Graphene Oxide Chemical Refining Screening to Improve Blood Compatibility of Graphene-Based Nanomaterials
Abstract
:1. Introduction
2. Materials and Methods
2.1. GO Synthesis
2.2. GO Reduction with Hydrazine (rGO)1–6
2.3. Esterification of GO (eGO)
2.4. Functionalization of GO with Cysteamine (cysGO)
2.5. GO Functionalization with Mercaptobenzoic Acid (mbaGO)
2.6. Ester GO Functionalization with Cysteamine (cys-eGO)
2.7. Methylation of Cysteamine Functionalized GO (met-cysGO)
2.8. Methylation of Cysteamine Functionalized GO Ester (met-cys-eGO)
2.9. Compounds Characterization
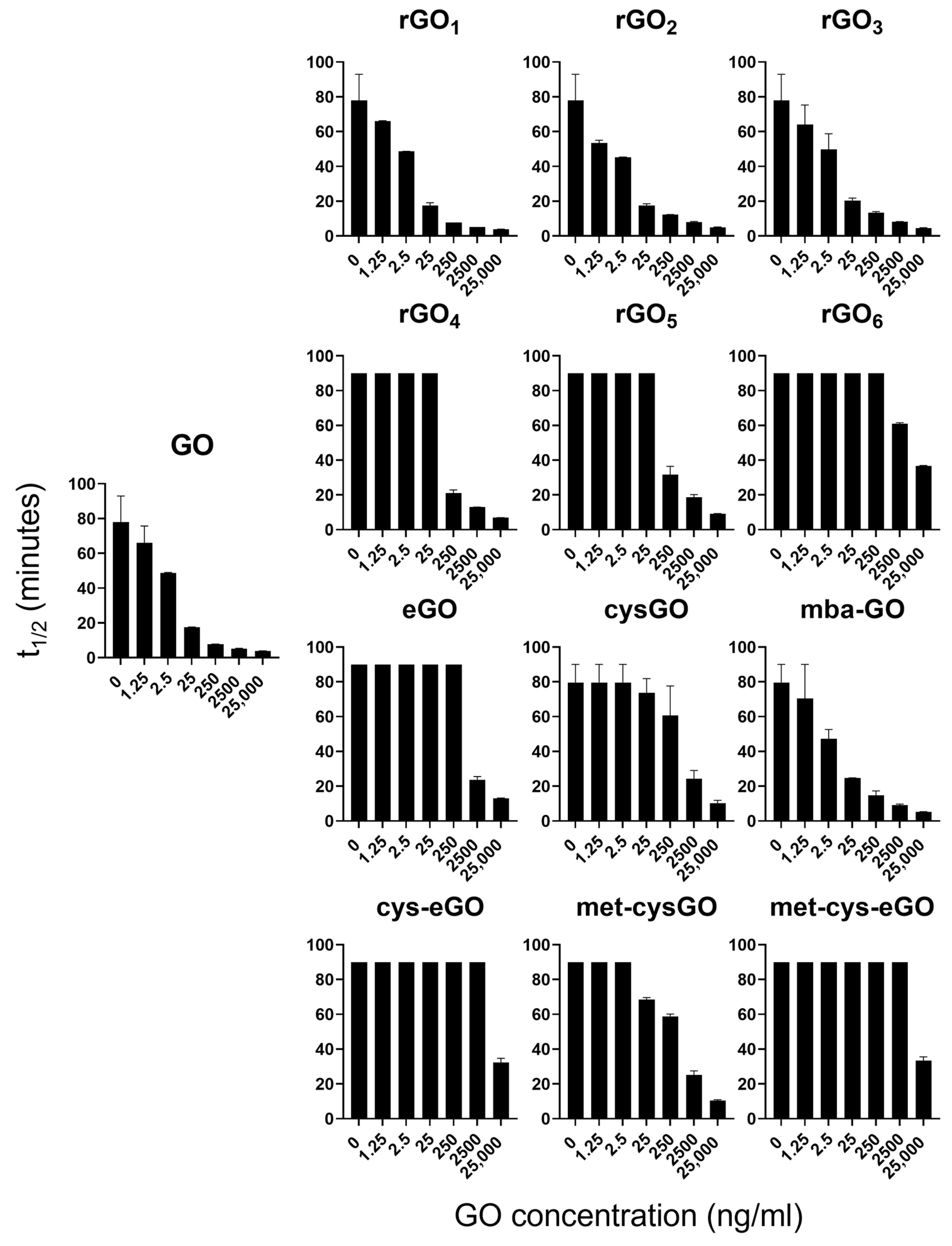
2.10. Procoagulant Activity Assay
2.11. Hemolytic Activity Assay
3. Results
3.1. Characterization of Synthesized GO
3.2. Synthesis of Reduced GO (rGO)
3.3. GO Covalent Functionalization
3.3.1. GO Esterification (eGO)
3.3.2. GO Functionalization with Cysteamine (cys-GO), Mercaptobenzoic Acid (mba-GO) and eGO with Cysteamine (cys-eGO)
3.3.3. Exhaustive Methylation of GO and GO Ester Functionalized with Cysteamine
3.4. Biocompatibility Assays
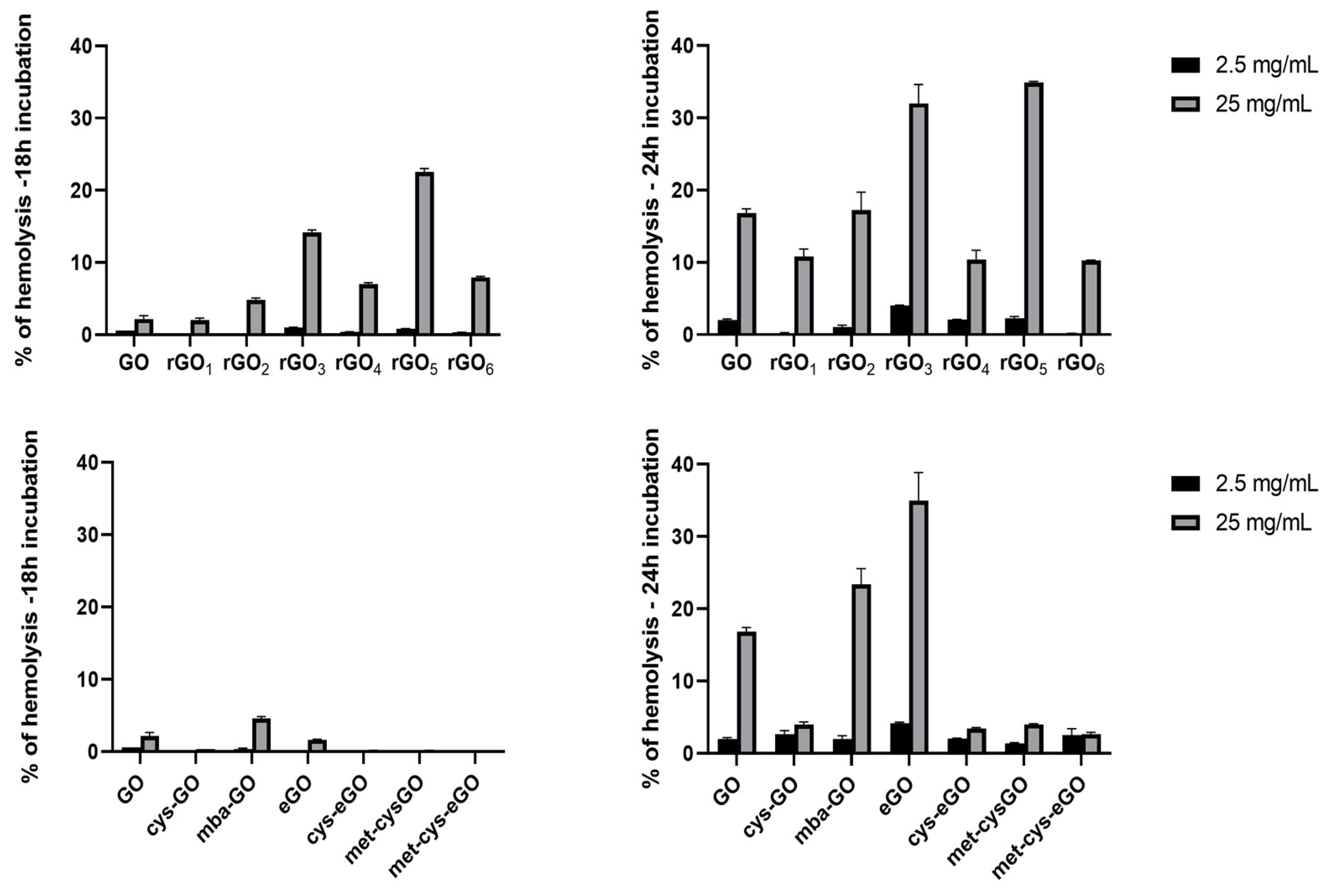
3.4.1. Hemolytic Activity
3.4.2. Pro-Coagulant Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Banerjee, A.N. Graphene and its derivatives as biomedical materials: Future prospects and challenges. Interface Focus 2018, 8, 20170056. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Chemical reduction of graphene oxide: A synthetic chemistry viewpoint. Chem. Soc. Rev. 2014, 43, 291–312. [Google Scholar] [CrossRef]
- Ou, L.; Song, B.; Liang, H.; Liu, J.; Feng, X.; Deng, B.; Sun, T.; Shao, L. Toxicity of graphene-family nanoparticles: A general review of the origins and mechanisms. Part. Fibre Toxicol. 2016, 13, 57. [Google Scholar] [CrossRef]
