Molecularly Imprinted Electrochemical Sensor Based on Poly (O-Phenylenediamine) for Sensitive Detection of Oxycodone in Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Procedure Preparation of MIP Receptor
3. Results
3.1. Electrochemical Characterization of the Imprinted Sensor Electrodes
3.2. Electrochemical Impedance Spectroscopy (EIS)
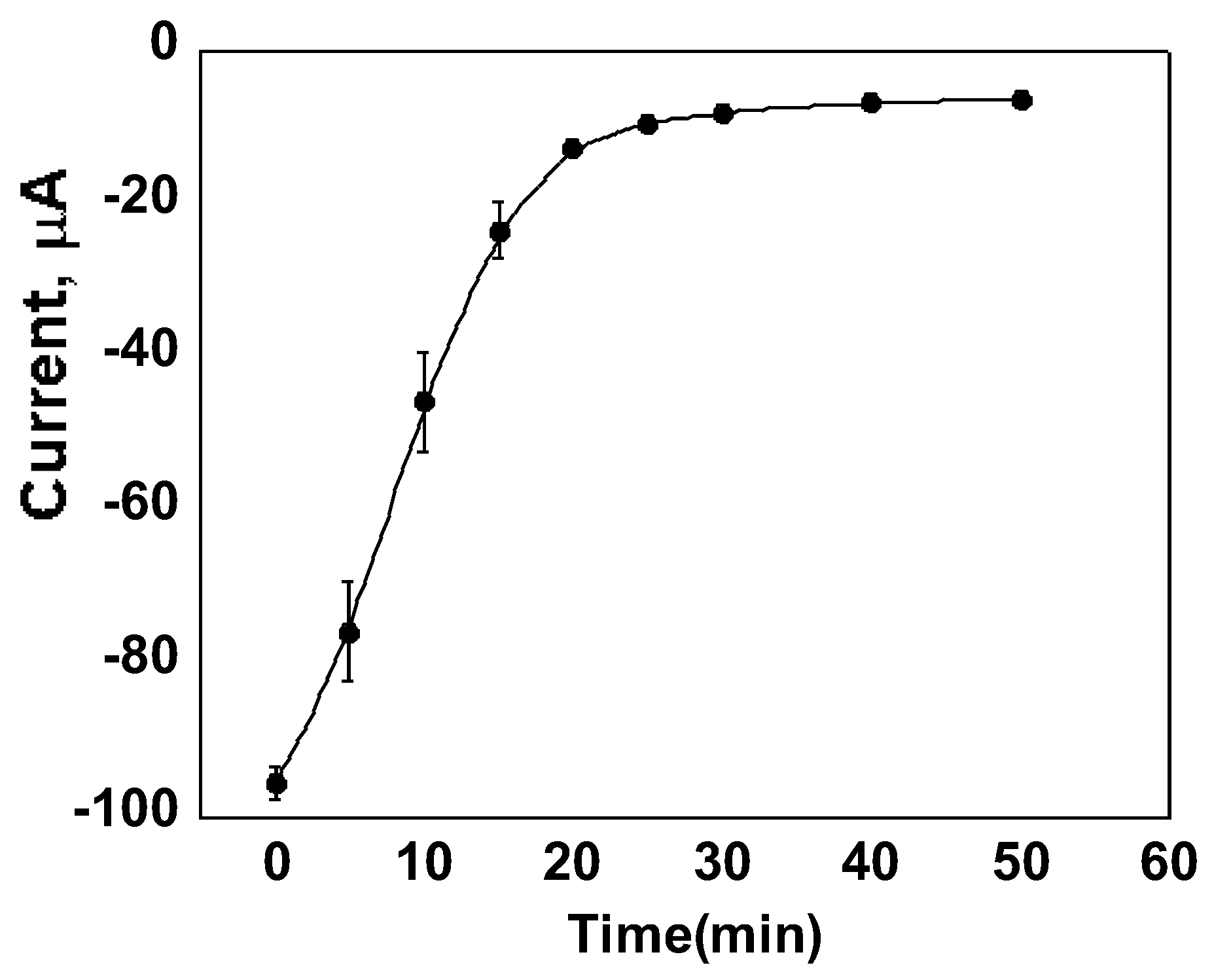
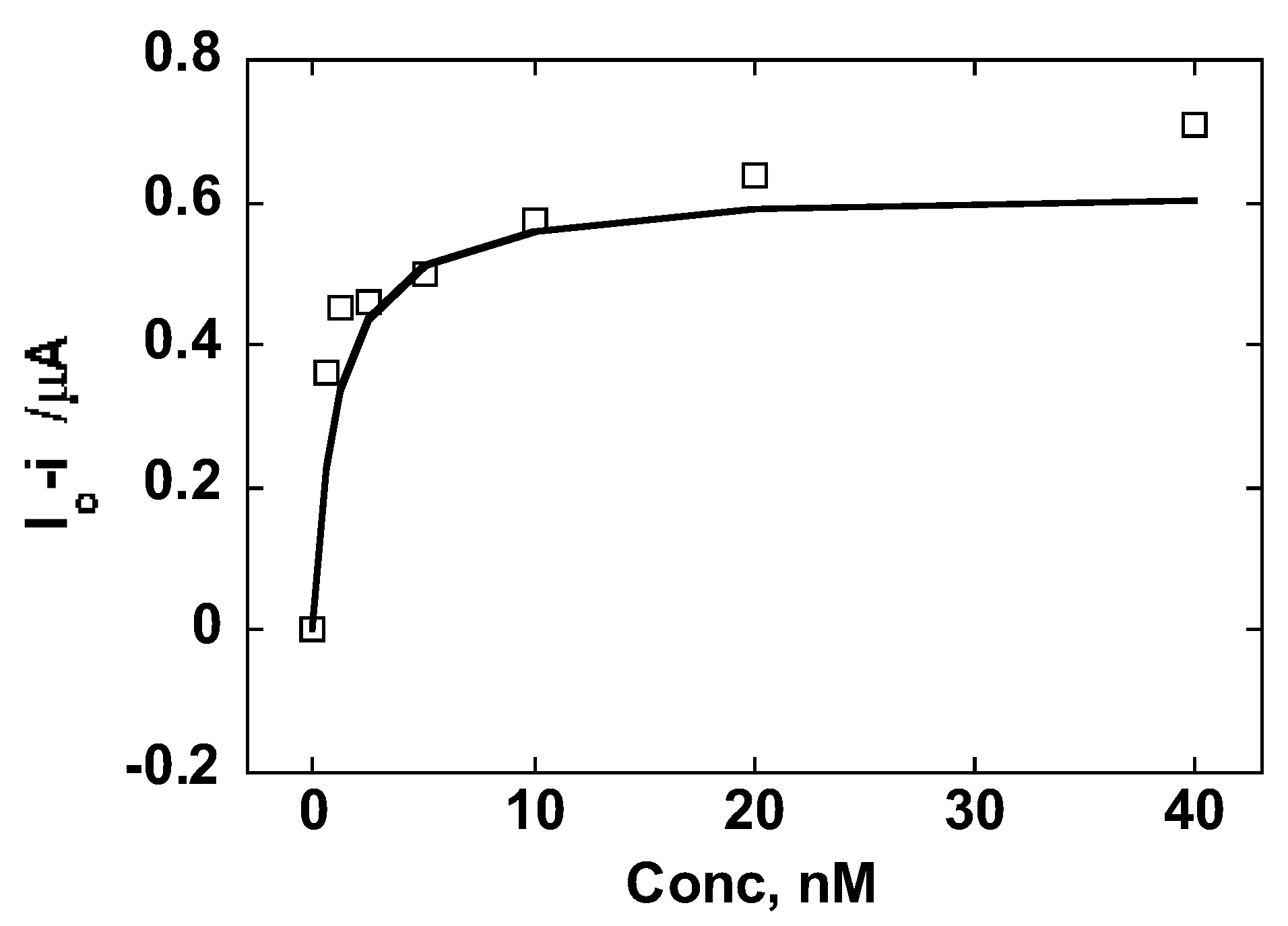
3.3. Calibration Curves and Binding Affinity of Oxycodone MIP
3.4. Interference Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rudd, R.A.; Aleshire, N.; Zibbell, J.E.; Gladden, R.M. Increases in Drug and Opioid Overdose Deaths—United States, 2000–2014. Am. J. Transplant. 2016, 16, 1323–1327. [Google Scholar] [CrossRef]
- Cajanus, K.; Neuvonen, M.; Koskela, O.; Kaunisto, M.A.; Neuvonen, P.J.; Niemi, M.; Kalso, E. Analgesic Plasma Concentrations of Oxycodone After Surgery for Breast Cancer—Which Factors Matter? Clin. Pharmacol. Ther. 2018, 103, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Protti, M.; Catapano, M.C.; Dekel, B.G.S.; Rudge, J.; Gerra, G.; Somaini, L.; Mandrioli, R.; Mercolini, L. Determination of oxycodone and its major metabolites in haematic and urinary matrices: Comparison of traditional and miniaturised sampling approaches. J. Pharm. Biomed. Anal. 2018, 152, 204–214. [Google Scholar] [CrossRef]
- Cheremina, O.; Bachmakov, I.; Neubert, A.; Brune, K.; Fromm, M.F.; Hinz, B. Simultaneous determination of oxycodone and its major metabolite, noroxycodone, in human plasma by high-performance liquid chromatography. Biomed. Chromatogr. 2005, 19, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Blackburn, J.; Brosseau, C.L. Quantitative Detection of Uric Acid by Electrochemical-Surface Enhanced Raman Spectroscopy Using a Multilayered Au/Ag Substrate. Anal. Chem. 2015, 87, 441–447. [Google Scholar] [CrossRef]
