Dry Reforming of Methane on Ni/Nanorod-CeO2 Catalysts Prepared by One-Pot Hydrothermal Synthesis: The Effect of Ni Content on Structure, Activity, and Stability
Abstract
:1. Introduction
2. Materials and Methods
3. Results
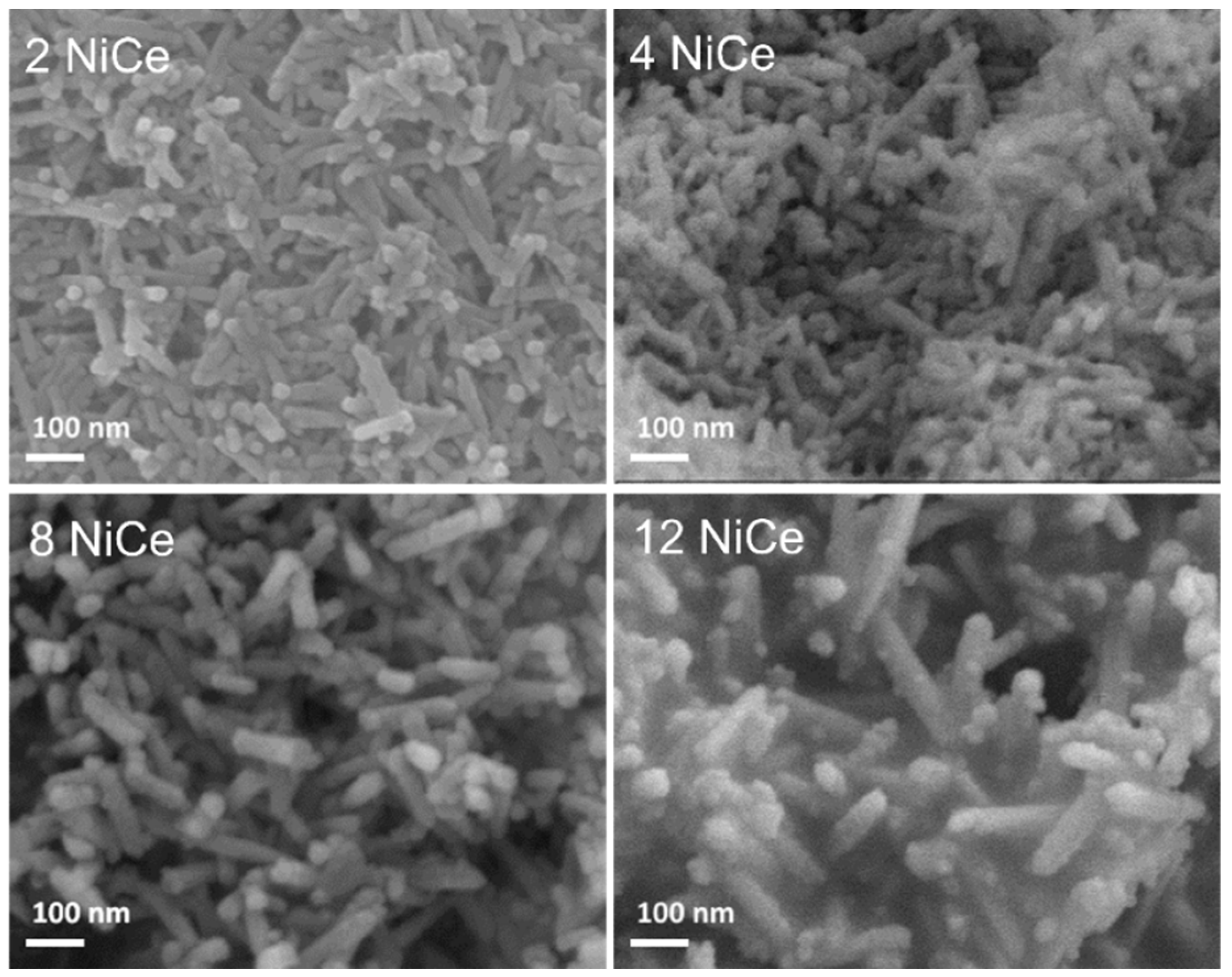
3.1. Morphology and Chemical Composition of Reduced NiCe Sample
3.2. Material Reducibility
3.3. Nickel Dispersion by H2-TPD
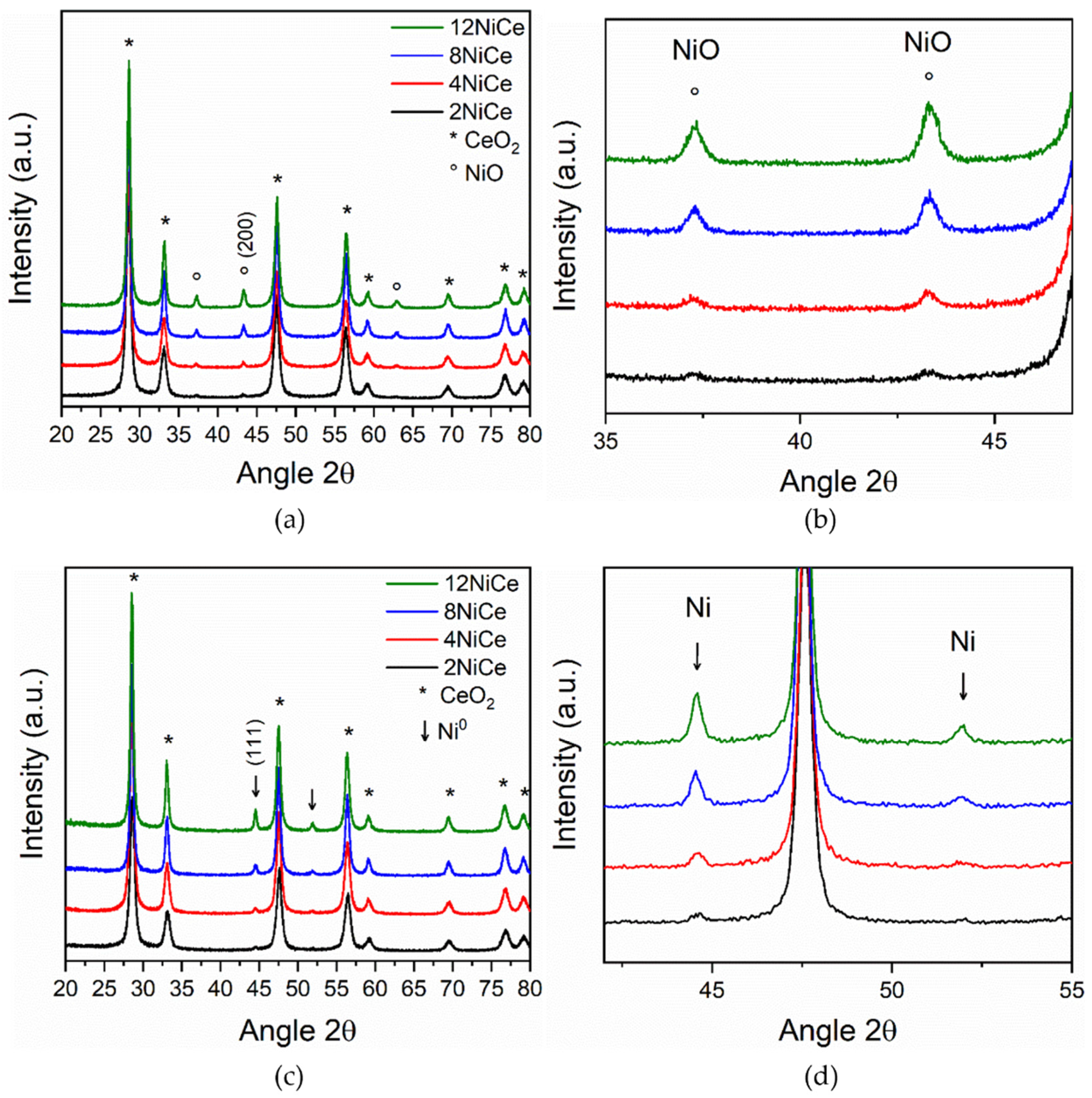
3.4. Structure and Morphology of Samples
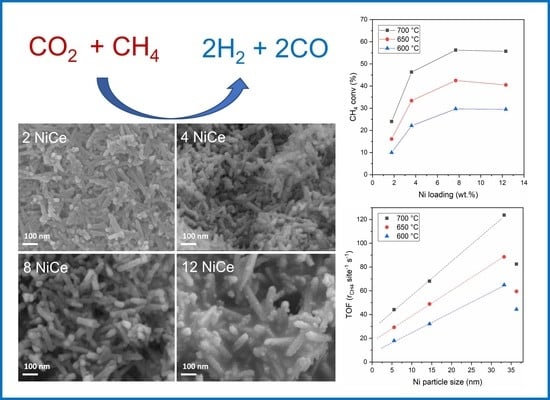
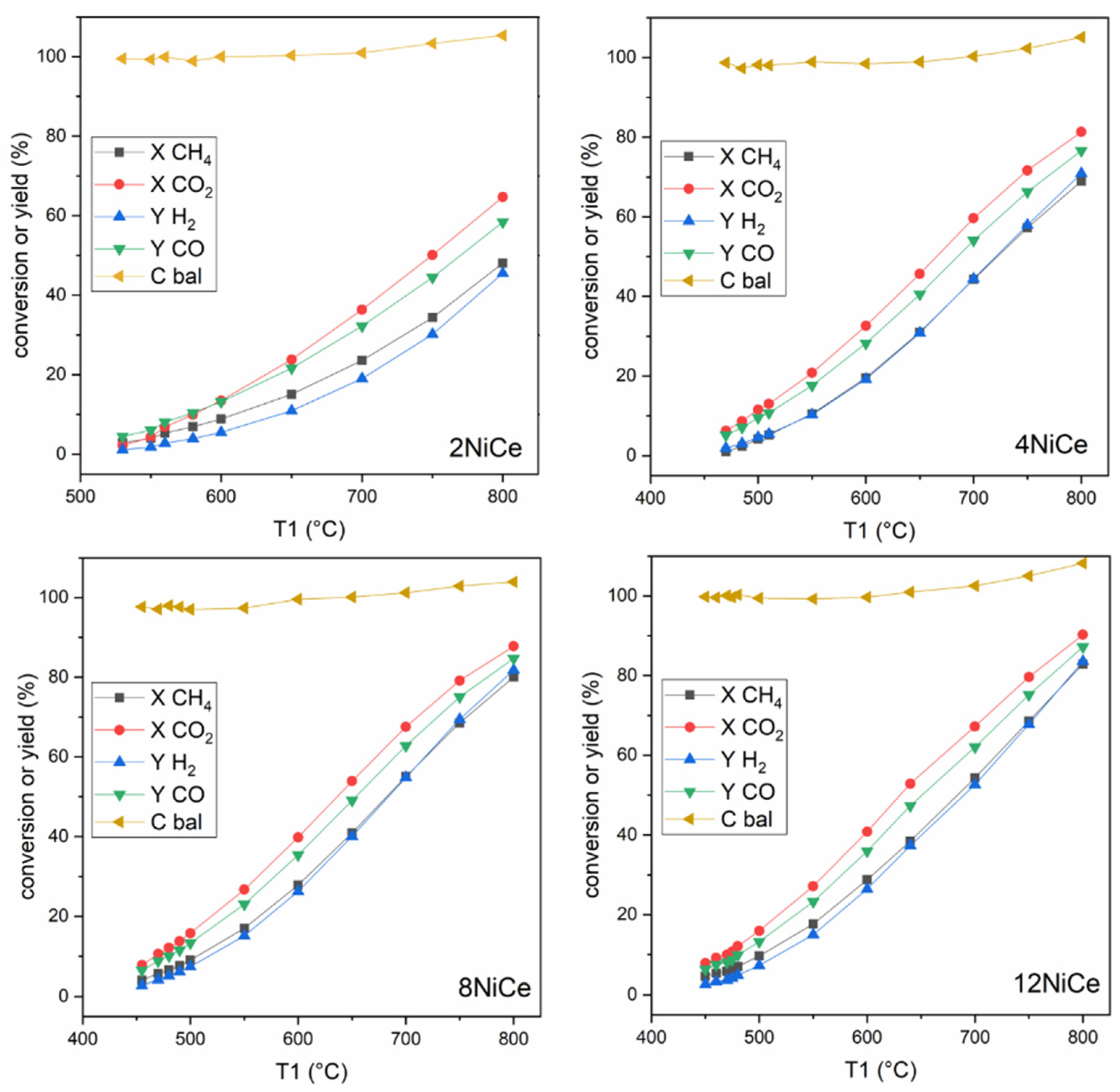
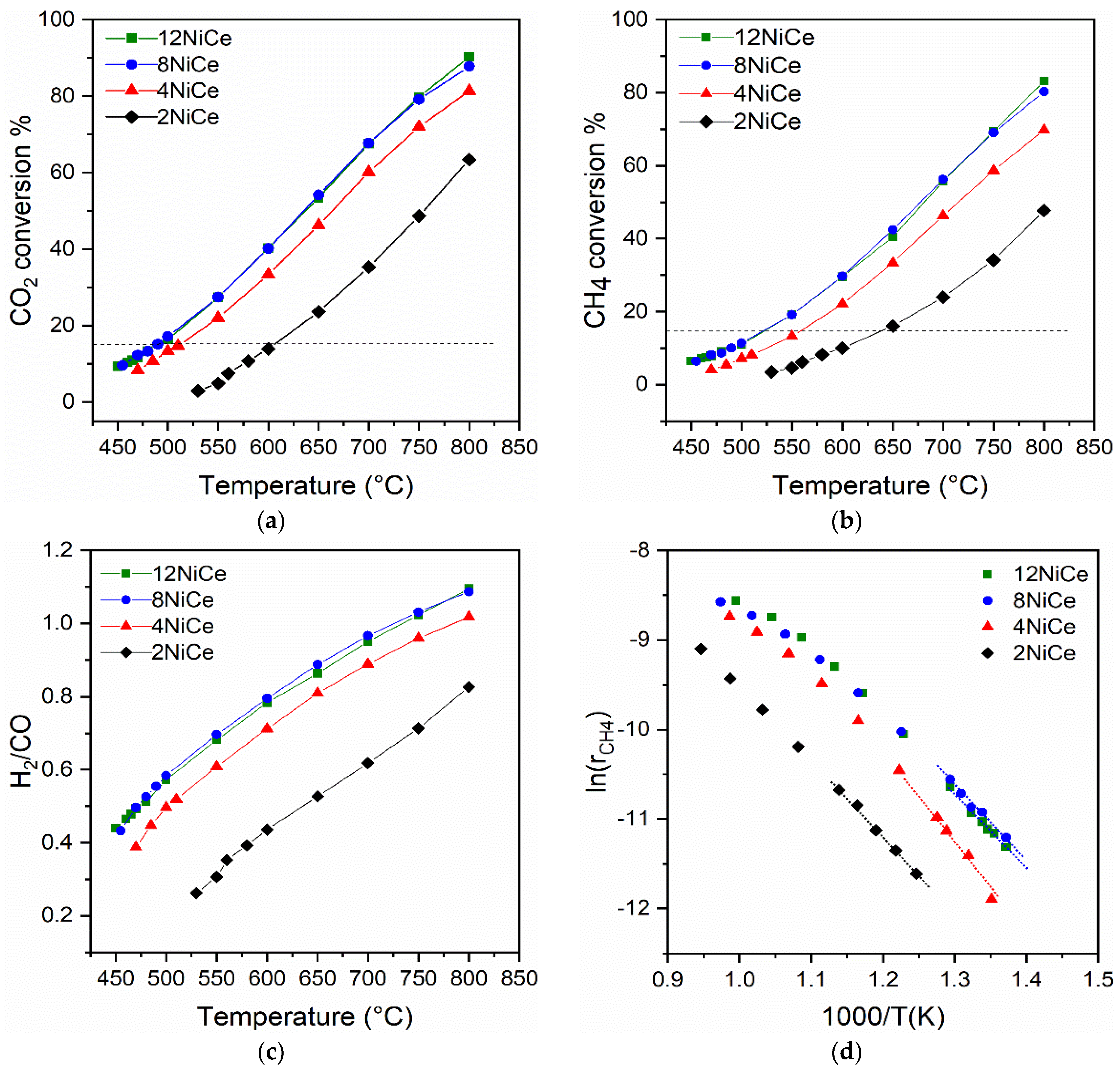
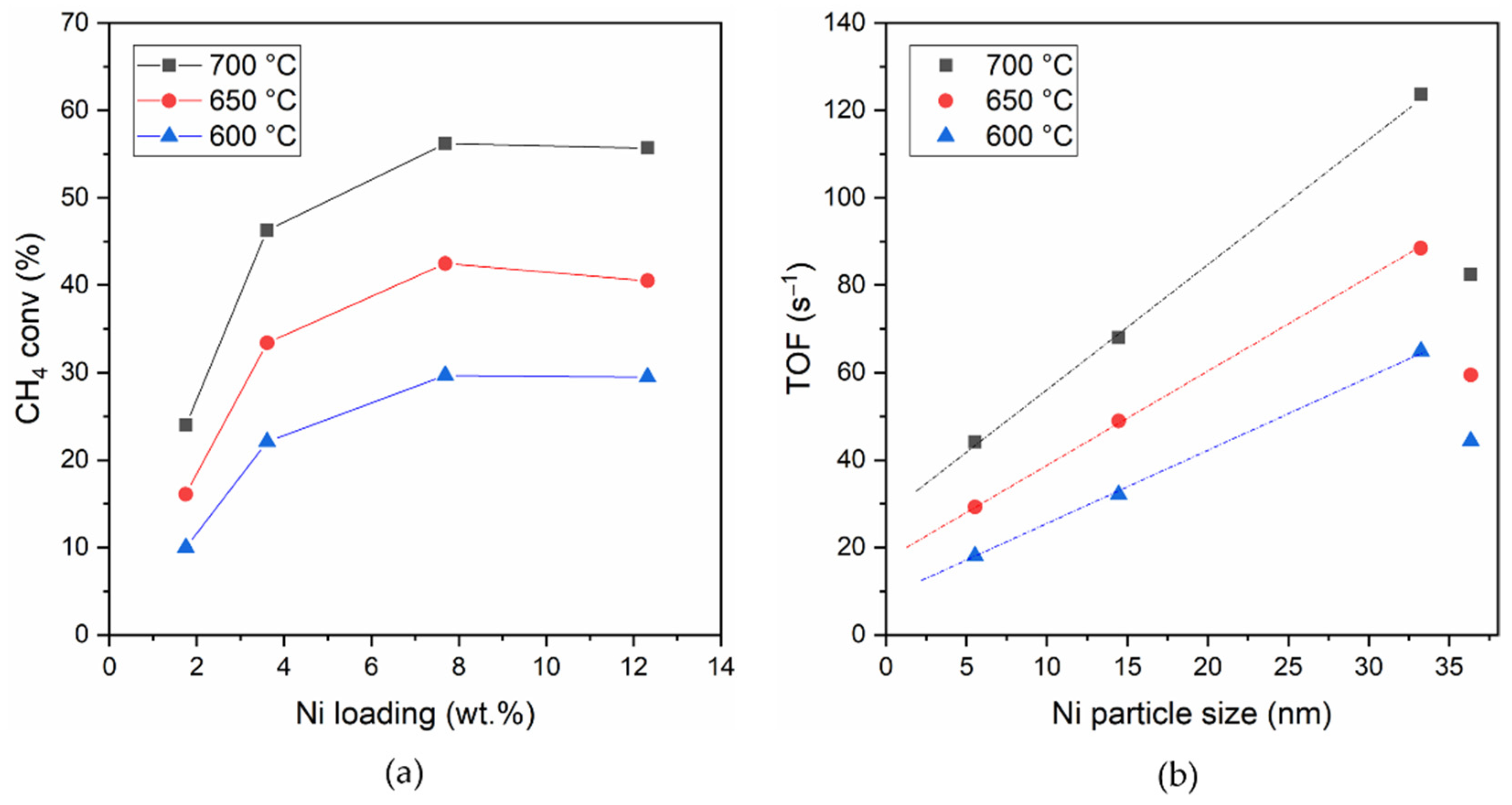
3.5. Catalytic Activity for Dry Reforming of Methane
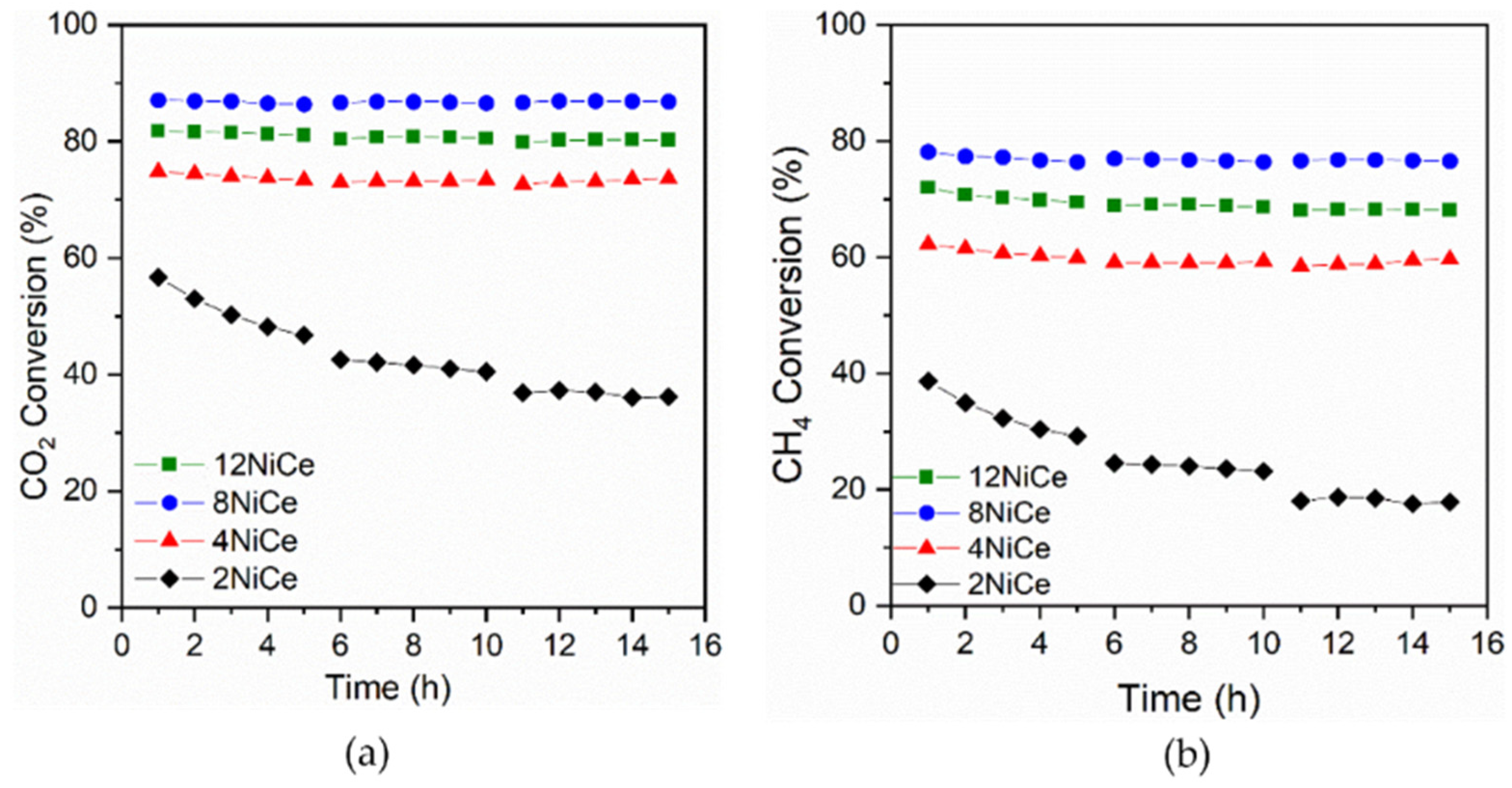
3.6. Catalyst Stability
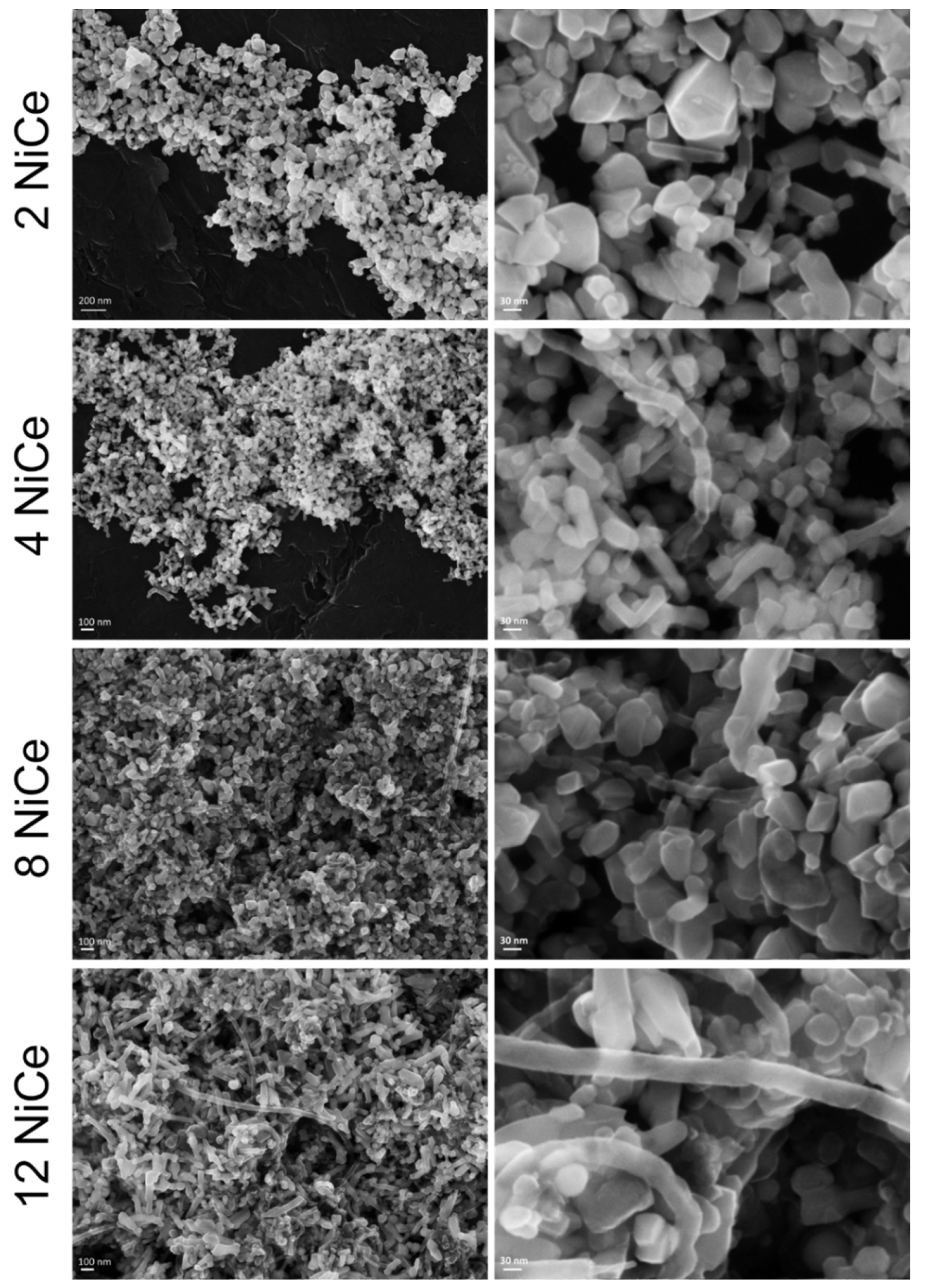
3.7. FE-SEM Characterization after TOS Experiment
4. Discussion
- (1)
- The activity increases with Ni content up to about Ni 8 wt.%;
- (2)
- The turnover frequency (TOF) for CH4 conversion increases linearly with the average Ni particle size in the range of 5.5–33 nm;
- (3)
- The H2/CO selectivity increases with nickel content due to the lower amount of produced CO;
- (4)
- Only the low-Ni-content sample (Ni 2 wt.%) is deactivated over time on stream.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bradford, M.C.J.; Vannice, M.A. CO2 Reforming of CH4. Catal. Rev. 1999, 41, 1–42. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, J.; Meng, Y.; Yan, F.; Aihemaiti, A. A Review of Recent Developments in Hydrogen Production via Biogas Dry Reforming. Energy Convers. Manag. 