- Glassman, P.M.; Hood, E.D.; Ferguson, L.T.; Zhao, Z.; Siegel, D.L.; Mitragotri, S.; Brenner, J.S.; Muzykantov, V.R. Red blood cells: The metamorphosis of a neglected carrier into the natural mothership for artificial nanocarriers. Adv. Drug Deliv. Rev. 2021, 178, 113992. [Google Scholar] [CrossRef]
- Moyes, R.B.; Kirch, H.; DeLoach, J.R. Enhanced biological activity of human recombinant interleukin 2 coupled to mouse red blood cells as evaluated using the mouse Meth A sarcoma model. Biotechnol. Appl. Biochem. 1996, 23, 29–36. [Google Scholar]
- Sabatino, R.; Antonelli, A.; Battistelli, S.; Schwendener, R.; Magnani, M.; Rossi, L. Macrophage depletion by free bisphosphonates and zoledronate-loaded red blood cells. PLoS ONE 2014, 9, e101260. [Google Scholar] [CrossRef] [PubMed]
- Magnani, M.; Pierigè, F.; Rossi, L. Erythrocytes as a novel delivery vehicle for biologics: From enzymes to nucleic acid-based therapeutics. Ther. Deliv. 2012, 3, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Glassman, P.M.; Villa, C.H.; Ukidve, A.; Zhao, Z.; Smith, P.; Mitragotri, S.; Russell, A.J.; Brenner, J.S.; Muzykantov, V.R. Vascular Drug Delivery Using Carrier Red Blood Cells: Focus on RBC Surface Loading and Pharmacokinetics. Pharmaceutics 2020, 12, 44. [Google Scholar] [CrossRef]
- Murciano, J.C.; Higazi, A.B.D.; Cines, D.B.; Muzykantov, V.R. Soluble urokinase receptor conjugated to carrier red blood cells binds latent pro-urokinase and alters its functional profile. J. Control. Release 2009, 139, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Tutwiler, V.; Litvinov, R.I.; Protopopova, A.; Nagaswami, C.; Villa, C.; Woods, E.; Abdulmalik, O.; Siegel, D.L.; Russell, J.E.; Muzykantov, V.R.; et al. Pathologically stiff erythrocytes impede contraction of blood clots. J. Thromb. Haemost. 