Study on the Sound Absorption Properties of Recycled Polyester Nonwovens through Alkaline Treatment and Dimple Processing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Alkaline Treatment
2.3. Dimple-Forming Process
2.4. Measurement of Properties for Sound-Absorbing Material
3. Results and Discussion
3.1. Rate of Change in Weight and Thickness by Alkaline Treatment
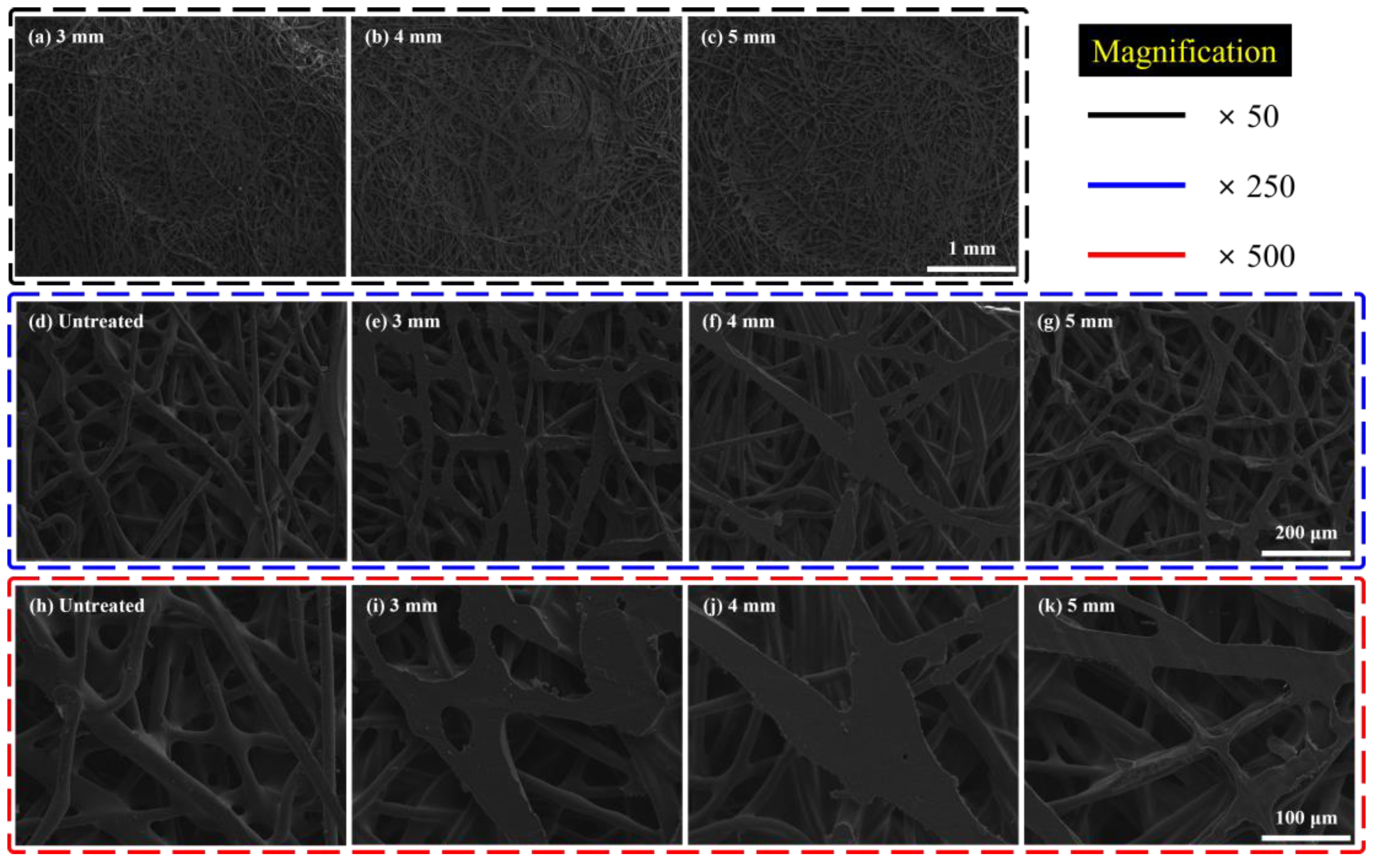
3.2. Analysis of Morphological Changes in Sound-Absorbing Materials during Alkaline Treatment and Dimple Processing
3.3. Air Permeability of Alkaline-Treated and Dimple-Processed Sound-Absorbing Materials
3.4. Adsorption and Desorption Pore Volume Changes of Sound-Absorbing Materials with Alkaline Treatment
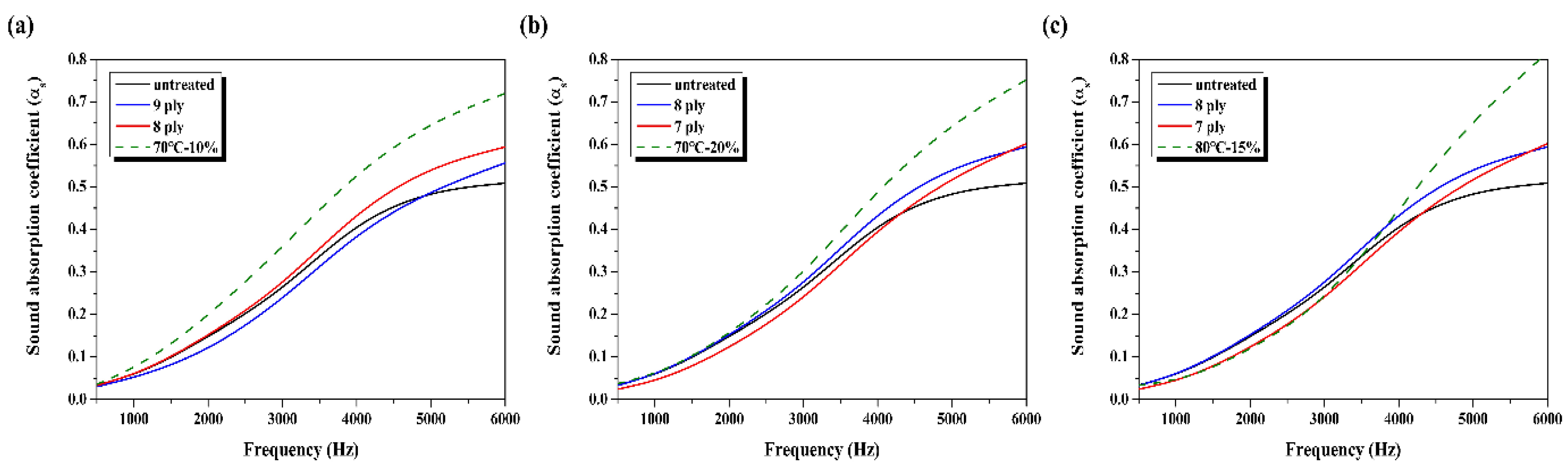
3.5. Changes in the Sound Absorption Coefficients of Alkaline-Treated and Dimple-Processed Sound-Absorbing Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, R.R.; Alok, K. Adoption of Electric Vehicle: A Literature Review and Prospects for Sustainability. J. Clean. Prod. 2020, 253, 119911. [Google Scholar] [CrossRef]
- Adu-Gyamfi, G.; Asamoah, A.N.; Obuobi, B.; Nketiah, E.; Zhang, M. Electric Mobility in an Oil-Producing Developing Nation: Empirical Assessment of Electric Vehicle Adoption. Technol. Forecast. Soc. Change 2024, 200, 123173. [Google Scholar] [CrossRef]
- Huang, H.B.; Wu, J.H.; Huang, X.R.; Ding, W.P.; Yang, M.L. A Novel Interval Analysis Method to Identify and Reduce Pure Electric Vehicle Structure-Borne Noise. J. Sound Vib. 2020, 475, 115258. [Google Scholar] [CrossRef]
- Hua, X.; Thomas, A.; Shultis, K. Recent Progress in Battery Electric Vehicle Noise, Vibration, and Harshness. Sci. Prog. 2021, 104, 00368504211005224. [Google Scholar] [CrossRef] [PubMed]
