an organic dye from aqueous solution. Chem. Eng. J. 2011, 173, 92–97. [Google Scholar] [CrossRef]
- Islam, M.R.; Ferdous, M.; Sujan, M.I.; Mao, X.; Zeng, H.; Azam, M.S. Recyclable Ag-decorated highly carbonaceous magnetic nanocomposites for the removal of organic pollutants. J. Colloid Interface Sci. 2020, 562, 52–62. [Google Scholar] [CrossRef]
- Chang, S.; Zhang, Q.; Lu, Y.; Wu, S.; Wang, W. High-efficiency and selective adsorption of organic pollutants by magnetic CoFe2O4/graphene oxide adsorbents: Experimental and molecular dynamics simulation study. Sep. Purif. Technol. 2020, 238, 119400. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, Y.; Liu, Y.; Zhang, W.; Wang, Y.; Guo, X.; Tang, X.; Zhang, Y.; Wang, Z.; Zhang, T. Magnetic Mn-Doped Fe3O4 hollow Microsphere/RGO heterogeneous Photo-Fenton Catalyst for high efficiency degradation of organic pollutant at neutral pH. Mater. Chem. Phys. 2019, 238, 121893. [Google Scholar] [CrossRef]
- Mishra, A. Study of organic pollutant removal capacity for magnetite@ graphene oxide nanocomposites. Vacuum 2018, 157, 524–529. [Google Scholar] [CrossRef]
- Bai, S.; Shen, X.; Zhong, X.; Liu, Y.; Zhu, G.; Xu, X.; Chen, K. One-pot solvothermal preparation of magnetic reduced graphene oxide-ferrite hybrids for organic dye removal. Carbon 2012, 50, 2337–2346. [Google Scholar] [CrossRef]
- Song, Z.J.; Ran, W.; Wei, F.Y. One-step approach for the synthesis of CoFe2O4 @rGO core-shell nanocomposites as efficient adsorbent for removal of organic pollutants. Water Sci. Technol. 2017, 75, 397–405. [Google Scholar] [CrossRef]

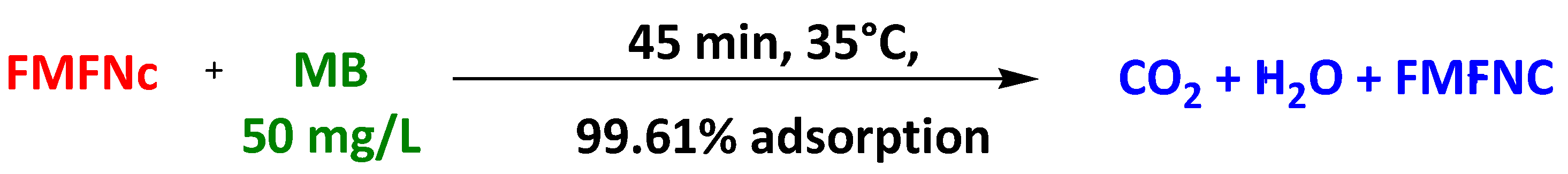


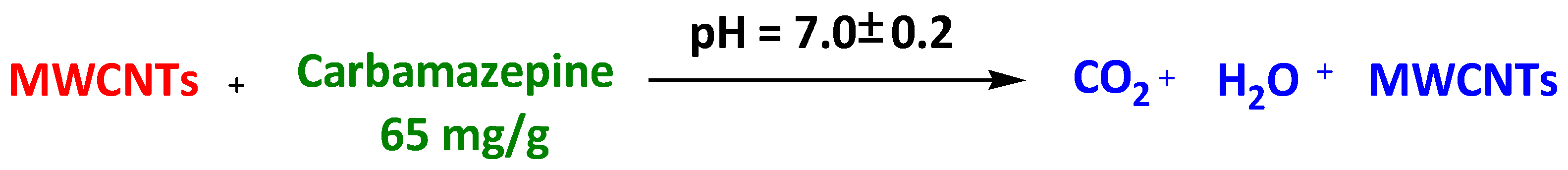
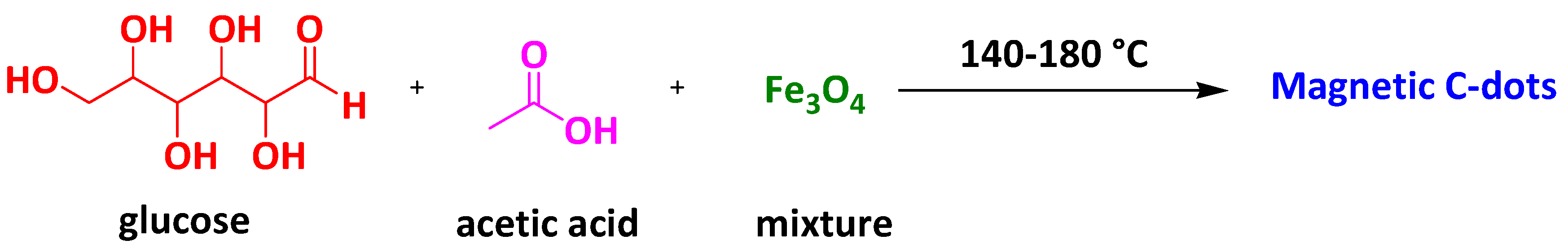







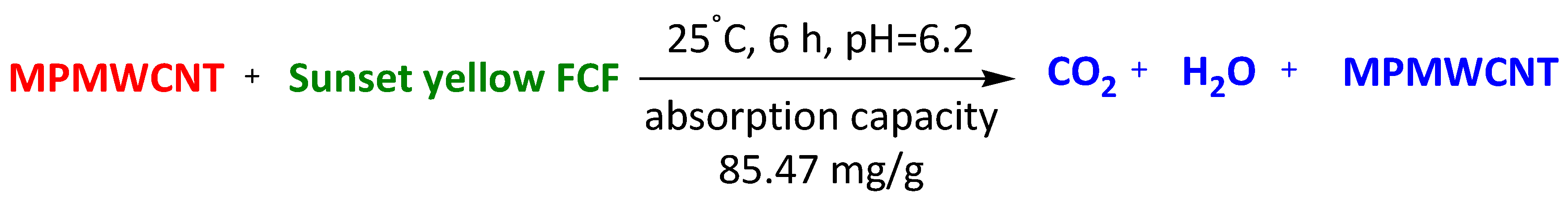