2021, 19, 1990–2001. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Kim, J.; Suja, V.C.; Kapate, N.; Gao, Y.; Guo, J.; Muzykantov, V.R.; Mitragotri, S. Red Blood Cell Anchoring Enables Targeted Transduction and Re-Administration of AAV-Mediated Gene Therapy. Adv. Sci. 2022, 9, e2201293. [Google Scholar] [CrossRef]
- Brenner, J.S.; Pan, D.C.; Myerson, J.W.; Marcos-Contreras, O.A.; Villa, C.H.; Patel, P.; Hekierski, H.; Chatterjee, S.; Tao, J.-Q.; Parhiz, H.; et al. Red blood cell-hitchhiking boosts delivery of nanocarriers to chosen organs by orders of magnitude. Nat. Commun. 2018, 9, 2684. [Google Scholar] [CrossRef]
- Sasidharan, A.; Panchakarla, L.S.; Sadanandan, A.R.; Ashokan, A.; Chandran, P.; Girish, C.M.; Menon, D.; Nair, S.V.; Rao, C.N.R.; Koyakutty, M. Hemocompatibility and macrophage response of pristine and functionalized graphene. Small 2012, 8, 1251–1263. [Google Scholar] [CrossRef]
- Ding, Z.; Zhang, Z.; Ma, H.; Chen, Y. In vitro hemocompatibility and toxic mechanism of graphene oxide on human peripheral blood T Lymphocytes and serum albumin. ACS Appl. Mater. Interfaces 2014, 6, 19797–19807. [Google Scholar] [CrossRef]
- Liao, K.H.; Lin, Y.S.; MacOsko, C.W.; Haynes, C.L. Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts. ACS Appl. Mater. Interfaces 2011, 3, 2607–2615. [Google Scholar] [CrossRef]
- Frimat, M.; Boudhabhay, I.; Roumenina, L.T. Hemolysis Derived Products Toxicity and Endothelium: Model of the Second Hit. Toxins 2019, 11, 660. [Google Scholar] [CrossRef]
- Qian, Q.; Nath, K.A.; Wu, Y.; Daoud, T.M.; Sethi, S. Hemolysis and Acute Kidney Failure. Am. J. Kidney Dis. 2010, 56, 780–784. [Google Scholar] [CrossRef]
