Influence of Osmotic Dehydration on Mass Transfer Kinetics and Quality Retention of Ripe Papaya (Carica papaya L) during Drying
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Osmotic Dehydration
2.3. Convective Drying and Storage
2.4. Determination of Mass Transfer Parameters
2.4.1. Water Loss
2.4.2. Solute Gain
2.4.3. Validation of Empirical Models for Osmotic Dehydration of Papaya
2.4.4. Adequacy of Empirical Models
2.4.5. Effective Diffusivity of Water
2.5. Physicochemical Analysis
2.5.1. Moisture Content
2.5.2. Water Activity
2.5.3. Rehydration
2.5.4. Shrinkage
2.5.5. Total Soluble Solid
2.6. Determination of Antioxidant Properties of Air-Dried Papaya
2.6.1. Preparation of Extract
2.6.2. Ascorbic Acid (Vitamin C)
2.6.3. Determination of Total Phenolic Content
2.6.4. Determination of 2, 2-diphenyl-1-picrylhydrazyl (DPPH) Activity
2.7. Statistical Analysis
3. Results and Discussion
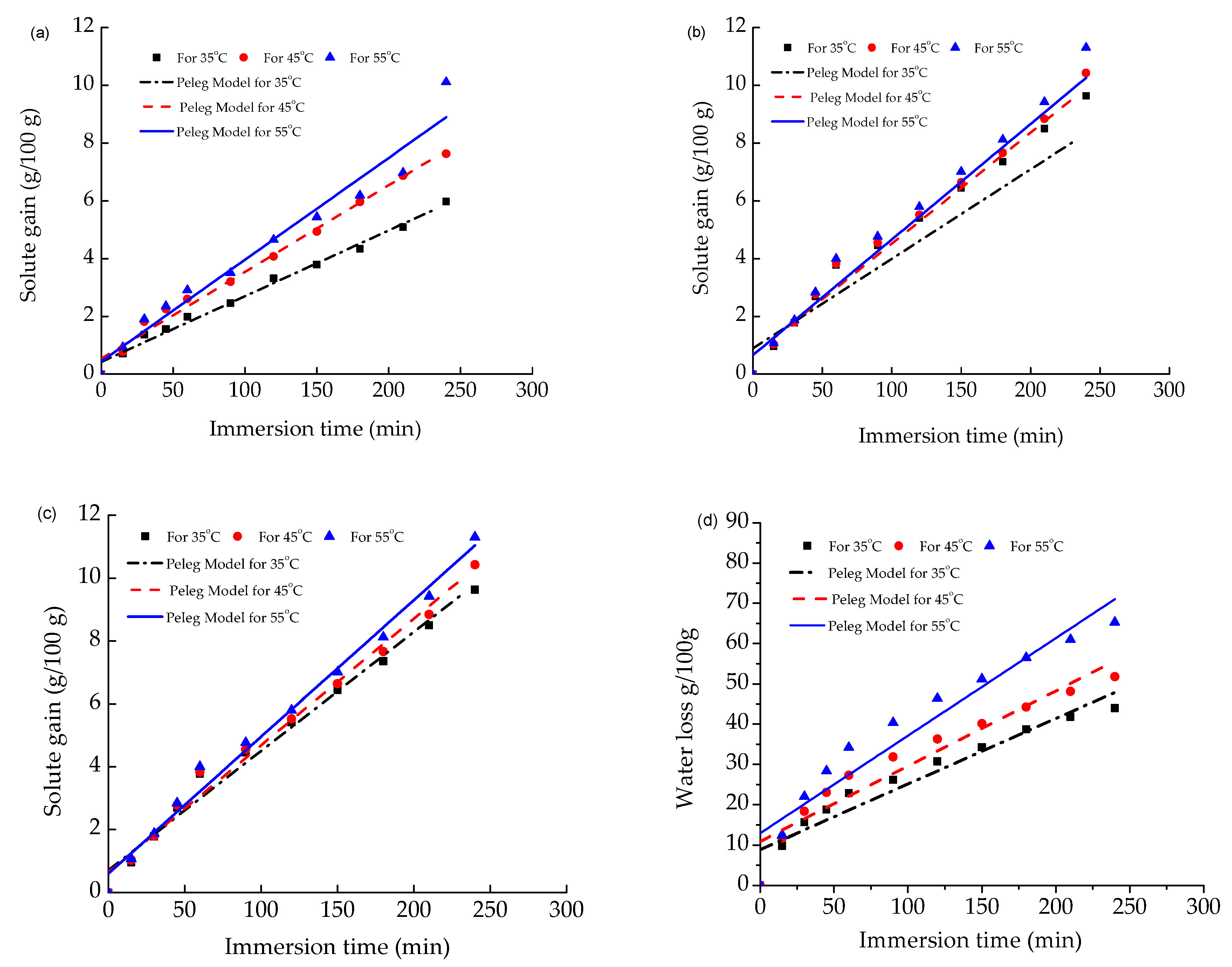
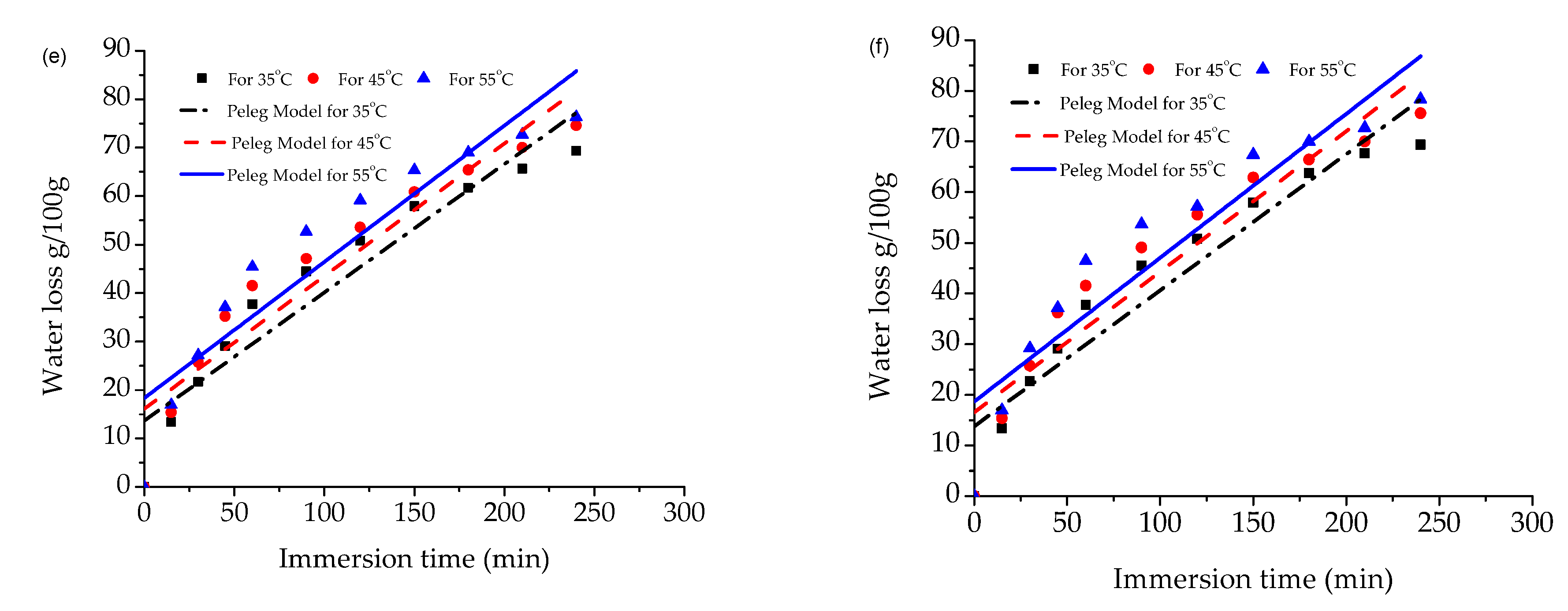
3.1. Water Loss and Solute Gain of Papaya
3.2. Validation of Empirical Models for Water Loss and Solute Gain
3.3. Effective Diffusivity of Water
3.4. Physicochemical Properties of Osmotically Pretreated Air-Dried Papaya
3.5. Effect of Osmotic Dehydration Pretreatments on the Bioactive Compounds of Air-Dried Papaya
3.5.1. Ascorbic Acid
3.5.2. Total Phenolic Content (TPC)
3.5.3. Antioxidant Activity (DPPH Assay %)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Udomkun, P.; Nagle, M.; Mahayothee, B.; Nohr, D.; Koza, A.; Müller, J. Influence of air drying properties on non-enzymatic browning, major bio-active compounds and antioxidant capacity of osmotically pretreated papaya. LWT Food Sci. Technol. 2015, 60, 914–922. [Google Scholar] [CrossRef]
- Dhall, R.K. Advances in edible coatings for fresh fruits and vegetables: A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Hasan, K. A Guide to Postharvest Handling of Fruits and Vegetables. Bangladesh. Available online: http://fpmu.gov.bd/agridrupal/sites/default/files/Postharvest Handling Guide.pdf (accessed on 10 January 2019).