- Chen, W.; Yang, L.; Yan, C.; Yao, B.; Lu, J.; Xu, J.; Liu, G. Surface-Confined Building of Au@Pt-Centered and Multi-G-Quadruplex/Hemin Wire-Surrounded Electroactive Super-nanostructures for Ultrasensitive Monitoring of Morphine. ACS Sens. 2020, 5, 2644–2651. [Google Scholar] [CrossRef]
- Prakash, V.; Garima; Prabhakar, N.; Kaur, G.; Diwan, A.; Mehta, S.; Sharma, S. Structural insight on thiourea doped graphene: An efficient electrochemical sensor for voltammetric detection of morphine in alcoholic and non-alcoholic beverages. Curr. Res. Green Sustain. Chem. 2022, 5, 100267. [Google Scholar] [CrossRef]
- Wester, N.; Mynttinen, E.; Etula, J.; Lilius, T.; Kalso, E.; Kauppinen, E.; Laurila, T.; Koskinen, J. Simultaneous Detection of Morphine and Codeine in the Presence of Ascorbic Acid and Uric Acid and in Human Plasma at Nafion Single-Walled Carbon Nanotube Thin-Film Electrode. ACS Omega 2019, 4, 17726–17734. [Google Scholar] [CrossRef]
- Yola, M.L.; Atar, N. Electrochemical Detection of Atrazine by Platinum Nanoparticles/Carbon Nitride Nanotubes with Molecularly Imprinted Polymer. Ind. Eng. Chem. Res. 2017, 56, 7631–7639. [Google Scholar] [CrossRef]
- Ahmed, S.; Ansari, A.; Haidyrah, A.S.; Chaudhary, A.A.; Imran, M.; Khan, A. Hierarchical Molecularly Imprinted Inverse Opal-Based Platforms for Highly Selective and Sensitive Determination of Histamine. ACS Appl. Polym. Mater. 2022, 4, 2783–2793. [Google Scholar] [CrossRef]
- Malik, M.I.; Shaikh, H.; Mustafa, G.; Bhanger, M.I. Recent Applications of Molecularly Imprinted Polymers in Analytical Chemistry. Sep. Purif. Rev. 2019, 48, 179–219. [Google Scholar] [CrossRef]
- Weng, C.-H.; Yeh, W.-M.; Ho, K.-C.; Lee, G.-B. A microfluidic system utilizing molecularly imprinted polymer films for amperometric detection of morphine. Sens. Actuators B Chem. 2007, 121, 576–582. [Google Scholar] [CrossRef]