- Palak, H.; Karagüzel Kayaoğlu, B. Analysis of the Effect of Production Parameters on Sound Absorption and Abrasion Resistance Performance of Needlepunched Nonwovens for Automotive Carpet Applications Using Taguchi Method. J. Ind. Text. 2021, 51, 714–739. [Google Scholar] [CrossRef]
- Liu, Z.; Fard, M.; Davy, J.L. Prediction of the Acoustic Effect of an Interior Trim Porous Material inside a Rigid-Walled Car Air Cavity Model. Appl. Acoust. 2020, 165, 107325. [Google Scholar] [CrossRef]
- Del Pizzo, L.G.; Teti, L.; Moro, A.; Bianco, F.; Fredianelli, L.; Licitra, G. Influence of Texture on Tyre Road Noise Spectra in Rubberized Pavements. Appl. Acoust. 2020, 159, 107080. [Google Scholar] [CrossRef]
- Singh, S.; Mohanty, A.R. HVAC Noise Control Using Natural Materials to Improve Vehicle Interior Sound Quality. Appl. Acoust. 2018, 140, 100–109. [Google Scholar] [CrossRef]
- Wang, Z.; Li, P.; Liu, H.; Yang, J.; Liu, S.; Xue, L. Objective Sound Quality Evaluation for the Vehicle Interior Noise Based on Responses of the Basilar Membrane in the Human Ear. Appl. Acoust. 2021, 172, 107619. [Google Scholar] [CrossRef]
- Ghorbani, K.; Hasani, H.; Zarrebini, M.; Saghafi, R. An Investigation into Sound Transmission Loss by Polypropylene Needle-Punched Nonwovens. Alex. Eng. J. 2016, 55, 907–914. [Google Scholar] [CrossRef]
- Chen, J.; Li, H.; Yan, Z.; Zhang, Z.; Gong, J.; Guo, Y.; Zhang, J. Simulation, Measurement and Optimization of Sound Absorption in Gradient Impedance Fiber-Based Composites. Measurement 2023, 221, 113499. [Google Scholar] [CrossRef]
- Cao, L.; Fu, Q.; Si, Y.; Ding, B.; Yu, J. Porous Materials for Sound Absorption. Compos. Commun. 2018, 10, 25–35. [Google Scholar] [CrossRef]
- Sharma, S.; Sudhakara, P.; Singh, J.; Singh, S.; Singh, G. Emerging Progressive Developments in the Fibrous Composites for Acoustic Applications. J. Manuf. Process. 2023, 102, 443–477. [Google Scholar] [CrossRef]
