The Role of Fullerenes in Neurodegenerative Disorders
Abstract
:1. Introduction to Fullerenes
2. Neuroprotective Effect of Fullerenes
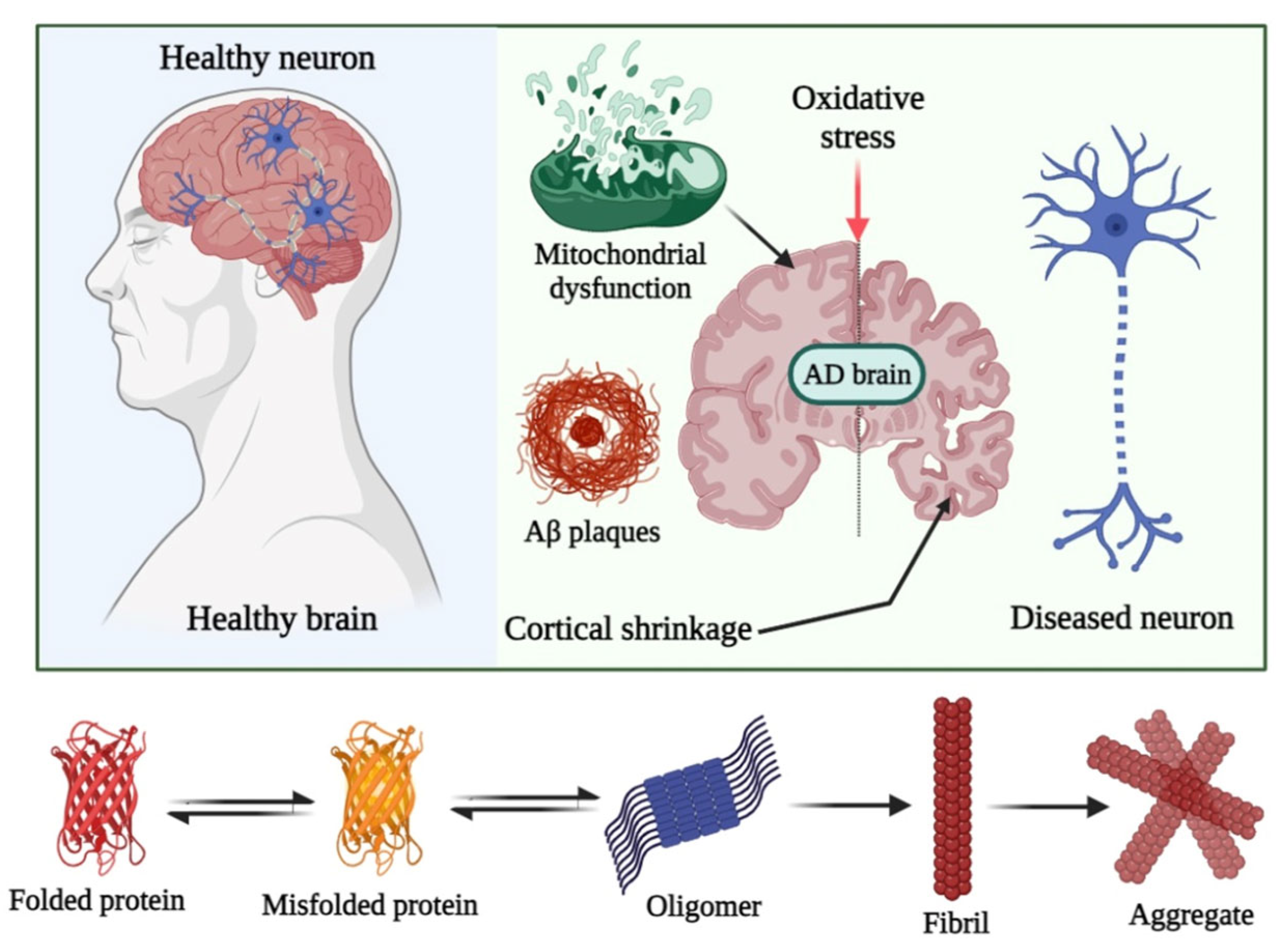
2.1. Potential of Fullerenes and Their Derivatives in Mitigating Amyloid-Associated Toxicity
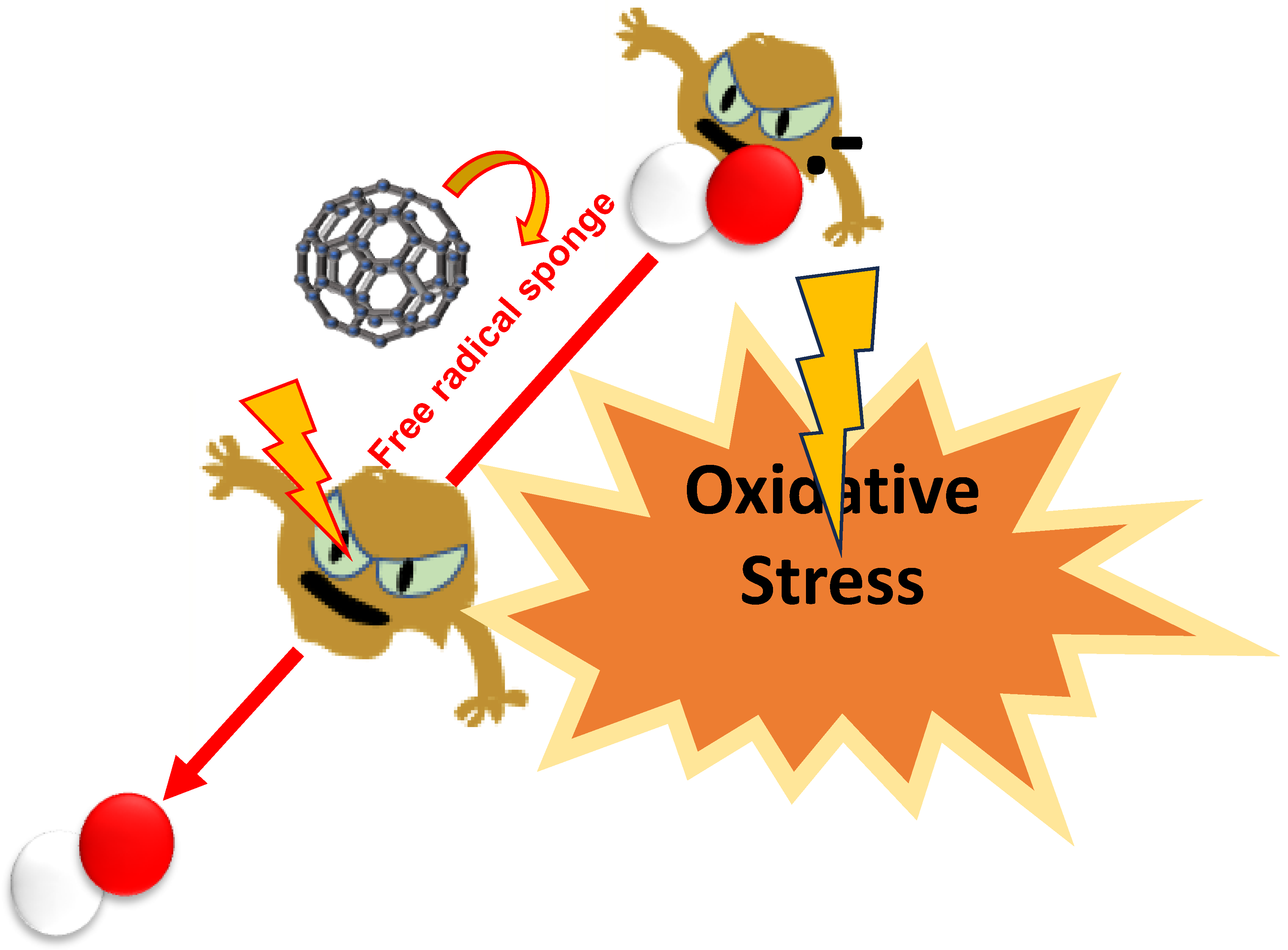
2.2. Potential of Fullerenes and Their Derivatives in Mitigating Oxidative Stress
3. Outlook and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Das, R.; Bandyopadhyay, R.; Pramanik, P. Carbon quantum dots from natural resource: A review. Mater. Today Chem. 2018, 8, 96–109. [Google Scholar] [CrossRef]
- Ehtesabi, H.; Hallaji, Z.; Nobar, S.N.; Bagheri, Z. Carbon dots with pH-responsive fluorescence: A review on synthesis and cell biological applications. Microchim. Acta 2020, 187, 150. [Google Scholar] [CrossRef]
- Yan, F.; Sun, Z.; Zhang, H.; Sun, X.; Jiang, Y.; Bai, Z. The fluorescence mechanism of carbon dots, and methods for tuning their emission color: A review. Microchim. Acta 2019, 186, 583. [Google Scholar] [CrossRef]
- Sharma, A.; Das, J. Small molecules derived carbon dots: Synthesis and applications in sensing, catalysis, imaging, and biomedicine. J. Nanobiotechnol. 2019, 17, 92. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresslhaus, G. Fullerenes and fullerene derived solids as electronic materials. Annu. Rev. Mater. Sci. 1995, 25, 487–523. [Google Scholar] [CrossRef]
- Castro, E.; Garcia, A.H.; Zavala, G.; Echegoyen, L. Fullerenes in biology and medicine. J. Mater. Chem. B 2017, 5, 6523–6535. [Google Scholar] [CrossRef]
- Singh, H.; Srivastava, M. Fullerenes: Synthesis, separation, characterization, reaction chemistry, and applications—A review. Energy Sources 1995, 17, 615–640. [Google Scholar] [CrossRef]
- Dugan, L.L.; Turetsky, D.M.; Du, C.; Lobner, D.; Wheeler, M.; Almli, C.R.; Shen, C.K.-F.; Luh, T.-Y.; Choi, D.W.; Lin, T.S.; et al. Carboxyfullerenes as neuroprotective agents. Proc. Natl. Acad. Sci. USA 1997, 94, 9434–9439. [Google Scholar] [CrossRef]
- Persson, T.; Popescu, B.O.; Cedazo-Minguez, A. Oxidative stress in Alzheimer’s disease: Why did antioxidant therapy fail? Oxidative Med. Cell. Longev. 2014, 2014, 427318. [Google Scholar] [CrossRef]
- Cummings, J.; Zhou, Y.; Lee, G.; Zhong, K.; Fonseca, J.; Cheng, F. Alzheimer’s disease drug development pipeline: 2023. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2013, 9, e12385. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2023 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2023, 19, 1598–1695. [Google Scholar] [CrossRef] [PubMed]
