Nanotheranostics: Platforms, Current Applications, and Mechanisms of Targeting in Breast and Prostate Cancers
Abstract
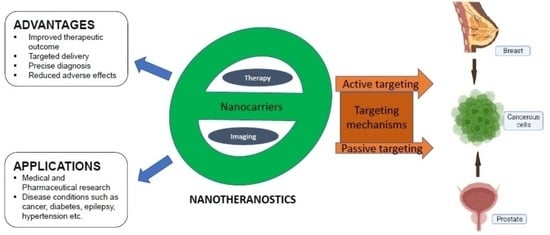
:1. Introduction
2. Nanotheranostic Platforms
2.1. Liposomes
2.2. Solid Lipid Nanoparticles
2.3. Polymers
2.4. Metallic and Magnetic Nanoparticles
2.5. Carbon Nanotubes
2.6. Dendrimers
2.7. Quantum Dots
3. Mechanisms of Targeting and Targeting Moieties/Ligands of Nanoparticles
3.1. Passive Targeting
3.2. Active Targeting
3.3. Transferrin
3.4. Hyaluronic Acid
3.5. Folic Acid
3.6. Peptides
3.7. Anisamide
3.8. Aptamers
4. Monoclonal Antibodies
5. Advantages and Applications of Nanotheranostics
| Nanoparticles | Therapeutic Agent | Diagnostic Agent | Cancer Type | Observation | References |
|---|---|---|---|---|---|
| Polystyrene | Doxorubicin | Cyanine dye | Breast | Improved therapeutic index in vitro and reduced tumor volume in vivo | [250] |
| Gold nanoclusters | Paclitaxel | Indocyanine green (ICG) | Breast | Suppression of tumor growth on mice breast cancer model | [251] |
| Graphene quantum dots | Doxorubicin | - | Breast | Synergistically enhanced anticancer strategy which provides treatment and diagnosis | [252] |
| PEG liposomes | Doxorubicin | Gadoteridol | Breast | Increased intratumor drug concentration and complete regression of lesion | [46,47] |
| Targeted PEG liposomes | Doxorubicin | Fluorescent probe (PFBT) | Breast | Inhibition of tumor-bearing mice | [253] |
| Micelles | Docetaxel | NIR probe DiR | Breast | Inhibition of tumor growth with little toxicity | [254] |
| Targeted PEG liposomes | Mitoxantrone | SPIONs | Breast | High cytotoxicity against MCF-7 human breast tumor cell line | [255] |
| Iron oxide | Curcumin | - | Breast | Display of strong anticancer properties compared to free curcumin | [256] |
| Poly(lactide-co-glycolic acid) PLGA | Resiquimod | Indocyanine green (ICG) | Prostate | Significant inhibition of PCa growth | [257] |
| Bovine serum albumin | Carbazitaxel | Gadolinium | Prostate | Lower hemolysis, similar tumor inhibition and enhanced cellular uptake in vitro compared with CBZ–Tween-80 injection | [258] |
| Gold | Aptamer/doxorubicin | - | Prostate | PSMA aptamer showed more potency against targeted LNCaP cell lines than non-targeted PC3 cells | [259] |
| Quantum dots | Aptamer/doxorubicin | - | Prostate | Targeted Qd-Apt (DOX) conjugate with reversible self-quenching properties | [77] |
5.1. Visualizing Drug Release
5.2. Visualizing Biodistribution in Real Time
5.3. Noninvasively Assessing Target Site Accumulation
5.4. Monitoring Drug Distribution at the Target Site
5.5. Facilitating Triggered Drug Release
5.6. Predicting Drug Responses
5.7. Evaluating Drug Efficacy Longitudinally
6. Limitations/Challenges of Nanotheranostics
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- NCI. Available online: https://www.cancer.gov/about-cancer/understanding (accessed on 30 July 2020).
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- WHO. Breast 2020. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/20-Breast-fact-sheet.pdf (accessed on 2 June 2021).
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2022. CA A Cancer J. Clin. 2022, 72, 524–541. [Google Scholar] [CrossRef]
- Altekruse, S.F.; Kosary, C.L.; Krapcho, M.; Neyman, N.; Aminou, R.; Waldron, W.; Ruhl, J.; Howlader, N.; Tatalovich, Z.; Cho, H.; et al. SEER Cancer Statistics Review, 1975–2007; National Cancer Institute: Bethesda, MD, USA, 2010. Available online: https://seer.cancer.gov/archive/csr/1975_2007/ (accessed on 17 May 2017).
- Sinn, H.P.; Kreipe, H. A Brief Overview of the WHO Classification of Breast Tumors, 4th Edition, Focusing on Issues and Updates from the 3rd Edition. Breast Care 2013, 8, 149–154. [Google Scholar] [CrossRef] [Green Version]
- Weigelt, B.; Peterse, J.L.; van’t Veer, L.J. Breast cancer metastasis: Markers and models. Nat. Rev. Cancer 2005, 5, 591–602. [Google Scholar] [CrossRef]
- Fragomeni, S.M.; Sciallis, A.; Jeruss, J.S. Molecular Subtypes and Local-Regional Control of Breast Cancer. Surg. Oncol. Clin. N. Am. 2018, 27, 95–120. [Google Scholar] [CrossRef] [PubMed]
- Bonotto, M.; Gerratana, L.; Poletto, E.; Driol, P.; Giangreco, M.; Russo, S.; Minisini, A.M.; Andreetta, C.; Mansutti, M.; Pisa, F.E.; et al. Measures of Outcome in Metastatic Breast Cancer: Insights from a Real-World Scenario. Oncology 2014, 19, 608–615. [Google Scholar] [CrossRef] [Green Version]
- Redig, A.J.; McAllister, S.S. Breast cancer as a systemic disease: A view of metastasis. J. Intern. Med. 2013, 274, 113–126. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Costello, L.C.; Franklin, R.B. A comprehensive review of the role of zinc in normal prostate function and metabolism; and its implications in prostate cancer. Arch. Biochem. Biophys. 2016, 611, 100–112. [Google Scholar] [CrossRef] [Green Version]
- Fujita, K.; Hayashi, T.; Matsushita, M.; Uemura, M.; Nonomura, N. Obesity, Inflammation, and Prostate Cancer. J. Clin. Med. 2019, 8, 201. [Google Scholar] [PubMed] [Green Version]
- Ferlay, J.; Shin, H.-R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, K.D.; Fidler-Benaoudia, M.; Keegan, T.H.; Hipp, H.S.; Jemal, A.; Siegel, R.L.; Dvm, A.J. Cancer statistics for adolescents and young adults, 2020. CA A Cancer J. Clin. 2020, 70, 443–459. [Google Scholar] [CrossRef]
- Perdonà, S.; Cavadas, V.; Di Lorenzo, G.; Damiano, R.; Chiappetta, G.; Del Prete, P.; Franco, R.; Azzarito, G.; Scala, S.; Arra, C.; et al. Prostate cancer detection in the ‘‘grey area” of prostate-specific antigen below 10 ng/ml: Head-to-head comparison of the updated PCPT calculator and Chun’s nomogram, two risk estimators incorporating prostate cancer antigen 3. Eur. Urol. 2011, 59, 81–87. [Google Scholar]
- Shinohara, K. Improving cancer detection by prostate biopsy: The role of core number and site. Nat. Rev. Endocrinol. 2006, 3, 526–527. [Google Scholar] [CrossRef]
- Naji, L.; Randhawa, H.; Sohani, Z.; Dennis, B.; Lautenbach, D.; Kavanagh, O.; Bawor, M.; Banfield, L.; Profetto, J. Digital Rectal Examination for Prostate Cancer Screening in Primary Care: A Systematic Review and Meta-Analysis. Ann. Fam. Med. 2018, 16, 149–154. [Google Scholar] [CrossRef]
- Catalona, W.J.; Smith, D.S.; Ratliff, T.L.; Dodds, K.M.; Coplen, D.E.; Yuan, J.J.; Petros, J.A.; Andriole, G.L. Measurement of Prostate-Specific Antigen in Serum as a Screening Test for Prostate Cancer. N. Engl. J. Med. 1991, 324, 1156–1161. [Google Scholar] [CrossRef]
- Schröder, F.H.; van der Cruijsen-Koeter, I.; de Koning, H.J.; Vis, A.N.; Hoedemaeker, R.F.; Kranse, R. Prostate cancer detection at low prostate specific antigen. J. Urol. 2000, 163, 806–812. [Google Scholar]
- Lee, K.H.; Kang, B.J.; Jeun, M.; Jang, G.H.; Song, S.H.; Jeong, I.G.; Kim, C.-S.; Searson, P.C. Diagnosis of prostate cancer via nanotechnological approach. Int. J. Nanomed. 2015, 10, 6555–6569. [Google Scholar] [CrossRef] [Green Version]
- Nie, S.; Xing, Y.; Kim, G.J.; Simons, J.W. Nanotechnology Applications in Cancer. Annu. Rev. Biomed. Eng. 2007, 9, 257–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melancon, M.P.; Stafford, R.J.; Li, C. Challenges to effective cancer nanotheranostics. J. Control. Release 2012, 164, 177–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falzone, L.; Salomone, S.; Libra, M. Evolution of Cancer Pharmacological Treatments at the Turn of the Third Millennium. Front. Pharmacol. 2018, 9, 1300. [Google Scholar] [CrossRef] [Green Version]
- Chinen, A.B.; Guan, C.M.; Ferrer, J.R.; Barnaby, S.N.; Merkel, T.J.; Mirkin, C.A. Nanoparticle Probes for the Detection of Cancer Biomarkers, Cells, and Tissues by Fluorescence. Chem. Rev. 2015, 115, 10530–10574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2016, 17, 20–37. [Google Scholar] [CrossRef] [Green Version]
- Banthia, P.; Gambhir, L.; Sharma, A.; Daga, D.; Kapoor, N.; Chaudhary, R.; Sharma, G. Nano to rescue: Repository of nanocarriers for targeted drug delivery to curb breast cancer. 3 Biotech 2022, 12, 1–23. [Google Scholar] [CrossRef]
- Funkhouser, J. Reinventing Pharma: The theranostic revolution. Curr. Drug Discov. 2002, 2, 17–19. [Google Scholar]
- Asem, H.; Malmström, E. Polymeric Nanoparticles Explored for Drug-Delivery Applications. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2018; pp. 315–331. [Google Scholar]
- Kröger, A.P.P.; Hamelmann, N.M.; Juan, A.; Lindhoud, S.; Paulusse, J.M.J. Biocompatible Single-Chain Polymer Nanoparticles for Drug Delivery—A Dual Approach. ACS Appl. Mater. Interfaces 2018, 10, 30946–30951. [Google Scholar] [CrossRef] [Green Version]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [Green Version]
- Rehman, A.U.; Akram, S.; Seralin, A.; Vandamme, T.; Anton, N. Lipid nanocarriers: Formulation, properties, and applications. In Smart Nanocontainers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 355–382. [Google Scholar]
