Review on Corrosion, Tribocorrosion and Osseointegration of Titanium Alloys as Biomaterials
Abstract
:1. Introduction
2. Corrosion of Titanium Alloys as Biomaterials
3. Tribocorrosion of Titanium Alloys as Biomaterials
4. Osseointegration of Titanium Alloys as Biomaterials
5. Conclusions and Future Perspectives
- Development of corrosion resistant porous β titanium alloys by 3D printing technique. This technique allows conducting controllable and precise fabrication to obtain materials with a suitable Young’s modulus and controlled porosity that provide channels for bone growth [106,107]. Nevertheless, due to their inhomogeneity and the presence of cavities, β titanium alloys may be less resistant to corrosion in the same way as porous titanium alloys fabricated by the powder metallurgy [108,109,110,111]. Overcoming this drawback and fabricating corrosion resistant β titanium alloys by 3D printing techniques could be the subject of further research;
- Development of soft computing techniques to design new β titanium alloys with suitable properties. Using soft computing techniques is the way to accelerate the development of new materials and reduce experimental studies [33]. CALPHAD (Calculation of PHAse Diagrams) approach that is a computational method to design new materials has been successfully used to design Ti-Zr-Ta alloys with high yield strength and low elastic modulus [112]. Other systems have been investigated by CALPHAD approach, among which include the two alloys Ti-Ni-Sn [113] and Ti-Zr-Sn [114]. In another work, the CALPHAD approach was combined with artificial intelligence algorithms to explore novel composition of the Ti-Nb-Zr-Sn system. This approach allowed the authors to determine suitable chemical compositions and temperatures for heat treatments that allow the formation of β phase while avoiding/minimizing formation of ω-phase [115];
- Concerning osseointegration, there exist many techniques using additive or substrative mater processes (physical and chemical deposition methods, laser texturing, chemical and mechanical etching, etc.) that allow modifying topography and/or wettability of the implant material. Despite numerous studies and publications, one can say that, currently, the effect of roughness on osseointegration is not clear and contradictory results are still published even though a beneficial effect of nanostructuring is widely reported.
Funding
Data Availability Statement
Conflicts of Interest
References
- Park, J.; Lakes, R.S. Biomaterials: An Introduction; Springer: New York, NY, USA, 2007; p. 11. [Google Scholar]
- Oldani, C.; Dominguez, A. Titanium as a Biometal for Implants. In Recent Advences in Arthroplasty; IntechOpen Book Series; Fokter, S., Ed.; IntechOpen: London, UK, 2012; pp. 149–161. [Google Scholar]
- Amukarimi, S.; Masoud Mozafari, M. Biodegradable magnesium-based biomaterials: An overview of challenges and opportunities. Med. Comm. 2021, 2, 123–144. [Google Scholar] [CrossRef]
- Huang, S.; Wang, B.; Zhang, X.; Lu, F.; Wang, Z.; Tian, S.; Li, D.; Yang, J.; Cao, F.; Cheng, L.; et al. High-purity weight-bearing magnesium screw: Translational application in the healing of femoral neck fracture. Biomaterials 2020, 238, 119829. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, D.; Ohodnicki, J.; Lee, B.; Roy, A.; Yao, R.; Chen, S.; Dong, Z.; Heineman, W.R.; Kumta, P.N. In vitro and in vivo evaluation of multi-phase ultra-high ductility Mg-Li-Zn alloys for cardiovascular stent applications. ACS Biomater. Sci. Eng. 2018, 4, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Shen, L.; Chen, J.; Zhang, X.; Kwak, M.; Wu, Y.; Fan, R.; Zhang, L.; Pei, J.; Yuan, G.; et al. A promising biodegradable magnesium alloy suitable for clinical vascular stent application. Sci. Rep. 2017, 7, 46343. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Li, Z.; Zhao, H.; Pan, Y.; Pavlinich, S.; Liu, X.; Li, X.; Zheng, Y.; Li, L. Microstructure, mechanical properties, corrosion behavior and biocompatibility of as-extruded biodegradable Mg–3Sn–1Zn–0.5Mn alloy. J. Mater. Sci. Technol. 2016, 32, 874–882. [Google Scholar] [CrossRef]