- Kuns, B.; Rosani, A.; Varghese, D. Memantine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Singh, R.; Sadiq, N.M. Cholinesterase Inhibitors. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Ghossein, N.; Kang, M.; Lakhkar, A.D. Anticholinergic Medications. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Männistö, P.T.; Kaakkola, S. Catechol-O-methyltransferase (COMT): Biochemistry, Molecular Biology, Pharmacology, and Clinical Efficacy of the New Selective COMT Inhibitors. Pharmacol. Rev. 1999, 51, 593. [Google Scholar]
- Alborghetti, M.; Nicoletti, F. Different Generations of Type-B Monoamine Oxidase Inhibitors in Parkinson’s Disease: From Bench to Bedside. Curr. Neuropharmacol. 2019, 17, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, K.; Yokoo, H.; Yoshida, M.; Tanaka, T.; Tanaka, M. Amantadine increases the extracellular dopamine levels in the striatum by re-uptake inhibition and by N-methyl-d-aspartate antagonism. Brain Res. 1994, 662, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, B.; Lebon, G. Human Adenosine A2A Receptor: Molecular Mechanism of Ligand Binding and Activation. Front. Pharmacol. 2017, 8, 898. [Google Scholar] [CrossRef]
- Choi, J.; Horner, K.A. Dopamine Agonists. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Elsori, D.; Rashid, G.; Khan, N.A.; Sachdeva, P.; Jindal, R.; Kayenat, F.; Sachdeva, B.; Kamal, M.A.; Babker, S.M.; Fahmy, S.A. Nanotube breakthroughs: Unveiling the potential of carbon nanotubes as a dual therapeutic arsenal for Alzheimer’s disease and brain tumors. Front. Oncol. 2023, 13, 1265347. [Google Scholar] [CrossRef]
- Ghosh, R.; Bag, J.; Datta, A.; Pramanick, A.; Abubakar, I.H. Functionalized carbon nanotubes—A boon in treating brain diseases. J. Appl. Pharm. Sci. 2023, 13, 032–039. [Google Scholar] [CrossRef]
- Joshi, R.; Missong, H.; Mishra, J.; Kaur, S.; Saini, S.; Kandimalla, R.; Reddy, P.H.; Babu, A.; Bhatti, G.K.; Bhatti, J.S. Nanotheranostics revolutionizing neurodegenerative diseases: From precision diagnosis to targeted therapies. J. Drug Deliv. Sci. Technol. 2023, 89, 105067. [Google Scholar] [CrossRef]
- Wei, M.; Yang, Z.; Li, S.; Le, W. Nanotherapeutic and Stem cell therapeutic strategies in neurodegenerative diseases: A promising therapeutic approach. Int. J. Nanomed. 2023, 18, 611–626. [Google Scholar] [CrossRef]
- Marsagishvili, L.G.; Bobylev, A.G.; Shpagina, M.D.; Troshin, P.A.; Podlubnaya, Z.A. Effect of fullerenes C 60 on X-protein amyloids. Biophysics 2009, 54, 135–138. [Google Scholar] [CrossRef]