- Bombelli, C.; Stringaro, A.; Borocci, S.; Bozzuto, G.; Colone, M.; Giansanti, L.; Sgambato, R.; Toccaceli, L.; Mancini, G.; Molinari, A. Efficiency of Liposomes in the Delivery of a Photosensitizer Controlled by the Stereochemistry of a Gemini Surfactant Component. Mol. Pharm. 2010, 7, 130–137. [Google Scholar] [CrossRef]
- Muthu, M.S.; Leong, D.T.; Mei, L.; Feng, S.-S. Nanotheranostics—Application and Further Development of Nanomedicine Strategies for Advanced Theranostics. Theranostics 2014, 4, 660–677. [Google Scholar] [CrossRef] [Green Version]
- Xing, H.; Hwang, K.; Lu, Y. Recent Developments of Liposomes as Nanocarriers for Theranostic Applications. Theranostics 2016, 6, 1336–1352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, Y.; Meng, X.; Lu, H.; Chang, H.; Dong, H.; Zhang, X. Light-triggered theranostic liposomes for tumor diagnosis and combined photodynamic and hypoxia-activated prodrug therapy. Biomaterials 2018, 185, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Karpuz, M.; Silindir-Gunay, M.; Ozer, A.Y.; Ozturk, S.C.; Yanik, H.; Tuncel, M.; Aydin, C.; Esendagli, G. Diagnostic and therapeutic evaluation of folate-targeted paclitaxel and vinorelbine encapsulating theranostic liposomes for non-small cell lung cancer. Eur. J. Pharm. Sci. 2020, 156, 105576. [Google Scholar] [CrossRef]
- Skupin-Mrugalska, P.; Sobotta, L.; Warowicka, A.; Wereszczynska, B.; Zalewski, T.; Gierlich, P.; Jarek, M.; Nowaczyk, G.; Kempka, M.; Gapinski, J.; et al. Theranostic liposomes as a bimodal carrier for magnetic resonance imaging contrast agent and photosensitizer. J. Inorg. Biochem. 2018, 180, 1–14. [Google Scholar] [CrossRef]
- Ren, W.; Chen, S.; Liao, Y.; Li, S.; Ge, J.; Tao, F.; Huo, Q.; Zhang, Y.; Zhao, Z. Near-infrared fluorescent carbon dots encapsulated liposomes as multifunctional nano-carrier and tracer of the anticancer agent cinobufagin in vivo and in vitro. Colloids Surfaces B Biointerfaces 2019, 174, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Grippin, A.J.; Wummer, B.; Wildes, T.; Dyson, K.; Trivedi, V.; Yang, C.; Sebastian, M.; Mendez-Gomez, H.R.; Padala, S.; Grubb, M.; et al. Dendritic Cell-Activating Magnetic Nanoparticles Enable Early Prediction of Antitumor Response with Magnetic Resonance Imaging. ACS Nano 2019, 13, 13884–13898. [Google Scholar] [CrossRef]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res Lett. 2021, 16, 173. [Google Scholar] [CrossRef]
- Markman, M. Pegylated liposomal doxorubicin in the treatment of cancers of the breast and ovary. Expert Opin. Pharmacother. 2006, 7, 1469–1474. [Google Scholar] [CrossRef]
- Malik, N.; Evagorou, E.G.; Duncan, R. Dendrimer-platinate: A novel approach to cancer chemotherapy. Anti-Cancer Drugs 1999, 10, 767–776. [Google Scholar] [CrossRef]
- Rizzitelli, S.; Giustetto, P.; Cutrin, J.; Castelli, D.D.; Boffa, C.; Ruzza, M.; Menchise, V.; Molinari, F.; Aime, S.; Terreno, E. Sonosensitive theranostic liposomes for preclinical in vivo MRI-guided visualization of doxorubicin release stimulated by pulsed low intensity non-focused ultrasound. J. Control. Release 2015, 202, 21–30. [Google Scholar] [CrossRef]
- Rizzitelli, S.; Giustetto, P.; Faletto, D.; Castelli, D.D.; Aime, S.; Terreno, E. The release of Doxorubicin from liposomes monitored by MRI and triggered by a combination of US stimuli led to a complete tumor regression in a breast cancer mouse model. J. Control. Release 2016, 230, 57–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yari, H.; Nkepang, G.; Awasthi, V. Surface Modification of Liposomes by a Lipopolymer Targeting Prostate Specific Membrane Antigen for Theranostic Delivery in Prostate Cancer. Materials 2019, 12, 756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayanan, N.K.; Nargi, D.; Randolph, C.; Narayanan, B.A. Liposome encapsulation of curcumin and resveratrol in combination reduces prostate cancer incidence in PTEN knockout mice. Int. J. Cancer 2009, 125, 1–8. [Google Scholar] [CrossRef]
- Thangapazham, R.L.; Puri, A.; Tele, S.; Blumenthal, R.; Maheshwari, R.K. Evaluation of a nanotechnology-based carrier for delivery of curcumin in prostate cancer cells. Int. J. Oncol. 2008, 32, 1119–1123. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.-Z.; Dai, D.-D.; Lu, C.-T.; Chen, L.-J.; Lin, M.; Shen, X.-T.; Li, X.-K.; Zhang, M.; Jiang, X.; Jin, R.-R.; et al. Epirubicin loaded with propylene glycol liposomes significantly overcomes multidrug resistance in breast cancer. Cancer Lett. 2013, 330, 74–83. [Google Scholar] [CrossRef]
- Fu, M.; Tang, W.; Liu, J.-J.; Gong, X.-Q.; Kong, L.; Yao, X.-M.; Jing, M.; Cai, F.-Y.; Li, X.-T.; Ju, R.-J. Combination of targeted daunorubicin liposomes and targeted emodin liposomes for treatment of invasive breast cancer. J. Drug Target. 2020, 28, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Sisay, B.; Abrha, S.; Yilma, Z.; Assen, A.; Molla, F.; Tadese, E.; Wondimu, A.; Gebre-Samuel, N.; Pattnaik, G. Cancer nanotheranostics: A new paradigm of simultaneous diagnosis and therapy. J. Drug Deliv. Ther. 2014, 4, 79–86. [Google Scholar] [CrossRef]
- Lima, A.M.; Pizzol, C.D.; Monteiro, F.B.; Creczynski-Pasa, T.B.; Andrade, G.P.; Ribeiro, A.O.; Perussi, J.R. Hypericin encapsulated in solid lipid nanoparticles: Phototoxicity and photodynamic efficiency. J. Photochem. Photobiol. B Biol. 2013, 125, 146–154. [Google Scholar] [CrossRef]
- Wong, H.L.; Bendayan, R.; Rauth, A.M.; Li, Y.; Wu, X.Y. Chemotherapy with anticancer drugs encapsulated in solid lipid nanoparticles. Adv. Drug Deliv. Rev. 2007, 59, 491–504. [Google Scholar] [CrossRef]
- Fathi, M.; Mozafari, M.; Mohebbi, M. Nanoencapsulation of food ingredients using lipid based delivery systems. Trends Food Sci. Technol. 2012, 23, 13–27. [Google Scholar] [CrossRef]
- Mehnert, W.; Mader, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef] [PubMed]
- Wissing, S.A.; Kayser, O.; Muller, R.H. Solid Lipid Nanoparticles for Parenteral Drug Delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.K.; Singh, B.; Jain, A.; Nirbhavane, P.; Sharma, R.; Tyagi, R.K.; Kushwah, V.; Jain, S.; Katare, O.P. Fucose decorated solid-lipid nanocarriers mediate efficient delivery of methotrexate in breast cancer therapeutics. Colloids Surfaces B Biointerfaces 2016, 146, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Eskiler, G.G.; Cecener, G.; Dikmen, G.; Egeli, U.; Tunca, B. Solid lipid nanoparticles: Reversal of tamoxifen resistance in breast cancer. Eur. J. Pharm. Sci. 2018, 120, 73–88. [Google Scholar] [CrossRef]
- Sharma, A.N.; Upadhyay, P.K.; Dewangan, H.K. Development, evaluation, pharmacokinetic and biodistribution estimation of resveratrol-loaded solid lipid nanoparticles for prostate cancer targeting. J. Microencapsul. 2022, 39, 563–574. [Google Scholar] [CrossRef]
- Akanda, M.H.; Rai, R.; Slipper, I.J.; Chowdhry, B.Z.; Lamprou, D.; Getti, G.; Douroumis, D. Delivery of retinoic acid to LNCap human prostate cancer cells using solid lipid nanoparticles. Int. J. Pharm. 2015, 493, 161–171. [Google Scholar] [CrossRef] [Green Version]
- Janib, S.M.; Moses, A.S.; MacKay, J.A. Imaging and drug delivery using theranostic nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 1052–1063. [Google Scholar] [CrossRef] [Green Version]
- Grossman, J.H.; McNeil, S.E. Nanotechnology in Cancer Medicine. Phys. Today 2012, 65, 38–42. [Google Scholar] [CrossRef]
- Gardel, M.L. Synthetic polymers with biological rigidity. Nature 2013, 493, 619. [Google Scholar] [CrossRef]
- Ogay, V.; Mun, E.A.; Kudaibergen, G.; Baidarbekov, M.; Kassymbek, K.; Zharkinbekov, Z.; Saparov, A. Progress and Prospects of Polymer-Based Drug Delivery Systems for Bone Tissue Regeneration. Polymers 2020, 12, 2881. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Houston, Z.H.; Simpson, J.D.; Chen, L.; Fletcher, N.L.; Fuchs, A.V.; Blakey, I.; Thurecht, K.J. Using Peptide Aptamer Targeted Polymers as a Model Nanomedicine for Investigating Drug Distribution in Cancer Nanotheranostics. Mol. Pharm. 2017, 14, 3539–3549. [Google Scholar] [CrossRef]
- Jia, H.-R.; Jiang, Y.-W.; Zhu, Y.-X.; Li, Y.-H.; Wang, H.-Y.; Han, X.; Yu, Z.-W.; Gu, N.; Liu, P.; Chen, Z.; et al. Plasma membrane activatable polymeric nanotheranostics with self-enhanced light-triggered photosensitizer cellular influx for photodynamic cancer therapy. J. Control. Release 2017, 255, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, X.; Chen, J.; Zhang, J.; Meng, F.; Deng, C.; Cheng, R.; Feijen, J.; Zhong, Z. Bioresponsive and fluorescent hyaluronic acid-iodixanol nanogels for targeted X-ray computed tomography imaging and chemotherapy of breast tumors. J. Control. Release 2016, 244, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Zhu, M.; Li, N.; Ao, M.; Li, Y.; Zhong, M.; Yuan, Q.; Chen, H.; Fan, Z.; Wang, Y.; et al. Imaging-guided synergistic targeting-promoted photo-chemotherapy against cancers by methotrexate-conjugated hyaluronic acid nanoparticles. Chem. Eng. J. 2020, 380, 122426. [Google Scholar] [CrossRef]