- Xia, D.; Liu, Y.; Wang, S.; Zeng, R.-C.; Liu, Y.; Zheng, Y.; Zhou, Y. In vitro and in vivo investigation on biodegradable Mg-Li-Ca alloys for bone implant application. Sci. China Mater. 2019, 62, 256–272. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, W.; Liu, J.; Wang, L.; Tang, Y.; Wang, K. A review on magnesium alloys for biomedical applications. Front. Bioeng. Biotechnol. 2022, 10, 953344. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, M.; Liu, Y.; Xie, J.; Wu, Y.; Yang, S. Effect of Heat Treatment on Microstructure and Properties of Ti-6Al-4V-0.5Si alloy. Proc. Manufact. 2019, 37, 592–598. [Google Scholar] [CrossRef]
- Zhao, Z.-Y.; Li, L.; Bai, P.K.; Jin, Y.; Wu, L.Y.; Li, J.; Guan, R.G.; Qu, H.Q. The Heat Treatment Influence on the Microstructure and Hardness of TC4 Titanium Alloy Manufactured via Selective Laser Melting. Materials 2018, 11, 1318. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.; Asokamania, R.; Gogia, A.K. Ti based biomaterials the ultimate choice for orthopaedic implants—A review. Prog. Mat. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Jaber Razavi Arab, S.; Aghajani, H. Wear Behavior of Pure Titanium Coated With WC-Co by the Use of Electrospark Deposition Method. J. Tribol. 2019, 141, 051605. [Google Scholar] [CrossRef]
- Takadoum, J. Tribological behaviour of alumina sliding on several kinds of materials. Wear 1993, 170, 285–290. [Google Scholar] [CrossRef]
- Weng, W.; Biesiekierski, A.; Li, Y.; Wen, C. Effects of selected metallic and interstitial elements on the microstructure and mechanical properties of beta titanium alloys for orthopedic applications. Materialia 2019, 6, 100323. [Google Scholar] [CrossRef]
- Maa, Y.; Du, Z.; Cui, X.; Cheng, G.; Liu, T.; Liu, G.; Gong, T.; Liu, H.; Wang, X.; Chen, Y. Effect of cold rolling process on microstructure and mechanical properties of high strength β titanium alloy thin sheets. Prog. Nat. Sci. Mater. Int. 2018, 28, 711–717. [Google Scholar] [CrossRef]
- Sidhu, S.S.; Singh, H.; Gepreel, M.A.H. A review on alloy design, biological response, and strengthening of β-titanium alloys as biomaterials. Mater. Sci. Eng. C 2021, 121, 111661. [Google Scholar] [CrossRef] [PubMed]
- Furuta, T.; Kuramoto, S.; Hwang, J.; Nishino, K.; Saito, T.; Niinomi, M. Mechanical properties and phase stability of Ti-Nb-Ta-Zr-O alloys. Mater. Trans. 2007, 48, 1124–1130. [Google Scholar] [CrossRef]
- Zhu, J.; Zeng, Q.; Fu, T. An updated review on TiNi alloy for biomedical applications. Corros. Rev. 2019, 37, 539–552. [Google Scholar] [CrossRef]
- Hu, T.; Chu, C.; Xin, Y.; Wu, S.; Yeung, K.W.; Chu, P.K. Corrosion products and mechanism on TiNi shape memory alloy in physiological environment. J. Mater. Res. 2010, 25, 350–358. [Google Scholar] [CrossRef]
- Teixeira, R.L.P.; De Lacerda, J.C.; Conceicao, I.C.; Da Silva, S.N.; Siqueira, G.O.; Moura Filha, F. The effects of niobium on the bioactivity of Ni-Ti-Al-Nb shape memory alloys. Arch. Metall. Mater. 2021, 66, 437–442. [Google Scholar]
- Jani, J.M.; Leary, M.; Subic, A.; Gibson, M. A review of shape memory alloy research, applications and opportunities. Mater. Des. 2014, 56, 1078–1113. [Google Scholar] [CrossRef]
- Wever, D.; Veldhuizen, A.; Sanders, M.; Schakenraad, J.; Van Horn, J. Cytotoxic, allergic and genotoxic activity of a nickel-titanium alloy. Biomaterials 1997, 18, 1115e20. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.H.; Chan, C.W.; Man, H.C.; Waugh, D.G.; Lawrence, J. NiTi shape memory alloy with enhanced wear performance by laser selective area nitriding for orthpeadic applications. Surf. Coat. Technol. 2017, 309, 1015–1022. [Google Scholar] [CrossRef]
- Nasakina, E.O.; Konushkin, S.V.; Baskakova, M.I.; Fedyuk, I.M.; Sergienko, K.V.; Baikin, A.S.; Kaplan, M.A.; Sevostyanov, M.A.; Kolmakov, A.G. The production of Thin wire of Ti-Nb-Ta-Zr shape memory alloy for medical devices. J. Mat. SC Eng. 2018, 7, 100403. [Google Scholar] [CrossRef]
- Li, Q.; Kong, L.; Xu, S.; Gong, H.; Li, Y. Corrosion resistance and cytocompatibility alloy for biomedical applications. J. Mat. Res. Technol. 2023, 26, 2352–2357. [Google Scholar] [CrossRef]
- Dzogbewu, T. Additive manufacturing of porrous Ti-based alloys for biomedical applications- A review. J. New Gener. Sci. 2017, 15, 277–294. [Google Scholar]
- Cheng, A.; Humayun, A.; Cohen, D.J.; Boyan, B.D.; Schwart, Z. Additively manufactured 3D porous Ti-6Al-4V constructs mimic trabecular bone structure and regulate osteoblast proliferation, differentiation and local factor production in a porosity and surface roughness dependent manner. Biofabrication 2014, 6, 045007. [Google Scholar] [CrossRef]
