Influence of Ethanol on the Acid-Induced Flocculation of Casein Micelles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Monitoring of Acid-Induced Coagulation of Milk
3. Results and Discussion
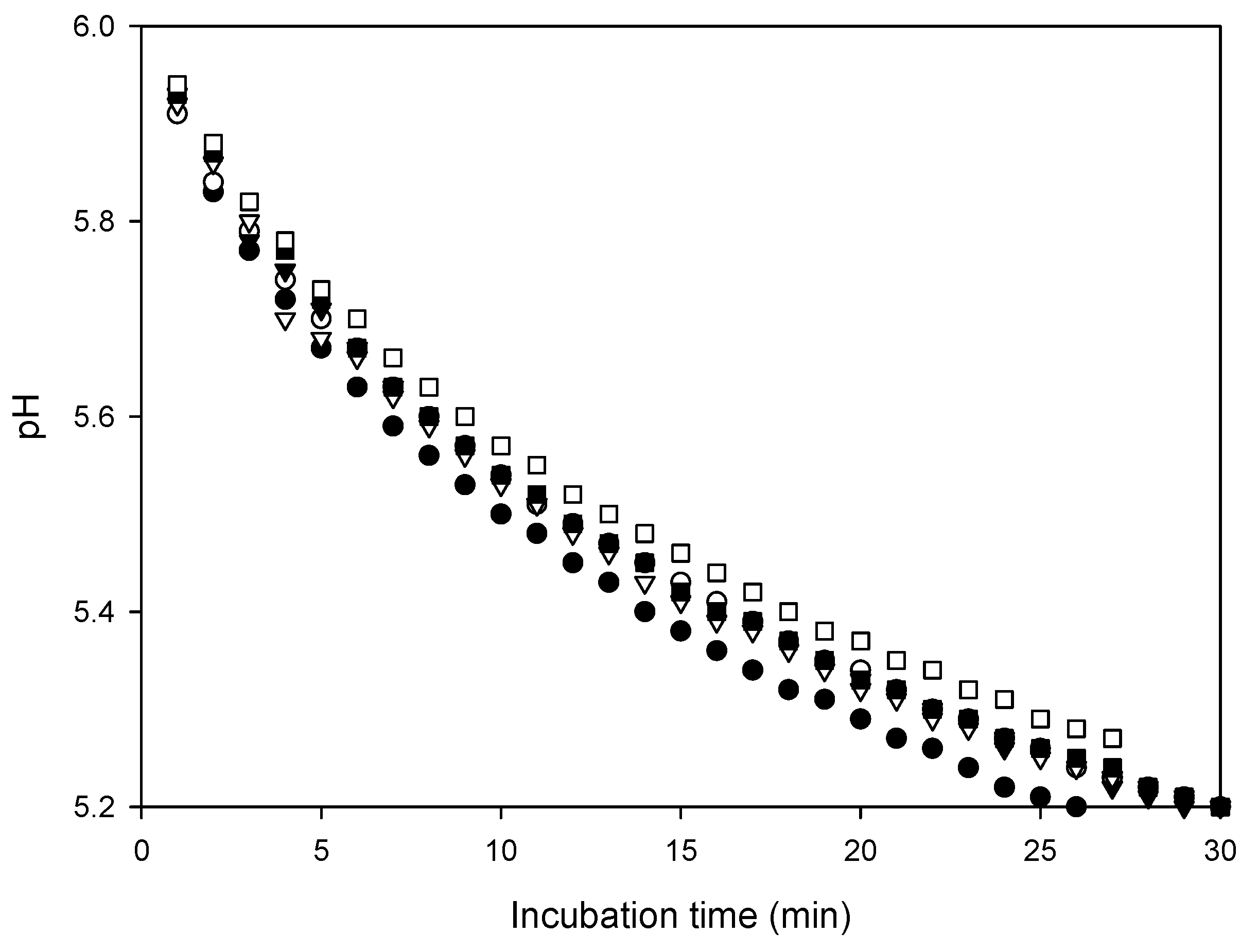
3.1. Influence of Ethanol on the Rate of Acidification of Milk
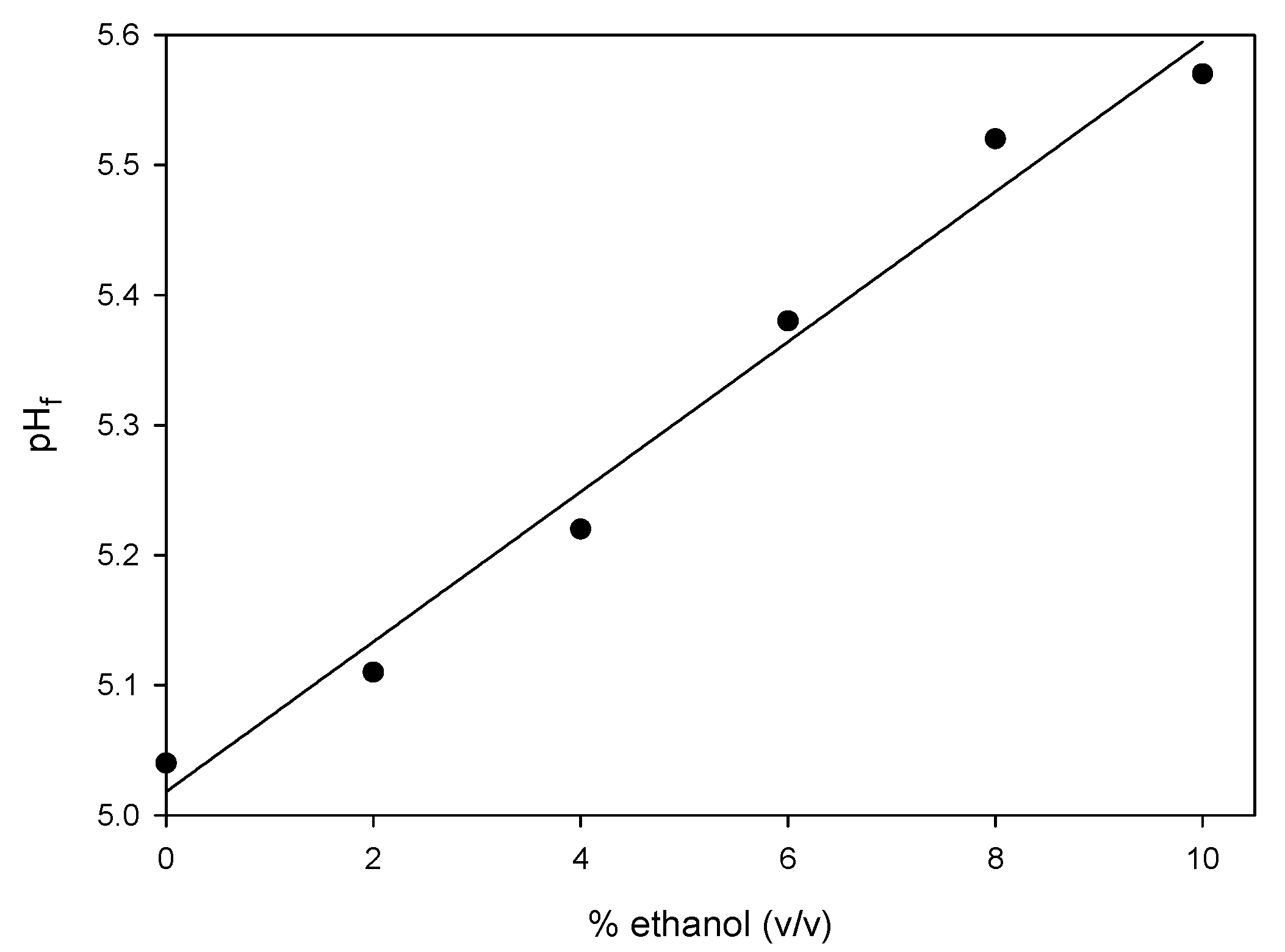
3.2. Influence of Ethanol on the Acid-Induced Coagulation of Milk
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Kruif, C.; Zhulina, E.B. κ-casein as a polyelectrolyte brush on the surface of casein micelles. Colloids Surf. A Physicochem. Eng. Asp. 1996, 117, 151–159. [Google Scholar] [CrossRef]
- Tuinier, R.; de Kruif, C.G. Stability of casein micelles in milk. J. Chem. Phys. 2002, 117, 1290–1295. [Google Scholar] [CrossRef]
- Dalgleish, D.G.; Corredig, M. The Structure of the Casein Micelle of Milk and Its Changes during Processing. Annu. Rev. Food Sci. Technol. 2012, 3, 449–467. [Google Scholar] [CrossRef] [PubMed]
- Huppertz, T.; Gazi, I.; Luyten, H.; Nieuwenhuijse, H.; Alting, A.; Schokker, E. Hydration of casein micelles and caseinates: Implications for casein micelle structure. Int. Dairy J. 2017, 74, 1–11. [Google Scholar] [CrossRef]
- de Kruif, C. Casein micelle interactions. Int. Dairy J. 1999, 9, 183–188. [Google Scholar] [CrossRef]
- De Kruif, C.G.; Roefs, S.P.F.M. Skim milk acidification at low temperatures: A model for the stability of casein micelles. Neth. Milk Dairy J. 1996, 50, 113–120. [Google Scholar]
- Huppertz, T.; Gazi, I. Caseins and casein micelles. In Understanding and Improving the Functional and Nutritional Properties of Milk; Burleigh Dodds Science Publishing Limited: Cambridge, UK, 2022; pp. 155–185. [Google Scholar]