- Mansur, A.A.; Caires, A.J.; Carvalho, S.M.; Capanema, N.S.; Carvalho, I.C.; Mansur, H.S. Dual-functional supramolecular nanohybrids of quantum dot/biopolymer/chemotherapeutic drug for bioimaging and killing brain cancer cells in vitro. Colloids Surfaces B Biointerfaces 2019, 184, 110507. [Google Scholar] [CrossRef]
- Shitole, A.A.; Sharma, N.; Giram, P.; Khandwekar, A.; Baruah, M.; Garnaik, B.; Koratkar, S. LHRH-conjugated, PEGylated, poly-lactide-co-glycolide nanocapsules for targeted delivery of combinational chemotherapeutic drugs Docetaxel and Quercetin for prostate cancer. Mater. Sci. Eng. C 2020, 114, 111035. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar]
- Libutti, S.K.; Paciotti, G.F.; Byrnes, A.A.; Alexander, H.R., Jr.; Gannon, W.E.; Walker, M.; Seidel, G.D.; Yuldasheva, N.; Tamarkin, L. Phase I and pharmacokinetic studies of CYT-6091, a novel PEGylated colloidal gold-rhTNF nanomedicine. Clin. Cancer Res. 2010, 16, 6139–6149. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Cao, Z.; Liu, R.; Liu, L.; Li, H.; Li, X.; Chen, Y.; Lu, C.; Liu, Y. AuNPs as an important inorganic nanoparticle applied in drug carrier systems. Artif. Cells Nanomed. Biotechnol. 2019, 47, 4222–4233. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Jiang, S.; Wang, J.; Li, X.; Wu, T.; Xu, P.; Santos-Oliveira, R.; Zhang, A. Radioactive Gold Nanoparticle in Two Forms (19879Au GNPs and 99mTc-GNPs) for Lung Cancer Antiproliferative Induction and Intralesional Imaging: A Proof of Concept. Anti-Cancer Agents Med. Chem. 2020, 20, 1648–1653. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Jeong, Y.Y.; Jon, S. A Drug-Loaded Aptamer−Gold Nanoparticle Bioconjugate for Combined CT Imaging and Therapy of Prostate Cancer. ACS Nano 2010, 4, 3689–3696. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Johnson, A.; Wang, X.; Li, H.; Erokwu, B.O.; Springer, S.; Lou, J.; Ramamurthy, G.; Flask, C.A.; Burda, C.; et al. Targeted Radiosensitizers for MR-Guided Radiation Therapy of Prostate Cancer. Nano Lett. 2020, 20, 7159–7167. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.; de Kock, M.; Engelbrecht, M.; Miles, X.; Slabbert, J.; Vandevoorde, C. Radiosensitization effect of gold nanoparticles in proton therapy. Front. Public Health. 2021, 9, 699822. [Google Scholar] [CrossRef] [PubMed]
- Bouché, M.; Hsu, J.C.; Dong, Y.C.; Kim, J.; Taing, K.; Cormode, D.P. Recent Advances in Molecular Imaging with Gold Nanoparticles. Bioconj. Chem. 2020, 31, 303–314. [Google Scholar] [CrossRef]
- Yang, S.-J.; Huang, C.-H.; Wang, C.-H.; Shieh, M.-J.; Chen, K.-C. The Synergistic Effect of Hyperthermia and Chemotherapy in Magnetite Nanomedicine-Based Lung Cancer Treatment. Int. J. Nanomed. 2020, 15, 10331–10347. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Wang, Y.; Zhu, W.; Li, G.; Ma, X.; Chen, S.; Tiwari, S.; Shi, K.; Zhang, S.; et al. Comprehensive understanding of magnetic hyperthermia for improving antitumor therapeutic efficacy. Theranostics 2020, 10, 3793–3815. [Google Scholar] [CrossRef]
- Ferreira, M.; Sousa, J.; Pais, A.; Vitorino, C. The Role of Magnetic Nanoparticles in Cancer Nanotheranostics. Materials 2020, 13, 266. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Wang, X.-Y.; Chen, Y.-L.; Liu, F.-Q.; Tan, M.-X.; Ao, M.; Yu, J.-H.; Ran, H.-T.; Wang, Z.-X. A light-controllable specific drug delivery nanoplatform for targeted bimodal imaging-guided photothermal/chemo synergistic cancer therapy. Acta Biomater. 2018, 80, 308–326. [Google Scholar] [CrossRef]
- Arndt, M.; Nairz, O.; Voss-Andreae, J.; Keller, C.; Van der Zouw, G.; Zeilinger, A. Wave-particle duality of C60. Nature 1999, 401, 680–682. [Google Scholar] [CrossRef]
- McDevitt, M.R.; Chattopadhyay, D.; Kappel, B.J.; Jaggi, J.S.; Schiffman, S.R.; Antczak, C.; Njardarson, J.T.; Brentjens, R.; Scheinberg, D.A. Tumor Targeting with Antibody-Functionalized, Radiolabeled Carbon Nanotubes. J. Nucl. Med. 2007, 48, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.E.; Gass, M.; Muller, K.; Skepper, J.N.; Midgley, P.A.; Welland, M. Direct imaging of single-walled carbon nanotubes in cells. Nat. Nanotechnol. 2007, 2, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Fubini, B.; Ghiazza, M.; Fenoglio, I. Physio-chemical features of engineered nanoparticles relevant to their toxicity. Nanotoxicology 2010, 4, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Fabbro, C.; Ali-Boucetta, H.; Da Ros, T.; Kostarelos, K.; Bianco, A.; Prato, M. Targeting carbon nanotubes against cancer. Chem. Commun. 2012, 48, 3911–3926. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, K.; Davis, C.; Sherlock, S.; Cao, Q.; Chen, X.; Dai, H. Drug Delivery with Carbon Nanotubes for In vivo Cancer Treatment. Cancer Res. 2008, 68, 6652–6660. [Google Scholar] [CrossRef] [Green Version]
- Burke, A.R.; Singh, R.N.; Carroll, D.L.; Wood, J.C.S.; D’Agostino, R.B.; Ajayan, P.M.; Torti, F.M.; Torti, S.V. The resistance of breast cancer stem cells to conventional hyperthermia and their sensitivity to nanoparticle-mediated photothermal therapy. Biomaterials 2012, 33, 2961–2970. [Google Scholar] [CrossRef] [Green Version]
- Das, M.; Datir, S.R.; Singh, R.P.; Jain, S. Augmented anticancer activity of a targeted, intracellularly activatable, theranostic nanomedicine based a fluorescent and radiolabeled, methotrxate-folic acid-multiwalled carbon nanotube conjugate. Mol. Pharm. 2013, 10, 2543–2557. [Google Scholar] [CrossRef]
- Shao, W.; Paul, A.; Rodes, L.; Prakash, S. A New Carbon Nanotube-Based Breast Cancer Drug Delivery System: Preparation and In Vitro Analysis Using Paclitaxel. Cell Biochem. Biophys. 2015, 71, 1405–1414. [Google Scholar] [CrossRef]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res Lett. 2014, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Yang, Y.; Sun, Y. Dendrimer Applications for Cancer Therapies. J. Phys. Conf. Ser. 2021, 1948, 012205. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, H.; Wu, Y. Dendrimer Advances for the Central Nervous System Delivery of Therapeutics. ACS Chem. Neurosci. 2014, 5, 2–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmerston Mendes, L.; Pan, J.; Torchilin, V.P. Dendrimers as Nanocarriers for Nucleic Acid and Drug Delivery in Cancer Therapy. Molecules 2017, 22, 1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menjoge, A.R.; Kannan, R.M.; Tomalia, D.A. Dendrimer-based drug and imaging conjugates: Design considerations for nanomedical applications. Drug Discov. Today 2010, 15, 171–185. [Google Scholar] [CrossRef]
- Kannan, R.M.; Nance, E.; Kannan, S.; Tomalia, D.A. Emerging concepts in dendrimer-based nanomedicine: From design principles to clinical applications. J. Intern. Med. 2014, 276, 579–617. [Google Scholar] [CrossRef]
- Ghosh, S.; Ghosal, K.; Mohammad, S.A.; Sarkar, K. Dendrimer functionalized carbon quantum dot for selective detection of breast cancer and gene therapy. Chem. Eng. J. 2019, 373, 468–484. [Google Scholar] [CrossRef]
- Guo, X.L.; Kang, X.X.; Wang, Y.Q.; Zhang, X.J.; Li, C.J.; Liu, Y.; Du, L.B. Codelivery of cisplatin and doxorubicin by covalently conjugating with polyamidoamine dendrimer for enhanced synergistic cancer therapy. Acta Biomater. 2019, 84, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Dorai, T.; Gehani, N.; Katz, A. Therapeutic potential of curcumin in human prostate cancer—I. curcumin induces apoptosis in both androgen-dependent and androgen-independent prostate cancer cells. Prostate Cancer Prostatic Dis. 2000, 3, 84–93. [Google Scholar] [CrossRef] [Green Version]
- Dorai, T.; Gehani, N.; Katz, A. Therapeutic potential of curcumin in human prostate cancer. II. Curcumin inhibits tyrosine kinase activity of epidermal growth factor receptor and depletes the protein. Mol. Urol. 2000, 4, 1–6. [Google Scholar]
- Dorai, T.; Cao, Y.C.; Dorai, B.; Buttyan, R.; Katz, A.E. Therapeutic potential of curcumin in human prostate cancer. III. Curcumin inhibits proliferation, induces apoptosis, and inhibits angiogenesis of LNCaP prostate cancer cells in vivo. Prostate 2001, 47, 293–303. [Google Scholar] [CrossRef]
- Davis, J.N.; Muqim, N.; Bhuiyan, M.; Kucuk, O.; Pienta, K.J.; Sarkar, F.H. Inhibition of prostate specific antigen expression by genistein in prostate cancer cells. Int. J. Oncol. 2000, 16, 1091–1098. [Google Scholar] [CrossRef]
- Chittasupho, C.; Anuchapreeda, S.; Sarisuta, N. CXCR4 targeted dendrimer for anti-cancer drug delivery and breast cancer cell migration inhibition. Eur. J. Pharm. Biopharm. 2017, 119, 310–321. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; HuangFu, M.; Xiao, Y.; Zhang, T.; Han, M.; Xu, D.; Li, F.; Ling, D.; Jin, Y.; et al. Pluronic-attached polyamidoamine dendrimer conjugates overcome drug resistance in breast cancer. Nanomedicine 2016, 11, 2917–2934. [Google Scholar] [CrossRef]
- Nottelet, B.; Darcos, V.; Coudane, J. Aliphatic polyesters for medical imaging and theranostic applications. Eur. J. Pharm. Biopharm. 2015, 97, 350–370. [Google Scholar] [CrossRef]