- Pattanayak, D.K.; Fukuda, A.; Matsushita, t.; Takemoto, M.; Fujibayashi, S.; Sasaki, K.; Nishida, N.; Nakamura, T.; Kokubo, T. Bioactive Ti metal analogous to human cancellous bone: Fabrication by selective laser melting and chemical treatments. Acta Bimateriala 2011, 7, 1398–1406. [Google Scholar] [CrossRef]
- Barbas, A.; Bonnet, A.S.; Lipinski, P.; Pesci, R.; Dubois, G. Development and mechanical characterization of porous substitutes. J. Mech. Beh. Biomed. Mat. 2012, 9, 34–44. [Google Scholar] [CrossRef]
- Ferraris, S.; Spriano, S. Porous titanium by additive manufacturing: A focus on surfaces for bone integration. Metals 2021, 11, 1343. [Google Scholar] [CrossRef]
- Matena, J.; Peterson, S.; Gieseke, M.; Kampmann, A.; Tesk, M.; Beyerbach, M.; Escobar, H.M.; Haferkamp, H.; Gellrich, N.-C.; Nolte, I. SLM produced porous titanium implant improvements for enhanced vascularization and osteoblast seeding. Int. J. Mol. Sci. 2015, 16, 7478–7492. [Google Scholar] [CrossRef]
- Traini, T.; Mangano, C.; Sammons, R.L.; Mangano, F.; Macchi, A.; Piatelli, A. Direct laser metal sintering as a new approach to fabrication of an isoelastic functionally graded material for manufacture of porous titanium dental implants. Materials 2008, 24, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Farber, E.; Orlov, A.; Borisov, E.; Repnin, A.; Kuzin, S.; Golubkov, N.; Popovich, A. TiNi alloy lattice Structures with negative poisson’s ratio: Computer simulation and experimental results. Metals 2022, 12, 1476. [Google Scholar] [CrossRef]
- Kobayashi, E.; Wang, T.J.; Doi, H.; Yoneyama, T.; H Hamanaka, H. Mechanical properties and corrosion resistance of Ti-6Al-7Nb alloy dental castings. J. Mater Sci. Mater Med. 1998, 9, 567–574. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedec applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.Y.; Scully, J.R. Corrosion and passivity of Ti-13%Nb-13%Cr in comparison to other biomedical implants alloys. Corrosion 1997, 53, 965–976. [Google Scholar] [CrossRef]
- Carquigny, S.; Takadoum, J.; Ivanescu, S. Corrosion and tribocorrosion study of 316L steel, Ti–6Al–4V and Ti–10Zr–10Nb–5Ta. Tribol. Mater. Surf. Interfaces 2019, 13, 112–119. [Google Scholar] [CrossRef]
- Li, Z.; Lai, W.; Wang, B.; Tong, X.; You, D.; Li, W.; Wang, X. A novel Ti42.5Zr42.5Nb5Ta10 multi-principal element alloy with excellent properties for biomedical applications. Intermettalics 2022, 151, 107731. [Google Scholar] [CrossRef]
- Khan, M.A.; Williams, R.L.; Williams, D.F. In-vitro corrosion and wear of titanium alloys in the biological environment. Biomaterials 1996, 17, 2117–2126. [Google Scholar] [CrossRef]
- Cheng, X.; Roscoe, S.G. Corrosion behavior of titanium in the presence of calcium phosphate and serum proteins. Biomaterials 2005, 26, 7350–7356. [Google Scholar] [CrossRef]
- Wang, W.; Mohammadi, F.; Alfantazi, A. Corrosion behaviour of niobium in phosphate buffered saline solutions with different concentrations of bovine serum albumin. Corros. Sci. 2012, 57, 11–21. [Google Scholar] [CrossRef]
- Williams, R.L.; Brown, S.A.; Merritt, K. Electrochemical studies on the influence of proteins on the corrosion of implant alloys. Biomaterials 1988, 9, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Runa, M.J.; Mathew, M.T.; Rocha, L.A. Tribocorrosion response of Ti6Al4V alloys commonly used in femoral stems. Tribol. Int. 2013, 68, 85–93. [Google Scholar] [CrossRef]
- Karimi, S.; Nickchi, T.; Alfantazi, A. Effects of bovine serum albumin on the corrosion behaviour of AISI 316L, Co–28Cr–6Mo, and Ti–6Al–4V alloys in phosphate buffered saline solutions. Cor. Sci. 2011, 53, 3262–3272. [Google Scholar] [CrossRef]
- Dragus, L.; Benea, L.; Simionescu, N.; Ravoiu, A.; Neaga, V. Effect of the inflammatory conditions and albumin presence on the corrosion behavior of grade 5 Titanium alloy in saliva biological solution. IOP Conf. Ser. Mater. Sci. Eng. 2019, 572, 012005. [Google Scholar] [CrossRef]
- Contu, F.; Elsener, B.; Böhni, H. Serum effect on the electrochemical behaviour of titanium, Ti-6A-14V and Ti-6Al-7Nb alloys in sulphuric acid and sodium hydroxide. Corros. Sci. 2004, 46, 2241–2254. [Google Scholar] [CrossRef]
- Hedberg, Y.S. Role of proteins in the degradation of relatively inert alloys in the human body. NPJ Mater Degrad. 2018, 2, 26. [Google Scholar] [CrossRef]