2018, 171, 133–155. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Panagiotopoulou, P.; Artemakis, G. A Review of Recent Efforts to Promote Dry Reforming of Methane (DRM) to Syngas Production via Bimetallic Catalyst Formulations. Appl. Catal. B: Environ. 2021, 296, 120210. [Google Scholar] [CrossRef]
- Aramouni, N.A.K.; Touma, J.G.; Tarboush, B.A.; Zeaiter, J.; Ahmad, M.N. Catalyst Design for Dry Reforming of Methane: Analysis Review. Renew. Sustain. Energy Rev. 2018, 82, 2570–2585. [Google Scholar] [CrossRef]
- Ranjekar, A.M.; Yadav, G.D. Dry Reforming of Methane for Syngas Production: A Review and Assessment of Catalyst Development and Efficacy. J. Indian Chem. Soc. 2021, 98, 100002. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, L.; Wang, S.; Mao, D.; Hu, C. Low-Temperature Catalytic CO2 Dry Reforming of Methane on Ni-Based Catalysts: A Review. Fuel Processing Technol. 2018, 169, 199–206. [Google Scholar] [CrossRef]
- Yusuf, M.; Farooqi, A.S.; Keong, L.K.; Hellgardt, K.; Abdullah, B. Contemporary Trends in Composite Ni-Based Catalysts for CO2 Reforming of Methane. Chem. Eng. Sci. 2021, 229, 116072. [Google Scholar] [CrossRef]
- Seo, H. Recent Scientific Progress on Developing Supported Ni Catalysts for Dry (CO2) Reforming of Methane. Catalysts 2018, 8, 110. [Google Scholar] [CrossRef] [Green Version]
