New Analytical Expressions of Concentrations in Packed Bed Immobilized-Cell Electrochemical Photobioreactor
Abstract
:1. Introduction
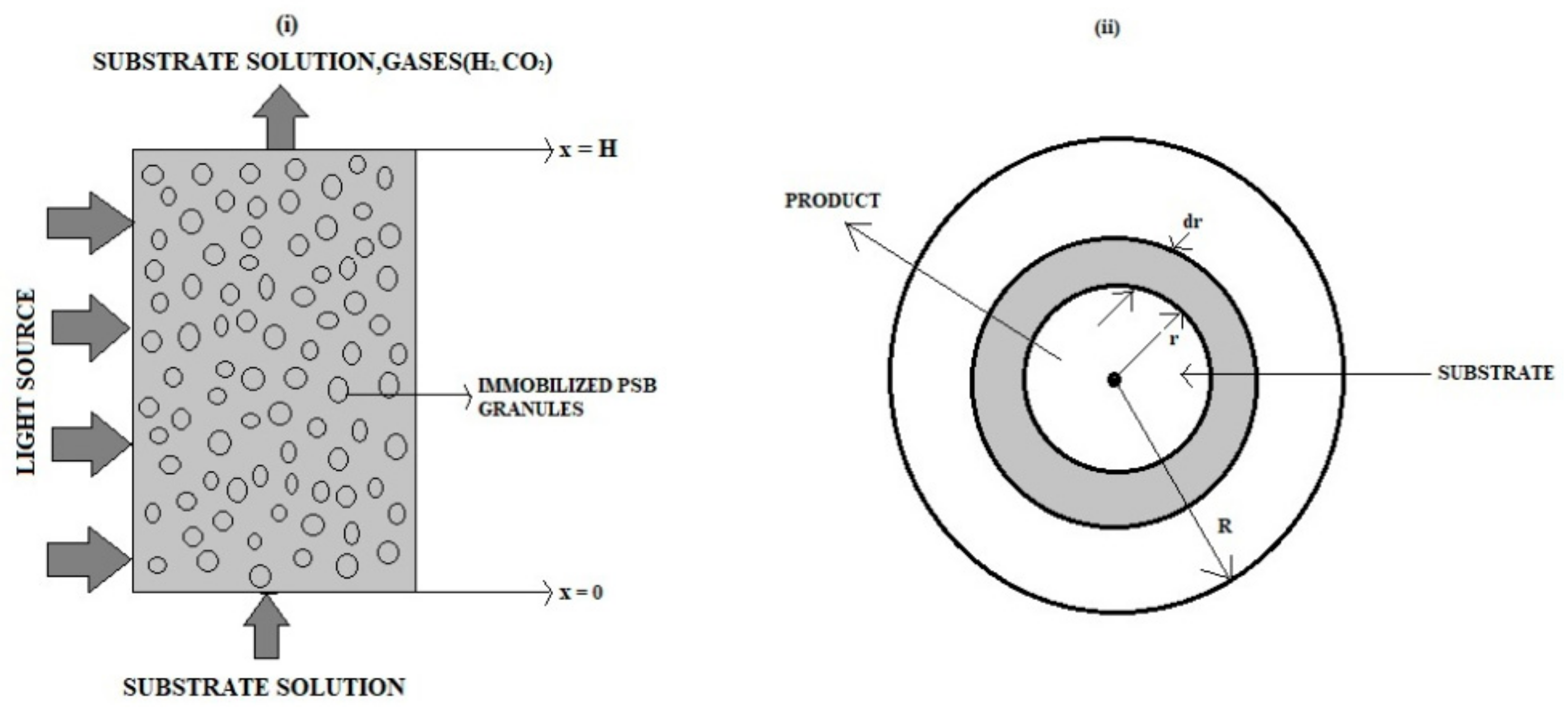
2. Formulation of the Problems
3. Analytical Expression of the Concentrations Using Akbari-Ganji’s Method
3.1. Concentration of Glucose (Substrate)
3.2. Concentration of Hydrogen (Product)
3.3. Normalized Steady-State Source Terms of Liquid and Gas Phases
4. Analytical Expression of the Concentrations Using Taylor Series Method
4.1. Concentration of Glucose (Substrate)
4.2. Concentration of Hydrogen (Product)
4.3. Normalized Steady-State Source Terms of Liquid and Gas Phases
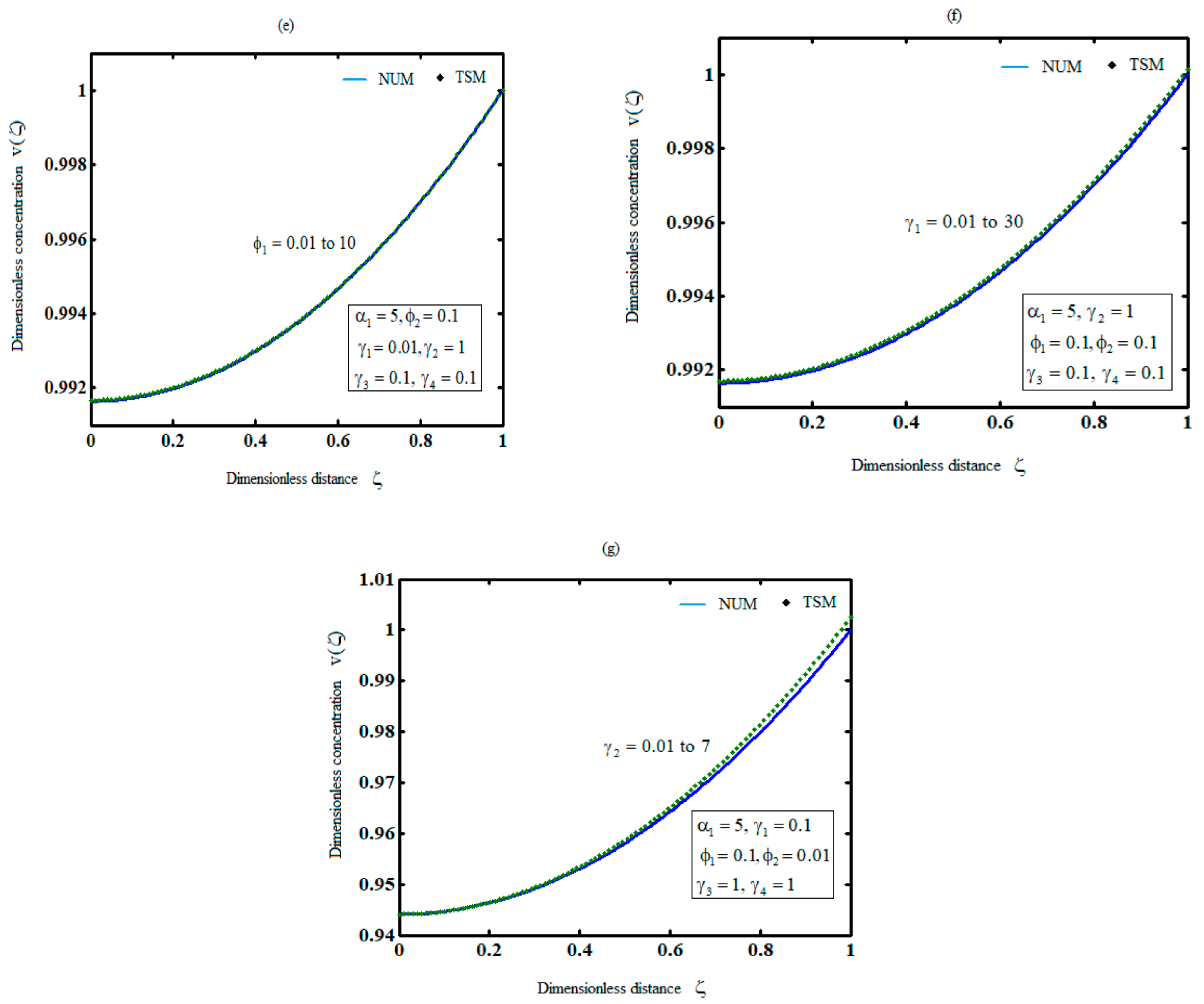
5. Comparison of Analytical Results with Previous Analytical Results and Numerical Simulation
6. Discussion
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Symbols
| Symbols | Description | Units |
| Local substrate concentration | kg/m3 | |
| Radius of the gel granule | m | |
| Diffusion coefficient | m2/s | |
| Absolute permeability | m2 | |
| Molecular weight | kg mol−1 | |
| Photobioreactor height | m | |
| Maintenance coefficient | h−1 | |
| Source item in mass conservation equation | ||
| Source item in species conservation equation | ||
| Specific area | m2/kg | |
| Growth associated kinetic constant for hydrogen production | None | |
| Non- growthassociated kinetic constant | h−1 | |
| Specific growth rate | h−1 | |
| Maximum specific growth rate | h−1 | |
| Dimensionless substrate concentration | None | |
| Dimensionless product concentration | None | |
| Dimensionless parameter | None | |
| Dimensionless parameter | None | |
| Dimensionless parameter | None | |
| Dimensionless parameter | None | |
| Dimensionless parameter | None | |
| Dimensionless distance | None | |
| Superscripts | ||
| S | Substrate | |
| H2 | Hydrogen | |
| CO2 | Carbon dioxide | |
| Subscripts | ||
| G | Gas phase | |
| l | Liquid phase | |
| Abbreviation | ||