- Takadoum, J. Materials and Surface Engineering in Tribology; Willey: New York, NY, USA, 2008; pp. 98–108. [Google Scholar]
- Da Silva Vieira Marques, I.; Fernanda Alfaro, M.; da Cruz, N.C.; Mesquita, M.F.; Takoudis, C.; Sukotjo, C.; Mathew, M.T.; Barao, V.A.R. Tribocorrosion behavior of biofunctional titanium oxide films produced by micro-arc oxidation: Synergism and mechanisms. J. Mech. Behav. Biomed. Mater. 2016, 60, 8–21. [Google Scholar] [CrossRef]
- Henry, P.; Takadoum, J.; Bercot, P. Corrosion of 316L stainless steel and TA6V4 alloy in H2SO4 media. Corros. Sci. 2009, 51, 1308–1314. [Google Scholar] [CrossRef]
- Takadoum, J.; Igartua, A. Phenomena of tribocorrosion in medical and industrial sectors. In Testing Tribocorrosion of Passivating Materials Supporting Research and Industrial Innovation; Celis, J.P., Ponthiaux, P., Eds.; Routledge: Leeds, UK, 2012; pp. 14–28. [Google Scholar]
- Longhofer, L.K.; Alexander Chong, A.; Strong, N.M.; Wooley, P.H.; Yang, S.Y. Specific material effects of wear-particle-induced inflammation and osteolysis at the boneeimplant interface: A rat model. J. Orthop. Transl. 2017, 8, 5–11. [Google Scholar]
- Hacisalihoglu, I.; Samancioglu, A.; Yildiz, F.; Purcek, G.; Alsaran, A. Tribocorrosion properties of different type titanium alloys in simulated body fluid. Wear 2015, 332–333, 679–686. [Google Scholar] [CrossRef]
- Hiromoto, S.; Mishler, S. The influence of proteins on the fretting–corrosion behaviour of a Ti6Al4V alloy. Wear 2006, 261, 1002–1011. [Google Scholar] [CrossRef]
- Brown, S.A.; Merritt, K. Fretting corrosion of plates and screws: An in vitro test method. In Corrosion and Degradation of Implant Materials: Second Symposium; Griffing, C.D., Ed.; ASTM: Philadelphia, PA, USA, 1985; pp. 105–116. [Google Scholar]
- Liamas, E.; Thomas, O.R.T.; Muñoz, A.I.; Zhang, Z.J. Tribocorrosion behaviour of pure titanium in bovine serum albumin solution: A multiscale study. J. Mech. Behav. Biomed. Mater. 2020, 102, 103511. [Google Scholar] [CrossRef] [PubMed]
- Balza, I.C.; Zujur, D.; Gil, L.; Subero, R.; Dominguez, E.; Delvasto, P.; Alvarez, J. Sandblasting as a surface modification technique on titanium alloys for biomedical applications: Abrasive particle behaviour. IOP Conf. Ser. Mater. Sci. Eng. 2013, 45, 012004. [Google Scholar] [CrossRef]
- Nicolas-Silvente, A.I.; Velasco-Ortega, E.; Ortiz-Garcia, I.; Monsalve-Guil, L.; Gil, J.; Jimenez-Guerra, A. Influence of the Titanium Implant Surface Treatment on the Surface Roughness and Chemical Composition. Materials 2020, 13, 314. [Google Scholar] [CrossRef]
- Velasco-Ortega, E.; Ortiz-Garcia, I.; Jimenez-Guerra, A.; Numez-Marquez, E.; Moreno-Munoz, J.; Rondon-Romero, J.-L.; Cabanillas-Balsera, D.; Mnsalve-Guil, L. Osseointegration of Sandblasted and Acid-Etched Implant Surfaces. A Histological and Histomorphometric Study in the Rabbit. Int. J. Mol. Sci. 2021, 22, 8507. [Google Scholar] [CrossRef]
- Velasco-Ortega, E.; Ortiz-García, I.; Jiménez-Guerra, A.; Loreto Monsalve-Guil, L.; Fernando Munoz-Guzón, F.; Perez, R.A.; Gil, F.J. Comparison between Sandblasted Acid-Etched and Oxidized Titanium Dental Implants: In Vivo Study. Int. J. Mol. Sci. 2019, 20, 3267. [Google Scholar] [CrossRef]
- Li, J.; Zhou, P.; Shokouh Attarilar, S.; Hongyuan Shi, H. Innovative Surface Modification Procedures to Achieve Micro/Nano-Graded Ti-Based Biomedical Alloys and Implants. Coatings 2021, 11, 647. [Google Scholar] [CrossRef]
- Bressan, E.; Sbricoli, L.; Guazzo, R.; Ilaria Tocco, I.; Roman, M.; Vindigni, V.; Edoardo Stellini, E.; Chiara Gardin, C.; Ferroni, L.; Sivolella, S.; et al. Nanostructured Surfaces of Dental Implants. Int. J. Mol. Sci. 2013, 14, 1918–1931. [Google Scholar] [CrossRef]
- Le Guehennec, L.; Martin, F.; Lopez Heredia, M.A.; Louarn, G.; Amouriq, Y.; Jacques Cousty, J.; Layrolle, P. Osteoblastic cell behavior on nanostructured metal implants. Nanomedicine 2008, 3, 61–71. [Google Scholar] [CrossRef]
- Böker, K.O.; Kleinwort, F.; Klein-Wiele, J.H.; Simon, P.; Jäckle, K.; Taheri, S.; Lehmann, W.; Schilling, A.F. Laser Ablated Periodic Nanostructures on Titanium and Steel Implants Influence Adhesion and Osteogenic Differentiation of Mesenchymal Stem Cells. Materials 2020, 13, 3526. [Google Scholar] [CrossRef]
- Dominguez-trujillo, C.; Peon, E.; Chicardi, E.; Perz, H.; Ridriguez-Ortiz, J.A.; Pavon, J.J.; Garcia-Couce, J.; Galvin, J.C.; Garcia-Moreno, F.; Torres, Y. Sol-gel deposition of hydroxyapatite coatings on porous titanium for biomedical applications. Surf. Coat. Technol. 2018, 333, 158–162. [Google Scholar] [CrossRef]