- Carvalho, M.R.; Reis, R.L.; Oliveira, J.M. Dendrimer nanoparticles for colorectal cancer applications. J. Mater. Chem. B 2020, 8, 1128–1138. [Google Scholar] [CrossRef]
- Granada-Ramirez, D.A.; Arias-Ceron, J.S.; Rodriguez-Fragoso, P.; Vazquez-Hernandez, F.; Luna-Arias, J.P.; Herrera-Perez, J.L.; Mendoza-Alvarez, J.G. Quantum dots for biomedical applications. In Nanobiomaterials; Narayan, R., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 411–436. [Google Scholar]
- Zhao, M.-X.; Zeng, E.-Z. Application of functional quantum dot nanoparticles as fluorescence probes in cell labeling and tumor diagnostic imaging. Nanoscale Res. Lett. 2015, 10, 171. [Google Scholar] [CrossRef] [Green Version]
- Ahar, M.J. A Review on Aptamer-Conjugated Quantum Dot Nanosystems for Cancer Imaging and Theranostic. J. Nanomed. Res. 2017, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.K.; Yan, J.; Liu, P.; Zhao, B.Y.; Cao, Y.; Zhang, X.F. A novel cancer nanotheranostics system based on quantum dots encapsulated by a polymer-prodrug with controlled release behavior. Aust. J. Chem. 2017, 70, 1302–1311. [Google Scholar] [CrossRef]
- AbdElhamid, A.S.; Helmy, M.W.; Ebrahim, S.M.; Bahey-El-Din, M.; Zayed, D.G.; Dein, E.A.Z.E.; El-Gizawy, S.; Elzoghby, A.O. Layer-by-layer gelatin/chondroitin quantum dots-based nanotheranostics: Combined rapamycin/celecoxib delivery and cancer imaging. Nanomedicine 2018, 13, 1707–1730. [Google Scholar] [CrossRef]
- Jiang, W.; Chen, J.; Gong, C.; Wang, Y.; Gao, Y.; Yuan, Y. Intravenous delivery of enzalutamide based on high drug loading multifunctional graphene oxide nanoparticles for castration-resistant prostate cancer therapy. J. Nanobiotechnol. 2020, 18, 50. [Google Scholar] [CrossRef]
- Gokarna, A.; Jin, L.-H.; Hwang, J.S.; Cho, Y.-H.; Lim, Y.T.; Chung, B.H.; Youn, S.H.; Choi, D.S.; Lim, J.H. Quantum dot-based protein micro- and nanoarrays for detection of prostate cancer biomarkers. Proteomics 2008, 8, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Samimi, S.; Ardestani, M.S.; Dorkoosh, F.A. Preparation of carbon quantum dots- quinic acid for drug delivery of gemcitabine to breast cancer cells. J. Drug Deliv. Sci. Technol. 2021, 61, 102287. [Google Scholar] [CrossRef]
- Sambi, M.; Bagheri, L.; Szewczuk, M.R. Current Challenges in Cancer Immunotherapy: Multimodal Approaches to Improve Efficacy and Patient Response Rates. J. Oncol. 2019, 2019, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, W.; Heo, Y.-J.; Han, D.K. New opportunities for nanoparticles in cancer immunotherapy. Biomater. Res. 2018, 22, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bregoli, L.; Movia, D.; Gavigan-Imedio, J.D.; Lysaght, J.; Reynolds, J.; Prina-Mello, A. Nanomedicine applied to translational oncology: A future perspective on cancer treatment. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 81–103. [Google Scholar] [CrossRef] [Green Version]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater. Sci. Eng. C 2019, 98, 1252–1276. [Google Scholar] [CrossRef]
- Lei, T.; Srinivasan, S.; Tang, Y.; Manchanda, R.; Nagesetti, A.; Fernandez-Fernandez, A.; McGoron, A.J. Comparing cellular uptake and cytotoxicity of targeted drug carriers in cancer cell lines with different drug resistance mechanisms. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 324–332. [Google Scholar] [CrossRef] [Green Version]
- Tahmasbi Rad, A.; Chen, C.W.; Aresh, W.; Xia, Y.; Lai, P.S.; Nieh, M.P. Combinational effects of active targeting, shape, and enhanced permeability and retention for cancer theranostic nanocarriers, ACS Appl. Mater. Interfaces 2019, 11, 10505–10519. [Google Scholar] [CrossRef]
- Bort, G.; Lux, F.; Dufort, S.; Crémillieux, Y.; Verry, C.; Tillement, O. EPR-mediated tumor targeting using ultrasmall-hybrid nanoparticles: From animal to human with theranostic AGuIX nanoparticles. Theranostics 2020, 10, 1319–1331. [Google Scholar] [CrossRef]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, J.; Li, C.; Qiao, H.; Hussain, Z. Functionalization of curcumin nanomedicines: A recent promising adaptation to maximize pharmacokinetic profile, specific cell internalization and anticancer efficacy against breast cancer. J. Nanobiotechnol. 2023, 21, 106. [Google Scholar] [CrossRef]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.; Shin, D.M. Therapeutic Nanoparticles for Drug Delivery in Cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef] [Green Version]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP–dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef] [Green Version]
- Peer, D.; Margalit, R. Fluoxetine and reversal of multidrug resistance. Cancer Lett. 2006, 237, 180–187. [Google Scholar] [CrossRef]
- Jain, R.K. Barriers to Drug Delivery in Solid Tumors. Sci. Am. 1994, 271, 58–65. [Google Scholar] [CrossRef]
- Lan, H.; Zhang, W.; Jin, K.; Liu, Y.; Wang, Z. Modulating barriers of tumor microenvironment through nanocarrier systems for improved cancer immunotherapy: A review of current status and future perspective. Drug Deliv. 2020, 27, 1248–1262. [Google Scholar] [CrossRef] [PubMed]
- Nahta, R. Molecular Mechanisms of Trastuzumab-Based Treatment in HER2-Overexpressing Breast Cancer. ISRN Oncol. 2012, 2012, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haberkorn, U.; Eder, M.; Kopka, K.; Babich, J.W.; Eisenhut, M. New Strategies in Prostate Cancer: Prostate-Specific Membrane Antigen (PSMA) Ligands for Diagnosis and Therapy. Clin. Cancer Res. 2016, 22, 9–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wüstemann, T.; Haberkorn, U.; Babich, J.; Mier, W. Targeting prostate cancer: Prostate-specific membrane antigen based diagnosis and therapy. Med. Res. Rev. 2019, 39, 40–69. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Garg, V.; Dureja, H.; Ling, C. Nanomedicine-based approaches for delivery of herbal compounds. Tradit Med Res. 2022, 7, 48. [Google Scholar] [CrossRef]
- Siwak, D.R.; Tari, A.M.; Lopez-Berestein, G. The Potential of Drug-carrying Immunoliposomes as Anticancer Agents: Commentary re: JW Park et al., Anti-HER2 Immunoliposomes: Enhanced Efficacy due to Targeted Delivery. Clin. Cancer Res. 2002, 8, 1172–1181. [Google Scholar]
- Ramesh, R.; Amreddy, N.; Muralidharan, R.; Babu, A.; Mehta, M.; Johnson, E.V.; Munshi, A.; Zhao, Y.D. Tumor-targeted and pH-controlled delivery of doxorubicin using gold nanorods for lung cancer therapy. Int. J. Nanomed. 2015, 10, 6773–6788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Wei, Y.; Zhai, S.; Chen, Q.; Xing, D. Dihydroartemisinin and transferrin dual-dressed nano-graphene oxide for a pH-triggered chemotherapy. Biomaterials 2015, 62, 35–46. [Google Scholar] [CrossRef]
- Santi, M.; Maccari, G.; Mereghetti, P.; Voliani, V.; Rocchiccioli, S.; Ucciferri, N.; Luin, S.; Signore, G. Rational Design of a Transferrin-Binding Peptide Sequence Tailored to Targeted Nanoparticle Internalization. Bioconj. Chem. 2017, 28, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.W.; Park, S.A.; Han, T.H.; Son, D.H.; Park, J.S.; Oh, S.J.; Moon, D.H.; Cho, K.-J.; Ahn, C.-H.; Byun, Y.; et al. In vivo tumor targeting and radionuclide imaging with self-assembled nanoparticles: Mechanisms, key factors, and their implications. Biomaterials 2007, 28, 1236–1247. [Google Scholar] [CrossRef] [PubMed]
- Lehner, R.; Wang, X.; Wolf, M.; Hunziker, P. Designing switchable nanosystems for medical application. J. Control. Release 2012, 161, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Ge, P.; Liu, Y.; Chen, Q.; Su, Z.; Du, Y.; Luo, S.; Zhao, X.; Cao, X.; Song, H.; Zhu, X. Transferrin receptors/magnetic resonance dual-targeted nanoplatform for precise chemo-photodynamic synergistic cancer therapy. Nanomed. Nanotechnol. Biol. Med. 2022, 39, 102467. [Google Scholar] [CrossRef]
- Ramalho, M.J.; Loureiro, J.A.; Coelho, M.A.N.; Pereira, M.C. Transferrin Receptor-Targeted Nanocarriers: Overcoming Barriers to Treat Glioblastoma. Pharmaceutics 2022, 14, 279. [Google Scholar] [CrossRef]
- Qi, X.; Wang, G.; Wang, P.; Pei, Y.; Zhang, C.; Yan, M.; Wei, P.; Tian, G.; Zhang, G. Transferrin protein corona-modifed CuGd core-shell nanoplatform for tumor targeting photothermal and chemodynamic synergistic therapies. ACS Appl. Mater Interfaces 2022, 14, 7659–7670. [Google Scholar] [CrossRef]
- Cycle, M.-C.; Jose, S.; Cinu, T.A.; Sebastian, R.; Shoja, M.H.; Aleykutty, N.A.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B. Transferrin-conjugated docetaxel–PLGA nanoparticles for tumor targeting: Influence on MCF-7 cell cycle. Polymers 2019, 11, 1905. [Google Scholar]
- Cui, Y.N.; Xu, Q.X.; Davoodi, P.; Wang, D.P.; Wang, C.H. Enhanced intracellular delivery and controlled drug release of magnetic PLGA nanoparticles modified with transferrin. Acta Pharmacol. Sin. 2017, 38, 943–953. [Google Scholar] [CrossRef] [Green Version]
- Lv, Q.; Li, L.-M.; Han, M.; Tang, X.-J.; Yao, J.-N.; Ying, X.-Y.; Li, F.-Z.; Gao, J.-Q. Characteristics of sequential targeting of brain glioma for transferrin-modified cisplatin liposome. Int. J. Pharm. 2013, 444, 1–9. [Google Scholar] [CrossRef]