- Cabaleiro-Lago, C.; Szczepankiewicz, O.; Linse, S. The effect of nanoparticles on amyloid aggregation depends on the protein stability and intrinsic aggregation rate. Langmuir 2012, 28, 1852–1857. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Kalhor, H.R.; Laurent, S.; Lynch, I. Protein fibrillation and nanoparticle interactions: Opportunities and challenges. Nanoscale 2013, 5, 2570–2588. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Lee, M. Fullerene inhibits β-amyloid peptide aggregation. Biochem. Biophys. Res. Commun. 2003, 303, 576–579. [Google Scholar] [CrossRef]
- Huy, P.D.Q.; Li, M.S. Binding of fullerenes to amyloid beta fibrils: Size matters. Phys. Chem. Chem. Phys. 2014, 16, 20030–20040. [Google Scholar] [CrossRef]
- Guo, J.; Li, J.; Zhang, Y.; Jin, X.; Liu, H.; Yao, X. Exploring the influence of carbon nanoparticles on the formation of β-sheet-rich oligomers of IAPP22–28 peptide by molecular dynamics simulation. PLoS ONE 2013, 8, e65579. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Qian, Z.; Wei, G. The inhibitory mechanism of a fullerene derivative against amyloid-β peptide aggregation: An atomistic simulation study. Phys. Chem. Chem. Phys. 2016, 18, 12582–12591. [Google Scholar] [CrossRef] [PubMed]
- Bednarikova, Z.; Huy, P.D.Q.; Mocanu, M.M.; Fedunova, D.; Li, M.S.; Gazova, Z. Fullerenol C 60 (OH) 16 prevents amyloid fibrillization of Aβ 40–in vitro and in silico approach. Phys. Chem. Chem. Phys. 2016, 18, 18855–18867. [Google Scholar] [CrossRef]
- Lei, J.; Qi, R.; Xie, L.; Xi, W.; Wei, G. Inhibitory effect of hydrophobic fullerenes on the β-sheet-rich oligomers of a hydrophilic GNNQQNY peptide revealed by atomistic simulations. RSC Adv. 2017, 7, 13947–13956. [Google Scholar] [CrossRef]
- Melchor, M.H.; Susana, F.G.; Francisco, G.S.; Norma, R.F.; Gustavo, B.I. Fullerenemalonates inhibit amyloid beta aggregation, in vitro and in silico evaluation. RSC Adv. 2018, 8, 39667–39677. [Google Scholar] [CrossRef]
- Sun, Y.; Kakinen, A.; Zhang, C.; Yang, Y.; Faridi, A.; Davis, T.P.; Ding, F. Amphiphilic surface chemistry of fullerenols is necessary for inhibiting the amyloid aggregation of alpha-synuclein NACore. Nanoscale 2019, 11, 11933–11945. [Google Scholar] [CrossRef]
- Podolski, I.Y.; Podlubnaya, Z.A.; Kosenko, E.A.; Mugantseva, E.A.; Makarova, E.G.; Marsagishvili, L.G.; Klochkov, V.K. Effects of hydrated forms of C60 fullerene on amyloid β-peptide fibrillization in vitro and performance of the cognitive task. J. Nanosci. Nanotechnol. 2007, 7, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Andujar, S.A.; Lugli, F.; Höfinger, S.; Enriz, R.D.; Zerbetto, F. Amyloid-β fibril disruption by C 60—Molecular guidance for rational drug design. Phys. Chem. Chem. Phys. 2012, 14, 8599–8607. [Google Scholar] [CrossRef]