- Yang, T.; Hu, L.; Xiong, X.; Petrů, M.; Noman, M.T.; Mishra, R.; Militký, J. Sound Absorption Properties of Natural Fibers: A Review. Sustainability 2020, 12, 8477. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, X.; Xin, F. Sound Absorption Performance of Helically Perforated Porous Metamaterials at High Temperature. Mater. Des. 2023, 225, 111437. [Google Scholar] [CrossRef]
- Liu, X.; Yu, C.; Xin, F. Gradually Perforated Porous Materials Backed with Helmholtz Resonant Cavity for Broadband Low-Frequency Sound Absorption. Compos. Struct. 2021, 263, 113647. [Google Scholar] [CrossRef]
- Kim, B.S.; Park, J. Double Resonant Porous Structure Backed by Air Cavity for Low Frequency Sound Absorption Improvement. Compos. Struct. 2018, 183, 545–549. [Google Scholar] [CrossRef]
- Dunne, R.; Desai, D.; Sadiku, R. A Review of the Factors That Influence Sound Absorption and the Available Empirical Models for Fibrous Materials. Acoust. Aust. 2017, 45, 453–469. [Google Scholar] [CrossRef]
- Li, X.; Liu, B.; Wu, Q. Enhanced Low-Frequency Sound Absorption of a Porous Layer Mosaicked with Perforated Resonator. Polymers 2022, 14, 223. [Google Scholar] [CrossRef]
- Huang, H.; Lim, T.C.; Wu, J.; Ding, W.; Pang, J. Multitarget Prediction and Optimization of Pure Electric Vehicle Tire/Road Airborne Noise Sound Quality Based on a Knowledge- and Data-Driven Method. Mech. Syst. Signal Process 2023, 197, 110361. [Google Scholar] [CrossRef]
- Kalinova, K. Sound Absorptive Light Comprising Nanofibrous Resonant Membrane Applicable in Room Acoustics. Build. Serv. Eng. Res. Technol. 2018, 39, 362–370. [Google Scholar] [CrossRef]
- Yang, D.; Wang, X.; Zhu, M. The Impact of the Neck Material on the Sound Absorption Performance of Helmholtz Resonators. J. Sound Vib. 2014, 333, 6843–6857. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, X.; Zhang, C.; Xin, F. A Novel Multiscale Porous Composite Structure for Sound Absorption Enhancement. Compos. Struct. 2021, 276, 114456. [Google Scholar] [CrossRef]