- Sun, T.; Wu, H.; Li, Y.; Huang, Y.; Yao, L.; Chen, X.; Han, X.; Zhou, Y.; Du, Z. Targeting transferrin receptor delivery of temozolomide for a potential glioma stem cell-mediated therapy. Oncotarget 2017, 8, 74451–74465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahoo, S.K.; Ma, W.; Labhasetwar, V. Efficacy of transferrin-conjugated paclitaxel-loaded nanoparticles in a murine model of prostate cancer. Int. J. Cancer 2004, 112, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Soe, Z.C.; Kwon, J.B.; Thapa, R.K.; Ou, W.; Nguyen, H.T.; Gautam, M.; Oh, K.T.; Choi, H.-G.; Ku, S.K.; Yong, C.S.; et al. Transferrin-Conjugated Polymeric Nanoparticle for Receptor-Mediated Delivery of Doxorubicin in Doxorubicin-Resistant Breast Cancer Cells. Pharmaceutics 2019, 11, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akanda, M.; Getti, G.; Nandi, U.; Mithu, S.; Douroumis, D. Bioconjugated solid lipid nanoparticles (SLNs) for targeted prostate cancer therapy. Int. J. Pharm. 2021, 599, 120416. [Google Scholar] [CrossRef]
- Luo, Z.; Dai, Y.; Gao, H. Development and application of hyaluronic acid in tumor targeting drug delivery. Acta Pharm. Sin. B 2019, 9, 1099–1112. [Google Scholar] [CrossRef]
- Cho, H.-J. Recent progresses in the development of hyaluronic acid-based nanosystems for tumor-targeted drug delivery and cancer imaging. J. Pharm. Investig. 2020, 50, 115–129. [Google Scholar] [CrossRef]
- Jiao, Y.; Pang, X.; Zhai, G. Advances in Hyaluronic Acid-Based Drug Delivery Systems. Curr. Drug Targets 2016, 17. [Google Scholar] [CrossRef]
- Narurkar, V.A.; Cohen, J.L.; Dayan, S.; Kaminer, M.S.; Rivkin, A.; Shamban, A.; Sykes, J.M.; Teller, C.F.; Weinkle, S.H.; Werschler, W.P.; et al. A Comprehensive approach to multimodal facial aesthetic treatment: Injection techniques and treatment characteristics from the HARMONY study. Dermatol. Surg. 2016, 42, S177–S191. [Google Scholar] [CrossRef]
- Bukhari, S.N.A.; Roswandi, N.L.; Waqas, M.; Habib, H.; Hussain, F.; Khan, S.; Sohail, M.; Ramli, N.A.; Thu, H.E.; Hussain, Z. Hyaluronic acid, a promising skin rejuvenating biomedicine: A review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int. J. Biol. Macromol. 2018, 120, 1682–1695. [Google Scholar] [CrossRef]
- Wickens, J.M.; Alsaab, H.O.; Kesharwani, P.; Bhise, K.; Amin, M.C.I.M.; Tekade, R.K.; Gupta, U.; Iyer, A.K. Recent advances in hyaluronic acid-decorated nanocarriers for targeted cancer therapy. Drug Discov. Today 2017, 22, 665–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senbanjo, L.T.; Chellaiah, M.A. CD44: A Multifunctional Cell Surface Adhesion Receptor Is a Regulator of Progression and Metastasis of Cancer Cells. Front. Cell Dev. Biol. 2017, 5, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basakran, N.S. CD44 as a potential diagnostic tumor marker. Saudi Med. J. 2015, 36, 273–279. [Google Scholar] [CrossRef] [PubMed]
- de la Rosa, J.M.R.; Tirella, A.; Tirelli, N. Receptor-Targeted Drug Delivery and the (Many) Problems We Know of: The Case of CD44 and Hyaluronic Acid. Adv. Biosyst. 2018, 2. [Google Scholar] [CrossRef]
- Seok, H.-Y.; Rejinold, N.S.; Lekshmi, K.M.; Cherukula, K.; Park, I.-K.; Kim, Y.-C. CD44 targeting biocompatible and biodegradable hyaluronic acid cross-linked zein nanogels for curcumin delivery to cancer cells: In vitro and in vivo evaluation. J. Control. Release 2018, 280, 20–30. [Google Scholar] [CrossRef]
- Lv, Y.; Xu, C.; Zhao, X.; Lin, C.; Yang, X.; Xin, X.; Zhang, L.; Qin, C.; Han, X.; Yang, L.; et al. Nanoplatform Assembled from a CD44-Targeted Prodrug and Smart Liposomes for Dual Targeting of Tumor Microenvironment and Cancer Cells. ACS Nano 2018, 12, 1519–1536. [Google Scholar] [CrossRef]
- Gibbs, P.; Clingan, P.R.; Ganju, V.; Strickland, A.H.; Wong, S.S.; Tebbutt, N.C.; Underhill, C.R.; Fox, R.M.; Clavant, S.P.; Leung, J.; et al. Hyaluronan-Irinotecan improves progression-free survival in 5-fluorouracil refractory patients with metastatic colorectal cancer: A randomized phase II trial. Cancer Chemother. Pharmacol. 2011, 67, 153–163. [Google Scholar] [CrossRef]
- Deng, X.W.; Cao, M.J.; Zhang, J.K.; Hu, K.; Yin, Z.; Zhou, Z.; Xiao, X.; Yang, Y.; Sheng, W.; Wu, Y.; et al. Hyaluronic Acid-Chitosan Nanoparticles for Co-Delivery of M1r-34a and Doxorubicin in Therapy against Triple Negative Breast Cancer. Biomaterials. 2014, 35, 4333–4344. [Google Scholar] [CrossRef]
- Xia, D.; Wang, F.; Pan, S.; Yuan, S.; Liu, Y.; Xu, Y. Redox/pH-Responsive Biodegradable Thiol-Hyaluronic Acid/Chitosan Charge-Reversal Nanocarriers for Triggered Drug Release. Polymers 2021, 13, 3785. [Google Scholar] [CrossRef]
- Vogus, D.R.; Evans, M.A.; Pusuluri, A.; Barajas, A.; Zhang, M.; Krishnan, V.; Nowak, M.; Menegatti, S.; Helgeson, M.E.; Squires, T.M.; et al. A hyaluronic acid conjugate engineered to synergistically and sequentially deliver gemcitabine and doxorubicin to treat triple negative breast cancer. J. Control. Release 2017, 267, 191–202. [Google Scholar] [CrossRef]
- Frigerio, B.; Bizzoni, C.; Jansen, G.; Leamon, C.P.; Peters, G.J.; Low, P.S.; Matherly, L.H.; Figini, M. Folate receptors and transporters: Biological role and diagnostic/therapeutic targets in cancer and other diseases. J. Exp. Clin. Cancer Res. 2019, 38, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Ke, J.; Zhou, X.E.; Yi, W.; Brunzelle, J.S.; Li, J.; Yong, E.-L.; Xu, H.E.; Melcher, K. Structural basis for molecular recognition of folic acid by folate receptors. Nature 2013, 500, 486–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assaraf, Y.G.; Leamon, C.P.; Reddy, J.A. The folate receptor as a rational therapeutic target for personalized cancer treatment. Drug Resist. Update 2014, 17, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Scaranti, M.; Cojocaru, E.; Banerjee, S.; Banerji, U. Exploiting the folate receptor α in oncology. Nat. Rev. Clin. Oncol. 2020, 17, 349–359. [Google Scholar] [CrossRef]
- Fernández, M.; Javaid, F.; Chudasama, V. Advances in targeting the folate receptor in the treatment/imaging of cancers. Chem. Sci. 2017, 9, 790–810. [Google Scholar] [CrossRef] [Green Version]
- Hao, Y.; Li, H.; Zhao, H.; Liu, Y.; Ge, X.; Li, X.; Chen, H.; Yang, A.; Zou, J.; Li, X.; et al. An intelligent nanovehicle armed with multifunctional navigation for precise delivery of Toll-like receptor 7/8 agonist and immunogenic cell death amplifers to eliminate solid tumors and trigger durable antitumor immunity. Adv. Healthcare Mater. 2022, 11, e2102739. [Google Scholar] [CrossRef]
- Janardhanam, L.S.L.; Bandi, S.P.; Venuganti, V.V.K. Functionalized LbL flm for localized delivery of STAT3 siRNA and oxaliplatin combination to treat colon cancer. ACS Appl. Mater. Interfaces 2022, 14, 10030–10046. [Google Scholar] [CrossRef]
- Kefayat, A.; Hosseini, M.; Ghahremani, F.; Jolfaie, N.A.; Rafienia, M. Biodegradable and biocompatible subcutaneous implants consisted of pH-sensitive mebendazole-loaded/folic acid-targeted chitosan nanoparticles for murine triple-negative breast cancer treatment. J. Nanobiotechnol. 2022, 20, 169. [Google Scholar]
- Sathiyaseelan, A.; Saravanakumar, K.; Manivasagan, P.; Jeong, M.S.; Jang, E.-S.; Wang, M.-H. Folic acid conjugated chitosan encapsulated palladium nanoclusters for NIR triggered photothermal breast cancer treatment. Carbohydr. Polym. 2022, 280, 119021. [Google Scholar] [CrossRef]
- LoRusso, P.M.; Edelman, M.J.; Bever, S.L.; Forman, K.M.; Pilat, M.; Quinn, M.F.; Li, J.; Heath, E.I.; Malburg, L.M.; Klein, P.J.; et al. Phase I Study of Folate Conjugate EC145 (Vintafolide) in Patients with Refractory Solid Tumors. J. Clin. Oncol. 2012, 30, 4011–4016. [Google Scholar] [CrossRef]
- Narmani, A.; Rezvani, M.; Farhood, B.; Darkhor, P.; Mohammadnejad, J.; Amini, B.; Refahi, S.; Goushbolagh, N.A. Folic acid functionalized nanoparticles as pharmaceutical carriers in drug delivery systems. Drug Dev. Res. 2019, 80, 404–424. [Google Scholar] [CrossRef]
- Ebrahimnejad, P.; Taleghani, A.S.; Asare-Addo, K.; Nokhodchi, A. An updated review of folate-functionalized nanocarriers: A promising ligand in cancer. Drug Discov. Today 2022, 27, 471–489. [Google Scholar] [CrossRef] [PubMed]
- Dhas, N.L.; Ige, P.P.; Kudarha, R.R. Design, optimization and in-vitro study of folic acid conjugated-chitosan functionalized PLGA nanoparticle for delivery of bicalutamide in prostate cancer. Powder Technol. 2015, 283, 234–245. [Google Scholar] [CrossRef]
- Patil, Y.; Shmeeda, H.; Amitay, Y.; Ohana, P.; Kumar, S.; Gabizon, A. Targeting of folate-conjugated liposomes with co-entrapped drugs to prostate cancer cells via prostate-specific membrane antigen (PSMA). Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Essa, D.; Kondiah, P.P.D.; Kumar, P.; Choonara, Y.E. Design of Chitosan-Coated, Quercetin-Loaded PLGA Nanoparticles for Enhanced PSMA-Specific Activity on LnCap Prostate Cancer Cells. Biomedicines 2023, 11, 1201. [Google Scholar] [CrossRef] [PubMed]