| AGM | Akbari-Ganji’s method | |
| ADM | Adomian decomposition method | |
| TSM | Taylor series method | |
| HPM | homotopy perturbation method | |
| PM | perturbation method | |
Appendix A. MATLAB Code for Numerical Solution of the Nonlinear Equations (9) and (10)
- function pdex4
- m = 2;
- x = linspace(0,1);
- t = linspace(0,10);
- sol = pdepe(m,@pdex4pde,@pdex4ic,@pdex4bc,x,t);
- u1 = sol(:,:,1);
- u2 = sol(:,:,2);
- %------------------------------------------------------------------
- figure
- plot(x,u1(end,:))
- title(‘u1(x,t)’)
- xlabel(‘Distance x’)
- ylabel(‘u1(x,1)’)
- %------------------------------------------------------------------
- figure
- plot(x,u2(end,:))
- title(‘u2(x,t)’)
- xlabel(‘Distance x’)
- ylabel(‘u2(x,2)’)
- % -----------------------------------------------------------------
- function [c,f,s] = pdex4pde(x,t,u,DuDx)
- c = [1; 1];
- f = [1; 1].* DuDx;
- a1 = 10; p1 = 30; p2 = 0.1; r1 = 0.1; r2 = 0.1; r3 = 0.1; r4 = 0.1; %
- F1 = −(((p1+r1)*u(1) + r2)/(1 + a1*u(1)));
- F2 = −(((p2+r3)*u(1) + r4)/(1 + a1*u(1)));
- S = [F1; F2];
- % -----------------------------------------------------------------
- function u0 = pdex4ic(x)
- u0 = [0; 0];
- % -----------------------------------------------------------------
- function [pl,ql,pr,qr] = pdex4bc(xl,ul,xr,ur,t)
- pl = [ul(1)-0; ul(2)-0];
- ql = [1; 1];
- pr = [ur(1)- 1; ur(2)-1];
- qr = [0; 0];
References
- Reiss, L.P.; Hanratty, T.J. Measurement of instantaneous rates of mass transfer to a small sink on a hall. AIChE J. 1962, 8, 245–247. [Google Scholar] [CrossRef]
- Relas, L.P.; Hanratty, T.J. An Experimental study of the unsteady nature of the viscous sublayer. AIChE J. 1963, 9, 154–160. [Google Scholar] [CrossRef]
- Shaw, P.V.; Hanratty, T.J. Fluctuations in the local rate of turbulent mass transfer to a pipe hall. AIChE J. 1964, 10, 475–482. [Google Scholar] [CrossRef]
- Shaw, P.V.; Reiss, L.P.; Hanratty, T.J. Rates of turbulent transfer to a pipe hall in the mass transfer entry region. AIChE J. 1963, 9, 362–364. [Google Scholar] [CrossRef]