- Kesavan, J.K.; Luisetto, I.; Tuti, S.; Meneghini, C.; Iucci, G.; Battocchio, C.; Mobilio, S.; Casciardi, S.; Sisto, R. Nickel Supported on YSZ: The Effect of Ni Particle Size on the Catalytic Activity for CO2 Methanation. J. CO2 Util. 2018, 23, 200–211. [Google Scholar] [CrossRef]
- Teh, L.P.; Setiabudi, H.D.; Timmiati, S.N.; Aziz, M.A.A.; Annuar, N.H.R.; Ruslan, N.N. Recent Progress in Ceria-Based Catalysts for the Dry Reforming of Methane: A Review. Chem. Eng. Sci. 2021, 242, 116606. [Google Scholar] [CrossRef]
- Moeini, S.; Battocchio, C.; Casciardi, S.; Luisetto, I.; Lupattelli, P.; Tofani, D.; Tuti, S. Oxidized Palladium Supported on Ceria Nanorods for Catalytic Aerobic Oxidation of Benzyl Alcohol to Benzaldehyde in Protic Solvents. Catalysts 2019, 9, 847. [Google Scholar] [CrossRef] [Green Version]
- Marconi, E.; Tuti, S.; Luisetto, I. Structure-Sensitivity of CO2 Methanation over Nanostructured Ni Supported on CeO2 Nanorods. Catalysts 2019, 9, 375. [Google Scholar] [CrossRef] [Green Version]
- Luisetto, I.; Tuti, S.; Di Bartolomeo, E. Co and Ni Supported on CeO2 as Selective Bimetallic Catalyst for Dry Reforming of Methane. Int. J. Hydrogen Energy 2012, 37, 15992–15999. [Google Scholar] [CrossRef]
- Luisetto, I.; Tuti, S.; Romano, C.; Boaro, M.; Di Bartolomeo, E.; Kesavan, J.K.; Kumar, S.S.; Selvakumar, K. Dry Reforming of Methane over Ni Supported on Doped CeO2: New Insight on the Role of Dopants for CO2 Activation. J. CO2 Util. 2019, 30, 63–78. [Google Scholar] [CrossRef]