- Oliveira, S.M.; Brandão, T.R.S.; Silva, C.L.M. Influence of Drying Processes and Pretreatments on Nutritional and Bioactive Characteristics of Dried Vegetables: A Review. Food Eng. Rev. 2016, 8, 134–163. [Google Scholar] [CrossRef]
- Pereira, L.M.; Ferrari, C.C.; Mastrantonio, S.D.S.; Rodrigues, A.C.C.; Hubinger, M.D. Kinetic aspects, texture, and color evaluation of some tropical fruits during osmotic dehydration. Dry. Technol. 2006, 24, 475–484. [Google Scholar] [CrossRef]
- Rekha, C.; Poornima, G.; Manasa, M.; Abhipsa, V.; Devi, J.P.; Kumar, H.T.V.; Kekuda, T.R.P. Ascorbic Acid, Total Phenol Content and Antioxidant Activity of Fresh Juices of Four Ripe and Unripe Citrus Fruits. Chem. Sci. Trans. 2012, 1, 303–310. [Google Scholar] [CrossRef]
- Gallo, J.A.Q.; Amaro, M.R.D. Application of Edible Coatings to Improve Shelf-life of Mexican Guava. Acta Hortic. 2003, 599, 589–594. [Google Scholar] [CrossRef]
- Fernandes, F.A.N.; Gallão, M.I.; Rodrigues, S. Effect of osmotic dehydration and ultrasound pre-treatment on cell structure: Melon dehydration. LWT Food Sci. Technol. 2008, 41, 604–610. [Google Scholar] [CrossRef]
- Yousefi, A.R.; Aghdam, K.; Chenar, M.P.; Niakousari, M. The Kinetics of Forced Convective Air-drying of papaya (Carica papaya L.) Slices Pretreated in Osmotic Solution. Iran. Food Sci. Technol. 2013, 9, 270–278. [Google Scholar]
- Mia, M.A.B. Shahi papaya—Digital Herbarium of Crop Plants. Available online: http://dhcrop.bsmrau.net/shahi-papaya/ (accessed on 21 March 2019).