- Bobylev, A.G.; Kornev, A.B.; Bobyleva, L.G.; Shpagina, M.D.; Fadeeva, I.S.; Fadeev, R.S.; Deryabin, D.G.; Balzarini, J.; Troshin, P.A.; Podlubnaya, Z.A. Fullerenolates: Metallated polyhydroxylated fullerenes with potent anti-amyloid activity. Org. Biomol. Chem. 2011, 9, 5714–5719. [Google Scholar] [CrossRef]
- Xie, L.; Luo, Y.; Lin, D.; Xi, W.; Yang, X.; Wei, G. The molecular mechanism of fullerene-inhibited aggregation of Alzheimer’s β-amyloid peptide fragment. Nanoscale 2014, 6, 9752–9762. [Google Scholar] [CrossRef]
- Zhou, X.; Xi, W.; Luo, Y.; Cao, S.; Wei, G. Interactions of a water-soluble fullerene derivative with amyloid-β protofibrils: Dynamics, binding mechanism, and the resulting salt-bridge disruption. J. Phys. Chem. B 2014, 118, 6733–6741. [Google Scholar] [CrossRef] [PubMed]
- Da Ros, T.; Prato, M. Medicinal chemistry with fullerenes and fullerene derivatives. Chem. Commun. 1999, 8, 663–669. [Google Scholar] [CrossRef]
- Christen, Y. Oxidative stress and Alzheimer disease. Am. J. Clin. Nutr. 2000, 71, 621S–629S. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef]
- Lee, C.M.; Huang, S.T.; Huang, S.H.; Lin, H.W.; Tsai, H.P.; Wu, J.Y.; Lin, C.M.; Chen, C.T. C60 fullerene-pentoxifylline dyad nanoparticles enhance autophagy to avoid cytotoxic effects caused by the β-amyloid peptide. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 107–114. [Google Scholar] [CrossRef]
- Lu, T.Y.; Kao, P.F.; Lee, C.M.; Huang, S.T.; Lin, C.M. C60 Fullerene Nanoparticle Prevents?—Amyloid Peptide Induced Cytotoxicity in Neuro 2A Cells. J. Food Drug Anal. 2011, 19, 17. [Google Scholar]
- Makarova, E.G.; Gordon, R.Y.; Podolski, I.Y. Fullerene C60 Prevents Neurotoxicity Induced by Intrahippocampal Microinjection of Amyloid-β Peptide. J. Nanosci. Nanotechnol. 2012, 12, 119–126. [Google Scholar] [CrossRef]
- Du, Z.; Gao, N.; Wang, X.; Ren, J.; Qu, X. Near-Infrared Switchable Fullerene-Based Synergy Therapy for Alzheimer’s Disease. Small 2018, 14, 1801852. [Google Scholar] [CrossRef]
- Liu, Z.; Zou, Y.; Zhang, Q.; Chen, P.; Liu, Y.; Qian, Z. Distinct binding dynamics, sites and interactions of fullerene and fullerenols with amyloid-β peptides revealed by molecular dynamics simulations. Int. J. Mol. Sci. 2019, 20, 2048. [Google Scholar] [CrossRef]