- Yang, T.; Mishra, R.; Horoshenkov, K.V.; Hurrell, A.; Saati, F.; Xiong, X. A Study of Some Airflow Resistivity Models for Multi-Component Polyester Fiber Assembly. Appl. Acoust. 2018, 139, 75–81. [Google Scholar] [CrossRef]
- Shao, X.; Yan, X. Sound Absorption Properties of Nanofiber Membrane-Based Multi-Layer Composites. Appl. Acoust. 2022, 200, 109029. [Google Scholar] [CrossRef]
- Miller, L.; Soulliere, K.; Sawyer-Beaulieu, S.; Tseng, S.; Tam, E. Challenges and Alternatives to Plastics Recycling in the Automotive Sector. Materials 2014, 7, 5883–5902. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Minemura, Y.; Nemoto, K.; Sugawara, H. Development of High-Performance All-Polyester Sound-Absorbing Materials. JSAE Rev. 1999, 20, 357–362. [Google Scholar] [CrossRef]
- Yao, Z.; Cai, J.; Wang, X.; Yang, Y.; He, Y. Application of Equivalent Diameter in Sound Absorption Performance Prediction of Non-Circular Sectional Polyester Fibers. Appl. Acoust. 2021, 182, 108238. [Google Scholar] [CrossRef]
- Volpe, V.; Lanzillo, M.S.; Molaro, A.; Affinita, G.; Pantani, R. Characterization of Recycled/Virgin Polyethylene Terephthalate Composite Reinforced with Glass Fiber for Automotive Applications. J. Compos. Sci. 2022, 6, 59. [Google Scholar] [CrossRef]
- Rutkevičius, M.; Austin, Z.; Chalk, B.; Mehl, G.H.; Qin, Q.; Rubini, P.A.; Stoyanov, S.D.; Paunov, V.N. Sound Absorption of Porous Cement Composites: Effects of the Porosity and the Pore Size. J. Mater. Sci. 2015, 50, 3495–3503. [Google Scholar] [CrossRef]
- Wang, W.; Liu, H.; Gu, W. A Novel Fabrication Approach for Improving the Mechanical and Sound Absorbing Properties of Porous Sound-Absorbing Ceramics. J. Alloys Compd. 2017, 695, 2477–2482. [Google Scholar] [CrossRef]