- Trovarelli, A.; Llorca, J. Ceria Catalysts at Nanoscale: How Do Crystal Shapes Shape Catalysis? ACS Catal. 2017, 7, 4716–4735. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, L.; Wang, W.; Zhao, Y.; Zhang, G.; Ma, X.; Gong, J. Morphology Control of Ceria Nanocrystals for Catalytic Conversion of CO2 with Methanol. Nanoscale 2013, 5, 5582. [Google Scholar] [CrossRef]
- Luisetto, I.; Stendardo, S.; Senthil Kumar, S.M.; Selvakumar, K.; Kesavan, J.K.; Iucci, G.; Pasqual Laverdura, U.; Tuti, S. One-Pot Synthesis of Ni0.05Ce0.95O2−δ Catalysts with Nanocubes and Nanorods Morphology for CO2 Methanation Reaction and in Operando DRIFT Analysis of Intermediate Species. Processes 2021, 9, 1899. [Google Scholar] [CrossRef]
- Du, X.; Zhang, D.; Shi, L.; Gao, R.; Zhang, J. Morphology Dependence of Catalytic Properties of Ni/CeO2 Nanostructures for Carbon Dioxide Reforming of Methane. J. Phys. Chem. C 2012, 116, 10009–10016. [Google Scholar] [CrossRef]
- Jomjaree, T.; Sintuya, P.; Srifa, A.; Koo-amornpattana, W.; Kiatphuengporn, S.; Assabumrungrat, S.; Sudoh, M.; Watanabe, R.; Fukuhara, C.; Ratchahat, S. Catalytic Performance of Ni Catalysts Supported on CeO2 with Different Morphologies for Low-Temperature CO2 Methanation. Catal. Today 2021, 375, 234–244. [Google Scholar] [CrossRef]
- Sakpal, T.; Lefferts, L. Structure-Dependent Activity of CeO2 Supported Ru Catalysts for CO2 Methanation. J. Catal. 2018, 367, 171–180. [Google Scholar] [CrossRef]
- Lei, Y.; Li, W.; Liu, Q.; Lin, Q.; Zheng, X.; Huang, Q.; Guan, S.; Wang, X.; Wang, C.; Li, F. Typical Crystal Face Effects of Different Morphology Ceria on the Activity of Pd/CeO2 Catalysts for Lean Methane Combustion. Fuel 2018, 233, 10–20. [Google Scholar] [CrossRef]