- Hu, W.; Peng, C.; Lv, M.; Li, X.; Zhang, Y.; Chen, N.; Fan, C.; Huang, Q. Protein corona-mediated mitigation of cytotoxicity of graphene oxide. ACS Nano 2011, 5, 3693–3700. [Google Scholar] [CrossRef]
- Duan, G.; Kang, S.G.; Tian, X.; Garate, J.A.; Zhao, L.; Ge, C.; Zhou, R. Protein corona mitigates the cytotoxicity of graphene oxide by reducing its physical interaction with cell membrane. Nanoscale 2015, 7, 15214–15224. [Google Scholar] [CrossRef]
- Ge, C.; Du, J.; Zhao, L.; Wang, L.; Liu, Y.; Li, D.; Yang, Y.; Zhou, R.; Zhao, Y.; Chai, Z.; et al. Binding of blood proteins to carbon nanotubes reduces cytotoxicity. Proc. Natl. Acad. Sci. USA 2011, 108, 16968–16973. [Google Scholar] [CrossRef]
- Singh, S.K.; Singh, M.K.; Kulkarni, P.P.; Sonkar, V.K.; Grácio, J.J.A.; Dash, D. Amine-modified graphene: Thrombo-protective safer alternative to graphene oxide for biomedical applications. ACS Nano 2012, 6, 2731–2740. [Google Scholar] [CrossRef]
- Bianco, A.; Cheng, H.M.; Enoki, T.; Gogotsi, Y.; Hurt, R.H.; Koratkar, N.; Kyotani, T.; Monthioux, M.; Park, C.R.; Tascon, J.M.; et al. All in the graphene family—A recommended nomenclature for two-dimensional carbon materials. Carbon 2013, 65, 1–6. [Google Scholar] [CrossRef]
- Palmieri, V.; Perini, G.; De Spirito, M.; Papi, M. Graphene oxide touches blood: In vivo interactions of bio-coronated 2D materials. Nanoscale Horiz. 2019, 4, 273–290. [Google Scholar] [CrossRef]
- De Silva, K.K.H.; Huang, H.H.; Joshi, R.K.; Yoshimura, M. Chemical reduction of graphene oxide using green reductants. Carbon 2017, 119, 190–199. [Google Scholar] [CrossRef]
- Almeida, J.P.M.; Chen, A.L.; Foster, A.; Drezek, R. In vivo biodistribution of nanoparticles. Nanomedicine 2011, 6, 815–835. [Google Scholar] [CrossRef]