- Urbaanski, W.; Marycz, K.; Krzak, J.; Pezowicz, C.; Dragan, S.F. Cytokine induction of sol-gel derived TiO2 and SiO2 coatings on metallic substrates after implantation to rat femur. Int. J. Nanomed. 2017, 12, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Goller, G. The effect of bond coat on mechanical properties of plasma sprayed bioglass-titanium coatings. Ceram. Int. 2004, 30, 351–355. [Google Scholar] [CrossRef]
- Lavenus, S.; Trichet, V.; Hoornartet, A.; Le Chevalier, S.; Louarn, G.; Layrolle, P. Cell differentiation and osseointegration influenced by nanoscale anodized titanium surfaces. Nanomedecine 2012, 7, 967–980. [Google Scholar] [CrossRef] [PubMed]
- Gabor, R.; Cvrcek, L.; Doubkova, M.; Nehasil, V.; Hlinka, J.; Unucka, P.; Buril, M.; Podeprelova, A.; Seidlerova, J.; Bacakova, L. Hybrid coatings for orthopaedic implants formed by physical vapour deposition and microarc oxidation. Mater. Des. 2022, 219, 110811. [Google Scholar] [CrossRef]
- Braceras, I.; Alava, J.I.; Onate, J.I.; Brizuela, M.; Garcia-Luis, A.; Garagorri, N.; Viviente, J.L.; Maeztu, M.A. Improved osseointegration in ion implantation treated dental implants. Surf. Coat. Technol. 2002, 158–159, 28–32. [Google Scholar] [CrossRef]
- Rautray, T.R.; Narayanan, R.; Kwon, T.Y.; Kim, K.H. Surface Modification of Titanium and Titanium Alloys by Ion Implantation. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 93, 581–591. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Wang, T.; Zhang, X.; Li, Z.; Ding, L.; Li, J.; Xiao, C.; Han, F.; Li, B. Enhanced osseointegration of titanium implants by surface modification with silicon doped titania nanotubes. Int. J. Nanomed. 2020, 15, 8583–8594. [Google Scholar]
- Lange, R.; Luthen, F.; Beck, U.; Baumann, A.; Nebel, B. Cell extracellular matrix interactions and physicochemical characteristics of titanium surfaces depend on the roughness of the material. Biomol. Eng. 2002, 19, 255–261. [Google Scholar] [CrossRef]
- Migita, S.; Yamaguchi, T. Initial adhesion behavior of osteoblast on titanium with sub-micron scale roughness. Recent Prog. Mater. 2020, 2. [Google Scholar] [CrossRef]
- Boyen, B.D.; Lossdorfer, S.; Wang, L.; Zhao, G.; Lohmann, C.H.; Cochran, D.L.; Schwartz, Z. Osteoblasts generate an osteogenic microenvironment when gown on surface with rough microtopographies. Eur. Cell Mat. 2003, 6, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Majhy, B.; Priyadarshini, P.; Sen, A.K. Effect of surface energy and roughness on cell adhesion and growth- facile surface modification for enhance cell culture. RSC Adv. 2021, 11, 15467. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, Z.; Raz, P.; Zhao, G.; Barak, Y.; Tauber, M.; Yao, H.; Boyan, B.D. Effect of Micrometer-Scale Roughness of the Surface of Ti6Al4V Pedicle Screws in Vitro and in Vivo. J. Bone Jt. Surg. Am. 2008, 90, 2485–2498. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.C.; Stanford, C.M.; Wightman, J.P.; Draughn, R.A.; Zaharias, R. Characterizations of titanium implant surfaces. III. J. Biomed. Mater. Res. 1994, 28, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Zareidoost, A.; Yousef, M.; Ghaseme, B.; Amanzadeh, A. The relationship of surface roughness and cell response of chemical surface modification of titanium. J. Mater. Sci. Med. 2012, 23, 1479–1488. [Google Scholar] [CrossRef] [PubMed]
- Sammons, R.L.; Lumbikanonda, N.; Gross, M.; Cantzler, P. Comparison of osteoblast spreading on microstructured dental implant surfaces and cell behaviour in an explant model of osseointegration. A scanning electron microscopic study. Clin. Oral. Implan. Res. 2005, 16, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; McLachlan, T.; Olivares-Navarette, R.; Cai, Y.; Berner, S.; Tannenbaum, R.; Schwartz, Z.; Sandhage, K.H.; Boyan, B.D. The effects of combined on micron-/submicron-scale surface roughness and nanoscale features on cell profileration and differentiation. J. Biomater. 2011, 32, 3395–3403. [Google Scholar] [CrossRef]
- Rupp, F.; Scheideler, L.; Olshanska, N.; de Wild, M.; Wieland, M.; Geis-Gerstorfer, J. Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. J. Biomed. Mater. Res. A 2006, 72, 323–334. [Google Scholar] [CrossRef]