- Ozen, B.F.; Dock, L.L.; Ozdemir, M.; Floros, J.D. Processing factors affecting the osmotic dehydration of diced green peppers. Int. J. Food Sci. Technol. 2002, 37, 497–502. [Google Scholar] [CrossRef]
- Singh, B.; Kumar, A.; Gupta, A.K. Study of mass transfer kinetics and effective diffusivity during osmotic dehydration of carrot cubes. J. Food Eng. 2007, 79, 471–480. [Google Scholar] [CrossRef]
- Peleg, M. An Empirical Model for the Description Moisture Sorption Curves. J. Food Sci. 1988, 53, 1216–1217. [Google Scholar] [CrossRef]
- Lazarides, H.N.; Mavroudis, N.E. Kinetics of osmotic dehydration of a highly shrinking vegetable tissue in a salt-free medium. J. Food Eng. 1996, 30, 61–74. [Google Scholar] [CrossRef]
- Crank, J. Mathematics of Diffusion, 2nd ed.; Oxford University Press: London, UK, 1975; pp. 104–254. [Google Scholar]
- Sharma, G.; Prasad, S. Effective moisture diffusivity of garlic cloves undergoing microwave-convective drying. J. Food Eng. 2004, 65, 609–617. [Google Scholar] [CrossRef]
- McMinn, W.A.M.; McLoughlin, C.M.; Magee, T.R.A. Thin-Layer Modeling of Microwave, Microwave-Convective, and Microwave-Vacuum Drying of Pharmaceutical Powders. Dry. Technol. 2005, 23, 513–532. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2004. [Google Scholar]
- Arabhosseini, A.; Huisman, W.; van Boxtel, A.; Müller, J. Modeling of the equilibrium moisture content (EMC) of tarragon (Artemisia dracunculus L.). Int. J. Food Eng. 2005, 1. [Google Scholar] [CrossRef]
- Doymaz, İ. Drying kinetics, rehydration and colour characteristics of convective hot-air drying of carrot slices. Heat Mass Transf. 2017, 53, 25–35. [Google Scholar] [CrossRef]
- Garcia, C.C.; Caetano, L.C.; de Souza Silva, K.; Mauro, M.A. Influence of edible coating on the drying and quality of papaya (Carica papaya). Food Bioprocess Technol. 2014, 7, 2828–2839. [Google Scholar] [CrossRef]
- Saikia, S.; Mahnot, N.K.; Mahanta, C.L. Effect of Spray Drying of Four Fruit Juices on Physicochemical, Phytochemical and Antioxidant Properties. J. Food Process. Preserv. 2015, 39, 1656–1664. [Google Scholar] [CrossRef]
- Ranganna, S. 2,6 Dichlorophenol Indophenol Visual Titration Method. In Handbook of Analysis and Quality Control for Fruit and Vegetable Products; Tata McGraw-Hill: New Delhi, India, 1986; pp. 105–106. [Google Scholar]
- Slinkard, K.; Singleton, V. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]
- Mundada, M.; Hathan, B.S.; Maske, S. Mass Transfer Kinetics during Osmotic Dehydration of Pomegranate Arils. J. Food Sci. 2011, 76, E31–E39. [Google Scholar] [CrossRef]
- Cichowska, J.; Figiel, A.; Stasiak-RóZáNska, L.; Witrowa-Rajchert, D. Modeling of Osmotic Dehydration of Apples in Sugar Alcohols and Dihydroxyacetone (DHA) Solutions. Foods 2019, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Assis, F.R.; Morais, R.M.; Morais, A.M. Mathematical Modelling of Osmotic Dehydration Kinetics of Apple Cubes. J. Food Process. Preserv. 2017, 41, e12895. [Google Scholar] [CrossRef]