- Sprong, T.; Stikkelbroeck, N.; van der Ley, P.; Steeghs, L.; van Alphen, L.; Klein, N.; Netea, M.G. Contributions of Neisseria meningitidis LPS and non-LPS to proinflammatory cytokine response. J. Leukoc. Biol. 2001, 70, 283–288. [Google Scholar] [CrossRef]
- Liu, Z.; Robinson, J.T.; Tabakman, S.M.; Yang, K.; Dai, H. Carbon materials for drug delivery & cancer therapy. Mater. Today 2011, 14, 316–323. [Google Scholar]
- Wang, Y.; Li, Z.; Wang, J.; Li, J.; Lin, Y. Graphene and graphene oxide: Biofunctionalization and applications in biotechnology. Trends Biotechnol. 2011, 29, 205–212. [Google Scholar] [CrossRef]
- Chung, C.; Kim, Y.K.; Shin, D.; Ryoo, S.R.; Hong, B.H.; Min, D.H. Biomedical applications of graphene and graphene oxide. Acc. Chem. Res. 2013, 46, 2211–2224. [Google Scholar] [CrossRef]
- Lundqvist, M.; Stigler, J.; Elia, G.; Lynch, I.; Cedervall, T.; Dawson, K.A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270. [Google Scholar] [CrossRef]
- Kenry Loh, K.P.; Lim, C.T. Molecular interactions of graphene oxide with human blood plasma proteins. Nanoscale 2016, 8, 9425–9441. [Google Scholar] [CrossRef]
- Wang, M.; Gustafsson, O.J.R.; Siddiqui, G.; Javed, I.; Kelly, H.G.; Blin, T.; Yin, H.; Kent, S.J.; Creek, D.J.; Kempe, K.; et al. Human plasma proteome association and cytotoxicity of nano-graphene oxide grafted with stealth polyethylene glycol and poly(2-ethyl-2-oxazoline). Nanoscale 2018, 10, 10863–10875. [Google Scholar] [CrossRef]
- Caputo, D.; Papi, M.; Coppola, R.; Palchetti, S.; Digiacomo, L.; Caracciolo, G.; Pozzi, D. A protein corona-enabled blood test for early cancer detection. Nanoscale 2017, 9, 349–354. [Google Scholar] [CrossRef]