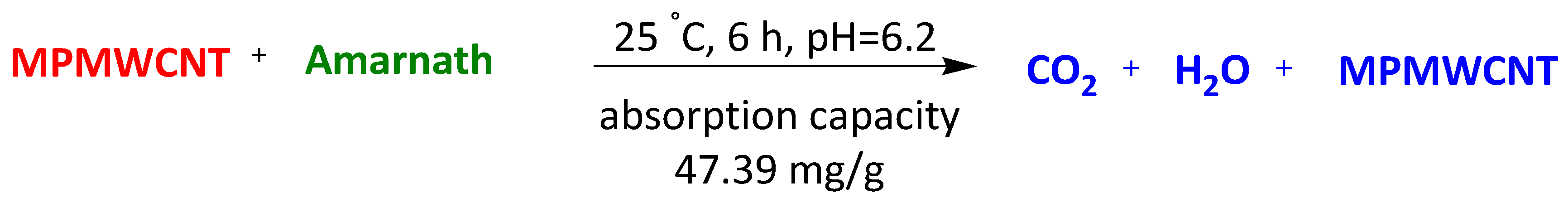





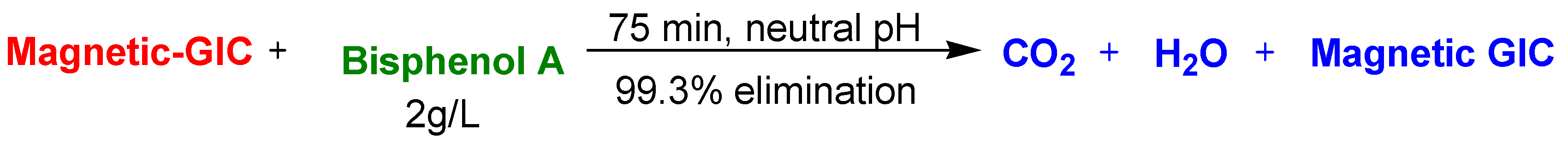
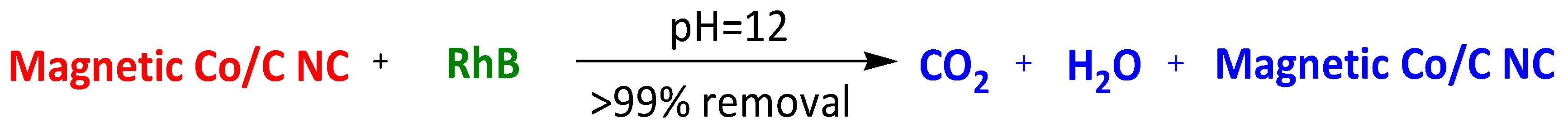



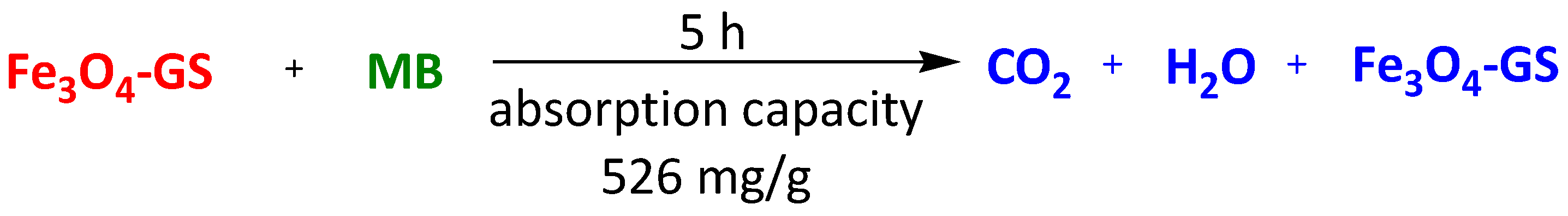
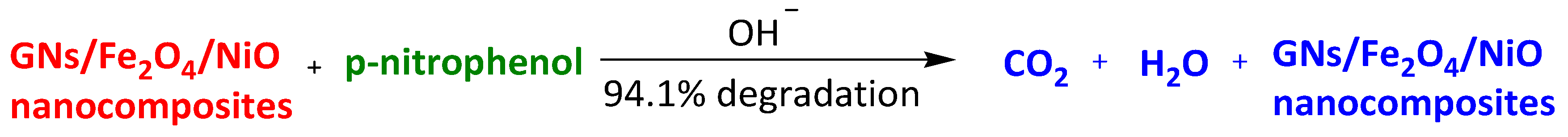

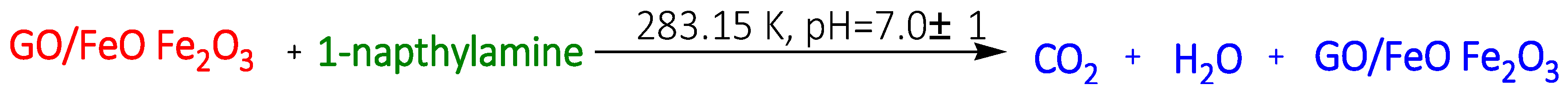


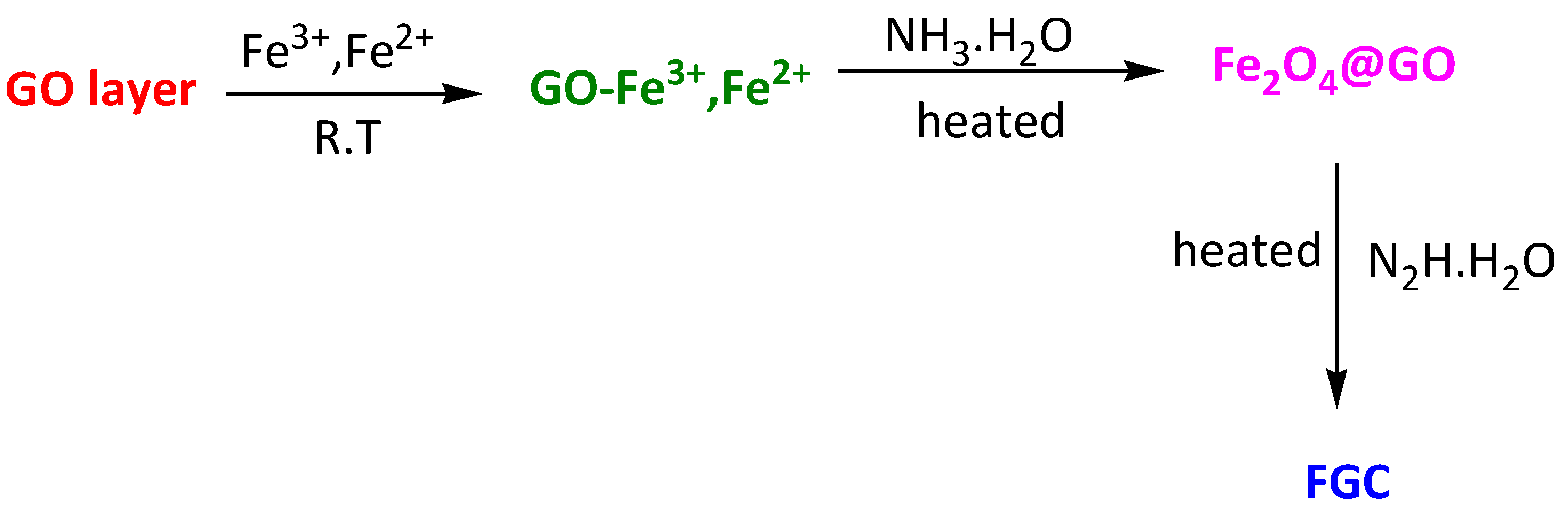
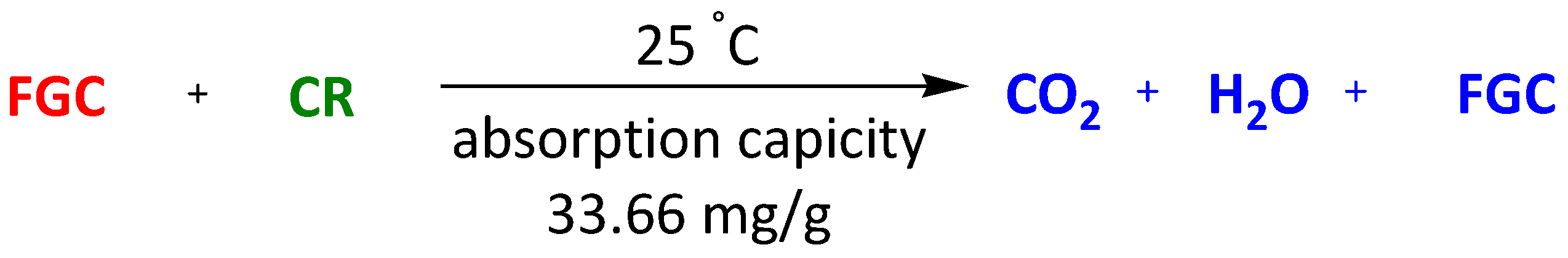
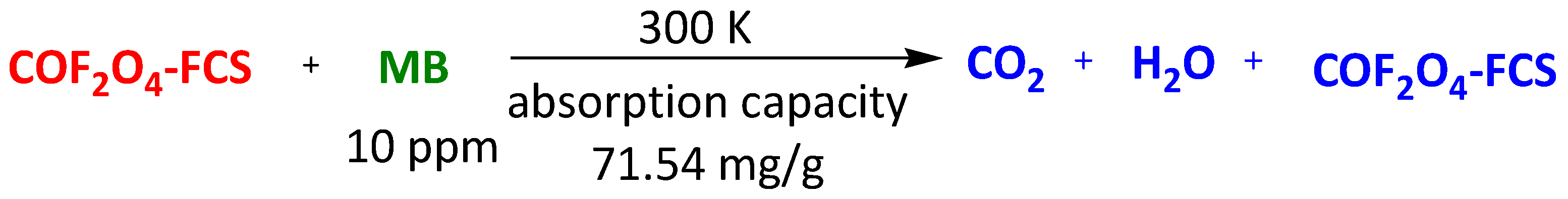
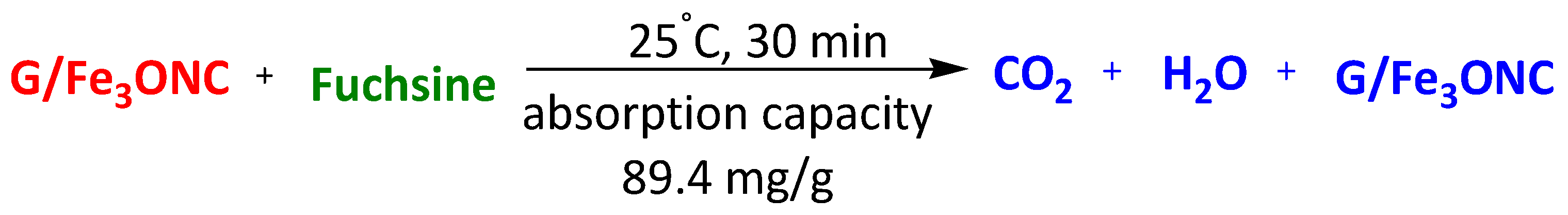


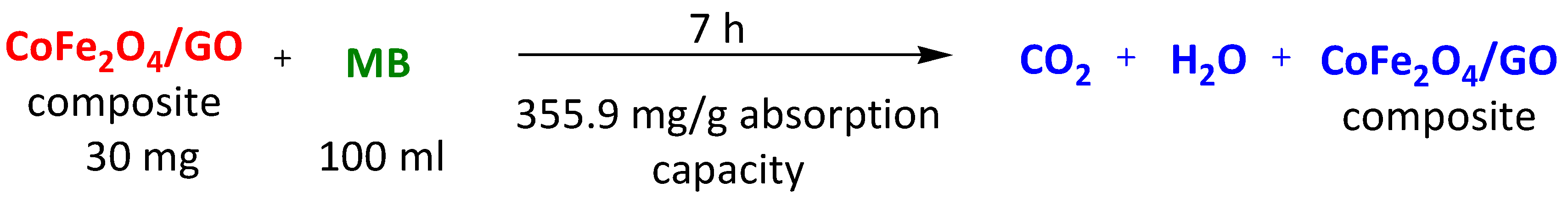
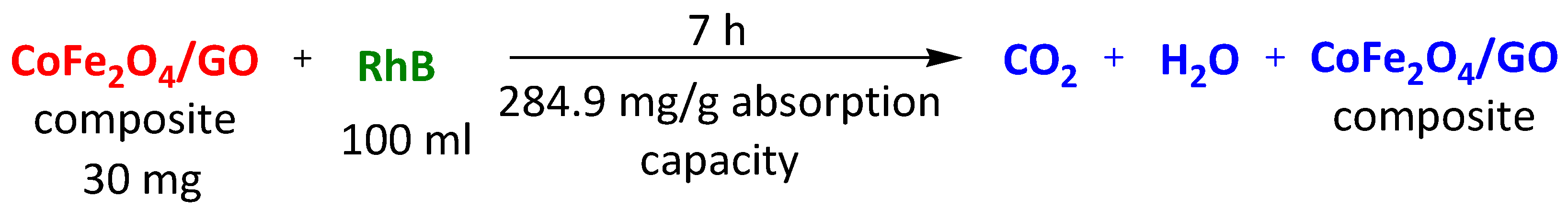


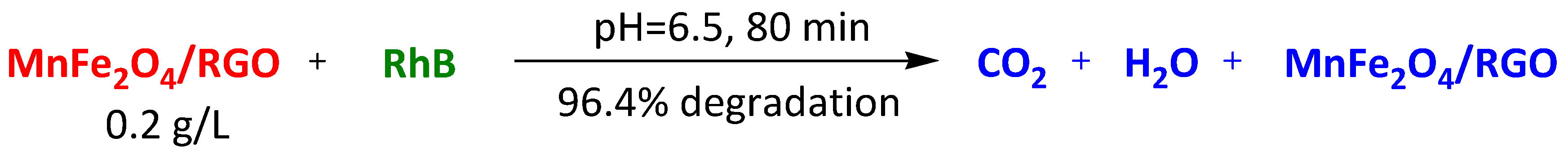


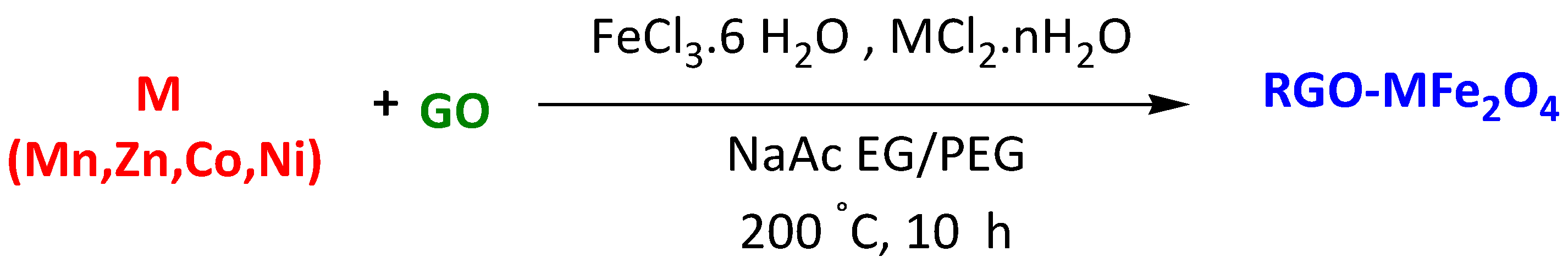
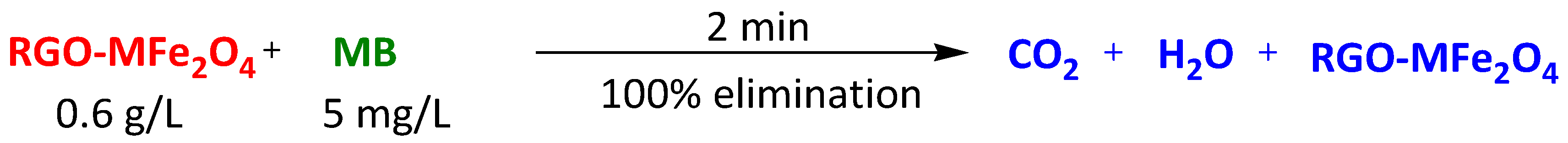


| Type | Nanoparticles/Composites | Type of Organic Pollutants | Time | Absorption/ Elimination Efficiency of Nanomaterial | Ref. |
|---|---|---|---|---|---|
| Magnetic fullerene nanocomposites | Functionalized Magnetic Fullerene Nanocomposites | Methylene Blue Acid Blue 25 | 45 min 45 min | 99.6% 97.01% | [88] |