- Ren, Z.; Peng, F.; Li, J.; Liang, X.; Chen, B. Morphology-Dependent Properties of Cu/CeO2 Catalysts for the Water-Gas Shift Reaction. Catalysts 2017, 7, 48. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Zhu, S.; Dong, M.; Wang, J.; Fan, W. Ru/CeO2 Catalyst with Optimized CeO2 Morphology and Surface Facet for Efficient Hydrogenation of Ethyl Levulinate to γ-Valerolactone. J. Catal. 2020, 389, 60–70. [Google Scholar] [CrossRef]
- Nath, D.; Singh, F.; Das, R. X-Ray Diffraction Analysis by Williamson-Hall, Halder-Wagner and Size-Strain Plot Methods of CdSe Nanoparticles- a Comparative Study. Mater. Chem. Phys. 2020, 239, 122021. [Google Scholar] [CrossRef]
- Atzori, L.; Cutrufello, M.G.; Meloni, D.; Cannas, C.; Gazzoli, D.; Monaci, R.; Sini, M.F.; Rombi, E. Highly Active NiO-CeO2 Catalysts for Synthetic Natural Gas Production by CO2 Methanation. Catal. Today 2018, 299, 183–192. [Google Scholar] [CrossRef]
- Fedorova, V.; Simonov, M.; Valeev, K.; Bespalko, Y.; Smal, E.; Eremeev, N.; Sadovskaya, E.; Krieger, T.; Ishchenko, A.; Sadykov, V. Kinetic Regularities of Methane Dry Reforming Reaction on Nickel-Containing Modified Ceria–Zirconia. Energies 2021, 14, 2973. [Google Scholar] [CrossRef]
- Santoro, M.; Luisetto, I.; Tuti, S.; Licoccia, S.; Romano, C.; Notargiacomo, A.; Di Bartolomeo, E. Nickel-Based Structured Catalysts for Indirect Internal Reforming of Methane. Appl. Sci. 2020, 10, 3083. [Google Scholar] [CrossRef]