- Jolls, K.R.; Hanratty, T.J. Use of electrochemical techniques to study mass transfer rates and local skin friction to a sphere in a dumped bed. AIChE J. 1969, 15, 199. [Google Scholar] [CrossRef]
- Karabelas, A.J.; Wegner, T.H.; Hanratty, T.J. Use of asymptotic relations to correlate mass transfer data in packed beds. Chem. Eng. Sci. 1971, 26, 1581–1589. [Google Scholar] [CrossRef]
- Tepe, O.; Dursun, A.Y. Combined effects of external mass transfer and biodegradation rates on removal of phenol by immobilized Ralstonia eutropha in a packed bed reactor. J. Hazard. Mater. 2008, 151, 9–16. [Google Scholar] [CrossRef]
- Nath, S.; Chand, S. Mass transfer and biochemical reaction in immobilized cell packed bed reactors: Correlation of experiment with theory. J. Chem. Technol. Biotechnol. 1996, 66, 286–292. [Google Scholar] [CrossRef]
- Banerjee, I.; Modak, J.M.; Bandopadhyay, K.; Das, D.; Maiti, B.R. Mathematical model for evaluation of mass transfer limitations in phenol biodegradation by immobilized Pseudomonas putida. J. Biotechnol. 2001, 87, 211–223. [Google Scholar] [CrossRef]
- Wang, J.L.; Wan, W. Kinetic models for fermentative hydrogen production: A review. Int. J. Hydrog. Energy 2009, 34, 3313–3323. [Google Scholar] [CrossRef]
- Palazzi, E.; Perego, P.; Fabiano, B. Mathematical modelling and optimization of hydrogen continuous production in a fixed bed bioreactor. Chem. Eng. Sci. 2002, 57, 3819–3830. [Google Scholar] [CrossRef]
- Liao, Q.; Liu, D.M.; Ye, D.D.; Zhu, X.; Lee, D.J. Mathematical modeling of two-phase flow and transport in an immobilized-cell photobioreactor. Int. J. Hydrog. Energy 2011, 36, 13936–13948. [Google Scholar] [CrossRef]
- Shirejini, S.Z.; Fattahi, M. Mathematical modeling and analytical solution of two-phase flow transport in an immobilized-cell photobioreactor using the homotopy perturbation method (HPM). Int. J. Hydrog. Energy 2016, 41, 18405–18417. [Google Scholar] [CrossRef]