- Horne, D.S. Ethanol stability. In Advanced Dairy Chemistry, 3rd ed.; Fox, P.F., McSweeney, P.L.H., Eds.; Kluwer Academic: New York, NY, USA; Plenum Publishers: New York, NY, USA, 2003; Volume 1, pp. 975–999. [Google Scholar]
- De Kruif, C.G. Skim milk acidification. J. Colloid Interface Sci. 1997, 185, 19–25. [Google Scholar] [CrossRef] [PubMed]
- E O’Connell, J.; Saracino, P.; Huppertz, T.; Uniake, T.; de Kruif, C.G.; Kelly, A.L.; Fox, P.F. Influence of ethanol on the rennet-induced coagulation of milk. J. Dairy Res. 2006, 73, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Vasbinder, A.J.; van Mill, P.J.J.M.; Bot, A.; de Kruif, C.G. Acid-induced gelation of heat-treated milk studied by diffusing wave spectroscopy. Colloids Surf. B 2001, 21, 245–250. [Google Scholar] [CrossRef]
- Jukes, T.; Schmidt, C.L. The Apparent Dissociation Constants of Certain Amino Acids and Related Substances in Water-Ethanol Mixtures. J. Biol. Chem. 1934, 105, 359–371. [Google Scholar] [CrossRef]
- Butler, R.A.; Vega, C.A.; Bates, R.G. Solvent effects on the dissociation of weak acids of three charge types in water/N-methylacetamide media. J. Solut. Chem. 1980, 9, 293–301. [Google Scholar] [CrossRef]
- Lucey, J.A.; Gorry, C.; Fox, P.F. Acid-base buffering properties of heated milk. Milchwissenschaft 1993, 48, 438–441. [Google Scholar]
- Tung, M.S.; Lin, C.; Chow, T.H.; Sung, P. The Effect of Ethanol on the Solubility of Hydroxyapatite in the System Ca(OH)2-H3PO4-H2O at 25 °C and 33 °C. In Hydroxyapatite and Related Materials; Brown, P.W., Con-stantz, B., Eds.; CRC Press: Baco Raton, FL, USA, 1994; pp. 145–151. [Google Scholar] [CrossRef]
- Dalgleish, D.; Alexander, M.; Corredig, M. Studies of the acid gelation of milk using ultrasonic spectroscopy and diffusing wave spectroscopy. Food Hydrocoll. 2004, 18, 747–755. [Google Scholar] [CrossRef]