- Klein, M.O.; Bijelic, A.; Ziebart, T.; Koch, F.; Kammerer, P.W.; Wielend, M.; Konerding, M.A.; Al-Nawas, B. Submicron scale- structured hydrophilic titanium surfaces promote early osteogenic gene response for cell adhesion and cell differentiation. Clin. Implant. Dent. Relat. Res. 2013, 15, 166–175. [Google Scholar] [CrossRef]
- Migita, S.; Okuyama, S.; Araki, K. Sub-micrometer scale surface roughness of titanium reduces fibroblast function. J. Appl. Biomat. Funct. Mater. 2016, 14, 65–69. [Google Scholar] [CrossRef]
- Migita, S.; Araki, K. Effect of nanometer scale surface roughness of titanium for osteoblast function. Bioengineering 2017, 4, 162–170. [Google Scholar] [CrossRef]
- Sugar, P.; Ludrovcova, B.; Hubalek Kalbacova, M.; Sugarova, J.; Sahul, M.; Jaroslav Kovacik, J. Laser Surface Modification of Powder Metallurgy-Processed Ti-Graphite Composite Which Can Enhance Cells’ Osteo-Differentiation. Materials 2021, 14, 6067. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Wennerberg, A. Oral implant surfaces: Part 1-review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int. J. Prosthodont. 2000, 17, 536–543. [Google Scholar]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Ranella, A.; Barberoglou, M.; Bakogianni, S.; Fotakis, C.; Stratakis, E. Tuning cell adhesion by controlling the roughness and wettability of 3D micro/nano silicon structures. Acta Biomater. 2010, 6, 2711–2720. [Google Scholar] [CrossRef] [PubMed]
- Nyoung, D.; Ko, W.; Rae, H.; Jin, S.; Lee, D.; Ho, S.; Haeng, J.; Woo, Y.; Grace, L.; Lee, D.; et al. Titanium dental implants surface-immobilized with gold nanoparticles as osteoinductive agents for rapid osseointegration. J. Colloid Interface Sci. 2016, 469, 129–137. [Google Scholar]
- Salou, L.; Hoornaert, A.; Stanovici, J.; Briand, S.; Louarn, G.; Layrolle, P. Comparative bone tissue integration of nanostructured and microroughened dental implants. Nonomedicine 2015, 10, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Lavenu, S.; Berreur, M.; Trichet, V.; Pilet, P.; Louarn, G.; Layolle, P. Adhesion and osteogenic differentiation of human Mesenchymal stem cells on titanium nanopores. Eur. Cel. Mater. 2011, 22, 84–96. [Google Scholar] [CrossRef]
- Gittens, R.A.; Olivares-Navarrete, R.; Schwartz, Z.; Barbara, D.; Boyan, B.D. Implant Osseointegration and the Role of Microroughness and Nanostructures: Lessons for Spine Implants. Acta Biomater. 2014, 10, 3363–3371. [Google Scholar] [CrossRef]
- Trueba, P.; Giner, M.; Rodríguez, A.; Beltran, A.M.; Amado, J.M.; Montoya-García, M.J.; Rodríguez-Albelo, L.M.; Torres, Y. Tribo-mechanical and cellular behavior of superficially modified porous titanium samples using femtosecond laser. Surf. Coat. Technol. 2021, 422, 127555. [Google Scholar] [CrossRef]
- Wu, Y.N.; Law, J.B.K.; He, A.Y.; Low, H.Y.; Hui, J.H.P.; Lim, C.T.; Yang, Z.; Lee, E.H. Substrate topography determines the fate of chondrogenesis from human mesenchymal stem cells resulting in specific cartilage phenotype formation. Nanomedicine 2014, 10, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Lackington, W.-A.; Schweizer, P.; Khokhlova, M.; Cancellieri, C.; Guimond, S.; Chopard-Lallier, A.L.; Hofstetter, J.; Schmutz, P.; Maeder, X.; Rottmar, M. Femtosecond Laser-Texturing the Surface of Ti-Based Implants to Improve Their Osseointegration Capacity. Adv. Mater. Interfaces 2022, 9, 2201164. [Google Scholar] [CrossRef]
- Marticorena, M.; Corti, G.; Olmedo, D.; Guglielmotti, M.B.; Duhalde, S. Laser surface modification of Ti implants to improve osseointegration. J. Phys. Conf. Ser. 2007, 59, 662–665. [Google Scholar] [CrossRef]
- Coathup, M.J.; Blunn, G.W.; Mirhosseini, N.; Erskine, K.; Liu, Z.; Garrod, D.R.; Li, L. Controlled Laser Texturing of Titanium Results in Reliable Osteointegration. J Orthop. Res. 2017, 35, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Zwahr, C.; Welle, A.; Weingärtner, T.; Heinemann, C.; Kruppke, B.; Gulow, N.; Holthaus, M.G.; Andrés Fabián Lasagni, A.F. Ultrashort Pulsed Laser Surface Patterning of Titanium to Improve Osseointegration of Dental Implants. Adv. Eng. Mater. 2019, 21, 1900639. [Google Scholar] [CrossRef]
- Zwahr, C.; Günther, D.; Brinkmann, T.; Gulow, N.; Oswald, S.; Holthaus, M.G.; Lasagni, A.F. Laser Surface Pattering of Titanium for Improving the Biological Performance of Dental Implants. Adv. Healthc. Mater. 2017, 6, 201600858. [Google Scholar] [CrossRef]