- Varvoutis, G.; Lykaki, M.; Stefa, S.; Binas, V.; Marnellos, G.E.; Konsolakis, M. Deciphering the Role of Ni Particle Size and Nickel-Ceria Interfacial Perimeter in the Low-Temperature CO2 Methanation Reaction over Remarkably Active Ni/CeO2 Nanorods. Appl. Catal. B: Environ. 2021, 297, 120401. [Google Scholar] [CrossRef]
- Lustemberg, P.G.; Mao, Z.; Salcedo, A.; Irigoyen, B.; Ganduglia-Pirovano, M.V.; Campbell, C.T. Nature of the Active Sites on Ni/CeO2 Catalysts for Methane Conversions. ACS Catal. 2021, 10, 10604–10613. [Google Scholar] [CrossRef]
- Wu, H.C.; Chang, Y.C.; Wu, J.H.; Lin, J.H.; Lin, I.K.; Chen, C.S. Methanation of CO2 and Reverse Water Gas Shift Reactions on Ni/SiO2 Catalysts: The Influence of Particle Size on Selectivity and Reaction Pathway. Catal. Sci. Technol. 2015, 5, 4154–4163. [Google Scholar] [CrossRef]



| Sample | Nominal Content (wt.%) | EDS Analysis (wt.%) | Shape of Nanorods (nm) | |||
|---|---|---|---|---|---|---|
| Ni | Ce | Ni | Ce | Length | Width | |
| 2NiCe | 1.75 | 79.96 | 1.7 | 84.3 | 75–225 | 20–30 |
| 4NiCe | 3.61 | 77.66 | 4.4 | 83.1 | 75–250 | 20–35 |
| 8NiCe | 7.69 | 73.44 | 7.3 | 68.1 | 100–250 | 30–40 |
| 12NiCe | 12.32 | 68.64 | 13.4 | 73.9 | 150–300 | 30–50 |
| Sample | Ni (mmol g−1) | Ce (mmol g−1) | H2 Consumption (mmol g−1) | H2/Ni (mol/mol) | H2 Consumption for Ce Reduction (mmol g−1) | Ni0 (%) | Ce3+ (%) |
|---|---|---|---|---|---|---|---|
| r-CeO2 | 0 | 5.81 | - | - | 0.67 | - | 23 |