- Tung, M.S.; O’Farrell, T.J. The effect of ethanol on the solubility of dicalcium phosphate dehydrate in the system Ca(OH)2-H3PO4-H2O at 37 °C. J. Mol. Liq. 1993, 56, 237–243. [Google Scholar] [CrossRef]
- Horne, D.S. Steric effects in the coagulation of milk by ethanol. Biopolymers 1984, 23, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Horne, D.S. Steric stabilization and casein micelle stability. J. Colloid Interface Sci. 1986, 111, 250–260. [Google Scholar] [CrossRef]
- Horne, D.S.; Davidson, C.M. The effect of environmental conditions on the steric stabil-zation of casein micelles. Colloid Polym. Sci. 1986, 264, 727–734. [Google Scholar] [CrossRef]
- Huppertz, T.; De Kruif, C.G. Ethanol stability of cross-linked casein micelles. Int. Dairy J. 2007, 17, 436–441. [Google Scholar] [CrossRef]
- O’Connell, J.E.; Kelly, A.L.; Auty, M.A.E.; Fox, P.F.; De Kruif, K.G. Etha-nol-dependent heat-induced dissociation of casein micelles. J. Agric. Food Chem. 2001, 49, 4420–4423. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, J.E.; Kelly, A.L.; Fox, P.F.; De Kruif, K.G. Mechanism for etha-nol-dependent heat-induced dissociation of casein micelles. J. Agric. Food Chem. 2001, 49, 4424–4428. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huppertz, T. Influence of Ethanol on the Acid-Induced Flocculation of Casein Micelles. Dairy 2022, 3, 693-699. https://doi.org/10.3390/dairy3030047
Huppertz T. Influence of Ethanol on the Acid-Induced Flocculation of Casein Micelles. Dairy. 2022; 3(3):693-699. https://doi.org/10.3390/dairy3030047
Chicago/Turabian StyleHuppertz, Thom. 2022. "Influence of Ethanol on the Acid-Induced Flocculation of Casein Micelles" Dairy 3, no. 3: 693-699. https://doi.org/10.3390/dairy3030047