| Functionalized Magnetic Fullerene Nanocomposites | Ciprofloxacin | 153 min | 65 mg/L | [89] | |
| Magnetic Carbon-dot Nanocomposites | Carbon-dot and magnetite-modified magnetic carbon nanotubes | Carbamazepine | 3 h | 65 mg/g | [90] |
| Magnetic C-Dots | Methylene Blue | 30 min | 83% | [91] | |
| Magnetic Carbon nanotube nanocomposites | Carbon nanotubes-incorporated MIL-88B-Fe | Phenol | 30 min | 55% | [93] |
| Magnetic CNTs functionalized with polyethyleneimine | Alizarin Red S | 40 min | 94.6% | [95] | |
| Multiwalled carbon nanotubes | Rose Bengal | 30 min | 100% | [94] | |
| Magnetic Carbon-nanotube– Cyclodextrin composite | Methylene Blue | 25 h | 196.5 mg/g | [95] | |
| Magnetic graphitized MWCNTs modified with Chitosan | Crystal Violet | 100 min | 94.56–100% | [96] | |
| Magnetic polymers multiwall carbon nanotube nanocomposite | Orange (II) Sunset yellow FCF Amarnath | 6 h 6 h 6 h | 67.57 mg/g 85.47 mg/g 47.39 mg/g | [97] | |
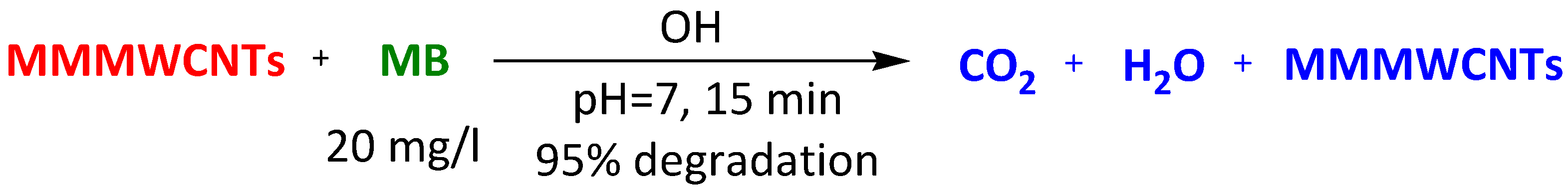
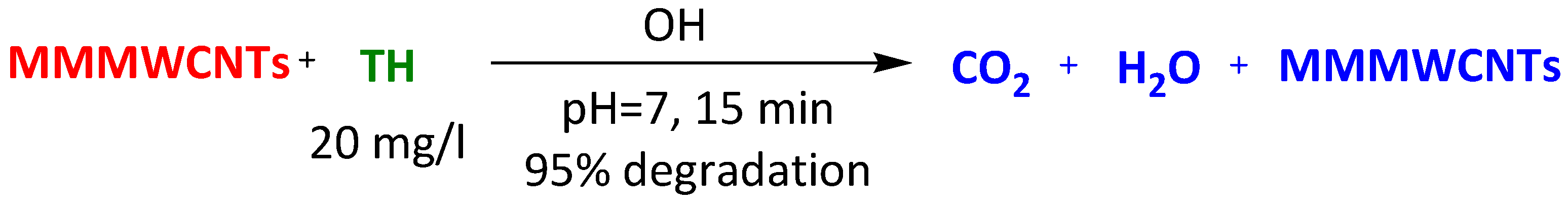
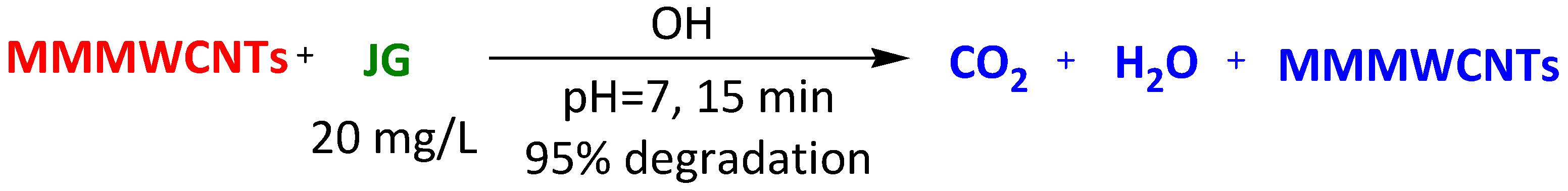
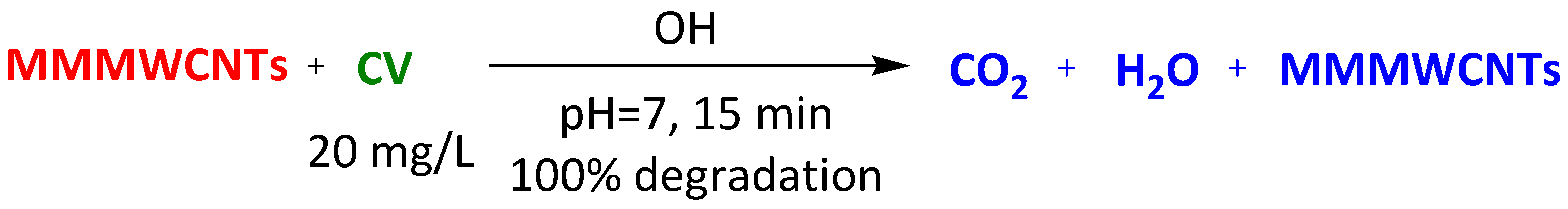
| Magnetic-modified multiwalled carbon nanotubes | Methylene Blue Thioflavin Janus Green Crystal Violet | 15 min 15 min 15 min 15 min | 95% 95% 95% 100% | [98] | |
| Magnetic carbon nanotubes modified with guar gum GG-MWCNT-Fe3O4 | Methylene Blue Neutral Red | 120 min 20 min | 37.4 mg/L 28.9 mg/L | [99] | |
| Magnetic multiwalled carbon nanotubes | Methylene Blue Neutral Red | 60 min 60 min | 42.3 mg/g 77.5 mg/g | [100] | |
| Magnetic Graphite Nanocomposites | Magnetic graphite intercalation compounds as persulfate activators | Bisphenol A | 75 min | 99.3% | [101] |