- Lang, N.P.; Salvi, G.E.; Huynha-Ba, G.; Ivanovski, S.; Donos, N.; Bosshardt, D.D. Early osseointegration to hydrophilic and hydrophobic implant surfaces in humans. Cli. Oral Implant. Res. 2011, 22, 349–356. [Google Scholar] [CrossRef]
- Sartoretto, S.C.; Calasans-Maia, J.; da Costa, Y.O.; Louro, R.S.; Monica, J.M.G.; Calasans-Maia, D. Accelerated healing period with hydrophilic implant placed in sheep tibia. Braz. Dent. J. 2017, 28, 559–565. [Google Scholar] [CrossRef]
- Arima, Y.; Iwata, H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials 2007, 28, 3074–3082. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of metallic biomaterials: A review. Materials 2019, 12, 407. [Google Scholar] [CrossRef]
- Shen, Y.W.; Tsai, Y.S.; Hsu, J.T.; Shie, M.Y.; Huang, H.L.; Fuh, L.J. Biomechanical Analyses of Porous Designs of 3D-Printed Titanium Implant for Mandibular Segmental Osteotomy Defects. Materials 2022, 15, 576. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Jiang, W.; Ma, X.; Wang, Z.; Sah, R.L.; Wang, J.; Sun, Y. Nanoscale Morphologies on the Surface of 3D-Printed Titanium Implants for Improved Osseointegration: A Systematic Review of the Literature. Int. J. Nanomed. 2023, 18, 4171–4191. [Google Scholar] [CrossRef] [PubMed]
- Gai, X.; Bai, Y.; Li, S.; Wang, L.; Ai, S.; Hao, Y.; Yang, R.; Dai, K. Review on Corrosion Characteristics of Porous Titanium Alloys Fabricated by Additive Manufacturing. J. Shanghai Jiaotong Univ. 2021, 26, 416–430. [Google Scholar] [CrossRef]
- Fojt, J.; Joska, L.; Málek, J. Corrosion behaviour of porous Ti–39Nb alloy for biomedical applications. Corros. Sci. 2013, 71, 78–83. [Google Scholar] [CrossRef]
- Xu, W.; Lu, X.; Zhang, B.; Liu, C.; Lv, S.; Yang, S.; Qu, X. Effects of Porosity on Mechanical Properties and Corrosion Resistances of PM-Fabricated Porous Ti-10Mo Alloy. Metals 2018, 8, 188. [Google Scholar] [CrossRef]
- Tjandra, J.; Alabort, E.; Barba, D.; Pedrazzini, S. Corrosion, fatigue and wear of additively manufactured Ti alloys for orthopaedic implants. Mater. Sci. Technol. 2023, 39, 2951–2965. [Google Scholar] [CrossRef]
- Wu, R.; Yi, Q.; Lei, S.; Dai, Y.; Lin, J. Design of Ti-Zr-Ta Alloys with Low Elastic Modulus Reinforced by Spinodal Decomposition. Coatings 2022, 12, 756. [Google Scholar] [CrossRef]
- Gürth, M.; Grytsiv, A.; Vrestal, J.; Romaka, V.V.; Giester, G.; Bauer, E.; Rogel, P. On the constitution and thermodynamic modelling of the system Ti–Ni–Sn. RSC Adv. 2015, 5, 92270–92291. [Google Scholar] [CrossRef]
- Tan, J.; Xu, G.; Tao, X.; Chen, F.; Cui, Y.; Zhou, L. CALPHAD assessment of bio-oriented Ti–Zr–Sn system and experimental validation in Ti/Zr-rich alloys. Calphad 2019, 67, 101686. [Google Scholar] [CrossRef]
- Jha, R.; Dulikravich, G.S. Discovery of New Ti-Based Alloys Aimed at Avoiding/Minimizing Formation of α” and ω-Phase Using CALPHAD and Artificial Intelligence. Metals 2021, 11, 15. [Google Scholar] [CrossRef]
- Raimbault, O.; Benayoun, S.; Karine Anselme, K.; Mauclair, C.; Bourgade, T.; Kietzig, A.-M.; Girard-Lauriault, P.-L.; Valette, S.; Donnet, C. The effects of femtosecond laser-textured Ti-6Al-4V on wettability and cell response. Mater. Sci. Eng. C 2016, 69, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Zhang, Y.; Hu, R.; Shi, B.; Zhang, L.; Huang, Q.; Yang, Y.; Tang, P.; Lin, C. Advanced surface engineering of titanium materials for biomedical applications: From static modification to dynamic responsive regulation. Bioact. Mater. 2023, 27, 15–57. [Google Scholar] [CrossRef] [PubMed]
- Lupi, S.M.; Torchia, M.; Rizzo, S. Biochemical Modification of Titanium Oral Implants: Evidence from In Vivo Studies. Materials 2021, 14, 2798. [Google Scholar] [CrossRef] [PubMed]

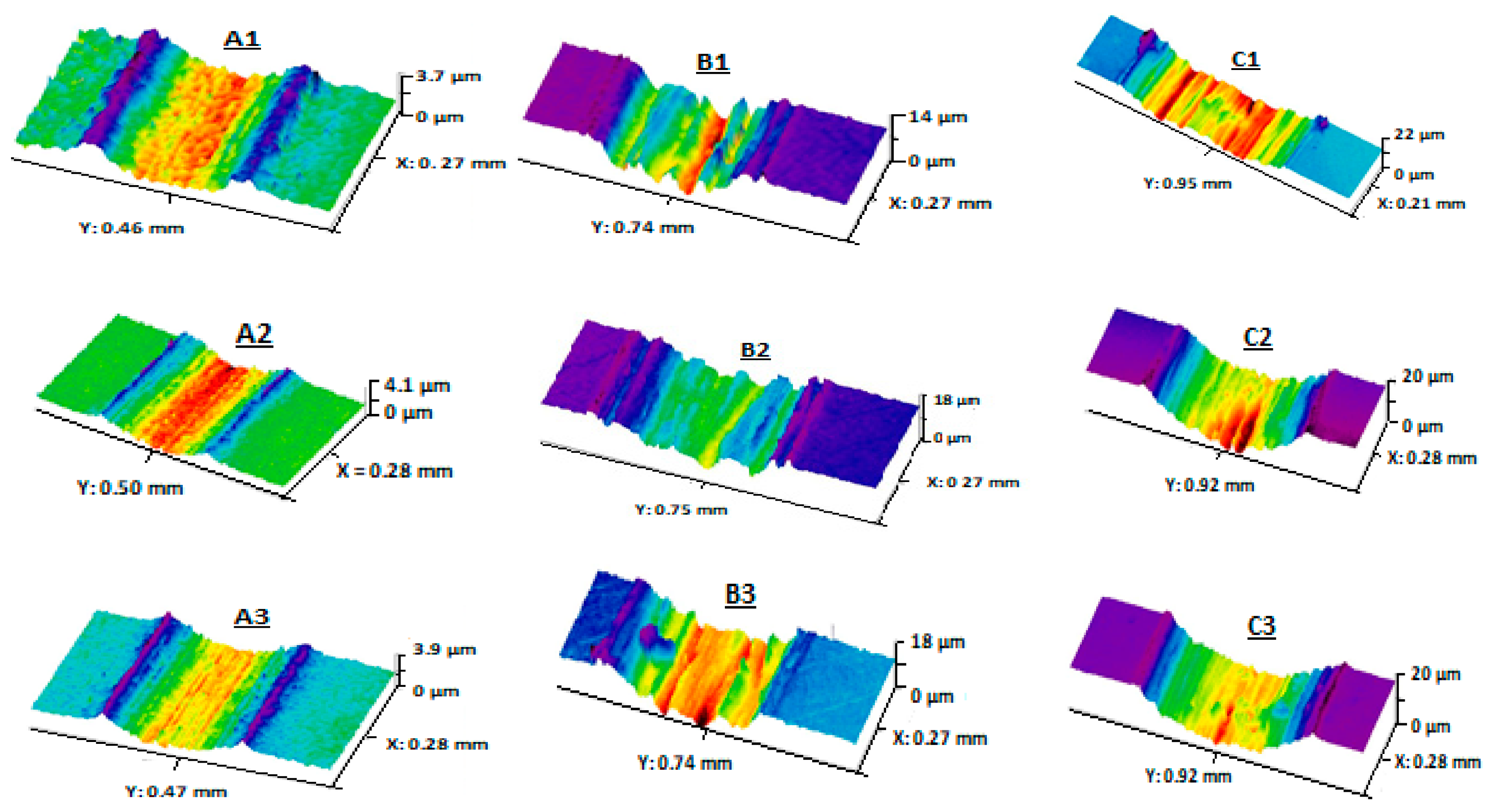
 ), (Ringer’s solution:
), (Ringer’s solution:  ), (PBS:
), (PBS:  ), (PBS + 1 g/L BSA:
), (PBS + 1 g/L BSA:  ), (PBS + 5 g/L BSA:
), (PBS + 5 g/L BSA:  ). 1: 316 L, 2: Ti-6Al-4V, 3: Ti-10Zr-10Nb-5Ta. Adapted with permission from [38]. Copyright 2019 Taylor & Francis Ltd.
). 1: 316 L, 2: Ti-6Al-4V, 3: Ti-10Zr-10Nb-5Ta. Adapted with permission from [38]. Copyright 2019 Taylor & Francis Ltd.
 ), (Ringer’s solution:
), (Ringer’s solution:  ), (PBS:
), (PBS:  ), (PBS + 1 g/L BSA:
), (PBS + 1 g/L BSA:  ), (PBS + 5 g/L BSA:
), (PBS + 5 g/L BSA:  ). 1: 316 L, 2: Ti-6Al-4V, 3: Ti-10Zr-10Nb-5Ta. Adapted with permission from [38]. Copyright 2019 Taylor & Francis Ltd.