- Lu, X.; Xu, P.; Ding, H.M.; Yu, Y.S.; Huo, D.; Ma, Y.Q. Tailoring the component of protein corona via simple chemistry. Nat. Commun. 2019, 10, 4520. [Google Scholar] [CrossRef]
- Gómez-Navarro, C.; Weitz, R.T.; Bittner, A.M.; Scolari, M.; Mews, A.; Burghard, M.; Kern, K. Electronic transport properties of individual chemically reduced graphene oxide sheets. Nano Lett. 2007, 7, 4520. [Google Scholar] [CrossRef]
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef]
- Robinson, J.T.; Zalalutdinov, M.; Baldwin, J.W.; Snow, E.S.; Wei, Z.; Sheehan, P.; Houston, B.H. Wafer-scale reduced graphene oxide films for nanomechanical devices. Nano Lett. 2008, 8, 3441–3445. [Google Scholar] [CrossRef]
- Luong, N.D.; Sinh, L.H.; Johansson, L.S.; Campell, J.; Seppälä, J. Functional graphene by thiol-ene click chemistry. Chem. A Eur. J. 2015, 21, 3183–3186. [Google Scholar] [CrossRef]
- Dimiev, A.M.; Alemany, L.B.; Tour, J.M. Graphene oxide. Origin of acidity, its instability in water, and a new dynamic structural model. ACS Nano 2013, 7, 576–588. [Google Scholar] [CrossRef]
- Shruthi Vighnesha, K.M.; Sandhya Sangeetha, D.N.; Selvakumar, M. Synthesis and Characterization of Reduced Graphene Oxide- Polyaniline Composite for Supercapacitor Applications. Surf. Eng. Appl. Electrochem. 2018, 54, 359–366. [Google Scholar] [CrossRef]
- Ilinskaya, A.N.; Dobrovolskaia, M.A. Nanoparticles and the blood coagulation system. Part II: Safety concerns. Nanomedicine 2013, 8, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Yu, Y.; Shen, C.; Jiao, Y.; Zhou, C. Impact of graphene oxide on the structure and function of important multiple blood components by a dose-dependent pattern. J. Biomed. Mater. Res. Part. A 2015, 103, 2006–2014. [Google Scholar] [CrossRef] [PubMed]