| Acid-resistant Magnetic Co/C nanocomposite | Rhodamine B Malachite Green | 30 min 30 min | >99% >99% | [102] | |
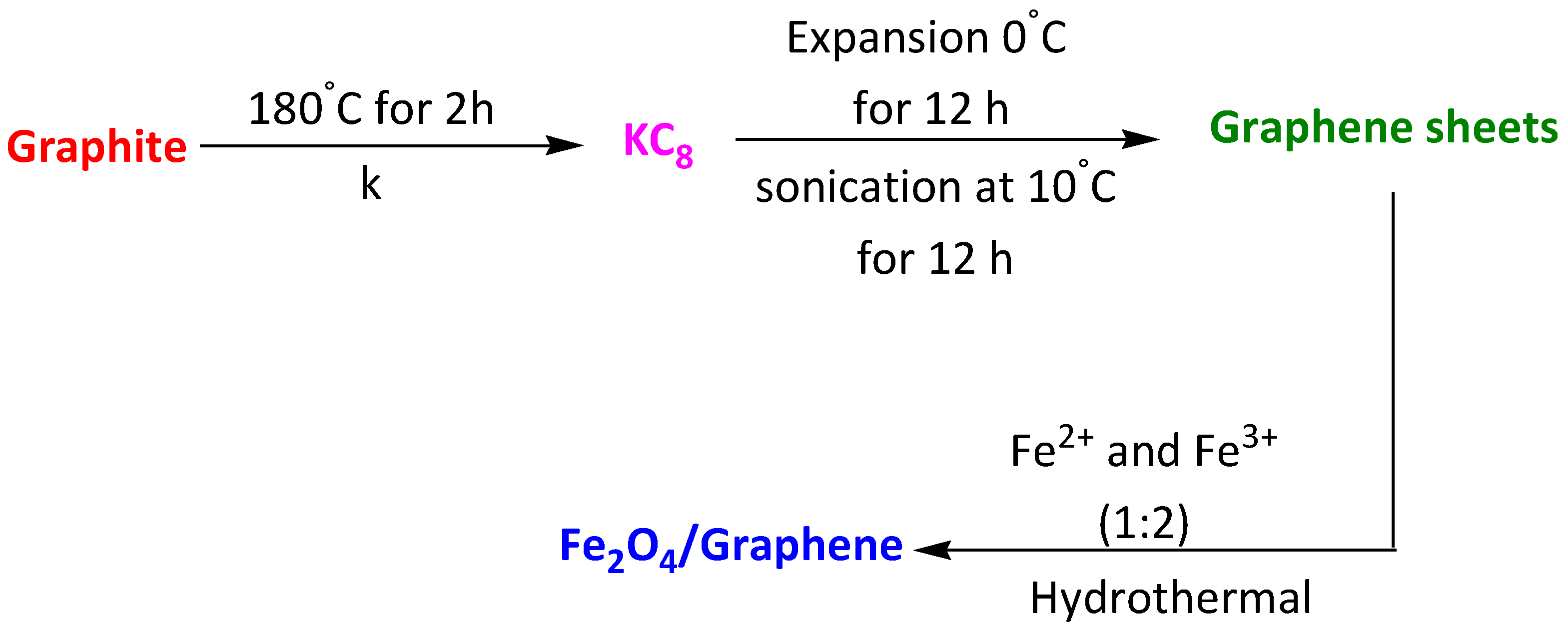
| Magnetic Fe2O4–graphite composite | Levofloxacin | 15 min | 80% | [103] | |
| Magnetic Graphene Nanocomposites | Fe2O4/porous graphene nanocomposite | Methyl Violet | 5 min | 460 mg/g | [104] |
| Magnetic sponge of graphene (Fe3O4-GS) | Methyl blue | 5 h | 526 mg/g | [105] | |
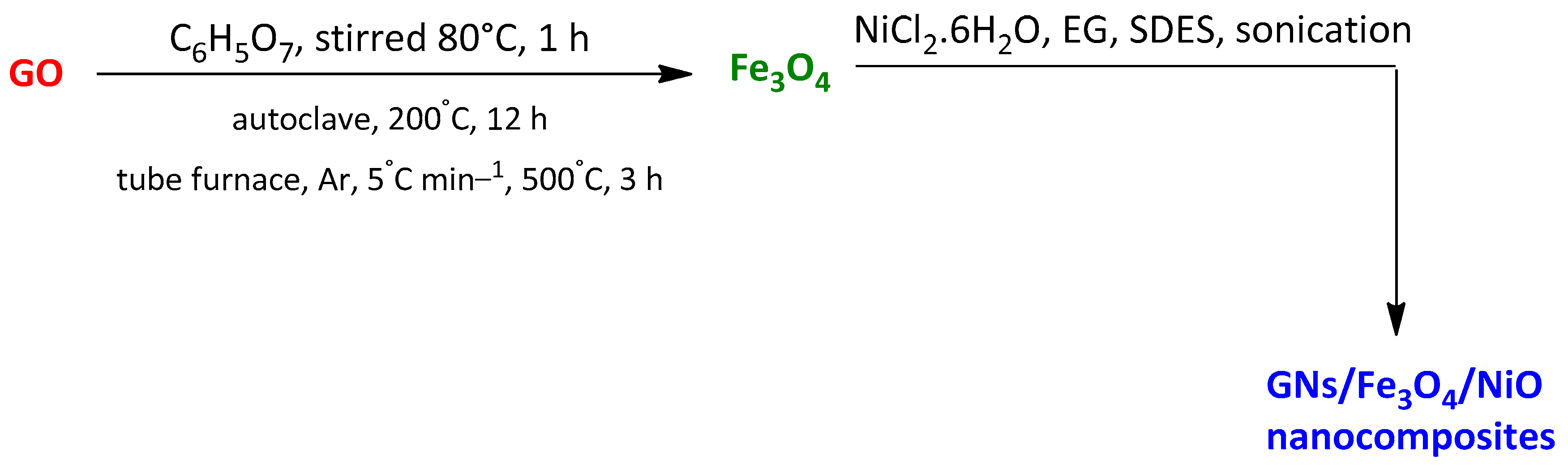
| Magnetic photocatalyst of graphene with Fe3O4 and NiO GNs/Fe3O4/NiO nanocomposite | p-Nitrophenol Rhodamine B | 20 min | 94.1% 86.7% | [106] | |
| Reduced graphene Oxide (GO) on Fe oxide (GO/FeO.Fe2O3) | 1-Naphthylamine 1-Napthol Napthalene | 1.45 mmol/g 1.13 mmol/g 1.05 mmol/g | [107] | ||
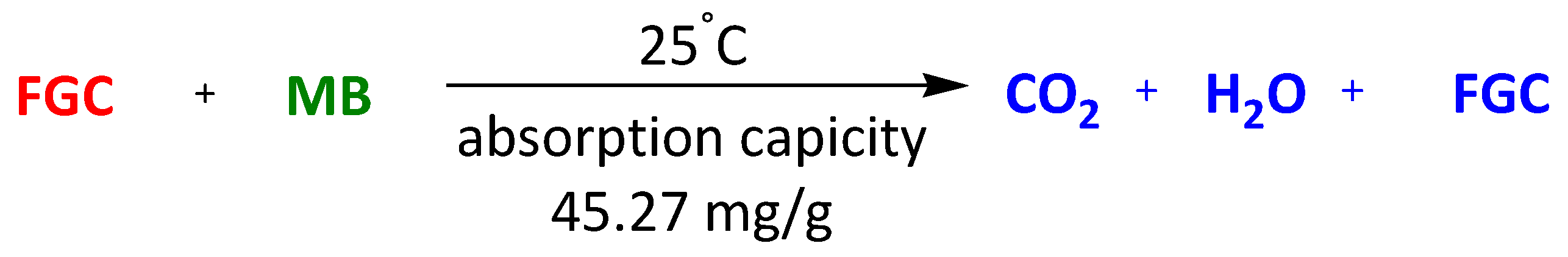
| Magnetic composite Fe3O4@graphene | Methylene Blue Congo Red | 30 min 30 min | 45.27 mg/g 33.66 mg/g | [108] | |