- Siposova, K.; Petrenko, V.I.; Ivankov, O.I.; Musatov, A.; Bulavin, L.A.; Avdeev, M.V.; Kyzyma, O.A. Fullerenes as an effective amyloid fibrils disaggregating nanomaterial. ACS Appl. Mater. Interfaces 2020, 12, 32410–32419. [Google Scholar] [CrossRef]
- Tiwari, P.; Tiwari, S. Detection and modulation of neurodegenerative processes using graphene-based nanomaterials: Nanoarchitectonics and applications. Adv. Colloid Interface Sci. 2023, 311, 102824. [Google Scholar] [CrossRef]
- Ivkovic, S.; Koruga, D. Role of fullerenols derivative 3HFWC in the treatment of Alzheimer’s disease. Neural Regen. Res. 2024, 19, 1641–1642. [Google Scholar] [CrossRef]
- Mehta, P.; Shende, P. Collation of fullerenes and carbon nanotubes with genistein for synergistic anti-Alzheimer’s activity by amyloid-β deaggregation. J. Drug Deliv. Sci. Technol. 2023, 91, 105205. [Google Scholar] [CrossRef]
- Jia, L.; Chen, M.; Yang, S. Functionalization of fullerene materials toward applications in perovskite solar cells. Mater. Chem. Front. 2020, 4, 2256–2282. [Google Scholar] [CrossRef]
- Afreen, S.; Muthoosamy, K.; Manickam, S.; Hashim, U. Functionalized fullerene (C60) as a potential nanomediator in the fabrication of highly sensitive biosensors. Biosens. Bioelectron. 2015, 63, 354–364. [Google Scholar] [CrossRef]
- Speranza, G. The role of functionalization in the applications of carbon materials: An overview. C 2019, 5, 84. [Google Scholar] [CrossRef]
- Maciel, C.; Fileti, E.E.; Rivelino, R. Assessing the solvation mechanism of C60 (OH) 24 in aqueous solution. Chem. Phys. Lett. 2011, 507, 244–247. [Google Scholar] [CrossRef]
- Pal, T.; Mukherjee, S.; Mondal, A. Nanoscale drug delivery and tissue engineering for neurodegenerative diseases. In Nanostructured Materials for Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2023; pp. 581–590. [Google Scholar]
- Sumner, S.C.; Fennell, T.R.; Snyder, R.W.; Taylor, G.F.; Lewin, A.H. Distribution of carbon-14 labeled C60 ([14C] C60) in the pregnant and in the lactating dam and the effect of C60 exposure on the biochemical profile of urine. J. Appl. Toxicol. Int. J. 2010, 30, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhu, L.; Li, Y.; Duan, Z.; Chen, W.; Alvarez, P.J. Developmental toxicity in zebrafish (Danio rerio) embryos after exposure to manufactured nanomaterials: Buckminsterfullerene aggregates (nC60) and fullerol. Environ. Toxicol. Chem. Int. J. 2007, 26, 976–979. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Deng, R.; Li, J.; Li, H.; Xu, Z.; Zhang, L.; Feng, L.; Shu, C.; Zhen, M.; Wang, C. Oral [60] fullerene reduces neuroinflammation to alleviate Parkinson’s disease via regulating gut microbiome. Theranostics 2023, 13, 4936. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Oguri, I.; Yamakoshi, Y.N.; Miyata, N. Novel harmful effects of [60] fullerene on mouse embryos in vitro and in vivo. FEBS Lett. 1996, 393, 139–145. [Google Scholar] [CrossRef]