- Falade, K.O.; Igbeka, J.C.; Ayanwuyi, F.A. Kinetics of mass transfer, and colour changes during osmotic dehydration of watermelon. J. Food Eng. 2007, 80, 979–985. [Google Scholar] [CrossRef]
- Ciurzyńska, A.; Cichowska, J.; Kowalska, H.; Czajkowska, K.; Lenart, A. Osmotic dehydration of Braeburn variety apples in the production of sustainable food products. Int. Agrophys. 2018, 32, 141–146. [Google Scholar] [CrossRef]
- Chiralt, A.; Talens, P. Physical and chemical changes induced by osmotic dehydration in plant tissues. J. Food Eng. 2005, 67, 167–177. [Google Scholar] [CrossRef]
- Silva, K.S.; Garcia, C.C.; Amado, L.R.; Mauro, M.A. Effects of Edible Coatings on Convective Drying and Characteristics of the Dried Pineapple. Food Bioprocess Technol. 2015, 8, 1465–1475. [Google Scholar] [CrossRef]
- Kaushal, P.; Sharma, H.K. Osmo-convective dehydration kinetics of jackfruit (Artocarpus heterophyllus). J. Saudi Soc. Agric. Sci. 2016, 15, 118–126. [Google Scholar] [CrossRef]
- Azoubel, P.M.; Murr, F.E.X. Mass transfer kinetics of osmotic dehydration of cherry tomato. J. Food Eng. 2004, 61, 291–295. [Google Scholar] [CrossRef]
- Tang, X.; Nail, S.L.; Pikal, M.J. Freeze-drying process design by manometric temperature measurement: Design of a smart freeze-dryer. Pharm. Res. 2005, 22, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Park, K.J.; Bin, A.; Pedro, F.; Brod, R.; Hae, T.; Brandini, K. Osmotic dehydration kinetics of pear D’ anjou (Pyrus communis L.). J. Food Eng. 2002, 52, 293–298. [Google Scholar] [CrossRef]
- El-Aouar, Â.A.; Azoubel, P.M.; Murr, F.E.X. Drying kinetics of fresh and osmotically pre-treated papaya (Carica papaya L.). J. Food Eng. 2003, 59, 85–91. [Google Scholar] [CrossRef]
- Sperber, W.H. Influence of Water Activity on Foodborne Bacteria-A Review. J. Food Prot. 1983, 46, 142–150. [Google Scholar] [CrossRef]
- Bchir, B.; Besbes, S.; Karoui, R.; Attia, H.; Paquot, M.; Blecker, C. Effect of Air-Drying Conditions on Physico-chemical Properties of Osmotically Pre-treated Pomegranate Seeds. Food Bioprocess Technol. 2012, 5, 1840–1852. [Google Scholar] [CrossRef]
- Biosci, I.J.; Akbarian, M.; Moayedi, F.; Ghasemkhani, N.; Ghaseminezhad, A. Impact of antioxidant edible coatings and osmotic dehydration on shrinkage and colour of “Quince” dried by hot air. Int. J. Biosci. 2014, 4, 27–33. [Google Scholar]
- Okpala, L.C.; Ekechi, C.A. Rehydration characteristics of dehydrated West African pepper (Piper guineense) leaves. Food Sci. Nutr. 2014, 2, 664–668. [Google Scholar] [CrossRef]
- El-Ishaq, A.; Obirinakem, S. Effect of Temperature and Storage on Vitamin C Content in Fruits Juice. Int. J. Chem. Biomol. Sci. 2015, 1, 17–21. [Google Scholar]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]

| Osmotic Solution Concentration (°Brix) | Parameters | ||||
|---|---|---|---|---|---|