| Nanocomposites of magnetic CoFe2O4 functionalized graphene sheets (CoFe2O4-FGS) | Methyl Orange | 60 min | 71.54 mg/g | [109] | |
| Graphene-based magnetic nanocomposite (G/Fe3O4) | Fuchsine | 30 min | 89.4 mg/g | [110] | |
| Magnetic graphene oxide nanocomposites | Magnetic RCNT-CD | 4-Nitrophenol Methylene Blue | 10 min 10 min | 84% 96% | [111] |
| Magnetic CoFe2O4/GO | Methylene Blue Rhodamine B Methyl Orange | 7 h 7 h 7 h | 355.9 mg/g 284.9 mg/g 53.0 mg/g | [112] | |
| Mn-doped Fe3O4 hollow microspheres on rGO | Rhodamine B | 80 min | 96.4% | [113] | |
| GO sheets with Fe3O4 nanoparticles | Rhodamine 6G | 5 min | 89% | [114] | |
| Reduced graphene oxide (RGO)-supported ferrite (MFe2O4, M = Mn, Zn, Co, Ni) | Methylene Blue Rhodamine B | 2 min 2 min | 100% 92% | [115] | |
| Nanocomposite containing CoFe2O4 and reduced graphene oxide | Methyl Orange | 2 h | 263 mg/g | [116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anjaneyulu, B.; Rana, R.; Versha; Afshari, M.; Carabineiro, S.A.C. The Use of Magnetic Porous Carbon Nanocomposites for the Elimination of Organic Pollutants from Wastewater. Surfaces 2024, 7, 120-142. https://doi.org/10.3390/surfaces7010009
Anjaneyulu B, Rana R, Versha, Afshari M, Carabineiro SAC. The Use of Magnetic Porous Carbon Nanocomposites for the Elimination of Organic Pollutants from Wastewater. Surfaces. 2024; 7(1):120-142. https://doi.org/10.3390/surfaces7010009
Chicago/Turabian StyleAnjaneyulu, Bendi, Ravi Rana, Versha, Mozhgan Afshari, and Sónia A. C. Carabineiro. 2024. "The Use of Magnetic Porous Carbon Nanocomposites for the Elimination of Organic Pollutants from Wastewater" Surfaces 7, no. 1: 120-142. https://doi.org/10.3390/surfaces7010009