- Kim, K.Y.; Doh, S.J.; Im, J.N.; Jeong, W.Y.; An, H.J.; Lim, D.Y. Effects of Binder Fibers and Bonding Processes on PET Hollow Fiber Nonwovens for Automotive Cushion Materials. Fibers Polym. 2013, 14, 639–646. [Google Scholar] [CrossRef]
- Sivri, Ç.; Haji, A. Surface Coating of Needle-Punched Nonwovens with Meltblown Nonwovens to Improve Acoustic Properties. Coatings 2022, 12, 1092. [Google Scholar] [CrossRef]
- Čorak, I.; Tarbuk, A.; Đorđević, D.; Višić, K.; Botteri, L. Sustainable Alkaline Hydrolysis of Polyester Fabric at Low Temperature. Materials 2022, 15, 1530. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, W.; Zhang, J. Alkali Resistance of Poly(Ethylene Terephthalate) (PET) and Poly(Ethylene Glycol-Co-1,4-Cyclohexanedimethanol Terephthalate) (PETG) Copolyesters: The Role of Composition. Polym. Degrad. Stab. 2015, 120, 232–243. [Google Scholar] [CrossRef]
- Kalauni, K.; Pawar, S.J. A Review on the Taxonomy, Factors Associated with Sound Absorption and Theoretical Modeling of Porous Sound Absorbing Materials. J. Porous Mater. 2019, 26, 1795–1819. [Google Scholar] [CrossRef]
- Kim, H.S.; Ma, P.S.; Kim, B.K.; Kim, S.R.; Lee, S.H. Near-Field Effects on the Sound Transmission and Absorption of Elastic Micro-Perforated Plates in Impedance Tubes. J. Sound Vib. 2021, 499, 116001. [Google Scholar] [CrossRef]
- Corredor-Bedoya, A.C.; Acuña, B.; Serpa, A.L.; Masiero, B. Effect of the Excitation Signal Type on the Absorption Coefficient Measurement Using the Impedance Tube. Appl. Acoust. 2021, 171, 107659. [Google Scholar] [CrossRef]
- Kumagai, S.; Hirahashi, S.; Grause, G.; Kameda, T.; Toyoda, H.; Yoshioka, T. Alkaline Hydrolysis of PVC-Coated PET Fibers for Simultaneous Recycling of PET and PVC. J. Mater. Cycles Waste Manag. 2018, 20, 439–449. [Google Scholar] [CrossRef]