| 2NiCe | 0.30 | 5.71 | 0.89 | 2.98 | 0.59 | 100 | 21 |
| 4NiCe | 0.61 | 5.60 | 1.15 | 1.86 | 0.53 | 100 | 19 |
| 8NiCe | 1.31 | 5.36 | 1.76 | 1.34 | 0.45 | 100 | 17 |
| 12NiCe | 2.10 | 5.09 | 2.19 | 1.04 | 0.09 | 100 | 3.4 |
| Sample | Peak Area (Counts) | Desorbed H2 (µmol g−1) | dNiTPD (nm) | DNiTPD (%) | |||
|---|---|---|---|---|---|---|---|
| α | β | Total | Total | Ni-H | |||
| 2NiCe-R | 5904 | 8582 | 10,5817 | 199 | 27 | 5.5 | 18 |
| 4NiCe-R | 4829 | 6022 | 78,538 | 155 | 21 | 14 | 7.0 |
| 8NiCe-R | 4278 | 5906 | 67,573 | 132 | 20 | 33 | 3.0 |
| 12NiCe-R | 5523 | 9075 | 67,766 | 135 | 29 | 36 | 2.8 |
| Sample | Scherrer Low | Size–Strain Plot | Williamson–Hall Plot | Scherrer Equation | |||||
|---|---|---|---|---|---|---|---|---|---|
| a (Å) | dCeO2 (nm) | εS-S (×10−3) | R2 | dCeO2 (nm) | εW-H (×10−3) | R2 | dNiO (nm) | dNi (nm) | |
| r-CeO2 | 5.41 | 11 | 17 | 0.990 | 14 | 1.7 | 0.799 | ||
| 2NiCe | 5.41 | 14 | 16 | 0.996 | 16 | 1.9 | 0.896 | 13 | - |
| 4NiCe | 5.41 | 15 | 17 | 0.997 | 19 | 2.1 | 0.922 | 16 | - |
| 8NiCe | 5.40 | 25 | 19 | 0.972 | 40 | 3.1 | 0.99 | 19 | - |
| 12NiCe | 5.40 | 23 | 17 | 0.980 | 32 | 2.5 | 0.810 | 20 | - |
| 2NiCe-R | 5.40 | 28 | 8.9 | 0.988 | 32 | 0.9 | 0.927 | - | 21 |
| 4NiCe-R | 5.41 | 30 | 8.9 | 0.993 | 40 | 1.3 | 0.929 | - | 33 |
| 8NiCe-R | 5.41 | 45 | 11 | 0.977 | 73 | 1.9 | 0.978 | - | 30 |
| 12NiCe-R | 5.41 | 43 | 11 | 0.953 | 87 | 2.2 | 0.953 | - | 34 |
| Sample | S.A.1 (m2 g−1) | Pore Volume (cm3 g−1) | Average Pore Diameter 2 (nm) |
|---|---|---|---|