- Parvathaneni, V.; Shukla, S.K.; Gupta, V. Development and Characterization of Folic Acid-Conjugated Amodiaquine-Loaded Nanoparticles–Efficacy in Cancer Treatment. Pharmaceutics 2023, 15, 1001. [Google Scholar] [CrossRef] [PubMed]
- Faghfuri, E.; Sagha, M.; Faghfouri, A.H. The Cytotoxicity Effect of Curcumin Loaded Folic Acid Conjugated-Nanoparticles on Breast Cancer Cells and Its Association with Inhibition of STAT3 Phosphorylation. J. Clust. Sci. 2022, 33, 2037–2044. [Google Scholar] [CrossRef]
- Reda, A.; Hosseiny, S.; El-Sherbiny, I.M. Next-generation nanotheranostics targeting cancer stem cells. Nanomedicine 2019, 14, 2487–2514. [Google Scholar] [CrossRef]
- Jiang, Z.; Guan, J.; Qian, J.; Zhan, C. Peptide ligand-mediated targeted drug delivery of nanomedicines. Biomater. Sci. 2019, 7, 461–471. [Google Scholar] [CrossRef]
- Mousavizadeh, A.; Jabbari, A.; Akrami, M.; Bardania, H. Cell targeting peptides as smart ligands for targeting of therapeutic or diagnostic agents: A systematic review. Colloids Surfaces B Biointerfaces 2017, 158, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Genta, I.; Chiesa, E.; Colzani, B.; Modena, T.; Conti, B.; Dorati, R. GE11 Peptide as an Active Targeting Agent in Antitumor Therapy: A Minireview. Pharmaceutics 2017, 10, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahmatkeshan, M.; Gheybi, F.; Rezayat, S.M.; Jaafari, M.R. Improved drug delivery and therapeutic efficacy of PEgylated liposomal doxorubicin by targeting anti-HER2 peptide in murine breast tumor model. Eur. J. Pharm. Sci. 2016, 86, 125–135. [Google Scholar] [CrossRef]
- Bandekar, A.; Zhu, C.; Gomez, A.; Menzenski, M.Z.; Sempkowski, M.; Sofou, S. Masking and Triggered Unmasking of Targeting Ligands on Liposomal Chemotherapy Selectively Suppress Tumor Growth in Vivo. Mol. Pharm. 2012, 10, 152–160. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Ruan, L.-M.; Mao, W.-W.; Wang, J.-Q.; Shen, Y.-Q.; Sui, M.-H. Preparation of RGD-modified Long Circulating Liposome Loading Matrine, and its in vitro Anti-cancer Effects. Int. J. Med. Sci. 2010, 7, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Dai, W.; Yang, T.; Wang, X.; Wang, J.; Zhang, X.; Zhang, Q. PHSCNK-Modified and doxorubicin-loaded liposomes as a dual targeting system to integrin-overexpressing tumor neovasculature and tumor cells. J. Drug Target. 2009, 18, 254–263. [Google Scholar] [CrossRef]
- Chang, C.-C.; Liu, D.-Z.; Lin, S.-Y.; Liang, H.-J.; Hou, W.-C.; Huang, W.-J.; Chang, C.-H.; Ho, F.-M.; Liang, Y.-C. Liposome encapsulation reduces cantharidin toxicity. Food Chem. Toxicol. 2008, 46, 3116–3121. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.-Y.; Hsiao, J.-K.; Wang, Y.-P.; Lan, C.-H.; Wu, H.-C. Peptide-conjugated nanoparticles for targeted imaging and therapy of prostate cancer. Biomaterials 2016, 99, 1–15. [Google Scholar] [CrossRef]
- Nica, V.; Marino, A.; Pucci, C.; Sen, O.; Emanet, M.; De Pasquale, D.; Carmignani, A.; Petretto, A.; Bartolucci, M.; Lauciello, S.; et al. Cell-Membrane-Coated and Cell-Penetrating Peptide-Conjugated Trimagnetic Nanoparticles for Targeted Magnetic Hyperthermia of Prostate Cancer Cells. ACS Appl. Mater. Interfaces 2023, 15, 30008–30028. [Google Scholar] [CrossRef]
- Dasargyri, A.; Kumin, C.D.; Leroux, J.C. Targeting Nanocarriers with anisamide: Fact or artifact? Adv. Mater. 2017, 29, 1603451. [Google Scholar] [CrossRef]
- van Waarde, A.; Rybczynska, A.A.; Ramakrishnan, N.K.; Ishiwata, K.; Elsinga, P.H.; Dierckx, R.A. Potential applications for sigma receptor ligands in cancer diagnosis and therapy. Biochim. Biophys. Acta Biomembr. 2015, 1848, 2703–2714. [Google Scholar] [CrossRef] [Green Version]
- Luan, X.; Rahme, K.; Cong, Z.; Wang, L.; Zou, Y.; He, Y.; Yang, H.; Holmes, J.D.; O’Driscoll, C.M.; Guo, J. Anisamide-targeted PEGylated gold nanoparticles designed to target prostate cancer mediate: Enhanced systemic exposure of siRNA, tumour growth suppression and a synergistic therapeutic response in combination with paclitaxel in mice. Eur. J. Pharm. Biopharm. 2019, 137, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Liu, C.; Wang, N.; Zhou, H.; Chen, H.; Qiao, W. Anisamide-modified dual-responsive drug delivery system with MRI capacity for cancer targeting therapy. J. Mol. Liq. 2021, 340, 116889. [Google Scholar] [CrossRef]
- Jalilian, M.; Derakhshandeh, K.; Kord, M.; Lashani, H. Targeting Solid Lipid Nanoparticles with Anisamide for Docetaxel Delivery to Prostate Cancer: Preparation, Optimization, and In-vitro Evaluation. Iran. J. Pharm. Res. 2021, 20, 327–338. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Rahme, K.; Guo, J.; Holmes, J.D.; O’Driscoll, C.M. Anisamide-targeted gold nanoparticles for siRNA delivery in prostate cancer–synthesis, physicochemical characterisation and in vitro evaluation. J. Mater. Chem. B 2016, 4, 2242–2252. [Google Scholar]
- Guo, J.; Ogier, J.R.; Desgranges, S.; Darcy, R.; Caitriona, O. Anisamide-targeted cyclodextrin nanoparticles for siRNA delivery to prostate tumours in mice. Biomaterials. 2012, 33, 7775–7784. [Google Scholar] [CrossRef] [Green Version]
- Xi, Z.; Huang, R.; Deng, Y.; He, N. Progress in Selection and Biomedical Applications of Aptamers. J. Biomed. Nanotechnol. 2014, 10, 3043–3062. [Google Scholar] [CrossRef]
- Moosavian, S.A.; Sahebkar, A. Aptamer-functionalized liposomes for targeted cancer therapy. Cancer Lett. 2019, 448, 144–154. [Google Scholar] [PubMed]
- Taghavi, S.; Ramezani, M.; Alibolandi, M.; Abnous, K.; Taghdisi, S.M. Chitosan-modified PLGA nanoparticles tagged with 5TR1 aptamer for in vivo tumor-targeted drug delivery. Cancer Lett. 2017, 400, 1–8. [Google Scholar] [CrossRef]
- Bahreyni, A.; Alibolandi, M.; Ramezani, M.; Sadeghi, A.S.; Abnous, K.; Taghdisi, S.M. A novel MUC1 aptamer-modified PLGA-epirubicin-PβAE-antimir-21 nanocomplex platform for targeted co-delivery of anticancer agents in vitro and in vivo. Colloids Surf. B Biointerfaces 2019, 175, 231–238. [Google Scholar]
- Liu, Z.; Duan, J.-H.; Song, Y.-M.; Ma, J.; Wang, F.-D.; Lu, X.; Yang, X.-D. Novel HER2 Aptamer Selectively Delivers Cytotoxic Drug to HER2-positive Breast Cancer Cells in Vitro. J. Transl. Med. 2012, 10, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagalkot, V.; Farokhzad, O.C.; Langer, R.; Jon, S. An Aptamer–Doxorubicin Physical Conjugate as a Novel Targeted Drug-Delivery Platform. Angew. Chem. Int. Ed. 2006, 45, 8149–8152. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Qian, W.; Tao, W.; Zhou, Y.; Xue, B. Delivery of Curcumin Nanoliposomes Using Surface Modified with CD133 Aptamers for Prostate Cancer. Drug Des. Dev. Ther. 2019, 13, 4021–4033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.S.; Lee, G.H.; Jeong, H.Y.; Park, Y.S.; Kim, D.E. Efficient and Specific Co-Delivery of Vimentin siRNA and Doxorubicin with Aptamosomes for Combination Cancer Therapy. Mol. Ther. 2012, 20, S158. [Google Scholar]
- Lee, G.H.; Jeong, H.Y.; Park, Y.S.; Kim, D.E.; Kim, K.S. Prostate Cancer Cell-Specific siRNA and Drug Co-Delivery with Aptamer-Functionalized Liposomes (Aptamosomes). Mol. Ther. 2013, 21, S63. [Google Scholar]
- Dhar, S.; Gu, F.X.; Langer, R.; Farokhzad, O.C.; Lippard, S.J. Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA–PEG nanoparticles. Proc. Natl. Acad. Sci. USA 2008, 105, 17356–17361. [Google Scholar] [CrossRef]
- Bala, J.; Bhaskar, A.; Varshney, A.; Singh, A.K.; Dey, S.; Yadava, P. In vitro selected RNA aptamer recognizing glutathione induces ROS mediated apoptosis in the human breast cancer cell line MCF 7. RNA Biol. 2011, 8, 101–111. [Google Scholar] [CrossRef] [Green Version]
- Yuhan, J.; Zhu, L.; Zhu, L.; Huang, K.; He, X.; Xu, W. Cell-specific aptamers as potential drugs in therapeutic applications: A review of current progress. J. Control. Release 2022, 346, 405–420. [Google Scholar] [CrossRef]
- Soundararajan, S.; Chen, W.; Spicer, E.K.; Courtenay-Luck, N.; Fernandes, D.J. The Nucleolin Targeting Aptamer AS1411 Destabilizes Bcl-2 Messenger RNA in Human Breast Cancer Cells. Cancer Res. 2008, 68, 2358–2365. [Google Scholar] [CrossRef] [Green Version]
- Ibarra, L.E.; Camorani, S.; Agnello, L.; Pedone, E.; Pirone, L.; Chesta, C.A.; Palacios, R.E.; Fedele, M.; Cerchia, L. Selective Photo-Assisted Eradication of Triple-Negative Breast Cancer Cells through Aptamer Decoration of Doped Conjugated Polymer Nanoparticles. Pharmaceutics 2022, 14, 626. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Liu, L.; Zhu, Z.; Ouyang, G.; An, Y.; Zhao, C.; Yang, C.J. In Vitro Selection of DNA Aptamers for Metastatic Breast Cancer Cell Recognition and Tissue Imaging. Anal. Chem. 2014, 86, 6596–6603. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-F.; Lin, Y.-W.; Lin, Z.-H.; Chang, H.-T. Aptamer-modified gold nanoparticles for targeting breast cancer cells through light scattering. J. Nanoparticle Res. 2009, 11, 775–783. [Google Scholar] [CrossRef]