- Youn, S.; Hee Park, C. Development of Breathable Janus Superhydrophobic Polyester Fabrics Using Alkaline Hydrolysis and Blade Coating. Text. Res. J. 2019, 89, 959–974. [Google Scholar] [CrossRef]
- Soltani, P.; Norouzi, M. Prediction of the Sound Absorption Behavior of Nonwoven Fabrics: Computational Study and Experimental Validation. J. Sound Vib. 2020, 485, 115607. [Google Scholar] [CrossRef]


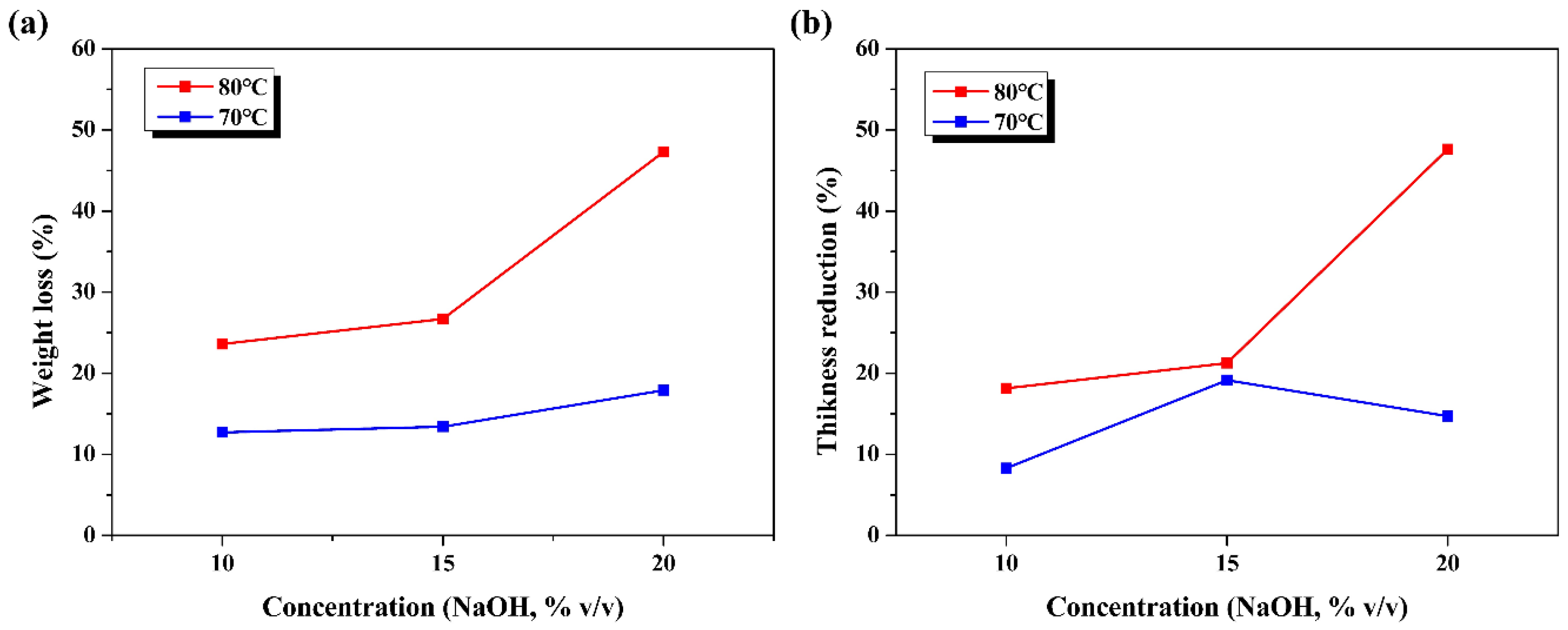





| Treatment Condition | Rate of Change | ||
|---|---|---|---|
| Temperature (°C) | NaOH Concentration (v/v%) | Weight Loss (%) | Thickness Reduction (%) |
| 70 | 10 | 12.66 | 8.29 |
| 15 | 13.40 | 19.15 | |
| 20 | 26.69 | 14.70 | |
| 80 | 10 | 17.89 | 18.15 |
| 15 | 23.57 | 21.25 | |
| 20 | 47.33 | 47.63 | |
| Treatment Condition | Volume of Pores of Change | |
|---|---|---|
| Temperature (°C) | NaOH Concentration (v/v%) | Adsorption Cumulative Volume of Pores (cm3/g) |
| 0 | 0 | 0.00058 |
| 70 | 10 | 0.00174 |
| 15 | 0.00093 | |
| 20 | 0.00205 | |
| 80 | 10 | 0.00145 |
| 15 | 0.00366 | |
| 20 | 0.00152 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, G.C.; Park, J.J.; Kang, E.H.; Lee, S.Y.; Huh, Y.; Lee, S.G. Study on the Sound Absorption Properties of Recycled Polyester Nonwovens through Alkaline Treatment and Dimple Processing. Surfaces 2024, 7, 238-250. https://doi.org/10.3390/surfaces7020016
Yu GC, Park JJ, Kang EH, Lee SY, Huh Y, Lee SG. Study on the Sound Absorption Properties of Recycled Polyester Nonwovens through Alkaline Treatment and Dimple Processing. Surfaces. 2024; 7(2):238-250. https://doi.org/10.3390/surfaces7020016
Chicago/Turabian StyleYu, Gyeong Cheol, Jeong Jin Park, Eun Hye Kang, Sun Young Lee, Youl Huh, and Seung Goo Lee. 2024. "Study on the Sound Absorption Properties of Recycled Polyester Nonwovens through Alkaline Treatment and Dimple Processing" Surfaces 7, no. 2: 238-250. https://doi.org/10.3390/surfaces7020016