- Rahmani, M.E.; Ansari, M.; Kazemipour, M.; Nateghi, M. Selective extraction of morphine from biological fluids by magnetic molecularly imprinted polymers and determination using UHPLC with diode array detection. J. Sep. Sci. 2017, 41, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Lanza, F.; Sellergren, B. The application of molecular imprinting technology to solid phase extraction. Chromatographia 2001, 53, 599–611. [Google Scholar] [CrossRef]
- Pardieu, E.; Cheap, H.; Vedrine, C.; Lazerges, M.; Lattach, Y.; Garnier, F.; Remita, S.; Pernelle, C. Molecularly imprinted conducting polymer based electrochemical sensor for detection of atrazine. Anal. Chim. Acta 2009, 649, 236–245. [Google Scholar] [CrossRef]
- Malitesta, C.; Palmisano, F.; Torsi, L.; Zambonin, P.G. Glucose fast-response amperometric sensor based on glucose oxidase immobilized in an electropolymerized poly(o-phenylenediamine) film. Anal. Chem. 1990, 62, 2735–2740. [Google Scholar] [CrossRef]
- Li, J.; Jiang, F.; Wei, X. Molecularly Imprinted Sensor Based on an Enzyme Amplifier for Ultratrace Oxytetracycline Determination. Anal. Chem. 2010, 82, 6074–6078. [Google Scholar] [CrossRef]
- Feng, L.; Liu, Y.; Tan, Y.; Hu, J. Biosensor for the determination of sorbitol based on molecularly imprinted electrosynthesized polymers. Biosens. Bioelectron. 2004, 19, 1513–1519. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Wei, X.; Tao, H.; Pan, H. Molecularly Imprinted Electrochemical Luminescence Sensor Based on Signal Amplification for Selective Determination of Trace Gibberellin A3. Anal. Chem. 2012, 84, 9951–9955. [Google Scholar] [CrossRef]
- Erdem, A.; Muti, M.; Karadeniz, H.; Congur, G.; Canavar, E. Electrochemical monitoring of indicator-free DNA hybridization by carbon nanotubes–chitosan modified disposable graphite sensors. Colloid. Surfaces B Biointerfaces 2012, 95, 222–228. [Google Scholar] [CrossRef]