- Ganji, D.; Hosseini, M.; Shayegh, J. Some nonlinear heat transfer equations solved by three approximate methods. Int. Commun. Heat Mass Transf. 2007, 34, 1003–1016. [Google Scholar] [CrossRef]
- He, J.H. Homotopy perturbation technique. Comput. Methods Appl. Mech. Eng. 1999, 178, 257–262. [Google Scholar] [CrossRef]
- He, J.H. Variational iteration method for autonomous ordinary differential systems. Appl. Math. Comput. 2000, 114, 115–123. [Google Scholar] [CrossRef]
- Ghafoori, S.; Motevalli, M.; Nejad, M.G.; Shakeri, F.; Ganji, D.D.; Jalaal, M. Efficiency of differential transformation method for nonlinear oscillation: Comparison with HPM and VIM. Curr. Appl. Phys. 2011, 11, 965–971. [Google Scholar] [CrossRef]
- Liao, S.J. The Proposed Homotopy Analysis Technique for the Solution of Nonlinear Problems. Ph.D. Thesis, Shanghai Jiao Tong University, Shanghai, China, 1992. [Google Scholar]
- Liao, S.J. Beyond Perturbation: Introduction to Homotopy Analysis Method; Chapman & Hall/CRC: Boca Raton, FL, USA, 2003. [Google Scholar]
- Arikoglu, A.; Ozkol, I. Solution of difference equations by using differential transform method. Appl. Math. Comput. 2006, 174, 1216–1228. [Google Scholar] [CrossRef]
- Soltanalizadeh, B. Differential transformation method for solving one-space-dimensional telegraph equation. Comput. Appl. Math. 2011, 30, 639–653. [Google Scholar] [CrossRef]
- Wazwaz, A.M. A reliable modification of Adomian decomposition method. Appl. Math. Comput. 1999, 102, 77–86. [Google Scholar] [CrossRef]
- AlRasheed, N.N. Adaptation of Taylor’s Formula for Solving System of Differential Equations. Nonlinear Differ. Equ. App. 2016, 4, 95–107. [Google Scholar] [CrossRef]
- El-Ajou, A.; Abu Arqub, O.; Al-Smadi, M. A general form of the generalized Taylor’s formula with some applications. Appl. Math. Comput. 2015, 256, 851–859. [Google Scholar] [CrossRef]