- Neklyudov, V.V.; Khafizov, N.R.; Sedov, I.A.; Dimiev, A.M. New insights into the solubility of graphene oxide in water and alcohols. Phys. Chem. Chem. Phys. 2017, 19, 17000–17008. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, Y.; Bidram, E.; Zarrabi, A.; Amini, A.; Cheng, C. Graphene oxide and its derivatives as promising In-vitro bio-imaging platforms. Sci. Rep. 2020, 10, 18052. [Google Scholar] [CrossRef]
- Mirrahimi, M.; Alamzadeh, Z.; Beik, J.; Sarikhani, A.; Mousavi, M.; Irajirad, R.; Khani, T.; Davani, E.S.; Farashahi, A.; Ardakani, T.S.; et al. A 2D nanotheranostic platform based on graphene oxide and phase-change materials for bimodal CT/MR imaging, NIR-activated drug release, and synergistic thermo-chemotherapy. Nanotheranostics 2022, 6, 350–364. [Google Scholar] [CrossRef]
- Liu, L.; Ma, Q.; Cao, J.; Gao, Y.; Han, S.; Liang, Y.; Zhang, T.; Song, Y.; Sun, Y. Recent progress of graphene oxide-based multifunctional nanomaterials for cancer treatment. Cancer Nano 2021, 12, 18. [Google Scholar]
- Park, S.; An, J.; Potts, J.R.; Velamakanni, A.; Murali, S.; Ruoff, R.S. Hydrazine-reduction of graphite- and graphene oxide. Carbon 2011, 49, 3019–3023. [Google Scholar] [CrossRef]
- Park, S.; Hu, Y.; Hwang, J.O.; Lee, E.S.; Casabianca, L.B.; Cai, W.; Potts, J.R.; Ha, H.W.; Chen, S.; Oh, J. Chemical structures of hydrazine-treated graphene oxide and generation of aromatic nitrogen doping. Nat. Commun. 2012, 3, 638–645. [Google Scholar] [CrossRef]
- Pan, D.C.; Myerson, J.W.; Brenner, J.S.; Patel, P.N.; Anselmo, A.C.; Mitragotri, S.; Muzykantov, V. Nanoparticle Properties Modulate Their Attachment and Effect on Carrier Red Blood Cells. Sci. Rep. 2018, 8, 1615. [Google Scholar] [CrossRef]