| r-CeO2 | 94 | 0.40 | 19 |
| 2NiCe-R | 73 | 0.49 | 32 |
| 4NiCe-R | 65 | 0.68 | 39 |
| 8NiCe-R | 34 | 0.28 | 32 |
| 12NiCe-R | 28 | 0.21 | 30 |
| Sample | Ea app (kJ mol) | CH4 Conversion (%) | TOF (CH4) (s−1) | ||||
|---|---|---|---|---|---|---|---|
| 450–650 °C | 600 °C | 650 °C | 700 °C | 600 °C | 650 °C | 700 °C | |
| 2NiCe | 71.8 | 10.0 | 16.1 | 24.0 | 18.1 | 29.3 | 44.2 |
| 4NiCe | 86.6 | 22.1 | 33.4 | 46.3 | 32.1 | 48.9 | 68.1 |
| 8NiCe | 67.6 | 29.7 | 42.5 | 56.2 | 65.0 | 88.5 | 123.7 |
| 12NiCe | 73.5 | 29.5 | 40.5 | 55.7 | 44.4 | 59.5 | 82.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuti, S.; Luisetto, I.; Pasqual Laverdura, U.; Marconi, E. Dry Reforming of Methane on Ni/Nanorod-CeO2 Catalysts Prepared by One-Pot Hydrothermal Synthesis: The Effect of Ni Content on Structure, Activity, and Stability. Reactions 2022, 3, 333-351. https://doi.org/10.3390/reactions3030025
Tuti S, Luisetto I, Pasqual Laverdura U, Marconi E. Dry Reforming of Methane on Ni/Nanorod-CeO2 Catalysts Prepared by One-Pot Hydrothermal Synthesis: The Effect of Ni Content on Structure, Activity, and Stability. Reactions. 2022; 3(3):333-351. https://doi.org/10.3390/reactions3030025
Chicago/Turabian StyleTuti, Simonetta, Igor Luisetto, Umberto Pasqual Laverdura, and Eleonora Marconi. 2022. "Dry Reforming of Methane on Ni/Nanorod-CeO2 Catalysts Prepared by One-Pot Hydrothermal Synthesis: The Effect of Ni Content on Structure, Activity, and Stability" Reactions 3, no. 3: 333-351. https://doi.org/10.3390/reactions3030025