- Hassan, E.M.; Mohamed, A.; DeRosa, M.C.; Willmore, W.G.; Hanaoka, Y.; Kiwa, T.; Ozaki, T. High-sensitivity detection of metastatic breast cancer cells via terahertz chemical microscopy using aptamers. Sensors Actuators B Chem. 2019, 287, 595–601. [Google Scholar] [CrossRef]
- Tang, L.; Yang, X.; Dobrucki, L.W.; Chaudhury, I.; Yin, Q.; Yao, C.; Lezmi, S.; Helferich, W.G.; Fan, T.M.; Cheng, J. Aptamer-Functionalized, Ultra-Small, Monodisperse Silica Nanoconjugates for Targeted Dual-Modal Imaging of Lymph Nodes with Metastatic Tumors. Angew. Chem. Int. Ed. 2012, 51, 12721–12726. [Google Scholar] [CrossRef]
- Lakhin, A.V.; Tarantul, V.Z.; Gening, L.V. Aptamers: Problems, solutions and prospects. Acta Naturae 2013, 5, 34–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, F.; Wen, N.; Xiao, D.; Yan, J.; Xiong, H.; Cai, S.; Liu, Z.; Liu, Y. Aptamer-Based Targeted Drug Delivery Systems: Current Potential and Challenges. Curr. Med. Chem. 2020, 27, 2189–2219. [Google Scholar] [CrossRef] [PubMed]
- Pecetta, S.; Finco, O.; Seubert, A. Quantum leap of monoclonal antibody (mAb) discovery and development in the COVID-19 era. Semin. Immunol. 2020, 50, 101427. [Google Scholar] [CrossRef]
- Santos-Neto, J.F.; Oliveira, F.O.; Hodel, K.V.S.; Fonseca, L.M.S.; Badaró, R.; Machado, B.A.S. Technological Advancements in Monoclonal Antibodies. Sci. World J. 2021, 2021, 1–19. [Google Scholar] [CrossRef]
- Gklinos, P.; Papadopoulou, M.; Stanulovic, V.; Mitsikostas, D.; Papadopoulos, D. Monoclonal Antibodies as Neurological Therapeutics. Pharmaceuticals 2021, 14, 92. [Google Scholar] [CrossRef]
- Devita, V.T.; Hellman, S.; Rosenberg, S.A. Cancer: Principles and Practice of Oncology, 6th ed.; Lippincott Williams & Wilkins Publishers: Philadelphia, PA, USA, 2001. [Google Scholar]
- Boulianne, G.L.; Hozumi, N.; Shulman, M.J. Production of functional chimaeric mouse/human antibody. Nature 1984, 312, 643–646. [Google Scholar] [CrossRef]
- Jones, P.T.; Dear, P.H.; Foote, J.; Neuberger, M.S.; Winter, G. Replacing the complementarity-determining regions in a human antibody with those from a mouse. Nature 1986, 321, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.-M.; Hwang, Y.-C.; Liu, I.-J.; Lee, C.-C.; Tsai, H.-Z.; Li, H.-J.; Wu, H.-C. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020, 27, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, S. Advances in the production of human monoclonal antibodies. Antib. Technol. J. 2011, 1, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Nelson, A.L.; Dhimolea, E.; Reichert, J.M. Development trends for human monoclonal antibody therapeutics. Nat. Rev. Drug Discov. 2010, 9, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Finn, O.J. Human Tumor Antigens Yesterday, Today, and Tomorrow. Cancer Immunol. Res. 2017, 5, 347–354. [Google Scholar] [CrossRef] [Green Version]
- Hazin, J.; Moldenhauer, G.; Altevogt, P.; Brady, N.R. A novel method for measuring cellular antibody uptake using imaging flow cytometry reveals distinct uptake rates for two different monoclonal antibodies targeting L1. J. Immunol. Methods 2015, 423, 70–77. [Google Scholar] [CrossRef]
- Ribatti, D. from the discovery of monoclonal antibodies to their therapeutic application: An historical reappraisal. Immunol. Lett. 2014, 161, 96–99. [Google Scholar] [CrossRef]
- Li, S.; Schmitz, K.R.; Jeffrey, P.D.; Wiltzius, J.J.W.; Kussie, P.; Ferguson, K.M. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell 2005, 7, 301–311. [Google Scholar] [CrossRef] [Green Version]
- Patel, D.; Bassi, R.; Hooper, A.; Prewett, M.; Hicklin, D.J.; Kang, X. Anti-epidermal growth factor receptor monoclonal antibody cetuximab inhibits EGFR/HER-2 heterodimerization and activation. Int. J. Oncol. 2009, 34, 25–32. [Google Scholar] [CrossRef]
- Chen, J.S.; Lan, K.; Hung, M.C. Strategies to Target HER2/Neu Overexpression for Cancer Therapy. Drug Resist. Updates 2003, 6, 129–136. [Google Scholar]
- Vallabhajosula, S.; Nikolopoulou, A.; Jhanwar, Y.S.; Kaur, G.; Tagawa, S.T.; Nanus, D.M.; Bander, N.H.; Goldsmith, S.J. Radioimmunotherapy of metastatic prostate cancer with 177Lu-DOTAhuJ591 anti prostate specific membrane antigen specific monoclonal antibody. Curr. Radiopharm. 2016, 9, 44–53. [Google Scholar] [PubMed]
- McLaughlin, P.; Grillo-López, A.J.; Link, B.K.; Levy, R.; Czuczman, M.S.; Williams, M.E.; Heyman, M.R.; Bence-Bruckler, I.; White, C.A.; Cabanillas, F.; et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: Half of patients respond to a four-dose treatment program. J. Clin. Oncol. 1998, 16, 2825–2833. [Google Scholar] [CrossRef] [PubMed]
- Benavente, S.; Huang, S.; Armstrong, E.A.; Chi, A.; Hsu, K.T.; Wheeler, D.L.; Harari, P.M. Establishment and Characterization of a Model of Acquired Resistance to Epidermal Growth Factor Receptor Targeting Agents in Human Cancer Cells. Clin. Cancer Res. 2009, 15, 1585–1592. [Google Scholar] [PubMed] [Green Version]
- Ahmad, A. Current Updates on Trastuzumab Resistance in HER2 Overexpressing Breast Cancers. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2019; Volume 1152, pp. 217–228. [Google Scholar] [CrossRef]
- Valabrega, G.; Montemurro, F.; Aglietta, M. Trastuzumab: Mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann. Oncol. 2007, 18, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Gennari, R.; Menard, S.; Fagnoni, F.; Ponchio, L.; Scelsi, M.; Tagliabue, E.; Castiglioni, F.; Villani, L.; Magalotti, C.; Gibelli, N.; et al. Pilot Study of the Mechanism of Action of Preoperative Trastuzumab in Patients with Primary Operable Breast Tumors Overexpressing HER2. Clin. Cancer Res. 2004, 10, 5650–5655. [Google Scholar] [CrossRef] [Green Version]
- Kominsky, S.L.; Hobeika, A.C.; Lake, F.; Torres, B.; Johnson, H.M. Down-regulation of neu/HER-2 by interferon-gamma in prostate cancer cells. Cancer Res 2000, 60, 3904–3908. [Google Scholar] [PubMed]
- Shi, Y.; Fan, X.; Meng, W.; Deng, H.; Zhang, N.; An, Z. Engagement of Immune Effector Cells by Trastuzumab Induces HER2/ERBB2 Downregulation in Cancer Cells through STAT1 Activation. Breast Cancer Res. 2014, 16, 1–11. [Google Scholar]
- Teicher, B.A.; Chari, R.V. Antibody Conjugate Therapeutics: Challenges and Potential. Clin. Cancer Res. 2011, 17, 6389–6397. [Google Scholar] [CrossRef] [Green Version]
- Kalash, R.S.; Lakshmanan, V.K.; Cho, C.; Park, I.K. Biomaterials Nanoarchitectonics; William Andrew Publishing: Cambridge, MA, USA, 2016; pp. 197–215. [Google Scholar]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic. Bioeng. Transl. Med. 2016, 1, 10–29. [Google Scholar]
- Medavenkata, S.P.; Akshatha, H.S. Nano Theranostics—A Breakthrough in Cancer Diagnosis and Treatment and Regulations of Nano Technology Products. Int. J. Pharm. Sci. Res. 2018, 9, 3136–3149. [Google Scholar] [CrossRef]
- Cano-Cortes, M.V.; Navarro-Marchal, S.A.; Ruiz-Blas, M.P.; Diaz-Mochon, J.J.; Marchal, J.A.; Sanchez-Martin, R.M. A versatile theranostic nanodevice based on an orthogonal bioconjugation strategy for efficient targeted treatment and monitoring of triple negative breast cancer. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102120. [Google Scholar] [CrossRef]
- Liu, R.; Hu, C.; Yang, Y.; Zhang, J.; Gao, H. Theranostic nanoparticles with tumor-specific enzyme-triggered size reduction and drug release to perform photothermal therapy for breast cancer treatment. Acta Pharm. Sin. B 2019, 9, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Ko, N.R.; Nafiujjaman, M.; Lee, J.S.; Lim, H.-N.; Lee, Y.-K.; Kwon, I.K. Graphene quantum dot-based theranostic agents for active targeting of breast cancer. RSC Adv. 2017, 7, 11420–11427. [Google Scholar] [CrossRef] [Green Version]
- Ma, M.; Lei, M.; Tan, X.; Tan, F.; Li, N. Theranostic liposomes containing conjugated polymer dots and doxorubicin for bio-imaging and targeted therapeutic delivery. RSC Adv. 2016, 6, 1945–1957. [Google Scholar] [CrossRef]
- Ma, M.; Zhang, X.; Hao, Y.; Liu, N.; Yin, Z.; Wang, L. A novel lipid-based nanomicelle of docetaxel: Evaluation of antitumor activity and biodistribution. Int. J. Nanomed. 2012, 7, 3389–3398. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Zhang, L.; Song, C.; Zhu, D. Design of multifunctional magnetic iron oxide nanoparticles/mitoxantrone-loaded liposomes for both magnetic resonance imaging and targeted cancer therapy. Int. J. Nanomed. 2014, 9, 4055–4066. [Google Scholar] [CrossRef] [Green Version]
- Yallapu, M.M.; Othman, S.F.; Curtis, E.T.; Bauer, N.A.; Chauhan, N.; Kumar, D.; Jaggi, M.; Chauhan, S.C. Curcumin-loaded magnetic nanoparticles for breast cancer therapeutics and imaging applications. Int. J. Nanomed. 2012, 7, 1761–1779. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.; Li, C.; Xu, N.; Watanabe, M.; Xue, R.; Xu, A.; Araki, M.; Sun, R.; Liu, C.; Nasu, Y.; et al. Dual-Functional PLGA Nanoparticles Co-Loaded with Indocyanine Green and Resiquimod for Prostate Cancer Treatment. Int. J. Nanomed. 2021, 16, 2775–2787. [Google Scholar] [CrossRef]