- Pires, C.K.; Reis, B.F.; Galhardo, C.X.; Martelli, P.B. A Multicommuted Flow Procedure for the Determination of Cholesterol in Animal Blood Serum by Chemiluminescence. Anal. Lett. 2003, 36, 3011–3024. [Google Scholar] [CrossRef]
- Randviir, E.P.; Banks, C.E. Electrochemical impedance spectroscopy: An overview of bioanalytical applications. Anal. Methods 2013, 5, 1098–1115. [Google Scholar] [CrossRef]
- Metters, J.P.; Kadara, R.O.; Banks, C.E. New directions in screen printed electroanalytical sensors: An overview of recent developments. Analyst 2011, 136, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Afkhami, A.; Gomar, F.; Madrakian, T. CoFe2O4 nanoparticles modified carbon paste electrode for simultaneous detection of oxycodone and codeine in human plasma and urine. Sens. Actuators B Chem. 2016, 233, 263–271. [Google Scholar] [CrossRef]
- Mynttinen, E.; Wester, N.; Lilius, T.; Kalso, E.; Mikladal, B.; Varjos, I.; Sainio, S.; Jiang, H.; Kauppinen, E.I.; Koskinen, J.; et al. Electrochemical Detection of Oxycodone and Its Main Metabolites with Nafion-Coated Single-Walled Carbon Nanotube Electrodes. Anal. Chem. 2020, 92, 8218–8227. [Google Scholar] [CrossRef]
- Ansari, S.; Masoum, S. A hybrid imprinted polymer based on magnetic graphene oxide and carbon dots for ultrasonic assisted dispersive solid-phase microextraction of oxycodone. Microchem. J. 2021, 164, 105988. [Google Scholar] [CrossRef]
- Minhas, R.S.; Rudd, D.A.; Al Hmoud, H.Z.; Guinan, T.M.; Kirkbride, K.P.; Voelcker, N.H. Rapid Detection of Anabolic and Narcotic Doping Agents in Saliva and Urine By Means of Nanostructured Silicon SALDI Mass Spectrometry. ACS Appl. Mater. Interfaces 2020, 12, 31195–31204. [Google Scholar] [CrossRef]
- Le, N.L.; Reiter, A.; Tomlinson, K.; Jones, J.; Moore, C. The Detection of Oxycodone in Meconium Specimens. J. Anal. Toxicol. 2005, 29, 54–57. [Google Scholar] [CrossRef]