- He, C.H.; Shen, Y.; Ji, F.Y.; He, J.H. Taylor series solution for Fractal Bratu-Type equation arising in electrospinning process. Fractals 2020, 28, 2050011. [Google Scholar] [CrossRef]
- Akbari, M.R.; Ganji, D.D.; Nimafar, M.; Ahmadi, A.R. Significant progress in solution of nonlinear equations at displacement of structure and heat transfer extended surface by new AGM approach. Front. Mech. Eng. 2014, 9, 390–401. [Google Scholar] [CrossRef]
- Akbari, M. Nonlinear Dynamic in Engineering by Akbari-Ganji’s Method; Xlibris Corporation: Bloomington, IN, USA, 2015. [Google Scholar]
- Ganji, D.D.; Talarposhti, R.A. Numerical and Analytical Solutions for Solving Nonlinear Equations in Heat Transfer; Technology and Engineering, IGI Global: Hershey, PA, USA, 2017. [Google Scholar]
- Sheikholeslami, M.; Ganji, D.D. Applications of Semi-Analytical Methods for Nanofluid Flow and Heat Transfer; Elsevier: Oxford, UK, 2018. [Google Scholar]
- Praveen, T.; Rajendran, L. Theoretical analysis through mathematical modeling of two-phase flow transport in an immobilized-cell photobioreactor. Chem. Phys. Lett. 2015, 625, 193–201. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeyabarathi, P.; Abukhaled, M.; Kannan, M.; Rajendran, L.; Lyons, M.E.G. New Analytical Expressions of Concentrations in Packed Bed Immobilized-Cell Electrochemical Photobioreactor. Electrochem 2023, 4, 447-459. https://doi.org/10.3390/electrochem4040029
Jeyabarathi P, Abukhaled M, Kannan M, Rajendran L, Lyons MEG. New Analytical Expressions of Concentrations in Packed Bed Immobilized-Cell Electrochemical Photobioreactor. Electrochem. 2023; 4(4):447-459. https://doi.org/10.3390/electrochem4040029
Chicago/Turabian StyleJeyabarathi, Ponraj, Marwan Abukhaled, Murugesan Kannan, Lakshmanan Rajendran, and Michael E. G. Lyons. 2023. "New Analytical Expressions of Concentrations in Packed Bed Immobilized-Cell Electrochemical Photobioreactor" Electrochem 4, no. 4: 447-459. https://doi.org/10.3390/electrochem4040029