- Wan, Z.; Xie, F.; Wang, L.; Zhang, G.; Zhang, H. Preparation and Evaluation of Cabazitaxel-Loaded Bovine Serum Albumin Nanoparticles for Prostate Cancer. Int. J. Nanomed. 2020, 15, 5333–5344. [Google Scholar] [CrossRef]
- Bagalkot, V.; Zhang, L.; Levy-Nissenbaum, E.; Jon, S.; Kantoff, P.W.; Langer, R.; Farokhzad, O.C. Quantum Dot−Aptamer Conjugates for Synchronous Cancer Imaging, Therapy, and Sensing of Drug Delivery Based on Bi-Fluorescence Resonance Energy Transfer. Nano Lett. 2007, 7, 3065–3070. [Google Scholar] [CrossRef]
- Viglianti, B.L.; Abraham, S.A.; Michelich, C.R.; Yarmolenko, P.S.; MacFall, J.R.; Bally, M.B.; Dewhirst, M.W. In vivo monitoring of tissue pharmacokinetics of liposome/drug using MRI: Illustration of targeted delivery. Magn. Reson. Med. 2004, 51, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Viglianti, B.L.; Ponce, A.M.; Michelich, C.R.; Yu, D.; Abraham, S.A.; Sanders, L.; Yarmolenko, P.S.; Schroeder, T.; MacFall, J.R.; Barboriak, D.P.; et al. Chemodosimetry of in vivo tumor liposomal drug concentration using MRI. Magn. Reson. Med. 2006, 56, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Ponce, A.M.; Viglianti, B.L.; Yu, D.; Yarmolenko, P.S.; Michelich, C.R.; Woo, J.; Bally, M.B.; Dewhirst, M.W. Magnetic Resonance Imaging of Temperature-Sensitive Liposome Release: Drug Dose Painting and Antitumor Effects. Gynecol. Oncol. 2007, 99, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Seymour, L.W.; Ferry, D.R.; Anderson, D.; Hesslewood, S.; Julyan, P.J.; Poyner, R.; Doran, J.; Young, A.M.; Burtles, S.; Kerr, D.J. Hepatic drug targeting: Phase I evaluation of polymer-bound doxorubicin. J. Clin. Oncol. 2002, 20, 1668–1676. [Google Scholar] [PubMed]
- Van Dongen, G.A.M.S.; Visser, G.W.M.; Lub-De Hooghe, M.N.; De Vries, E.G.; Perk, L.R. Immuno-PET: A navigator in monoclonal antibody development and applications. Oncologist 2007, 12, 1379–1389. [Google Scholar]
- Wang, D.; Miller, S.C.; Sima, M.; Parker, D.; Buswell, H.; Goodrich, K.C.; Kopečková, P.; Kopeček, J. The Arthrotropism of Macromolecules in Adjuvant-Induced Arthritis Rat Model: A Preliminary Study. Pharm. Res. 2004, 21, 1741–1749. [Google Scholar] [CrossRef]
- Mulder, W.J.M.; Strijkers, G.; Van Tilborg, G.A.F.; Griffioen, A.W.; Nicolay, K. Lipid-based nanoparticles for contrast-enhanced MRI and molecular imaging. NMR Biomed. 2006, 19, 142–164. [Google Scholar] [CrossRef]
- Mulder, W.J.M.; Strijkers, G.J.; Habets, J.W.; Bleeker, E.J.W.; Schaft, D.W.J.; Storm, G.; Koning, G.A.; Griffioen, A.W.; Nicolay, K. MR molecular imaging and fluorescence microscopy for identification of activated tumor endothelium using a bimodal lipidic nanoparticle. FASEB J. 2005, 19, 2008–2010. [Google Scholar] [CrossRef]
- Langereis, S.; Keupp, J.; van Velthoven, J.L.; de Roos, I.H.; Burdinski, D.; Pikkemaat, J.A.; Grull, H. A temperature-sensitive liposomal 1H CEST and 19F contrast agent for MR image-guided drug delivery. J. Am. Chem. Soc. 2009, 131, 1380–1381. [Google Scholar]
- Terreno, E.; Cabella, C.; Carrera, C.; Castelli, D.D.; Mazzon, R.; Rollet, S.; Stancanello, J.; Visigalli, M.; Aime, S. from Spherical to Osmotically Shrunken Paramagnetic Liposomes: An Improved Generation of LIPOCEST MRI Agents with Highly Shifted Water Protons. Angew. Chem. Int. Ed. 2007, 46, 966–968. [Google Scholar] [CrossRef]
- de Smet, M.; Langereis, S.; van den Bosch, S.V.D.; Grüll, H. Temperature-sensitive liposomes for doxorubicin delivery under MRI guidance. J. Control. Release 2010, 143, 120–127. [Google Scholar] [CrossRef] [PubMed]
- AshaRrani, P.V.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and Genotoxicity of Silver Nanoparticles in Human Cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef]
- Lammers, T.; Rizzo, L.Y.; Storm, G.; Kiessling, F. Personalized Nanomedicine. Clin. Cancer Res. 2012, 18, 4889–4894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrington, K.J.; Mohammadtaghi, S.; Uster, P.S.; Glass, D.; Peters, A.M.; Vile, R.G.; Stewart, J.S. Effective targeting of solid tumors in patients with locally advanced cancers by radiolabeled pegylated liposomes. Clin. Cancer Res. 2001, 7, 243–254. [Google Scholar] [PubMed]
- Harris, L.; Batist, G.; Belt, R.; Rovira, D.; Navari, R.; Azamia, N.; Welles, L.; Winer, E.; TLC D-99 Study Group. Liposome-encapsulated doxorubicin compared with conventional doxorubicin in a randomized multicentre trial as first-line therapy of metastatic breast carcinoma. Cancer 2002, 94, 25–36. [Google Scholar] [PubMed]
- Lison, D.; Muller, J. Lung and Systemic Responses to Carbon Nanotubes (CNT) in Mice. Toxicol. Sci. 2008, 101, 179–180, author reply, pp. 181−182. [Google Scholar]
- Choi, S.-J.; Oh, J.-M.; Choy, J.-H. Toxicological effects of inorganic nanoparticles on human lung cancer A549 cells. J. Inorg. Biochem. 2009, 103, 463–471. [Google Scholar] [CrossRef]
- Kamaruzman, N.I.; Aziz, N.A.; Poh, C.L.; Chowdhury, E.H. Oncogenic Signaling in Tumorigenesis and Applications of siRNA Nanotherapeutics in Breast Cancer. Cancers 2019, 11, 632. [Google Scholar] [CrossRef] [Green Version]
- Muthu, M.S.; Rajesh, C.V.; Mishra, A.; Singh, S. Stimulus-responsive targeted nanomicelles for effective cancer therapy. Nanomedicine 2009, 4, 657–667. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, Y.; Liang, X.-J. Theranostic Nanoparticles Engineered for Clinic and Pharmaceutics. Accounts Chem. Res. 2011, 44, 1114–1122. [Google Scholar] [CrossRef]




| Nanoparticles | Drug | Applications | Clinical Phase | CT Identifiers |
|---|---|---|---|---|
| Liposomes | IVAC_W_bre1_uID and IVAC_M_uID | Breast cancer | 1 | NCT 02316457 |
| Quantum dots | Veldoreotide | Breast cancer | 1 | NCT 04138342 |
| Liposomes | Daunorubicin | Breast cancer | 1 | NCT 00004207 |
| Magnetic | - | Prostate cancer | Early phase 1 | NCT 02033447 |
| Silica | 64Cu-NOTA-PSMAi-PEG-Cy5.5-C’ dots | Prostate cancer | 1 | NCT 04167969 |
| Polymer | CRLX101 and enzalutamide | Prostate cancer | 2 | NCT 03531827 |
| Polymer | BIND-014 | Prostate cancer | 2 | NCT 01812746 |
| Polymer | PTX and Durvalumab with or without neoantigen vaccine | Breast cancer | 2 | NCT 03606967 |
| Polymer | DOX, cyclophosphamide and filgrastim followed by PTX | Breast cancer | 2 | NCT 00407888 |
| Polymer | Carboplatin and nab-paclitaxel with or without vorinostat | Breast cancer | 2 | NCT 00616967 |
| Polymer | PTX and cyclophosphamide | Breast cancer | 2 | NCT 00629499P |
| Hafnium oxide | - | Prostate cancer | 1 and 2 | NCT 02805894 |
| Albumin bound | PTX and cyclophosphamide | Breast cancer | 2 | NCT 00629499 |
| Albumin stabilized | PTX, gemcitabine and bevacizumab | Breast cancer | 2 | NCT 00662129 |
| Magnetic | Superparamagnetic iron oxide | Breast cancer | 1 and 2 | NCT 05359783 |
| ProductTM | Company | Nanoparticle | Drug | Indication | Approval (Year) |
|---|---|---|---|---|---|
| Doxil | Ortho Biotech (Bridgewater, NJ, United States) | Liposome | Doxorubicin | Breast cancer | FDA (1995) |
| Caelyx | Schering-Plough (Newton, NJ, United States) | Liposome | Doxorubicin | Breast cancer | EMA (1996) |
| Myocet | Teva UK (Castleford UK) | Liposome | Doxorubicin | Breast cancer | EMA (2000) |
| Lipo-Dox | Sun Pharmaceuticals (Princeton, NJ, United States) | Liposome | Doxorubicin | Breast cancer | Taiwan (1998) |
| Eligard | Recordati Industria Chimicae Farmaceutica (Milan, Italy) | Polymer | Leuprorelin acetate | Prostate cancer | FDA (2002) |
| Abraxane | American Biosciences, Inc. (Blauvelt, NY, United States) | Albumin | Paclitaxel | Breast cancer | FDA (2005) |
| Lipusu | Liposome | Paclitaxel | Breast cancer | EMA (2013) | |
| Nano Therm | Magforce (Berlin, Gemany) | Metallic | Fe2O3 | Prostate cancer, Prostrate cancer | EMA (2013) |
| Kadcyla | Genentech (San Francisco, CA, United States) | Trastuzumab linked to DM1 via thioether linker MCC | DM1 | Breast cancer | FDA, EMA (2013) |
| Pazenir | Ratiopharm GmbH (Ulm, Germany) | Albumin | Paclitaxel | Breast cancer | EMA (2019) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akpa, P.A.; Peter, I.E.; Onwuka, A.M.; Obi, B.C.; Akunne, M.O.; Nworu, C.S.; Ejikeme, P.M.; Akunne, T.C.; Attama, A.A.; Akah, P.A. Nanotheranostics: Platforms, Current Applications, and Mechanisms of Targeting in Breast and Prostate Cancers. J. Nanotheranostics 2023, 4, 346-383. https://doi.org/10.3390/jnt4030016
Akpa PA, Peter IE, Onwuka AM, Obi BC, Akunne MO, Nworu CS, Ejikeme PM, Akunne TC, Attama AA, Akah PA. Nanotheranostics: Platforms, Current Applications, and Mechanisms of Targeting in Breast and Prostate Cancers. Journal of Nanotheranostics. 2023; 4(3):346-383. https://doi.org/10.3390/jnt4030016
Chicago/Turabian StyleAkpa, Paul A., Ikechukwu E. Peter, Akachukwu M. Onwuka, Bonaventure C. Obi, Maureen O. Akunne, Chukwuemeka S. Nworu, Paul M. Ejikeme, Theophine C. Akunne, Anthony A. Attama, and Peter A. Akah. 2023. "Nanotheranostics: Platforms, Current Applications, and Mechanisms of Targeting in Breast and Prostate Cancers" Journal of Nanotheranostics 4, no. 3: 346-383. https://doi.org/10.3390/jnt4030016