- Zhang, M.; Jin, C.; Nie, Y.; Ren, Y.; Hao, N.; Xu, Z.; Dong, L.; Zhang, J.X.J. Silver nanoparticle on zinc oxide array for label-free detection of opioids through surface-enhanced raman spectroscopy. RSC Adv. 2021, 11, 11329–11337. [Google Scholar] [CrossRef]
- Kazemi, R.; Potts, E.I.; Dick, J.E. Quantifying Interferent Effects on Molecularly Imprinted Polymer Sensors for Per- and Polyfluoroalkyl Substances (PFAS). Anal. Chem. 2020, 92, 10597–10605. [Google Scholar] [CrossRef]






| Electrode-Based | |||
|---|---|---|---|
| Modified Electrodes | Linear Range | Detection Limit | Reference |
| CoFe2O4/CPE | 0.06–38 uM | 0.05 uM | [24] |
| Nafion/SWCNT | 0.5–10 μM | 85 nM | [25] |
| o-Phenylenediamine polymer imprint | 0.4–5 nM | 1.8 nM | This work |
| Other methods | |||
| HPLC-UV | 1–2000 ng/mL | 2.67 ng/mL | [26] |
| nano-Si-based SALDI-MS. | 0–100 ng/mL | 1.56 ng/mL | [27] |
| ELISA combine with GC-MS | 0 to 1000 ng/g | 50 ng/g | [28] |
| Silver NPs on zinc oxide, SERS | 900 ug/mL to 90 ng/mL | 90 ng/ mL | [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charkravarthula, P.; Mugweru, A. Molecularly Imprinted Electrochemical Sensor Based on Poly (O-Phenylenediamine) for Sensitive Detection of Oxycodone in Water. Electrochem 2023, 4, 435-446. https://doi.org/10.3390/electrochem4040028
Charkravarthula P, Mugweru A. Molecularly Imprinted Electrochemical Sensor Based on Poly (O-Phenylenediamine) for Sensitive Detection of Oxycodone in Water. Electrochem. 2023; 4(4):435-446. https://doi.org/10.3390/electrochem4040028
Chicago/Turabian StyleCharkravarthula, Pranaya, and Amos Mugweru. 2023. "Molecularly Imprinted Electrochemical Sensor Based on Poly (O-Phenylenediamine) for Sensitive Detection of Oxycodone in Water" Electrochem 4, no. 4: 435-446. https://doi.org/10.3390/electrochem4040028