- Tavano, R.; Segat, D.; Reddi, E.; Kos, J.; Rojnik, M.; Kocbek, P.; Iratni, S.; Scheglmann, D.; Colucci, M.; Echevarria, I.M.R.; et al. Procoagulant properties of bare and highly PEGylated vinyl-modified silica nanoparticles. Nanomedicine 2010, 5, 881–896. [Google Scholar] [CrossRef]

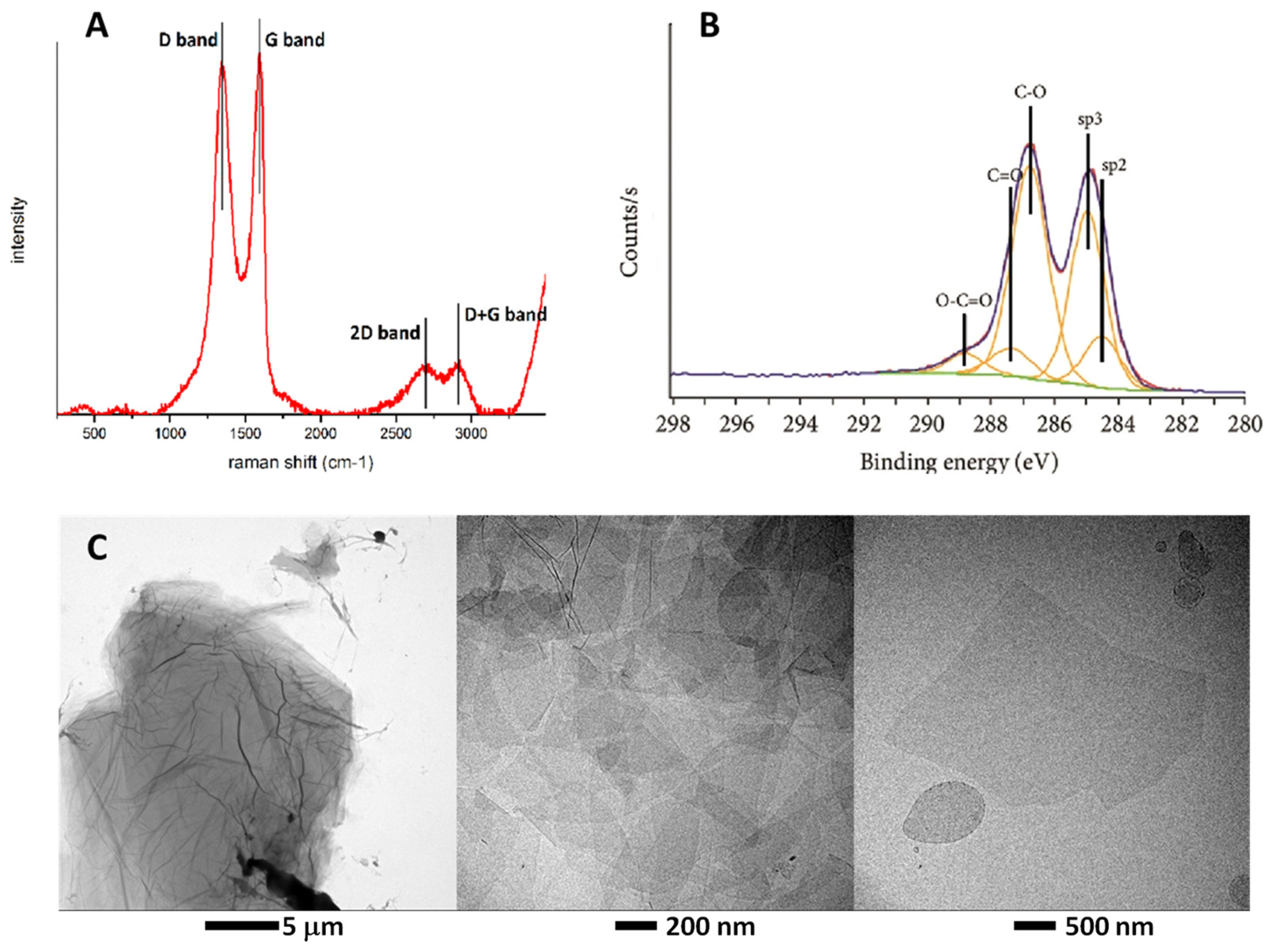
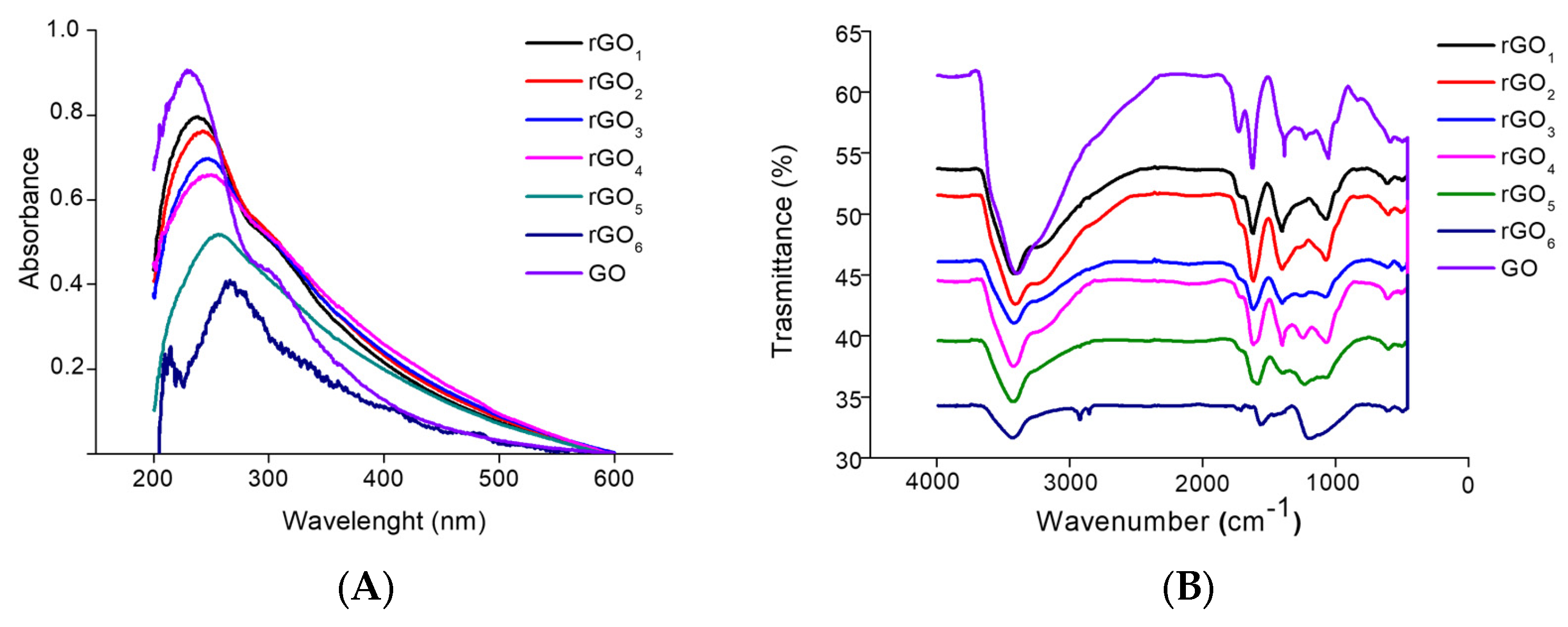

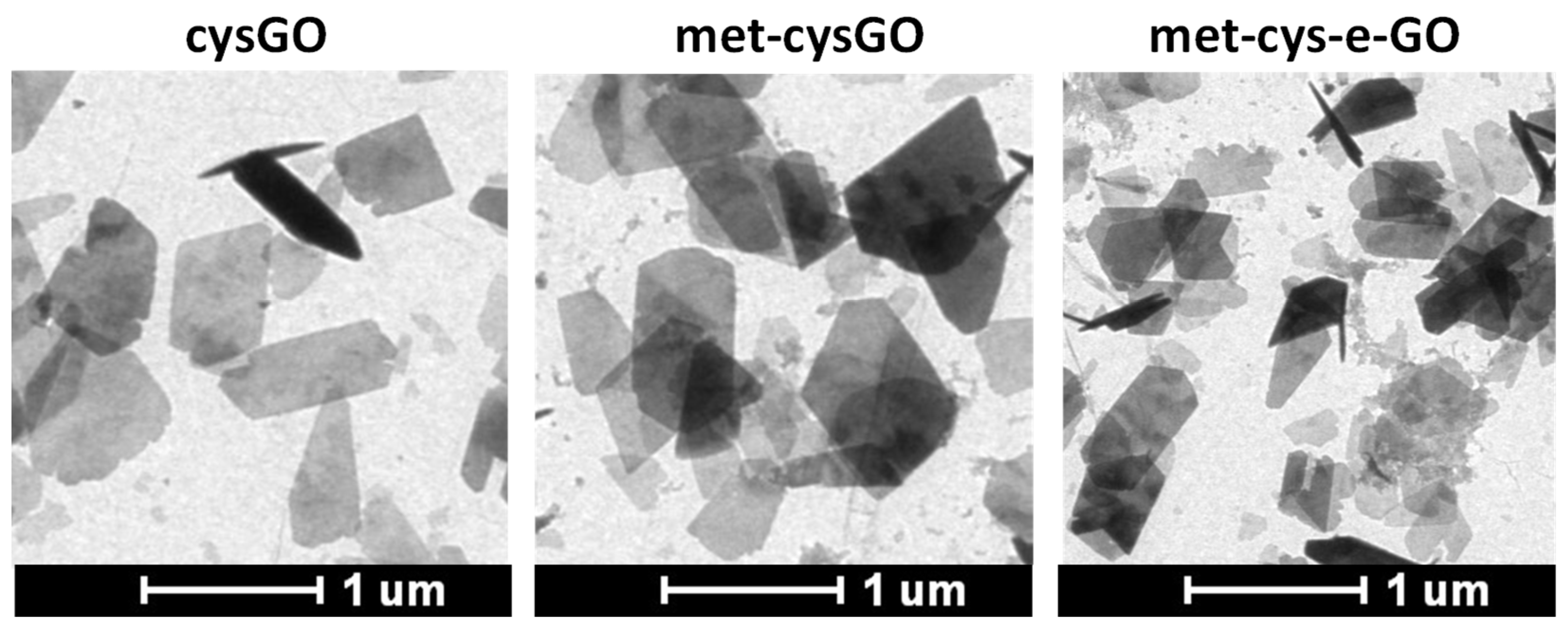

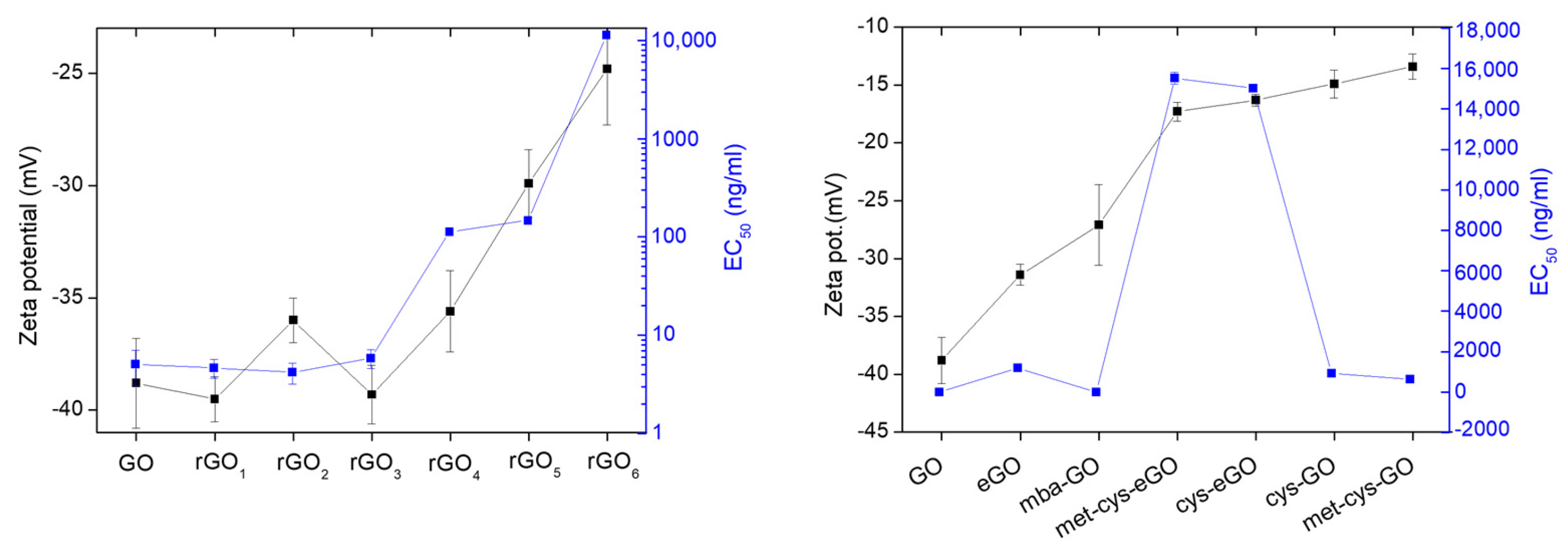
| Ζ-Potential (mV) | C/O Molar Ratio | |
|---|---|---|
| GO | −38.8 ± 2 | 1.31 |
| eGO | −31.4 ± 0.9 | 1.58 |
| Ζ-Potential (mV) | C/O Molar Ratio | N/S Molar Ratio | |
|---|---|---|---|
| GO | −38.8 ± 2 | 1.31 | - |
| cysGO | −16.3 ± 1.2 | 1.64 | 1.05 |
| cys-eGO | −13.2 ± 0.5 | 1.71 | 1.08 |
| mbaGO | −27.1 ± 0.5 | 2.32 | - |
| ζ-Potential (mV) | C/O Molar Ratio | N/S Molar Ratio | |
|---|---|---|---|
| cysGO | −16.3 ± 1.2 | 1.64 | 1.05 |
| met-cysGO | −13.4 ± 1.1 | 1.76 | 1.09 |
| cys-eGO | −13.2 ± 0.5 | 1.71 | 1.08 |
| met-cys-eGO | −12.3 ± 0.8 | 1.95 | 0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pieretti, F.; Moretto, A.; Papini, E.; Tavano, R. Graphene Oxide Chemical Refining Screening to Improve Blood Compatibility of Graphene-Based Nanomaterials. J. Nanotheranostics 2024, 5, 13-28. https://doi.org/10.3390/jnt5010002
Pieretti F, Moretto A, Papini E, Tavano R. Graphene Oxide Chemical Refining Screening to Improve Blood Compatibility of Graphene-Based Nanomaterials. Journal of Nanotheranostics. 2024; 5(1):13-28. https://doi.org/10.3390/jnt5010002
Chicago/Turabian StylePieretti, Fabio, Alessandro Moretto, Emanuele Papini, and Regina Tavano. 2024. "Graphene Oxide Chemical Refining Screening to Improve Blood Compatibility of Graphene-Based Nanomaterials" Journal of Nanotheranostics 5, no. 1: 13-28. https://doi.org/10.3390/jnt5010002