| Solution Temperature (°C) | Moisture Content (%) | Total Soluble Solid (°Brix) | Water Loss (g/100 g of Fresh Fruit) | Solute Gain (g/100 g of Fresh Fruit) | |
| 40 | 35 | 79.39 ± 0.02 a | 21.60 ± 0.20 d | 43.98 ± 0.02 h | 5.97 ± 0.04 e |
| 45 | 75.49 ± 0.32 b | 27.03 ± 0.06 c | 51.75 ± 0.03 g | 7.63 ± 0.05 d | |
| 55 | 72.35 ± 0.09 c | 33.10 ± 0.10 a | 65.30 ± 0.01 f | 10.11 ± 0.01 b | |
| 50 | 35 | 72.11 ± 0.09 c | 26.47 ± 0.12 c | 65.30 ± 0.04 f | 7.90 ± 0.03 d |
| 45 | 71.12 ± 0.11 c | 34.10 ± 0.10 a | 66.82 ± 0.02 e | 9.80 ± 0.02 c | |
| 55 | 67.35 ± 0.07 d | 31.03 ± 0.06 b | 70.17 ± 0.05 c | 10.30 ± 0.03 b | |
| 60 | 35 | 64.80 ± 0.02 e | 32.00 ± 0.10 b | 69.33 ± 0.02 d | 9.63 ± 0.02 c |
| 45 | 60.16 ± 0.05 f | 31.93 ± 0.12 b | 74.57 ± 0.03 b | 10.43 ± 0.04 b | |
| 55 | 57.59 ± 0.05 g | 33.17 ± 0.06 a | 76.34 ± 0.04 a | 11.31 ± 0.02 a | |
| Osmotic Solution Conc. (°Brix) | Temp. (°C) | Water Loss | Solute Gain | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| K | R2 | χ2 | RMSE | E% | K | R2 | χ2 | RMSE | E% | ||
| 40 | 35 | 5.198 | 0.99 | 0.08 | 0.021 | 0.23 | 0.322 | 0.93 | 1.21 | 1.022 | 16.58 |
| 45 | 4.899 | 0.99 | 0.04 | 0.087 | 7.65 | 0.422 | 0.92 | 3.21 | 1.011 | 14.29 | |
| 55 | 4.562 | 0.99 | 0.07 | 0.042 | 0.20 | 0.477 | 0.87 | 4.78 | 2.013 | 26.90 | |
| 50 | 35 | 4.765 | 0.99 | 0.12 | 0.043 | 8.96 | 0.469 | 0.96 | 1.11 | 0.540 | 8.10 |
| 45 | 4.401 | 0.99 | 0.11 | 0.056 | 2.16 | 0.540 | 0.93 | 3.56 | 0.873 | 14.59 | |
| 55 | 4.220 | 0.98 | 0.41 | 0.076 | 2.82 | 0.558 | 0.92 | 4.11 | 2.160 | 16.02 | |
| 60 | 35 | 4.297 | 0.99 | 0.02 | 0.020 | 1.93 | 0.537 | 0.93 | 2.12 | 1.021 | 13.60 |
| 45 | 3.329 | 0.99 | 0.05 | 0.012 | 1.78 | 0.591 | 0.92 | 3.67 | 2.011 | 16.68 | |
| 55 | 2.839 | 0.99 | 0.06 | 0.019 | 5.49 | 0.596 | 0.91 | 4.32 | 2.212 | 18.23 | |
| Osmotic Solution Conc. (°Brix) | Temp. (°C) | Water Loss | Solute Gain | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K1 | K2 | R2 | χ2 | RMSE | E% | K1 | K2 | R2 | χ2 | RMSE | E% | ||
| 40 | 35 | 17.233 | 0.223 | 0.96 | 0.05 | 0.032 | 4.23 | 0.422 | 0.023 | 0.99 | 0.03 | 0.08 | 1.50 |
| 45 | 14.789 | 0.212 | 0.95 | 0.04 | 0.013 | 3.23 | 0.539 | 0.030 | 0.99 | 0.02 | 0.05 | 1.44 | |
| 55 | 12.332 | 0.245 | 0.95 | 0.07 | 0.021 | 2.87 | 0.439 | 0.035 | 0.96 | 0.01 | 0.25 | 5.06 | |
| 50 | 35 | 15.456 | 0.224 | 0.94 | 0.05 | 0.023 | 4.23 | 0.892 | 0.031 | 0.95 | 0.07 | 0.45 | 5.44 |
| 45 | 12.345 | 0.221 | 0.96 | 0.03 | 0.043 | 3.11 | 0.671 | 0.038 | 0.98 | 0.06 | 0.76 | 1.02 | |
| 55 | 11.435 | 0.203 | 0.98 | 0.07 | 0.024 | 3.78 | 0.661 | 0.040 | 0.98 | 0.01 | 0.03 | 0.38 | |
| 60 | 35 | 10.346 | 0.226 | 0.96 | 0.04 | 0.043 | 3.76 | 0.709 | 0.038 | 0.98 | 0.03 | 0.63 | 1.86 |
| 45 | 9.6743 | 0.178 | 0.97 | 0.07 | 0.011 | 2.67 | 0.646 | 0.040 | 0.99 | 0.02 | 0.04 | 1.05 | |
| 55 | 6.784 | 0.145 | 0.98 | 0.08 | 0.008 | 2.89 | 0.602 | 0.043 | 0.99 | 0.04 | 0.03 | 2.30 | |
| Osmotic Solution Concentration (°Brix) | Temperature (°C) | DWL × 10−9 ± Margin of Error (m2/s) | DSG × 10−9 ± Margin of Error (m2/s) |