| Neurodegenerative Disorder | Drug Class | Drug Indications | Mechanism of Action | Drug Name | Reference |
|---|---|---|---|---|---|
| Alzheimer’s Disease | Cholinesterase inhibitor | Increases cognitive function by increasing levels of acetylcholine | Prevents the hydrolysis of acetylcholine into acetate and choline | Donepezil Rivastigmine Galantamine | [12] |
| N-Methyl-D-Aspartate (NMDA) receptor antagonist | Regulates glutamate activity and prevent exitoxicity | Binds to NMDA receptors and reduces the influx of Ca+ to regulate glutamate activity | Memantine | [13] | |
| Parkinson’s Disease | Dopamine agonists | Increases dopamine availability | Activates dopamine receptors D2 and D3 receptors | Pramipexole Apomorphine Transdermal Ritigotine Ropinirole | [14] |
| Levodopa | Manage motor symptoms | Decarboxylases into dopamine | Carbidopa | [15] | |
| Monoamine oxidase type B (MAO-B) Inhibitors | Increases dopamine availability | Inhibit the deactivation of dopamine | Selegiline Rasagiline Safinamide | [16] | |
| Cathechol-O-Methyl transferase (COMT) Inhibitors | Manage motor symptoms when used with levodopa | Inhibit COMT activity to reduced the methylation of catecholamines | Tolcapone Entacapone Opicapone | [17] | |
| Adenosine 2A Antagonists | Manage motor symptoms when used with levodopa | Inhibit A2A receptor antagonists | Istradefylline | [18] | |
| Anticholinergics | Manage motor symptoms | Inhibit binding of neuro transmitter acetylcholine | Trihexyphenidyl Benztropine Orphenadrine Procyclidine Biperiden | [19] | |
| Amantadine | Used as a prophylactic while taking levodopa | Inhibits the re-uptake of N-methyl-D-aspartate antagonism | Gocovri Symmetrel | [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilson, D.L.; Ahlawat, J.; Narayan, M. The Role of Fullerenes in Neurodegenerative Disorders. J. Nanotheranostics 2024, 5, 1-12. https://doi.org/10.3390/jnt5010001
Wilson DL, Ahlawat J, Narayan M. The Role of Fullerenes in Neurodegenerative Disorders. Journal of Nanotheranostics. 2024; 5(1):1-12. https://doi.org/10.3390/jnt5010001
Chicago/Turabian StyleWilson, Daisy L., Jyoti Ahlawat, and Mahesh Narayan. 2024. "The Role of Fullerenes in Neurodegenerative Disorders" Journal of Nanotheranostics 5, no. 1: 1-12. https://doi.org/10.3390/jnt5010001