). 1: 316 L, 2: Ti-6Al-4V, 3: Ti-10Zr-10Nb-5Ta. Adapted with permission from [38]. Copyright 2019 Taylor & Francis Ltd.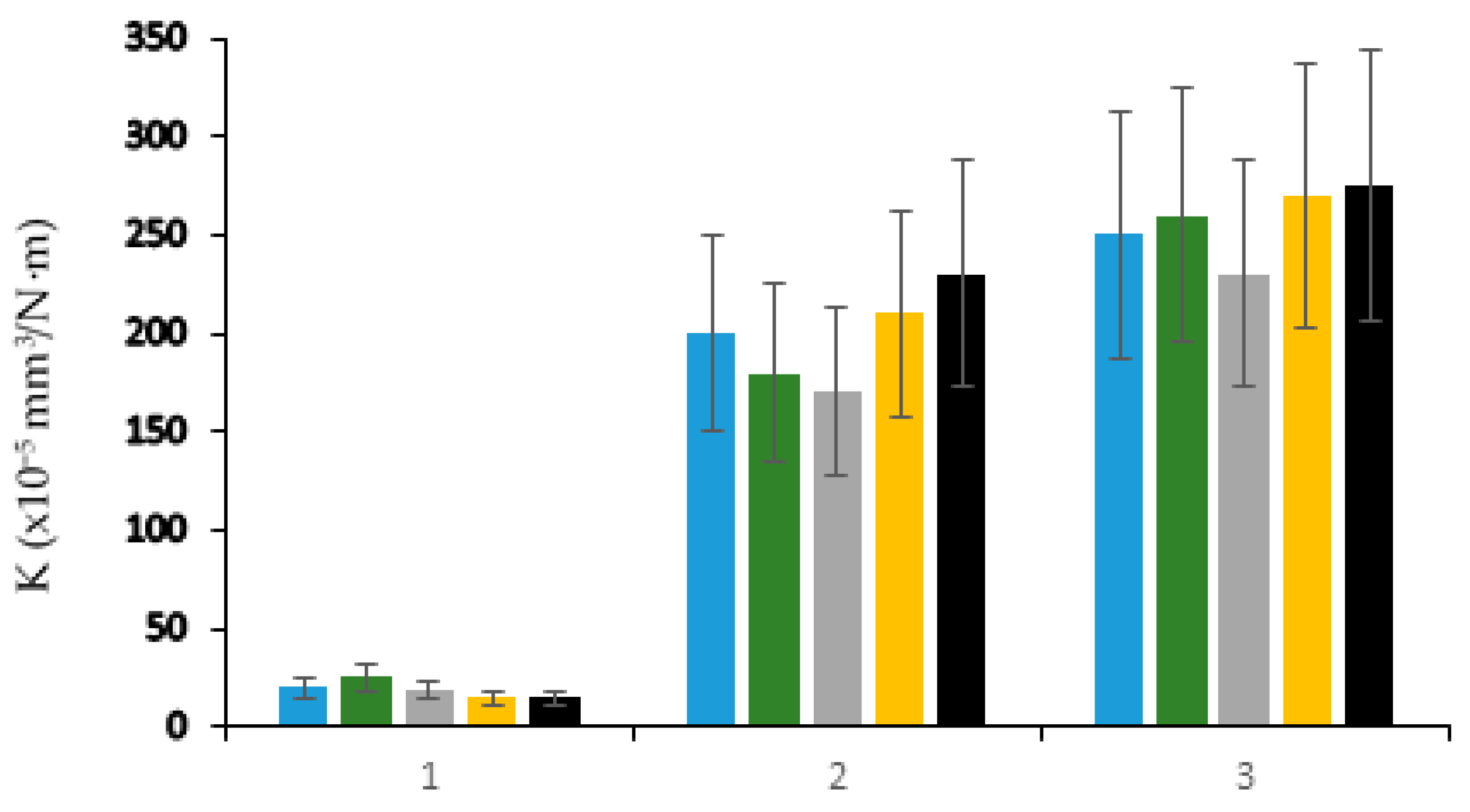
| Stainless Steel | Fe-18Cr-14Ni-2.5Mo Fe-21Cr-10Ni-3.5Mn-2.5Mo Fe-23Mn-21Cr-1Mo-1N |
| Cobalt-Based Alloys | Co-28Cr-6Mo Co-20Cr-15W-10Ni-1.5Mn Co-20Cr-15Ni-15Fe-7Mo-2Mn |
| Titanium and Titanium-Based Alloys | Ti Ti-6Al-4V Ti-6Al-7Nb Ti-13Nb-13Zr Ti-19Zr-11Nb-4Ta Ti-10Zr-10Nb-5Ta Ti-42.5Zr-5Nb-10Ta Ti-23Nb-0.7Ta-2Zr-1.2O Ti-32Nb-2Ta-3Zr-0.5O |
| Special Alloys | Zr-2.5Nb Ni-45Ti |
| Magnesium and Magnesium-Based Alloys | Mg Mg-3Zn-0.5Ca Mg-2.2Nd-0.1Zn-0.4Zr Mg-9Li-1Zn |
| Femoral Head | Acetabular Cup |
|---|---|
| CoCrMo alloy | Ultra-high molecular weight polyethylene (UHMWPE) |
| Partially stabilized zirconia | UHMWPE |
| Alumina | UHMWPE |
| CoCrMo alloy | CoCrMo alloy |
| Alumina | Alumina |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takadoum, J. Review on Corrosion, Tribocorrosion and Osseointegration of Titanium Alloys as Biomaterials. Corros. Mater. Degrad. 2023, 4, 644-658. https://doi.org/10.3390/cmd4040033
Takadoum J. Review on Corrosion, Tribocorrosion and Osseointegration of Titanium Alloys as Biomaterials. Corrosion and Materials Degradation. 2023; 4(4):644-658. https://doi.org/10.3390/cmd4040033
Chicago/Turabian StyleTakadoum, Jamal. 2023. "Review on Corrosion, Tribocorrosion and Osseointegration of Titanium Alloys as Biomaterials" Corrosion and Materials Degradation 4, no. 4: 644-658. https://doi.org/10.3390/cmd4040033