|---|---|---|---|
| 40 | 35 | 2.25 ± 0.032 | 3.01 ± 0.082 |
| 45 | 2.61 ± 0.012 | 3.31 ± 0.022 | |
| 55 | 2.89 ± 0.040 | 3.71 ± 0.032 | |
| 50 | 35 | 2.89 ± 0.011 | 3.94 ± 0.036 |
| 45 | 3.07 ± 0.022 | 4.77 ± 0.043 | |
| 55 | 3.28 ± 0.013 | 5.37 ± 0.065 | |
| 60 | 35 | 3.20 ± 0.014 | 4.20 ± 0.092 |
| 45 | 3.89 ± 0.015 | 4.89 ± 0.062 | |
| 55 | 4.31 ± 0.008 | 5.61 ± 0.027 |
| Osmotic Solution Concentration (°Brix) | Parameters | ||||
|---|---|---|---|---|---|
| Solution Temperature (°C) | Moisture Content (%) | Water Activity | Shrinkage (%) | Rehydration Ratio | |
| Control (without OD) | 7.45 ± 0.12 a | 0.37 ± 0.02 a | 90.02 | 3.53 ± 0.13 d | |
| 40 | 35 | 6.62 ± 0.04 b | 0.36 ± 0.02 a | 76.12 | 3.98 ± 0.04 d |
| 45 | 6.21 ± 0.11 b | 0.33 ± 0.02 a | 74.32 | 3.43 ± 0.07 d | |
| 55 | 5.11 ± 0.06 c | 0.33 ± 0.01 a | 74.56 | 3.12 ± 0.11 e | |
| 50 | 35 | 5.98 ± 0.15 b | 0.34 ± 0.02 a | 67.23 | 4.13 ± 0.08 c |
| 45 | 5.10 ± 0.01 c | 0.32 ± 0.01 b | 65.34 | 3.87 ± 0.12 d | |
| 55 | 4.14 ± 0.10 d | 0.31 ± 0.01 b | 64.76 | 3.64 ± 0.05 d | |
| 60 | 35 | 4.12 ± 0.04 d | 0.30 ± 0.02 b | 59.56 | 6.68 ± 0.51 a |
| 45 | 4.23 ± 0.01 d | 0.29 ± 0.01 b | 58.32 | 5.87 ± 0.12 b | |
| 55 | 3.67 ± 0.03 e | 0.29 ± 0.01 b | 57.03 | 6.12 ± 0.21 a | |
| Osmotic Solution Concentration (°Brix) | Osmotic Solution Temperature (°C) | Total Phenolic Content (mgGAE/100 g d.w.) | Ascorbic Acid (mg/100 g d.w.) | DPPH Inhibition (%) |
|---|---|---|---|---|
| Fresh | 73.33 ± 1.64 a | 42.14 ± 1.96 a | 91.39 ± 0.64 a | |
| 40 | 35 | 43.85 ± 2.14 b | 27.69 ±1.51 b | 42.59 ± 0.33 b |
| 45 | 41.85 ± 3.22 b | 22.30 ± 3.41 b | 36.05 ± 0.32 c | |
| 55 | 26.01 ± 1.06 c | 17.44 ± 3.49 c | 34.16 ± 0.28 d | |
| 50 | 35 | 45.81 ± 4.04 b | 29.30 ± 2.03 b | 48.09 ± 0.80 b |
| 45 | 49.57 ± 7.73 b | 26.95 ± 2.03 b | 39.99 ± 0.23 c | |
| 55 | 29.20 ± 4.18 c | 20.22 ± 1.84 c | 37.89 ± 0.05 d | |
| 60 | 35 | 67.47 ± 3.53 b | 36.56 ± 3.66 b | 50.99 ± 1.64 b |
| 45 | 65.09 ± 2.07 b | 30.62 ± 1.83 c | 46.04 ± 1.18 c | |
| 55 | 46.39 ± 3.91 c | 23.84 ± 2.06 d | 39.46 ± 1.67 d | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.Z.; Das, S.; Monalisa, K.; Sayem, A.S.M. Influence of Osmotic Dehydration on Mass Transfer Kinetics and Quality Retention of Ripe Papaya (Carica papaya L) during Drying. AgriEngineering 2019, 1, 220-234. https://doi.org/10.3390/agriengineering1020016
Islam MZ, Das S, Monalisa K, Sayem ASM. Influence of Osmotic Dehydration on Mass Transfer Kinetics and Quality Retention of Ripe Papaya (Carica papaya L) during Drying. AgriEngineering. 2019; 1(2):220-234. https://doi.org/10.3390/agriengineering1020016
Chicago/Turabian StyleIslam, Md Zohurul, Shuvo Das, Kamrunnaher Monalisa, and A.S.M. Sayem. 2019. "Influence of Osmotic Dehydration on Mass Transfer Kinetics and Quality Retention of Ripe Papaya (Carica papaya L) during Drying" AgriEngineering 1, no. 2: 220-234. https://doi.org/10.3390/agriengineering1020016






